Abstract
Purpose: Beneficial mechanisms of bone marrow cell (BMC) therapy for acute ST-segment elevation myocardial infarct (STEMI) are largely unknown in humans. Therefore, we evaluated the feasibility of serial positron emission tomography (PET) and MRI studies to provide insight into the effects of BMCs on the healing process of ischemic myocardial damage. Methods: Nineteen patients with successful primary reteplase thrombolysis (mean 2.4 h after symptoms) for STEMI were randomized for BMC therapy (2.9 × 106 CD34+ cells) or placebo after bone marrow aspiration in a double-blind, multi-center study. Three days post-MI, coronary angioplasty, and paclitaxel eluting stent implantation preceded either BMC or placebo therapy. Cardiac PET and MRI studies were performed 7–12 days after therapies and repeated after 6 months, and images were analyzed at a central core laboratory. Results: In BMC-treated patients, there was a decrease in [11C]-HED defect size (−4.9 ± 4.0 vs. −1.6 ± 2.2%, p = 0.08) and an increase in [18F]-FDG uptake in the infarct area at risk (0.06 ± 0.09 vs. −0.05 ± 0.16, p = 0.07) compared to controls, as well as less left ventricular dilatation (−4.4 ± 13.3 vs. 8.0 ± 16.7 mL/m2, p = 0.12) at 6 months follow-up. However, BMC treatment was inferior to placebo in terms of changes in rest perfusion in the area at risk (−0.09 ± 0.17 vs. 0.10 ± 0.17, p = 0.03) and infarct size (0.4 ± 4.2 vs. −5.1 ± 5.9 g, p = 0.047), and no effect was observed on ejection fraction (p = 0.37). Conclusion: After the acute phase of STEMI, BMC therapy showed only minor trends of long-term benefit in patients with rapid successful thrombolysis. There was a trend of more decrease in innervation defect size and enhanced glucose metabolism in the infarct-related myocardium and also a trend of less ventricular dilatation in the BMC-treated group compared to placebo. However, no consistently better outcome was observed in the BMC-treated group compared to placebo.
Keywords: stem cell therapy, myocardial infarct, PET, MRI
Introduction
Even optimal reperfusion therapy for acute ST-segment elevation myocardial infarction (STEMI) is not always effective in preventing left ventricular (LV) remodeling, heart failure, and compromised clinical outcome (Sutton and Sharpe, 2000), and no approaches are available in clinical practice to replace infarct scar with functional myocardium (Sutton and Sharpe, 2000; Losordo and Dimmeler, 2004; Wollert and Drexler, 2005; Rosenzweig, 2006). However, bone marrow-derived stem cell (BMC) therapy has been demonstrated to improve the recovery of LV function and reduce the infarct size after acute myocardial infarct (MI; Assmus et al., 2002; Strauer et al., 2002; Britten et al., 2003; Fernandez-Aviles et al., 2004; Wollert et al., 2004; Janssens et al., 2006; Schachinger et al., 2006a) but recent placebo-controlled, randomized trials have been partially controversial with regard to functional benefit after MI both in animals and humans (Janssens et al., 2006; Lunde et al., 2006; Meyer et al., 2006; de Silva et al., 2008; Hashemi et al., 2008; Herbots et al., 2009; Tendera et al., 2009). Therefore, it is not surprising that the mechanisms behind the beneficial effects are not fully elucidated (Jackson et al., 2001; Kocher et al., 2001; Orlic et al., 2001; Balsam et al., 2004; Murry et al., 2004; Janssens et al., 2006; Lunde et al., 2006) but paracrine factors seem to have a major role in the process (Yeghiazarians et al., 2009). Conflicting results have been explained by different timing of bone marrow harvesting and therapy, type and number of stem cells, and different methods of cell delivery to the myocardium at risk (Schachinger et al., 2006b).
Myocardial perfusion, metabolism, and innervation that all determine cardiac adaptation and remodeling following MI can be studied quantitatively in vivo using positron emission tomography (PET). Moreover, serial cardiac MRI is the reference standard for evaluating left and right ventricular function, wall motion and infarct size. In this FINCELL-INSIGHT sub-study we applied comprehensive cardiac imaging for evaluation of the long-term effects and the potential mechanisms of BMC therapy on the healing process of ischemic myocardial damage. The focus was targeted to changes in perfusion, metabolism, innervation, and cardiac volumetrics occurring after intracoronary infusion of BMCs early after acute ST-elevation MI.
Materials and Methods
Patients and study design
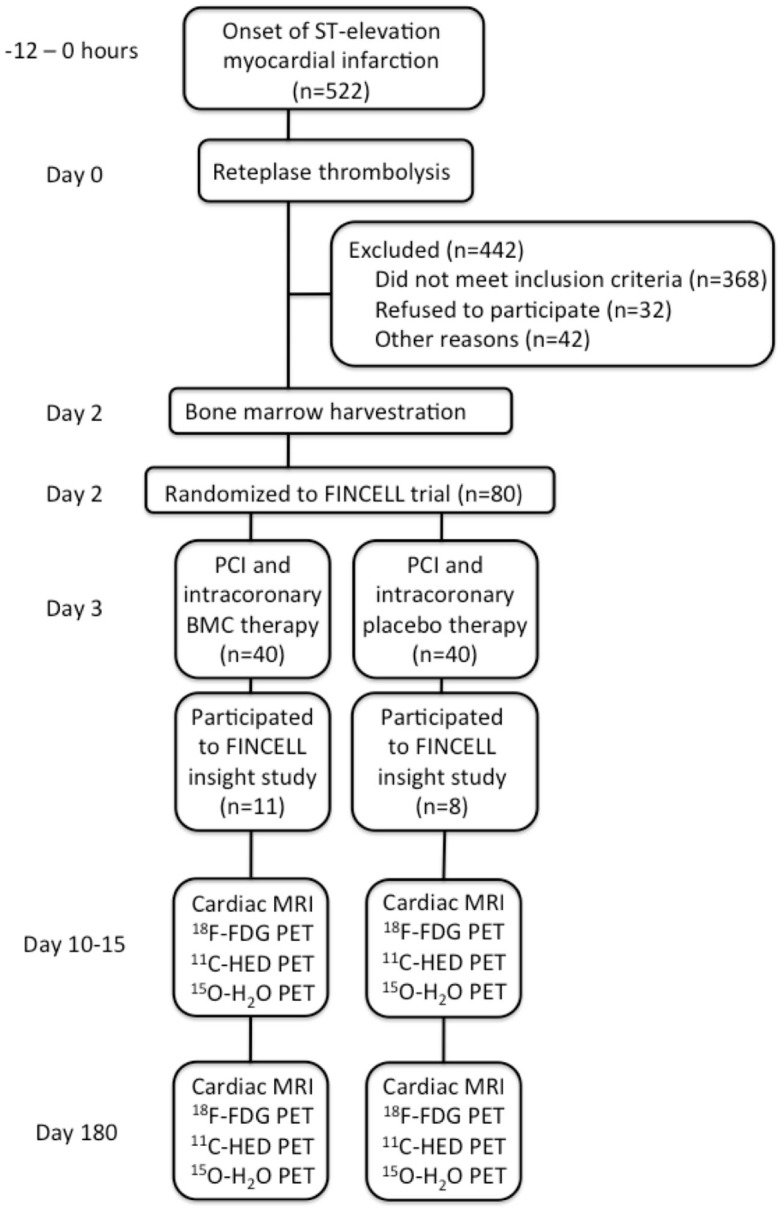
A total of 522 consecutive patients with STEMI treated with primary intravenous thrombolytic therapy admitted to the University Hospitals of Turku and Oulu, Finland, between October 2004 and February 2007 were screened for eligibility. Patients were considered eligible for the trial, if they were <75 years of age, had evidence of STEMI from an electrocardiogram (ECG), elevated troponin levels, thrombolytic therapy given within 12 h after the onset of symptoms, no need for urgent PCI immediately after thrombolysis, no cardiogenic shock, hemodynamic instability, or lack of resolution of ST-segment elevations after thrombolysis, no need for immediate coronary artery bypass graft surgery, and no severe coexisting condition that interfered with the ability of the patient to comply with the protocol. After exclusions, a total of 80 patients were included in the FINCELL trial (Huikuri et al., 2008), of which 19 random patients were included to FINCELL INSIGHT sub-study according to their willingness to participate in additional PET and MRI studies at Turku University Hospital. The 19 patients were randomized to receive either BMC (n = 11) or placebo (n = 8) treatment by a technician who did not participate in any other parts of the research protocol. The study flow chart is presented in Figure 1. The clinical and angiographic characterization of the study population is presented in Table 1. The study was carried out in accordance with the Declaration of Helsinki (2000) of the World Medical Association and was approved by The Ethical Committees of Turku and Northern Ostrobotnia Hospital District. All patients gave their written, informed consent prior to their inclusion in the study.
Figure 1.
Study flow chart.
Table 1.
Characteristics of the patients.
