Abstract
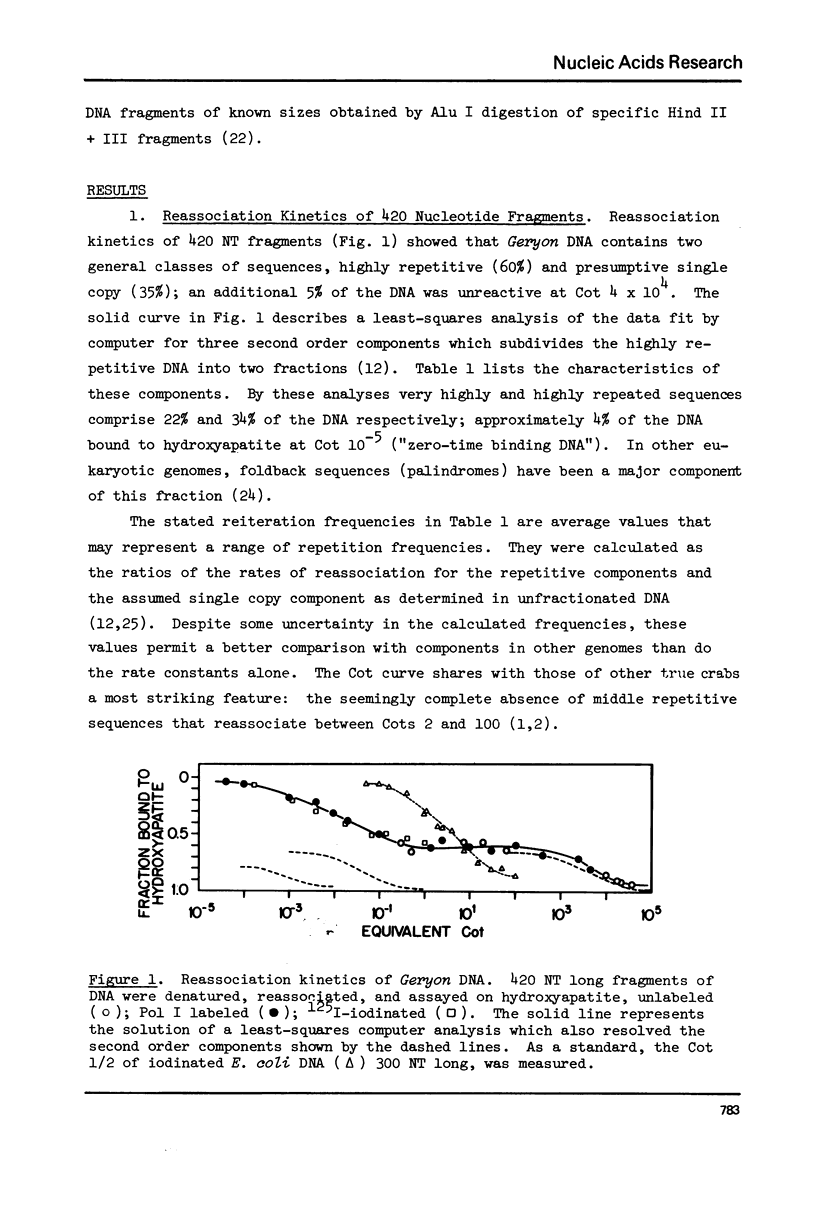