| BMC (n = 11) | Control (n = 8) | p | |
|---|---|---|---|
| Male gender, n (%) | 11 (100%) | 8 (100%) | 1.00 |
| Age, years | 56 ± 12 | 55 ± 9 | 0.89 |
| Body mass index, kg/m2 | 26 (26–28) | 26 (24–27) | 0.49 |
| Hypertension, n (%) | 5/11 (45%) | 2/8 (25%) | 0.63 |
| Hypercholesterolemia, n (%) | 3/11 (27%) | 3/8 (38%) | 1.00 |
| Diabetes mellitus, n (%) | 1/11 (9%) | 0/8 (0%) | 1.00 |
| Current smoking, n (%) | 3/11 (27%) | 3/8 (38%) | 0.61 |
| SEVERITY OF CAD n (%) | |||
| Previous AMI | 2 (18%) | 1 (13%) | 1.00 |
| Previous angina pectoris | 1/11 (9%) | 2/8 (25%) | 0.55 |
| 1-Vessel disease | 5/11 (46%) | 5/8 (63%) | 0.65 |
| 2-Vessel disease | 4/11 (36%) | 3/8 (38%) | 1.00 |
| 3-Vessel disease | 2/11 (18%) | 0/8 (0%) | 0.49 |
| INDEX EVENT AND PERCUTANEOUS CORONARY INTERVENTION | |||
| Symptoms to thrombolysis time, hours | 2.0 (1.2) | 2.9 (2.0) | 0.27 |
| Highest troponin value, μg/L | 2.3 (0.7–3.8) | 1.0 (1.0–2.0) | 0.37 |
| From reperfusion to study therapy, days | 3.2 (1.0) | 3.1 (1.2) | 0.84 |
| Infarct-related artery, n (%) | |||
| LAD | 3/11 (27%) | 4/8 (50%) | 0.38 |
| LCX | 2/11 (18%) | 3/8 (38%) | 0.60 |
| RCA | 6/11 (55%) | 1/8 (13%) | 0.15 |
| Target lesion stenosis, % | 80 (60–94) | 70 (59–99) | 0.34 |
| TIMI FLOW BEFORE PCI | |||
| 0 | 2/11 (18%) | 0/8 (0%) | 0.49 |
| 1 | 2/11 (18%) | 2/8 (25%) | 1.00 |
| 2 | 0/11 (0%) | 2/8 (25%) | 0.16 |
| 3 | 7/11 (64%) | 4/8 (50%) | 0.66 |
| TIMI FLOW AFTER PCI | |||
| 3 | 11/11 (100%) | 8/8 (100%) | 1.00 |
| Drug-eluting stents | 11/11 (100%) | 8/8 (100%) | 1.00 |
| GPIIb/IIIa inhibitor during acute PCI | 2/11 (18%) | 1/8 (13%) | 1.00 |
| LEFT VENTRICULAR PARAMETERS BY ANGIOGRAPHY BEFORE STUDY THERAPY | |||
| EF, % | 66.9 ± 5.2 | 55.1 ± 18.3 | 0.15 |
| EDV, mL | 161 ± 48 | 154 ± 32 | 0.74 |
| ESV, mL | 57.0 (38.5–61.7) | 53.6 (46.6–86.4) | 0.44 |
| STUDY THERAPY | |||
| Amount of injected CD34+ * 106 | 2.9 ± 2.0 | placebo | NA |
| Viability of cells, % | >95% | – | NA |
| CARDIAC MEDICATION AT DISCHARGE, n (%) | |||
| Aspirin | 11/11(100%) | 8/8 (100%) | 1.00 |
| Clopidogrel | 11/11(100%) | 8/8 (100%) | 1.00 |
| β-Blocker | 10/11 (91%) | 8/8 (100%) | 1.00 |
| ACE inhibitor or ATII blocker | 9/11 (82%) | 7/8 (88%) | 1.00 |
| Statin | 11/11(100%) | 8/8 (100%) | 1.00 |
| Diuretics | 1/11 (9%) | 6/8 (75%) | 0.006 |
| Nitrate | 5/11 (46%) | 2/8 (25%) | 0.63 |
| Calcium antagonists | 0/11 (0%) | 0/8 (0%) | 1.00 |
| Digitalis | 1/11 (9%) | 4/8 (50%) | 0.11 |
| CARDIAC MEDICATION AT 6 MONTH FOLLOW-UP, n (%) | |||
| Aspirin | 11/11 (100%) | 8/8 (100%) | 1.00 |
| Clopidogrel | 10/11 (91%) | 8/8 (100%) | 1.00 |
| β-Blocker | 10/11 (91%) | 8/8 (100%) | 1.00 |
| ACE inhibitor or ATII blocker | 9/11(82%) | 7/8 (88%) | 1.00 |
| Statin | 11/11(100%) | 8/8 (100%) | 1.00 |
| Diuretics | 1/11(9%) | 2/8 (25%) | 0.55 |
| Nitrate | 1/11(9%) | 2/8 (25%) | 0.55 |
| Calcium antagonists | 0/11 (0%) | 0/8 (0%) | 1.00 |
| Digitalis | 0/11 (0%) | 0/8 (0%) | 1.00 |
Continuous variables with normal distribution are expressed as mean ± SD and with a non-parametric distribution as median (lower-upper quartile), categorical variables as frequency (percent). Independent sample T-test combined to Levene’s test were applied for continuous variables with normal distribution, and Mann–Whitney U test for variables with non-parametric distribution. Chi-square/Fisher’s exact test was used for categorical variables as appropriate. All tests were performed two-tailed. CAD, coronary artery disease; AMI, acute myocardial infarction; LAD, left anterior descending coronary artery; LCX, left circumflex coronary artery; RCA, right coronary artery; PCI, percutaneous coronary intervention; GP, glycoprotein; EF, ejection fraction; EDV, end-diastolic volume; ESV, end-systolic volume; ACE, angiotensin-converting enzyme; AT, angiotensin receptor.
The time from the onset of symptoms to intravenous thrombolysis was 2.0 h (range 0.9–4.0 h) in the BMC group and 2.9 h (range 1.2–7.0 h) in controls (p = 0.27). Intravenous reteplase was used as a thrombolytic agent and the day of therapy was defined as day 0. At day 3 (3.2 vs. 3.1 days for BMC and control group), the pharmacological thrombolysis was followed by angioplasty and paclitaxel eluting stent implantation in both groups. After stent implantation, either BMC or placebo was injected distally into the affected artery using an “over the wire balloon,” and a short balloon occlusion at the time of injection was used to prevent acute cell loss. To confirm the blinded nature of the study both for the patients and investigators, all patients underwent bone marrow harvesting at the morning of angioplasty. The randomization code was opened after all imaging data were analyzed from all patients. Baseline cardiac function was measured by cine angiography at day 3 (same day as therapy). Cardiac MRI and PET studies were performed 7–12 days after BMC or placebo therapy and repeated after 6 months.
Bone marrow
A total of 40–80 mL of bone marrow was aspirated from the posterior iliac crest under local anesthesia in the morning of the PCI day. Mononuclear cells were isolated using Ficoll-Hypaque gradient centrifugation, and were washed, suspended, filtered, subjected to quality-control, and counted for CD34+ cells as described earlier (Huikuri et al., 2008). The BMC separation procedure took about 3 h and the cells were kept in +4°C until intracoronary injection was performed within 3 h after the procedure. The isolated BMC’s functional capacity was confirmed by a high mean number of 450 granulocyte–macrophage colony-forming units/plate. The placebo contained the patient’s own serum and heparinized saline.
Cardiac PET
All imaging studies were performed after the subject had fasted for 6 h and avoided alcohol and caffeine for 12 h. Heart rate, blood pressure, and ECG were monitored throughout the studies. The positron-emitting tracers [11C]HED, [15O]H2O, and [18F]FDG (Hamacher et al., 1986; Någren et al., 1995; Sipilä et al., 2001) were used with PET (GE Discovery STE System; GE Medical Systems, Milwaukee, WI, USA) and image acquisition protocols as previously described (Nuutila et al., 1995; Mäki et al., 1996; Koskenvuo et al., 2001; Pietilä et al., 2002). The subjects were lying in a supine position throughout the study. The total imaging time in the PET camera was 2 h and 20 min/time-point. All PET data were corrected for dead time, decay, and measured photon attenuation. Images were reconstructed with standard algorithms. The PET images were quantitatively analyzed using Carimas™ software (Nesterov et al., 2009) by two experienced readers blinded to therapy and other results. The total radiation dose from the PET studies was 15.4 mSv.
Carbon-11 labeled hydroxyephedrine ([11C]HED) is a false norepinephrine analog developed for the evaluation of presynaptic sympathetic innervation (Rosenspire et al., 1990; Schwaiger et al., 1990). [11C]HED shares the same uptake-1 and vesicular storage mechanisms with norepinephrine. The relative stability of [11C]HED makes it a suitable agent for mapping sympathetic neurons in the human heart. A bolus of [11C]HED (517 MBq, equivalent to radiation dose of 1.1 mSv) was injected intravenously over 60 s and a dynamic emission scan was acquired for 40 min. [11C]HED studies were analyzed using the retention index method (Pietilä et al., 2002).
Oxygen-15 labeled water was produced using a diffusion membrane technique in a continuously working water module (Hidex Radiowater Generator, Hidex Oy, Turku, Finland; Sipilä et al., 2001). To measure myocardial perfusion, [15O]H2O (950 MBq, equivalent to radiation dose of 0.9 mSv, given twice per time-point) was injected over 15 s at an infusion rate of 10 mL/min. A dynamic scan was performed for 4 min 40 s (14 × 5 s, 3 × 10 s, 3 × 20 s, and 4 × 30 s). After decay of the 15O radioactivity (10 min), the second [15O]-H2O scan was performed during adenosine (140 μg/min/kg) induced stress. Adenosine was started 2 min before the start of the scan and infused for a total of 6 min 40 s. The coronary flow reserve (CFR) was defined as the ratio of myocardial perfusion during adenosine infusion to perfusion at baseline.
To stabilize metabolic conditions for imaging euglycemic, an insulin clamp (1 mU/kg/min) was applied 60 min before the FDG-PET-study and continued until the end of the study (DeFronzo et al., 1979). During hyperinsulinemia, normoglycemia was maintained using 20% glucose infusion adjusted according to plasma glucose levels determined every 5–10 min from arterialized venous blood. Once steady state was achieved, [18F]FDG (255 MBq, equivalent to a radiation dose of 4.8 mSv) was injected and a dynamic scan was performed for 62 min giving 26 frames (12 × 15 s, 4 × 30 s, 2 × 120 s, 1 × 180 s, 4 × 300 s, and 3 × 600 s). Arterialized venous samples were drawn to measure radioactivity and calculate the input function for Patlak analysis.
Determining area at risk and reference area
For all imaging data, the LV was divided into 17 segments (Cerqueira et al., 2002). After blinded analysis of angiography, PET, and MRI, the myocardial segments were classified as belong either to area at risk or reference area according to the culprit lesion in the first angiography, but also considering wall motion abnormalities in the first MRI and echocardiography (results not shown). The segments were classified by consensus of four readers. In each patient the sum of segments behind the culprit lesion in the infarct-related artery was determined as the area at risk. The reference area was the sum of segments outside the area at risk not showing any wall motion abnormality. Segments with wall motion abnormality observed outside the myocardium supplied by the culprit artery were excluded from further analysis to avoid using areas of an old MI as reference areas. This individual categorization into area at risk and reference area was applied for all MRI and PET studies, both day 10–15 and 6 months after BMC therapy.
Calculating area at risk (ratio), perfusion reserve, PET defect size, and BMC treatment effect
Positron emission tomography data were analyzed quantitatively on 17 segmental bases. Thereafter, segmental PET values in an area at risk or in reference area were summed and divided by the number of segments belonging to that area. This individual mean PET value in an area at risk was normalized (to be comparable with others) by dividing it by the mean value in the reference area. These normalized PET values, namely area at risk (ratio) are presented in Table 2. Perfusion reserve in an area at risk was calculated by dividing adenosine flow by resting flow. To calculate PET defect size, polar maps generated with Carimas™ software v.1 were opened in the ImageJ (v.1.42q) environment (Rasband, 2009). To assess the defect size in PET, values less than 70% of the maximum were determined as positive for defect, and the size was expressed as a ratio of the defect’s size to the size of the whole LV. The basal halves of the posterior septal segments were not included into the defect area. The effect of treatment was defined as the difference between defect sizes at 6 month’s and day 10–15 after therapy, so that negative numbers correspond to beneficial effects of the treatment.
Table 2.
Effect of mononuclear stem cell therapy on myocardial innervation, perfusion, metabolism, and infarct size measured by cardiac PET and MRI.
| Day 10–15 | p | Day 180 | p | Δ Day 180 – Day 10–15 | p | ||
|---|---|---|---|---|---|---|---|
| MYOCARDIAL INNERVATION ([11C]HED-PET) | |||||||
| HED-retention index in area at risk (ratio) | BMC | 0.89 ± 0.10 | 0.06 | 0.94 ± 0.07 | 0.02 | 0.06 ± 0.08 | 0.31 |
| Control | 0.73 ± 0.19 | 0.75 ± 0.19 | 0.02 ± 0.08 | ||||
| HED-defect size (%) | BMC | 14.9 ± 9.4 | 0.10 | 10.0 ± 7.8 | 0.04 | −4.9 ± 4.0 | 0.08 |
| Control | 26.1 ± 14.2 | 24.5 ± 14.5 | −1.6 ± 2.2 | ||||
| MYOCARDIAL PERFUSION ([15O]H2O-PET) | |||||||
| Rest perfusion in area at risk (ratio) | BMC | 0.93 ± 0.15 | 0.62 | 0.84 ± 0.22 | 0.18 | −0.09 ± 0.17 | 0.03 |
| Control | 0.89 ± 0.20 | 0.99 ± 0.25 | 0.10 ± 0.17 | ||||
| Stress perfusion in area at risk (ratio) | BMC | 0.83 ± 0.16 | 0.90 | 0.91 ± 0.20 | 0.75 | 0.08 ± 0.29 | 0.88 |
| Control | 0.81 ± 0.29 | 0.88 ± 0.21 | 0.06 ± 0.17 | ||||
| Perfusion reserve in area at risk (ratio) | BMC | 1.96 ± 1.01 | 0.18 | 1.62 ± 0.85 | 0.84 | −0.33 ± 1.58 | 0.15 |
| Control | 1.27 ± 1.07 | 1.70 ± 0.80 | 0.43 ± 0.34 | ||||
| MYOCARDIAL METABOLISM ([18F]FDG-PET) | |||||||
| FDG uptake in area at risk (ratio) | BMC | 0.77 ± 0.09 | 0.47 | 0.84 ± 0.12 | 0.04 | 0.06 ± 0.09 | 0.07 |
| Control | 0.71 ± 0.22 | 0.66 ± 0.22 | −0.05 ± 0.16 | ||||
| FDG-defect size (%) | BMC | 16.2 ± 8.3 | 0.44 | 12.8 ± 10.6 | 0.27 | −3.3 ± 6.7 | 0.52 |
| Control | 21.1 ± 14.5 | 19.6 ± 14.8 | −1.5 ± 3.4 | ||||
| INFARCT SIZE (LATE ENHANCEMENT Gd-DTPA MRI) | |||||||
| Infarct size (g) | BMC | 6.7 ± 4.3 | 0.08 | 7.0 ± 7.2 | 0.15 | 0.4 ± 4.2 | 0.047 |
| Control | 24.4 ± 22.3 | 19.3 ± 17.6 | −5.1 ± 5.9 | ||||
| Infarct size (% of LV mass) | BMC | 5.0 ± 3.1 | 0.08 | 5.4 ± 5.8 | 0.15 | 0.4 ± 3.6 | 0.045 |
| Control | 19.3 ± 17.5 | 14.0 ± 13.3 | −5.3 ± 6.7 | ||||
[11C]HED, carbon-11 labeled hydroxyephedrine; [15O]H2O, oxygen-15 labeled water; [18F]FDG, fluorine-18 labeled fluoro-2-deoxy-d-glucose. Tracer activity ratios were calculated in each patient by dividing PET measurement in the area at risk with corresponding value in the reference area. BMC and placebo groups were compared using independent sample t-test combined to Levene’s test.
Cardiac MRI
All subjects underwent a MRI study at 1.5 T (Philips Gyroscan Intera Nova Dual MR, Philips Medical Systems, Best, The Netherlands) with a phased-array torso coil and a vector cardiographic method for ECG-gating. All acquisitions were obtained during breath holding in mid inspiration. Each MRI study consisted of cine imaging of both ventricles for volumetrics and wall motion at rest. Late enhancement (LE) imaging was applied for scar assessment. MRI cine frames were analyzed offline with commercial software (ViewForum 2003 release 4.1, Philips Medical Systems, Best, The Netherlands). An experienced reader, blinded to therapy, analyzed all MRI exams.
The left and right ventricles were covered by 8–12 slices at short axis (SA) orientation using a balanced turbo field echo (bTFE) pulse sequence. Additionally, at least six slices were acquired both in four-chamber and two-chamber orientations. Visual wall motion analysis was performed using a 17-segment model of the LV (Cerqueira et al., 2002), and each segment was visually graded as normal (1), hypokinetic (2), akinetic (3), dyskinetic (4), or aneurysmal (5). To calculate the wall motion score index (WMSI), these segmental numbers in the area at risk or reference area were summed and divided by the number of segments belonging to that area. Volumetrics were analyzed according to our previous validation (Koskenvuo et al., 2007), where LV end-diastolic volumes (EDV) and end-systolic volumes were indexed to body surface area (LVEDVI and LVESVI). bTFE pulse sequences were used with the following parameters: retrospective gating, repetition time/echo time (TR/TE) 3.4/1.2 ms, flip angle 60°, FOV 320–360 mm, acquisition matrix 192 × 256, reconstruction matrix 256 × 256, rFOV 100%, 30 phases/cardiac cycle, slice thickness 6.0 mm, and gap 0 mm.
A phase-sensitive inversion recovery T1–TFE pulse sequence was applied for LE imaging 10 min after injecting gadoterate meglumine (Dotarem 279.3 mg/mL, Guerbet, Roissy CdG, France) with a dose 0.3 mmol/kg. The following imaging parameters were used: prospective gating, TR/TE 4.0/1.2 ms, flip angle 15°, acquisition matrix 168 × 256, reconstruction matrix 256 × 256, FOV 320–360 mm, rFOV 100%, 8–15 slices/breath hold, slice thickness 6 mm, and gap 0 mm. TI was optimized individually using a TI-scout sequence. The whole LV was covered by LE images at SA orientation, and at least eight additional slices were acquired in both two- and four-chamber orientation to make it easier to distinguish healthy myocardium from scar. The scar mass was measured quantitatively from LE in grams as shown earlier (Mewton et al., 2011).
Statistical analyses
The Shapiro–Wilk test was applied to determine whether that data are normally distributed. Normally distributed, continuous variables are expressed as mean ± SD and non-parametric as median, and lower and upper quartiles. All categorical variables are depicted using relative frequency distributions. Characteristics of treated patients and controls were compared using the chi-square test for categorical variables if appropriate, otherwise Fisher’s exact test was used. Independent samples t-tests combined with Levene’s tests were used to test the significance of differences between groups for all continuous variables and Mann–Whitney U tests were used for non-parametric variables. Differences were considered significant if the two-sided p-value was <0.05. All analyses were performed using SPSS software package (Version 16.0; SPSS, Inc., Chicago, IL, USA).
Results
Patient groups
Bone marrow cell and control groups were relatively well balanced according to age, BMI, risk factors/concomitant disease, severity of coronary artery disease, and medication both at discharge and 6 month time-point (Table 1). Three patients had had earlier MI, two from BMC group, and one from control group. One patient in each group had the earlier infarct in the current culprit artery whereas one patient’s infarct (BMC group) located in the other segments and those were excluded from the final analysis. The differences existed at baseline use of diuretics, which was 9% in BMC group and 75% in controls (p = 0.006).
Therapy in general
BMC therapy failed to demonstrate consistently better recovery from MI compared to placebo at 6 months follow-up. The jeopardized myocardium behind the infarct-related artery, namely area at risk, consisted of 6.1 segments on average per patient, of which 4.4 segments had a wall motion abnormality. Fourteen out of 323 myocardial segments were excluded from final analysis because wall motion abnormality was not related to the culprit lesion (probably corresponding to the old MI or other significant coronary stenosis).
PET parameters
The positron-emitting tracer [11C]HED retention index and [18F]FDG uptake in the area at risk describe the depth of myocardial injury, whereas defect sizes assess the extent of damage with respect to the whole LV (infarct expansion). Table 2 presents detailed PET results.
Myocardial innervation by PET
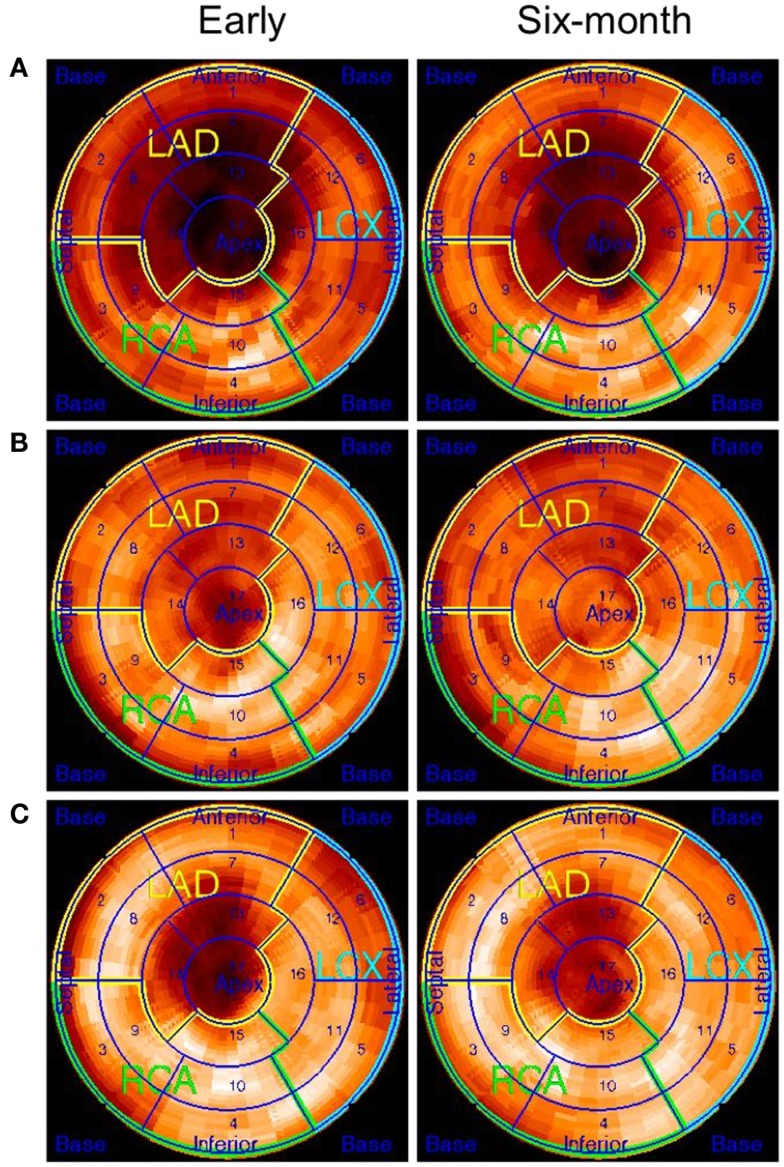
[11C]HED-retention index in the area at risk did not improve more in the BMC group vs. controls during follow-up (0.06 ± 0.08 vs. 0.02 ± 0.08, p = 0.31). However, there was a trend of more decrease in [11C]HED defect size (%) in the BMC-treated group compared to controls (−4.9 ± 4.0 vs. −1.6 ± 2.2%, p = 0.08). Figure 2 shows examples of [11C]HED polar plots from a control patient and two patients treated with BMCs.
Figure 2.
Polar plots of [11C]HED–PET studies at 1–2 weeks and 6 months after placebo or BMC therapy. (A) The patient with placebo treatment had no clear improvement at 6 months, (B) improvement in [11C]HED retention index in BMC-treated patient, and (C) slight improvement [11C]HED retention index in a patient with BMC therapy which was not as evident as in previous patient (B).
Myocardial perfusion by PET
Perfusion measured by [15O]H2O-PET did not differ in the area at risk, in rest or stress at an day 10–15 time-point between BMC and placebo groups (p = 0.62 and p = 0.90). However, rest perfusion in the area at risk adjusted to reference area decreased during follow-up in the BMC group and slightly increased in the placebo group (−0.09 ± 0.17 vs. 0.10 ± 0.17, p = 0.03). A small, non-significant decrease in perfusion reserve in the area at risk was observed in the BMC group compared to controls (−0.33 ± 1.58 vs. 0.43 ± 0.34, p = 0.15) during follow-up.
Myocardial glucose metabolism by PET
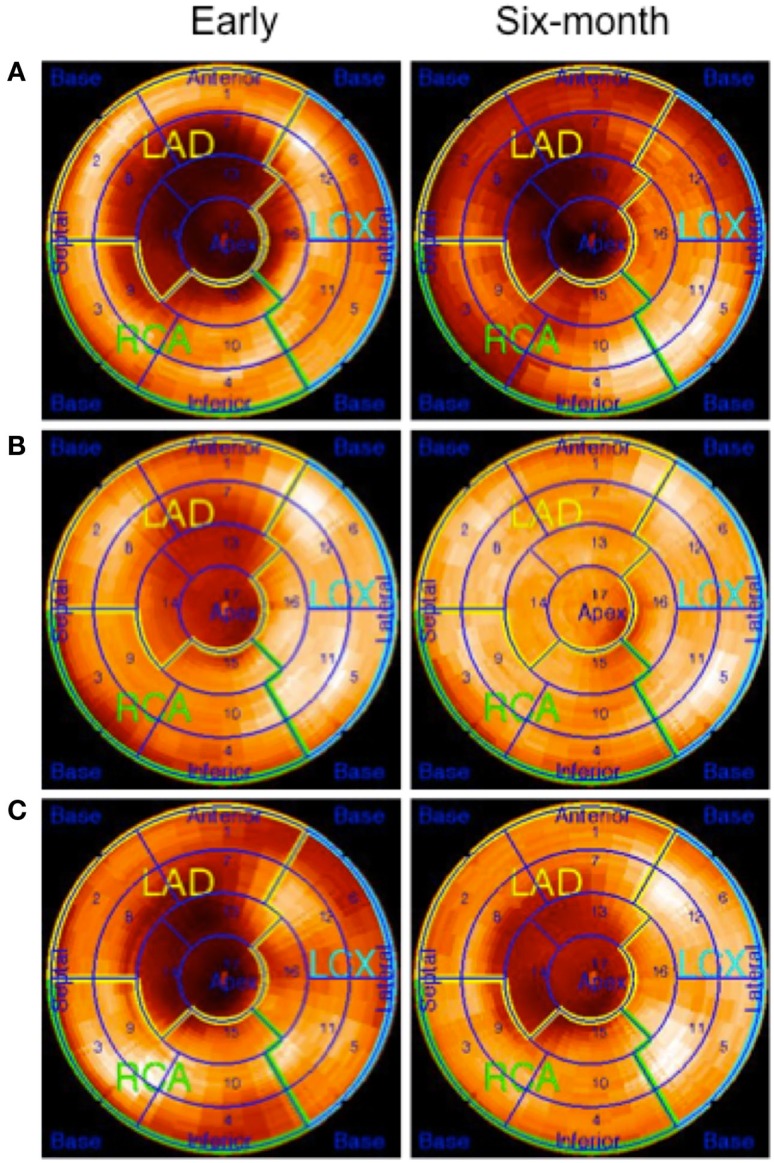
[18F]FDG uptake ratio or defect size at baseline did not differ between the BMC group and control group (0.77 ± 0.09 vs. 0.71 ± 0.22, p = 0.47 and 16.2 ± 8.3 vs. 21.1 ± 14.5, p = 0.44). At 6 month’s, the [18F]FDG uptake ratio was higher in the BMC group than in the control group (0.84 ± 0.12 vs. 0.66 ± 0.22, p = 0.04, respectively) and a trend of increasing glucose metabolism was found in the area at risk in the BMC group (p = 0.07). However, the decrease in FDG-defect size was not different between the groups (p = 0.52). Figure 3 shows examples of [18F]FDG polar plots from a control and two BMC-treated patients (patients are the same as in Figure 2).
Figure 3.
Polar plots of [18F]FDG-PET studies at 1–2 weeks and 6 months after placebo or BMC therapy. The patients are the same as in Figure 2. (A) The patient with placebo treatment has a large defect in the LAD region. The defect size is slightly reduced (34–27%) at 6 months, (B) a patient with BMC therapy has a moderate size defect in the LAD region at day 10–15 time-point, and the defect size was clearly reduced at 6 months follow-up (21–2%), and (C) a patient with BMC therapy has a moderate size defect in the LAD region and no clear change in the defect size (26–22%) was observed at 6 months follow-up.
Infarct size, cardiac function, and morphology by MRI
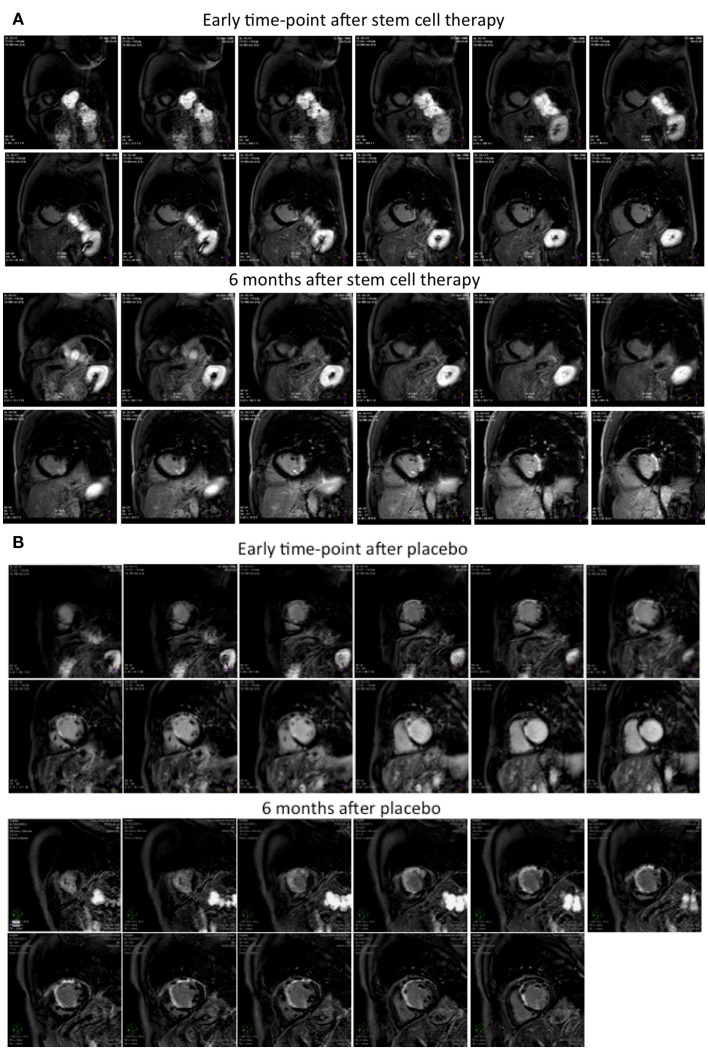
At LE MRI, the mean scar mass was 6.7 ± 4.3 g in the BMC group and 24.4 ± 22.3 g in controls at the day 10–15 time-point (p = 0.08) indicating a trend of slightly larger scars in the control group at day 10–15 phase. The scar mass decreased more in the control group than in the BMC group during 6 month follow-up (−5.1 ± 5.9 vs. 0.4 ± 4.2 g, p = 0.047), possibly because a larger scar size enables more recovery during follow-up. Figure 4 shows LE MRI images from BMC-treated (Figure 4A) and control (Figure 4B) patients at 1–2 week and 6 month time-points. LVEF was 53.6 ± 6.3% in BMC and 51.7 ± 12.7% in controls at 6 months (p = 0.71), indicating ejection fraction (EF) increases of 0.5 ± 3.7 and 2.9 ± 6.4% (p = 0.37) after first MRI evaluation (Table 3). A trend of less LV remodeling in the BMC group was observed when estimated by changes in LV end-diastolic (p = 0.12) and end-systolic (p = 0.19) volume indices at follow-up. Examples of cine MRI images are shown from a BMC-treated patient at 1–2 week (Online Resource 1) and at 6 month (Online Resource 2) time-points and from a control patient at 1–2 week (Online Resource 3) and at 6 month (Online Resource 4) time-points. A decreasing trend of cardiac index was detected in the BMC group compared to controls (−0.2 ± 0.4 vs. 0.4 ± 0.7 L/min/m2, p = 0.06). In the area at risk a systolic function parameter, namely WMSI, improved (decreased; −0.05 ± 0.23 vs. −0.09 ± 0.20) in BMC, and placebo groups at follow-up but neither therapy was better than the other (p = 0.73). No significant differences were observed between the groups in terms of right ventricular EF or volumes. Detailed data on the functional effects of BMC therapy on left and right heart are presented in Tables 3 and 4.
Figure 4.
(A,B) Late enhancement MRI images at 1–2 weeks and 6 months after placebo or BMC therapy. Late enhancement MRI images from (A) BMC and (B) placebo treated patients at day 10–15 (1–2 weeks) and 6 months time-points. Patient (A) had infarct size 8.8% at day 10–15 time-point and 11.3% at 6 months, and patient (B) infarct size was 40.3 and 31.6% at corresponding time-points.
Table 3.
Effect of mononuclear stem cell therapy in left ventricular function assessed by cardiac MRI and X-ray angiography.
| Baseline | Day 10–15 | Day 180 | Δ Day 10–15 vs. baseline | Δ Day 180 vs. baseline | Δ Day 180 vs. Day 10–15 | |
|---|---|---|---|---|---|---|
| LEFT VENTRICULAR EJECTION FRACTION (%) | ||||||
| BMC | 66.9 ± 5.2 | 53.1 ± 7.0 | 53.6 ± 6.3 | −13.8 ± 7.8 | −13.2 ± 7.4 | 0.5 ± 3.7 |
| Control | 55.1 ± 18.3 | 48.9 ± 11.8 | 51.7 ± 12.7 | −6.3 ± 7.6 | −3.4 ± 9.4 | 2.9 ± 6.4 |
| p-Value | 0.15 | 0.42 | 0.71 | 0.07 | 0.03 | 0.37 |
| LEFT VENTRICULAR END-DIASTOLIC VOLUME INDEX (mL/m2) | ||||||
| BMC | 78.0 ± 17.0 | 83.4 ± 19.2 | 79.0 ± 17.5 | 5.4 ± 21.7 | 1.0 ± 20.1 | −4.4 ± 13.3 |
| Control | 77.8 ± 20.2 | 87.3 ± 14.7 | 95.3 ± 24.0 | 9.5 ± 29.0 | 17.4 ± 38.6 | 8.0 ± 16.7 |
| p-Value | 0.99 | 0.66 | 0.14 | 0.75 | 0.29 | 0.12 |
| LEFT VENTRICULAR END-SYSTOLIC VOLUME INDEX (mL/m2) | ||||||
| BMC | 25.6 ± 6.0 | 39.7 ± 13.4 | 37.2 ± 12.7 | 14.1 ± 11.1 | 11.5 ± 11.6 | −2.5 ± 5.0 |
| Control | 33.7 ± 14.8 | 45.5 ± 15.7 | 47.6 ± 21.6 | 11.8 ± 11.9 | 13.9 ± 17.9 | 2.1 ± 8.2 |
| p-Value | 0.22 | 0.44 | 0.25 | 0.70 | 0.75 | 0.19 |
Left ventricular ejection fraction and volumes were measured using X-ray angiography at baseline (3 days post-MI, the day of therapy) and using cardiac MRI at day 10–15 and day 180 time-points after BMC (bone marrow mononuclear stem cell) therapy. All data is presented as mean ± SD. Independent sample t-test was used for comparison.
Table 4.
Effect of mononuclear stem cell therapy on left and right ventricular parameters measured by cardiac MRI.
| Day 10–15 | p | Day 180 | p | Δ Day 180-early | p | ||
|---|---|---|---|---|---|---|---|
| LEFT VENTRICLE | |||||||
| LV stroke volume index (mL/m2) | BMC | 43.6 ± 8.4 | 0.53 | 41.8 ± 6.0 | 0.45 | −1.8 ± 9.3 | 0.28 |
| Control | 41.1 ± 19 | 45.3 ± 11.2 | 4.2 ± 12.6 | ||||
| LV cardiac index (L/min/m2) | BMC | 2.3 ± 0.4 | 0.84 | 2.1 ± 0.2 | 0.04 | −0.2 ± 0.4 | 0.06 |
| Control | 2.3 ± 0.5 | 2.7 ± 0.7 | 0.4 ± 0.7 | ||||
| LV mass index (g/m2) | BMC | 66.2 ± 12.4 | 0.83 | 66.2 ± 6.8 | 0.32 | 0.0 ± 10.3 | 0.24 |
| Control | 65.0 ± 8.8 | 70.7 ± 11.4 | 5.7 ± 9.1 | ||||
| WMSI area at risk (score) | BMC | 1.67 ± 0.41 | 0.054 | 1.61 ± 0.39 | 0.06 | −0.05 ± 0.23 | 0.73 |
| Control | 2.26 ± 0.74 | 2.17 ± 0.72 | −0.09 ± 0.20 | ||||
| WMSI remote zone (score) | BMC | 1.06 ± 0.12 | 0.89 | 1.03 ± 0.06 | 0.95 | −0.04 ± 0.12 | 0.93 |
| Control | 1.06 ± 0.09 | 1.02 ± 0.10 | −0.03 ± 0.10 | ||||
| RIGHT VENTRICLE | |||||||
| RV–EF (%) | BMC | 62.1 ± 8.0 | 0.95 | 59.5 ± 8.3 | 0.53 | −2.6 ± 9.1 | 0.64 |
| Control | 61.8 ± 8.4 | 61.7 ± 5.7 | −0.1 ± 12.2 | ||||
| RV–EDVI (mL/m2) | BMC | 74.2 ± 28.1 | 0.35 | 68.4 ± 10.5 | 0.30 | −5.8 ± 23.6 | 0.12 |
| Control | 63.9 ± 10.9 | 75.5 ± 16.5 | 11.5 ± 19.2 | ||||
| RV–ESVI (mL/m2) | BMC | 28.3 ± 13.0 | 0.63 | 28.6 ± 8.8 | 0.95 | 0.3 ± 6.0 | 0.44 |
| Control | 25.9 ± 5.1 | 28.9 ± 6.9 | 3.0 ± 7.7 | ||||
LV, left ventricle; BMC, bone marrow cell; WMSI, wall motion score index; RV, right ventricle; EF, ejection fraction; EDVI, end-diastolic volume index; ESVI, end-systolic volume index. All data is presented as mean ± SD. Independent sample t-test was used for comparison.
Discussion
While several studies have investigated the effects of intracoronary BMC therapy on global LV function and hemodynamics, the present study is the first to evaluate the mechanistic insights and effects of BMCs on infarct-related myocardium by determining changes in perfusion, metabolism, innervation, and cardiac function occurring from the early initial phase to 6 months thereafter. Some recovery in jeopardized myocardium was also detected without BMCs, indicating the importance of the placebo-treated control group. The main effects of BMCs detected in the present study can be summarized as follows: (1) a trend of more decrease in the size of denervated myocardium; (2) minor enhancement of glucose metabolism in the area at risk; (3) potentially positive effect on LV remodeling; (4) mild negative effect on scar size and myocardial perfusion reserve in the area at risk. Thus, there were no gross differences in recovery between BMC and control groups at 6 months after acute STEMI with respect to numerous measured parameters.
Effect of BMC therapy on myocardial innervation
Reduced myocardial [11C]HED retention is associated with poor prognosis in chronic heart failure and it is even suggested that [11C]HED uptake is a better predictor of death than more common predictors of outcome such as EF or heart rate variability (Pietilä et al., 2001; Link and Caldwell, 2008). Our study evaluated for the first time the effect of stem cell therapy on cardiac innervation. [11C]HED uptake improved in both groups at 6 month follow-up following acute MI but BMC therapy was not superior to placebo (p = 0.31). However, we observed a positive trend of decreasing [11C]HED defect size in the BMC group (−4.9 ± 4.0 vs. −1.6 ± 2.2, p = 0.08).
Effect of BMC therapy on myocardial perfusion
The effect of BMC therapy on myocardial perfusion in the setting of acute MI has mostly been evaluated with SPECT (Strauer et al., 2002; Dobert et al., 2004; Bartunek et al., 2005; Lunde et al., 2006; Beeres et al., 2007; Meluzín et al., 2008). These studies show either reduction of perfusion defect (Strauer et al., 2002; Dobert et al., 2004; Bartunek et al., 2005) or unchanged perfusion improvement compared to controls (Lunde et al., 2006; Meluzín et al., 2008). However, the only study using PET for the quantification of myocardial perfusion showed no difference between BMC-treated patients and controls at 4 months follow-up (Janssens et al., 2006), which is consistent with our study. CFR provides insight into the integrity of both the epicardial conduit arteries and distal microvascular bed. The REPAIR-AMI sub study showed that BMC therapy was associated with complete restoration of intracoronary Doppler derived CFR (Erbs et al., 2007). However, coronary flow measurement may be misleading when fast deceleration time is not taken into account and a subtraction is not performed for reverse flow existing in late diastole due to permanent MI induced capillary damage (Saraste et al., 2007). Conversely, BMC therapy had no effect on PET measurement of perfusion reserve in the area at risk in our study nor in a larger placebo-controlled study with intracoronary infusion of mobilized peripheral blood stem cells (Kang et al., 2006).
Effect of BMC therapy on myocardial metabolism, viability, and infarct size
A total of five studies (including 128 patients) have used PET for evaluating the effect of stem cell therapy on FDG metabolism/viability in the setting of acute MI (Assmus et al., 2002; Chen et al., 2004; Dobert et al., 2004; Bartunek et al., 2005; Balogh et al., 2007; Beeres et al., 2007). Four of these studies used PET imaging only for BMC-treated patients and one had no baseline evaluation. All of these studies demonstrated increased 18F-FDG uptake in the infarct zone at 4–6 months follow-up. Our study showed a trend of improved 18F-FDG uptake in the area at risk during 6 months follow-up in the BMC group compared to placebo (p = 0.07), but FDG-defect size was comparable in the two groups (p = 0.52). Late-enhancement MRI describes viability in an alternative way, by detecting non-viable myocardium with the principle of “bright is dead,” which is regarded the most accurate technique for myocardial scar assessment. There are four earlier randomized studies with serial LE imaging after stem cell therapy for acute MI (Janssens et al., 2006; Lunde et al., 2006; Meyer et al., 2006; Dill et al., 2009). Janssens et al. (2006) demonstrated a reduction of scar size with BMCs (2.3 g more in a BMC group, p = 0.036) whereas the BOOST or REPAIR-AMI studies found no benefit of BMC therapy regarding scar size (Meyer et al., 2006; Dill et al., 2009). Notably, the ASTAMI study showed a trend of more scar reduction in placebo vs. BMC group (treatment effect −3.9 mL, p = 0.07; Lunde et al., 2006). The latter, negative findings are mainly consistent with our results as we found a trend of more scar reduction in the control group (−5.1 ± 5.9 vs. 0.4 ± 4.2 g p = 0.047). However, in this regard, a trend of larger infarcts in the control group (indicated by insignificantly lower EFs at baseline angiography and day 10–15 MRI) makes it more likely to also see more infarct reduction in the group with larger infarct. Regarding scar size measurements in our study, BMC therapy failed to demonstrate any benefit; the only change was the minor enhancement of glucose metabolism in the area at risk, which has uncertain clinical value.
Effect of BMC therapy on myocardial function and remodeling
MRI derived LV EFs were determined at day 10–15 and 6 months after intracoronary BMC transfer. X-ray cine angiography is likely to measure almost 9% higher EFs compared to MRI (Kondo et al., 2003) and therefore observed difference between EF in X-ray angiography at baseline to EF at day 10–15 MRI is mainly due to methodological differences and the relatively high baseline EF do not indicate very small MIs in this study. From day 10–15 to 6 months EF increase was not significant between the groups (0.5 ± 3.7% in BMC and 2.9 ± 6.4% in the control group, p = 0.37). Three recent meta-analyses applying different imaging methods for estimating changes in global EF after BMC therapy demonstrated improvements of 3.0, 3.7, and 4.8% (absolute change) in EF compared to the control group but not for EDV (p = 0.11, p = 0.39, and p = 0.41), which is a good measure of remodeling following MI (Abdel-Latif et al., 2007; Lipinski et al., 2007; Zhang et al., 2009). In many studies, echocardiography or angiography have been used for volumetric measurement, which is considered as a limitation when evaluating a small group of patients due to reproducibility issues. Cardiac MRI, as used in the current study, is the golden standard for determining ventricular volumes and EF. A total of eight randomized controlled studies (including 539 patients) have previously evaluated LV function and volumetrics using MRI after stem cell therapy for acute MI (Wollert et al., 2004; Janssens et al., 2006; Kang et al., 2006; Lunde et al., 2006; Meyer et al., 2006; Beeres et al., 2007; Dill et al., 2009; Hare et al., 2009). Only 3 of the 8 studies with serial cardiac MRI demonstrated improvement in global EF; (1) difference to placebo 6% (p = 0.0026; Wollert et al., 2004), (2) 5.3% (p = 0.046) at 6 months follow-up (Kang et al., 2006), and (3) difference to placebo 3.4% at 12 month follow-up, p = 0.003), whereas no improved EF was observed at 6 months and no significant effects on LV volumes at any time-point (Hare et al., 2009). Furthermore, none of the remaining five studies demonstrated positive trends (treatment effect >2% with a p < 0.15) toward better EF in the BMC group and only one study detected significant improvements in end-diastolic/systolic volume (ESV’s difference to placebo 11.9 mL, p = 0.04; Kang et al., 2006). One of the studies demonstrated a trend of lower EF in BMC group vs. placebo (difference 3.1%, p = 0.054; Lunde et al., 2006). Regarding EFs, our results are similar to observations by Janssens et al. and Lunde et al., and are at least partially explained by relatively fast reperfusion therapy. In these studies, the first reperfusion therapy was given within 4 h following acute STEMI and therefore may have resulted in a smaller infarct size and only modestly compromised EF at baseline as also observed in our study (Janssens et al., 2006; Lunde et al., 2006). In the REPAIR-AMI sub-study, the magnitude of LV contractile recovery was inversely related to the baseline EF and in the studies by Tendera et al. and Dill et al. the increase in EF in the BMC group was limited to patients with more depressed EFs (Schachinger et al., 2006a; Dill et al., 2009; Tendera et al., 2009). The REPAIR-AMI sub-study showed a trend of improved EF, EDV, and ESV (differences to placebo; 2.8%, p = 0.26; 14 mL, p = 0.12; and 13 mL, p = 0.08), respectively (Dill et al., 2009). In our study, a trend (p = 0.12) of LVEDVI improvement was observed in the BMC group (−4.4 mL/m2) at 6 months compared to the control group (8.0 mL/m2).
To overcome the insensitivity of global parameters of LV function, regional function was estimated using WMSI both in the area at risk and reference area. A slight improvement in WMSI was observed in the BMC and control groups within the measured time course, but no significant difference between the groups was found (p = 0.73). There are no earlier studies evaluating RV function following stem cell therapy for acute MI (some infarcts may extend into the right heart but this was not confirmed by LE imaging). Our study showed that BMC therapy had no remote effects on right heart function in this patient population.
BMC transplantation
According to a recent review of cardiac stem cell therapy in humans, neither the number of transplanted cells, nor cell type (bone marrow derived mononuclear vs. mesenchymal or circulating peripheral progenitor) had a significant effect on changes in EF or infarct size (Abdel-Latif et al., 2007). Interestingly, recent animal study proposed that mononuclear BMCs survive better at ischemic milieu compared to mesenchymal stem cells (van der Bogt et al., 2008) and in our study, mononuclear BMCs were used for therapy. However, so far the majority of transplanted cells die within 1–2 months after transplantation (Amsalem et al., 2007; van der Bogt et al., 2009), which is concordant with our finding that no major changes in cardiac parameters were detected during follow-up when compared to controls. It has been stated that poor survival pattern makes robust repopulation impossible and also limits protective paracrine action of the cells (van der Bogt et al., 2009).
The timing of cell delivery may also be important. In this study, BMCs were transferred 3 days after primary thrombolysis. The optimal time for cell delivery after myocardial infarction is unknown, but according to previous studies it has only a minor influence on outcome in the setting of acute MI (Bartunek et al., 2006; Abdel-Latif et al., 2007). Intracoronary cell infusion is used in most of the clinical studies for cell delivery and therefore it is unlikely to explain conflicting results between the studies. Moreover, intracoronary single-bolus BMC therapy is reported to be as effective as balloon-occlusion cell delivery (Doyle et al., 2007). Cell preparation and storage may also partly influence the discrepant results between the studies. In the present study, the BMCs were injected immediately after the bone marrow aspiration without storage and the colony-forming function of the cells was confirmed.
Limitations
A distinct difference between the present and previous studies is that we included patients treated with thrombolysis followed by later PCI, whereas all prior trials have assessed the efficacy of BMC therapy in patients treated with primary PCI. We performed cell transfer 7–12 days before the first PET and MRI studies, and therefore can not exclude potential cell-mediated effects arising beforehand and causing our protocol to underestimate therapeutic effects of BMC therapy. There are no consensus or good meta-analysis to reveal whether small or large infarct are more likely to benefit from stem cell therapy. Of the three largest clinical BMC trials (Lunde et al., 2006; Meluzín et al., 2006; Schachinger et al., 2006b), only REPAIR-AMI trial indicated that baseline EF correlated with EF change (p = 0.04) but the statistical analysis did not taken autocorrelation of the parameters into account, which should be considered as a major limitation. However, recent observations from small studies showed that large infarcts were less likely to get benefit from BMC therapy (Obradović et al., 2009; Traverse et al., 2010). Global LV function was quite preserved in our patient population, potentially improving the likelihood of a positive outcome. We used LV angiography instead of MRI for assessing cardiac function at baseline. A relatively small sample size together with a trend-type different EF and MI size at baseline may also limit the generalization of the results. Another potential confounding factor was the use of paclitaxel stents in all patients, which may influence the function of the injected cells itself. However, in the MAGIC Cell-3-DES study, drug-eluting stents were used for all patients and the authors found improved EF in BMC group vs. placebo (p = 0.04; Kang et al., 2006), suggesting that the stent is unlikely to explain the negative results in our study.
Conclusion
Cardiac [15O]H2O-, [18F]FDG- and [11C]HED- PET, and MRI offer attractive tools for measuring myocardium in detail after BMC transfer. A trend of improvement in metabolism and innervation defect may reflect slight recovery of jeopardized myocardium in BMC-treated patients. However, BMC therapy in infarct-related artery 3 days after successfully reperfused STEMI showed no consistently better outcome than placebo in this patient group. Therefore, larger studies are needed before trying to generalize the results from this pilot study.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at http://www.frontiersin.org/clinical_and_translational_physiology/10.3389/fphys.2012.00006/abstract
Online Resources
Short-axis cine MRI image (1 week) in BMC-treated patient. Short-axis cine MRI image stack at 1 week time-point from a patient with BMC therapy (same patient as in Figure 4A). End-diastolic volume was 192 mL and ejection fraction 41%.
Short-axis cine MRI image (6 months) in BMC-treated patient. Short-axis cine MRI image stack at 6 month from the same patient with BMC therapy as in Online Resource 1. End-diastolic volume was 204 mL and ejection fraction 43%.
Short-axis cine MRI image (1 week) in placebo treated patient. Short-axis cine MRI image stack at 1 week from a patient with placebo therapy. Same patient as in Figure 4B end-diastolic volume was 182 mL and ejection fraction 34%.
Short-axis cine MRI image (6 months) in placebo treated patient. Short axis cine MRI image stack at 6 month from the same patient with placebo therapy as in Online Resource 3. End-diastolic volume was 174 mL and ejection fraction 36%.
Acknowledgments
We would like to thank Ms Kirsi Kvist-Mäkelä and Tuija Vasankari for expert technical help. The study is part of the project “Molecular Imaging in Cardiovascular and Metabolic Research” which belongs to the Centre of Excellence program of the Academy of Finland. Financial support was also obtained from the Finnish Foundation for Cardiovascular Research, Helsinki, Finland, and the Foundation for the Northern Health Support, Boston Scientific Sverige AB, Stockholm, Sweden, The Hospital District of Southwest Finland. We thank Dr Tony Shepherd (Turku PET Centre) for linguistic revision.
References
- Abdel-Latif A., Bolli R., Tleyjeh I. M., Montori V. M., Perin E. C., Hornung C. A., Zuba-Surma E. K., Al-Mallah M., Dawn B. (2007). Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch. Intern. Med. 167, 989–997 10.1001/archinte.167.10.989 [DOI] [PubMed] [Google Scholar]
- Amsalem Y., Mardor Y., Feinberg M. S., Landa N., Miller L., Daniels D., Ocherashvilli A., Holbova R., Yosef O., Barbash I. M., Leor J. (2007). Iron-oxide labeling and outcome of transplanted mesenchymal stem cells in the infarcted myocardium. Circulation 116, I38–I45 10.1161/CIRCULATIONAHA.106.680231 [DOI] [PubMed] [Google Scholar]
- Assmus B., Schachinger V., Teupe C., Britten M., Lehmann R., Dobert N., Grunwald F., Aicher A., Urbich C., Martin H., Hoelzer D., Dimmeler S., Zeiher A. M. (2002). Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE-AMI). Circulation 106, 3009–3017 10.1161/01.CIR.0000043246.74879.CD [DOI] [PubMed] [Google Scholar]
- Balogh L., Czuriga I., Hunyadi J., Galuska L., Kristof E., Edes I. (2007). Effects of autologous bone marrow derived CD34+ stem cells on the left ventricular function following myocardial infarction. Orv. Hetil. 148, 243–249 10.1556/OH.2007.28006 [DOI] [PubMed] [Google Scholar]
- Balsam L. B., Wagers A. J., Christensen J. L., Kofidis T., Weissman I. L., Robbins R. C. (2004). Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature 428, 668–673 10.1038/nature02460 [DOI] [PubMed] [Google Scholar]
- Bartunek J., Vanderheyden M., Vandekerckhove B., Mansour S., De Bruyne B., De Bondt P., Van Haute I., Lootens N., Heyndrickx G., Wijns W. (2005). Intracoronary injection of CD133-positive enriched bone marrow progenitor cells promotes cardiac recovery after recent myocardial infarction: feasibility and safety. Circulation 112, I178–I183 [DOI] [PubMed] [Google Scholar]
- Bartunek J., Wijns W., Heyndrickx G. R., Vanderheyden M. (2006). Timing of intracoronary bone-marrow-derived stem cell transplantation after ST-elevation myocardial infarction. Nat. Clin. Pract. Cardiovasc. Med. 3(Suppl. 1), S52–S56 10.1038/ncpcardio0417 [DOI] [PubMed] [Google Scholar]
- Beeres S. L., Bengel F. M., Bartunek J., Atsma D. E., Hill J. M., Vanderheyden M., Penicka M., Schalij M. J., Wijns W., Bax J. J. (2007). Role of imaging in cardiac stem cell therapy. J. Am. Coll. Cardiol. 49, 1137–1148 10.1016/j.jacc.2006.10.072 [DOI] [PubMed] [Google Scholar]
- Britten M. B., Abolmaali N. D., Assmus B., Lehmann R., Honold J., Schmitt J., Vogl T. J., Martin H., Schachinger V., Dimmeler S., Zeiher A. M. (2003). Infarct remodeling after intracoronary progenitor cell treatment in patients with acute myocardial infarction (TOPCARE-AMI): mechanistic insights from serial contrast-enhanced magnetic resonance imaging. Circulation 108, 2212–2218 10.1161/01.CIR.0000095788.78169.AF [DOI] [PubMed] [Google Scholar]
- Cerqueira M. D., Weissman N. J., Dilsizian V., Jacobs A. K., Kaul S., Laskey W. K., Pennell D. J., Rumberger J. A., Ryan T., Verani M. S., American Heart Association Writing Group on Myocardial Segmentation and Registration for Cardiac Imaging (2002). Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the cardiac imaging committee of the council on clinical cardiology of the american heart association. Circulation 105, 539–542 10.1161/hc0402.102975 [DOI] [PubMed] [Google Scholar]
- Chen S. L., Fang W. W., Ye F., Liu Y. H., Qian J., Shan S. J., Zhang J. J., Chunhua R. Z., Liao L. M., Lin S., Sun J. P. (2004). Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am. J. Cardiol. 94, 92–95 10.1016/j.amjcard.2004.03.034 [DOI] [PubMed] [Google Scholar]
- de Silva R., Raval A. N., Hadi M., Gildea K. M., Bonifacino A. C., Yu Z. X., Yau Y. Y., Leitman S. F., Bacharach S. L., Donahue R. E., Read E. J., Lederman R. J. (2008). Intracoronary infusion of autologous mononuclear cells from bone marrow or granulocyte colony-stimulating factor-mobilized apheresis product may not improve remodelling, contractile function, perfusion, or infarct size in a swine model of large myocardial infarction. Eur. Heart J. 29, 1772–1782 10.1093/eurheartj/ehn216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R. A., Tobin J. D., Andres R. (1979). Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am. J. Physiol. 237, E214–E23 [DOI] [PubMed] [Google Scholar]
- Dill T., Schachinger V., Rolf A., Mollmann S., Thiele H., Tillmanns H., Assmus B., Dimmeler S., Zeiher A. M., Hamm C. (2009). Intracoronary administration of bone marrow-derived progenitor cells improves left ventricular function in patients at risk for adverse remodeling after acute ST-segment elevation myocardial infarction: results of the reinfusion of enriched progenitor cells and infarct remodeling in acute myocardial infarction study (REPAIR-AMI) cardiac magnetic resonance imaging substudy. Am. Heart J. 157, 541–547 10.1016/j.ahj.2008.11.011 [DOI] [PubMed] [Google Scholar]
- Dobert N., Britten M., Assmus B., Berner U., Menzel C., Lehmann R., Hamscho N., Schachinger V., Dimmeler S., Zeiher A. M., Grunwald F. (2004). Transplantation of progenitor cells after reperfused acute myocardial infarction: evaluation of perfusion and myocardial viability with FDG-PET and thallium SPECT. Eur. J. Nucl. Med. Mol. Imaging 31, 1146–1151 10.1007/s00259-004-1490-4 [DOI] [PubMed] [Google Scholar]
- Doyle B., Kemp B. J., Chareonthaitawee P., Reed C., Schmeckpeper J., Sorajja P., Russell S., Araoz P., Riederer S. J., Caplice N. M. (2007). Dynamic tracking during intracoronary injection of 18F-FDG-labeled progenitor cell therapy for acute myocardial infarction. J. Nucl. Med. 48, 1708–1714 10.2967/jnumed.107.042838 [DOI] [PubMed] [Google Scholar]
- Erbs S., Linke A., Schachinger V., Assmus B., Thiele H., Diederich K. W., Hoffmann C., Dimmeler S., Tonn T., Hambrecht R., Zeiher A. M., Schuler G. (2007). Restoration of microvascular function in the infarct-related artery by intracoronary transplantation of bone marrow progenitor cells in patients with acute myocardial infarction: the doppler substudy of the reinfusion of enriched progenitor cells and infarct remodeling in acute myocardial infarction (REPAIR-AMI) trial. Circulation 116, 366–374 10.1161/CIRCULATIONAHA.106.671545 [DOI] [PubMed] [Google Scholar]
- Fernandez-Aviles F., San Roman J. A., Garcia-Frade J., Fernandez M. E., Penarrubia M. J., de la Fuente L., Gomez-Bueno M., Cantalapiedra A., Fernandez J., Gutierrez O., Sanchez P. L., Hernandez C., Sanz R., Garcia-Sancho J., Sanchez A. (2004). Experimental and clinical regenerative capability of human bone marrow cells after myocardial infarction. Circ. Res. 95, 742–748 10.1161/01.RES.0000144798.54040.ed [DOI] [PubMed] [Google Scholar]
- Hamacher K., Coenen H. H., Stocklin G. (1986). Efficient stereospecific synthesis of no-carrier-added 2-[18F]-fluoro-2-deoxy-d-glucose using aminopolyether supported nucleophilic substitution. J. Nucl. Med. 27, 235–238 [PubMed] [Google Scholar]
- Hare J. M., Traverse J. H., Henry T. D., Dib N., Strumpf R. K., Schulman S. P., Gerstenblith G., DeMaria A. N., Denktas A. E., Gammon R. S., Hermiller J. B., Jr., Reisman M. A., Schaer G. L., Sherman W. (2009). A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J. Am. Coll. Cardiol. 54, 2277–2286 10.1016/j.jacc.2009.06.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemi S. M., Ghods S., Kolodgie F. D., Parcham-Azad K., Keane M., Hamamdzic D., Young R., Rippy M. K., Virmani R., Litt H., Wilensky R. L. (2008). A placebo controlled, dose-ranging, safety study of allogenic mesenchymal stem cells injected by endomyocardial delivery after an acute myocardial infarction. Eur. Heart J. 29, 251–259 10.1093/eurheartj/ehm559 [DOI] [PubMed] [Google Scholar]
- Herbots L., D’hooge J., Eroglu E., Thijs D., Ganame J., Claus P., Dubois C., Theunissen K., Bogaert J., Dens J., Kalantzi M., Dymarkowski S., Bijnens B., Belmans A., Boogaerts M., Sutherland G., Van de Werf F., Rademakers F., Janssens S. (2009). Improved regional function after autologous bone marrow-derived stem cell transfer in patients with acute myocardial infarction: a randomized, double-blind strain rate imaging study. Eur. Heart J. 30, 662–670 10.1093/eurheartj/ehn532 [DOI] [PubMed] [Google Scholar]
- Huikuri H. V., Kervinen K., Niemelä M., Ylitalo K., Säily M., Koistinen P., Savolainen E. R., Ukkonen H., Pietilä M., Airaksinen J. K., Knuuti J., Mäkikallio T. H., FINCELL Investigators (2008). Effects of intracoronary injection of mononuclear bone marrow cells on left ventricular function, arrhythmia risk profile, and restenosis after thrombolytic therapy of acute myocardial infarction. Eur. Heart J. 29, 2723–2732 10.1093/eurheartj/ehn436 [DOI] [PubMed] [Google Scholar]
- Jackson K. A., Majka S. M., Wang H., Pocius J., Hartley C. J., Majesky M. W., Entman M. L., Michael L. H., Hirschi K. K., Goodell M. A. (2001). Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J. Clin. Invest. 107, 1395–1402 10.1172/JCI12150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens S., Dubois C., Bogaert J., Theunissen K., Deroose C., Desmet W., Kalantzi M., Herbots L., Sinnaeve P., Dens J., Maertens J., Rademakers F., Dymarkowski S., Gheysens O., Van Cleemput J., Bormans G., Nuyts J., Belmans A., Mortelmans L., Boogaerts M., Van de Werf F. (2006). Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet 367, 113–121 10.1016/S0140-6736(05)67861-0 [DOI] [PubMed] [Google Scholar]
- Kang H. J., Lee H. Y., Na S. H., Chang S. A., Park K. W., Kim H. K., Kim S. Y., Chang H. J., Lee W., Kang W. J., Koo B. K., Kim Y. J., Lee D. S., Sohn D. W., Han K. S., Oh B. H., Park Y. B., Kim H. S. (2006). Differential effect of intracoronary infusion of mobilized peripheral blood stem cells by granulocyte colony-stimulating factor on left ventricular function and remodeling in patients with acute myocardial infarction versus old myocardial infarction: the MAGIC cell-3-DES randomized, controlled trial. Circulation 114, I145–51 10.1161/CIRCULATIONAHA.105.001107 [DOI] [PubMed] [Google Scholar]
- Kocher A. A., Schuster M. D., Szabolcs M. J., Takuma S., Burkhoff D., Wang J., Homma S., Edwards N. M., Itescu S. (2001). Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat. Med. 7, 430–436 10.1038/86498 [DOI] [PubMed] [Google Scholar]
- Kondo C., Fukushima K., Kusakabe K. (2003). Measurement of left ventricular volumes and ejection fraction by quantitative gated SPET, contrast ventriculography and magnetic resonance imaging: a meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 6, 851–858 10.1007/s00259-003-1146-9 [DOI] [PubMed] [Google Scholar]
- Koskenvuo J. W., Karra H., Lehtinen J., Niemi P., Parkka J., Knuuti J., Hartiala J. J. (2007). Cardiac MRI: accuracy of simultaneous measurement of left and right ventricular parameters using three different sequences. Clin. Physiol. Funct. Imaging 27, 385–393 10.1111/j.1475-097X.2007.00764.x [DOI] [PubMed] [Google Scholar]
- Koskenvuo J. W., Sakuma H., Niemi P., Toikka J. O., Knuuti J., Laine H., Komu M., Kormano M., Saraste M., Hartiala J. J. (2001). Global myocardial blood flow and global flow reserve measurements by MRI and PET are comparable. J. Magn. Reson. Imaging 13, 361–366 10.1002/jmri.1051 [DOI] [PubMed] [Google Scholar]
- Link J. M., Caldwell J. H. (2008). Diagnostic and prognostic imaging of the cardiac sympathetic nervous system. Nat. Clin. Pract. Cardiovasc. Med. 5(Suppl. 2), S79–S86 10.1038/ncpcardio1150 [DOI] [PubMed] [Google Scholar]
- Lipinski M. J., Biondi-Zoccai G. G., Abbate A., Khianey R., Sheiban I., Bartunek J., Vanderheyden M., Kim H. S., Kang H. J., Strauer B. E., Vetrovec G. W. (2007). Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: a collaborative systematic review and meta-analysis of controlled clinical trials. J. Am. Coll. Cardiol. 50, 1761–1767 10.1016/j.jacc.2007.07.041 [DOI] [PubMed] [Google Scholar]
- Losordo D. W., Dimmeler S. (2004). Therapeutic angiogenesis and vasculogenesis for ischemic disease: part II: cell-based therapies. Circulation 109, 2692–2697 10.1161/01.CIR.0000128595.79378.FA [DOI] [PubMed] [Google Scholar]
- Lunde K., Solheim S., Aakhus S., Arnesen H., Abdelnoor M., Egeland T., Endresen K., Ilebekk A., Mangschau A., Fjeld J. G., Smith H. J., Taraldsrud E., Grogaard H. K., Bjornerheim R., Brekke M., Muller C., Hopp E., Ragnarsson A., Brinchmann J. E., Forfang K. (2006). Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N. Engl. J. Med. 355, 1199–1209 10.1056/NEJMoa055706 [DOI] [PubMed] [Google Scholar]
- Mäki M., Luotolahti M., Nuutila P., Iida H., Voipio-Pulkki L. M., Ruotsalainen U., Haaparanta M., Solin O., Hartiala J., Härkonen R., Knuuti J. (1996). Glucose uptake in the chronically dysfunctional but viable myocardium. Circulation 93, 1658–1666 [DOI] [PubMed] [Google Scholar]
- Meluzín J., Janousek S., Mayer J., Groch L., Hornacek I., Hlinomaz O., Kala P., Panovsky R., Prasek J., Kaminek M., Stanicek J., Klabusay M., Koristek Z., Navratil M., Dusek L., Vinklarkova J. (2008). Three-, 6-, and 12-month results of autologous transplantation of mononuclear bone marrow cells in patients with acute myocardial infarction. Int. J. Cardiol. 128, 185–192 10.1016/j.ijcard.2007.04.098 [DOI] [PubMed] [Google Scholar]
- Meluzín J., Mayer J., Groch L., Janousek S., Hornácek I., Hlinomaz O., Kala P., Panovský R., Prásek J., Kamínek M., Stanícek J., Klabusay M., Korístek Z., Navrátil M., Dusek L., Vinklárková J. (2006). Autologous transplantation of mononuclear bone marrow cells in patients with acute myocardial infarction: the effect of the dose of transplanted cells on myocardial function. Am. Heart J. 152, 9–15 10.1016/j.ahj.2006.08.004 [DOI] [PubMed] [Google Scholar]
- Mewton N., Revel D., Bonnefoy E., Ovize M., Croisille P. (2011). Comparison of visual scoring and quantitative planimetry methods for estimation of global infarct size on delayed enhanced cardiac MRI and validation with myocardial enzymes. Eur. J. Radiol. 78, 87–92 10.1016/j.ejrad.2009.09.027 [DOI] [PubMed] [Google Scholar]
- Meyer G. P., Wollert K. C., Lotz J., Steffens J., Lippolt P., Fichtner S., Hecker H., Schaefer A., Arseniev L., Hertenstein B., Ganser A., Drexler H. (2006). Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months’ follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation 113, 1287–1294 10.1161/CIRCULATIONAHA.105.575118 [DOI] [PubMed] [Google Scholar]
- Murry C. E., Soonpaa M. H., Reinecke H., Nakajima H., Nakajima H. O., Rubart M., Pasumarthi K. B., Virag J. I., Bartelmez S. H., Poppa V., Bradford G., Dowell J. D., Williams D. A., Field L. J. (2004). Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature 428, 664–668 10.1038/nature02446 [DOI] [PubMed] [Google Scholar]
- Någren K., Muller L., Halldin C., Swahn C. G., Lehikoinen P. (1995). Improved synthesis of some commonly used PET radioligands by the use of [11C]methyl triflate. Nucl. Med. Biol. 22, 235–239 10.1016/0969-8051(94)00083-V [DOI] [PubMed] [Google Scholar]
- Nesterov S. V., Han C., Mäki M., Kajander S., Naum A. G., Helenius H., Lisinen I., Ukkonen H., Pietilä M., Joutsiniemi E., Knuuti J. (2009). Myocardial perfusion quantitation with 15O-labelled water PET: high reproducibility of the new cardiac analysis software (carimas). Eur. J. Nucl. Med. Mol. Imaging 36, 1594–1602 10.1007/s00259-009-1143-8 [DOI] [PubMed] [Google Scholar]
- Nuutila P., Mäki M., Laine H., Knuuti M. J., Ruotsalainen U., Luotolahti M., Haaparanta M., Solin O., Jula A., Koivisto V. A. (1995). Insulin action on heart and skeletal muscle glucose uptake in essential hypertension. J. Clin. Invest. 96, 1003–1009 10.1172/JCI118085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obradović S., Balint B., Romanovic R., Trifunovic Z., Rusovic S., Baskot B., Dopudja M., Trifunovic G., Rafajlovski S., Jung R., Gligic B. (2009). Influence of intracoronary injections of bone-marrow-derived mononuclear cells on large myocardial infarction outcome: quantum of initial necrosis is the key. Vojnosanit. Pregl. 66, 998–1004 10.2298/VSP0912998O [DOI] [PubMed] [Google Scholar]
- Orlic D., Kajstura J., Chimenti S., Jakoniuk I., Anderson S. M., Li B., Pickel J., McKay R., Nadal-Ginard B., Bodine D. M., Leri A., Anversa P. (2001). Bone marrow cells regenerate infarcted myocardium. Nature 410, 701–705 10.1038/35070587 [DOI] [PubMed] [Google Scholar]
- Pietilä M., Malminiemi K., Ukkonen H., Saraste M., Någren K., Lehikoinen P., Voipio-Pulkki L. M. (2001). Reduced myocardial carbon-11 hydroxyephedrine retention is associated with poor prognosis in chronic heart failure. Eur. J. Nucl. Med. 28, 373–376 10.1007/s002590000449 [DOI] [PubMed] [Google Scholar]
- Pietilä M., Malminiemi K., Vesalainen R., Jartti T., Teräs M., Någren K., Lehikoinen P., Voipio-Pulkki L. M. (2002). Exercise training in chronic heart failure: beneficial effects on cardiac (11)C-hydroxyephedrine PET, autonomic nervous control, and ventricular repolarization. J. Nucl. Med. 43, 773–779 [PubMed] [Google Scholar]
- Rasband W. S. (2009). ImageJ v1.42q. Bethesda, MD: U. S. National Institutes of Health [Google Scholar]
- Rosenspire K. C., Haka M. S., Van Dort M. E., Jewett D. M., Gildersleeve D. L., Schwaiger M., Wieland D. M. (1990). Synthesis and preliminary evaluation of carbon-11-meta-hydroxyephedrine: a false transmitter agent for heart neuronal imaging. J. Nucl. Med. 31, 1328–1334 [PubMed] [Google Scholar]
- Rosenzweig A. (2006). Cardiac cell therapy – mixed results from mixed cells. N. Engl. J. Med. 355, 1274–1277 10.1056/NEJMe068172 [DOI] [PubMed] [Google Scholar]
- Saraste A., Koskenvuo J. W., Saraste M., Pärkkä J., Toikka J., Naum A., Ukkonen H., Knuuti J., Airaksinen J., Hartiala J. (2007). Coronary artery flow velocity profile measured by transthoracic doppler echocardiography predicts myocardial viability after acute myocardial infarction. Heart 93, 456–457 10.1136/hrt.2006.094995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachinger V., Erbs S., Elsasser A., Haberbosch W., Hambrecht R., Holschermann H., Yu J., Corti R., Mathey D. G., Hamm C. W., Suselbeck T., Assmus B., Tonn T., Dimmeler S., Zeiher A. M., REPAIR-AMI Investigators (2006a). Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N. Engl. J. Med. 355, 1210–1221 10.1056/NEJMoa060186 [DOI] [PubMed] [Google Scholar]
- Schachinger V., Erbs S., Elsasser A., Haberbosch W., Hambrecht R., Holschermann H., Yu J., Corti R., Mathey D. G., Hamm C. W., Suselbeck T., Werner N., Haase J., Neuzner J., Germing A., Mark B., Assmus B., Tonn T., Dimmeler S., Zeiher A. M., REPAIR-AMI Investigators (2006b). Improved clinical outcome after intracoronary administration of bone-marrow-derived progenitor cells in acute myocardial infarction: final 1-year results of the REPAIR-AMI trial. Eur. Heart J. 27, 2775–2783 10.1093/eurheartj/ehl388 [DOI] [PubMed] [Google Scholar]
- Schwaiger M., Kalff V., Rosenspire K., Haka M. S., Molina E., Hutchins G. D., Deeb M., Wolfe E., Jr., Wieland D. M. (1990). Noninvasive evaluation of sympathetic nervous system in human heart by positron emission tomography. Circulation 82, 457–464 10.1161/01.CIR.82.2.457 [DOI] [PubMed] [Google Scholar]
- Sipilä H. T., Clark J. C., Peltola O., Teräs M. (2001). An automatic 15OH2O production system for heart and brain studies. J. Labelled Comp. Radiopharm. 44, S1066–S1088 [Google Scholar]
- Strauer B. E., Brehm M., Zeus T., Kostering M., Hernandez A., Sorg R. V., Kogler G., Wernet P. (2002). Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation 106, 1913–1918 10.1161/01.CIR.0000034046.87607.1C [DOI] [PubMed] [Google Scholar]
- Sutton M. G., Sharpe N. (2000). Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation 101, 2981–2988 [DOI] [PubMed] [Google Scholar]
- Tendera M., Wojakowski W., Ruzyllo W., Chojnowska L., Kepka C., Tracz W., Musialek P., Piwowarska W., Nessler J., Buszman P., Grajek S., Breborowicz P., Majka M., Ratajczak M. Z., REGENT Investigators (2009). Intracoronary infusion of bone marrow-derived selected CD34+CXCR4+ cells and non-selected mononuclear cells in patients with acute STEMI and reduced left ventricular ejection fraction: results of randomized, multicentre myocardial regeneration by intracoronary infusion of selected population of stem cells in acute myocardial infarction (REGENT) trial. Eur. Heart J. 30, 1313–1321 10.1093/eurheartj/ehp073 [DOI] [PubMed] [Google Scholar]
- Traverse J., McKenna D., Harvey K., Jorgenso B., Olson R., Bostrom N., Kadidlo D., Lesser J., Jagadeesan V., Garberich R., Henry T. (2010). Results of a phase 1, randomized, double-blind, placebo-controlled trial of bone marrow mononuclear stem cell administration in patients following ST-elevation myocardial infaction. Am. Heart J. 160, 428–434 10.1016/j.ahj.2010.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Bogt K. E., Schrepfer S., Yu J., Sheikh A. Y., Hoyt G., Govaert J. A., Velotta J. B., Contag C. H., Robbins R. C., Wu J. C. (2009). Comparison of transplantation of adipose tissue- and bone marrow-derived mesenchymal stem cells in the infarcted heart. Transplantation 87, 642–652 10.1097/TP.0b013e31819609d9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Bogt K. E., Sheikh A. Y., Schrepfer S., Hoyt G., Cao F., Ransohoff K. J., Swijnenburg R. J., Pearl J., Lee A., Fischbein M., Contag C. H., Robbins R. C., Wu J. C. (2008). Comparison of different adult stem cell types for treatment of myocardial ischemia. Circulation 118, S121–S129 10.1161/CIRCULATIONAHA.107.759480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollert K. C., Drexler H. (2005). Clinical applications of stem cells for the heart. Circ. Res. 96, 151–163 10.1161/01.RES.0000155333.69009.63 [DOI] [PubMed] [Google Scholar]
- Wollert K. C., Meyer G. P., Lotz J., Ringes-Lichtenberg S., Lippolt P., Breidenbach C., Fichtner S., Korte T., Hornig B., Messinger D., Arseniev L., Hertenstein B., Ganser A., Drexler H. (2004). Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet 364, 141–148 10.1016/S0140-6736(04)16626-9 [DOI] [PubMed] [Google Scholar]
- Yeghiazarians Y., Zhang Y., Prasad M., Shih H., Saini S. A., Takagawa J., Sievers R. E., Wong M. L., Kapasi N. K., Mirsky R., Koskenvuo J., Minasi P., Ye J., Viswanathan M. N., Angeli F. S., Boyle A. J., Springer M. L., Grossman W. (2009). Injection of bone marrow cell extract into infarcted hearts results in functional improvement comparable to intact cell therapy. Mol. Ther. 17, 1250–1256 10.1038/mt.2009.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S. N., Sun A. J., Ge J. B., Yao K., Huang Z. Y., Wang K. Q., Zou Y. Z. (2009). Intracoronary autologous bone marrow stem cells transfer for patients with acute myocardial infarction: a meta-analysis of randomised controlled trials. Int. J. Cardiol. 136, 178–185 10.1016/j.ijcard.2008.04.028 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Short-axis cine MRI image (1 week) in BMC-treated patient. Short-axis cine MRI image stack at 1 week time-point from a patient with BMC therapy (same patient as in Figure 4A). End-diastolic volume was 192 mL and ejection fraction 41%.
Short-axis cine MRI image (6 months) in BMC-treated patient. Short-axis cine MRI image stack at 6 month from the same patient with BMC therapy as in Online Resource 1. End-diastolic volume was 204 mL and ejection fraction 43%.
Short-axis cine MRI image (1 week) in placebo treated patient. Short-axis cine MRI image stack at 1 week from a patient with placebo therapy. Same patient as in Figure 4B end-diastolic volume was 182 mL and ejection fraction 34%.
Short-axis cine MRI image (6 months) in placebo treated patient. Short axis cine MRI image stack at 6 month from the same patient with placebo therapy as in Online Resource 3. End-diastolic volume was 174 mL and ejection fraction 36%.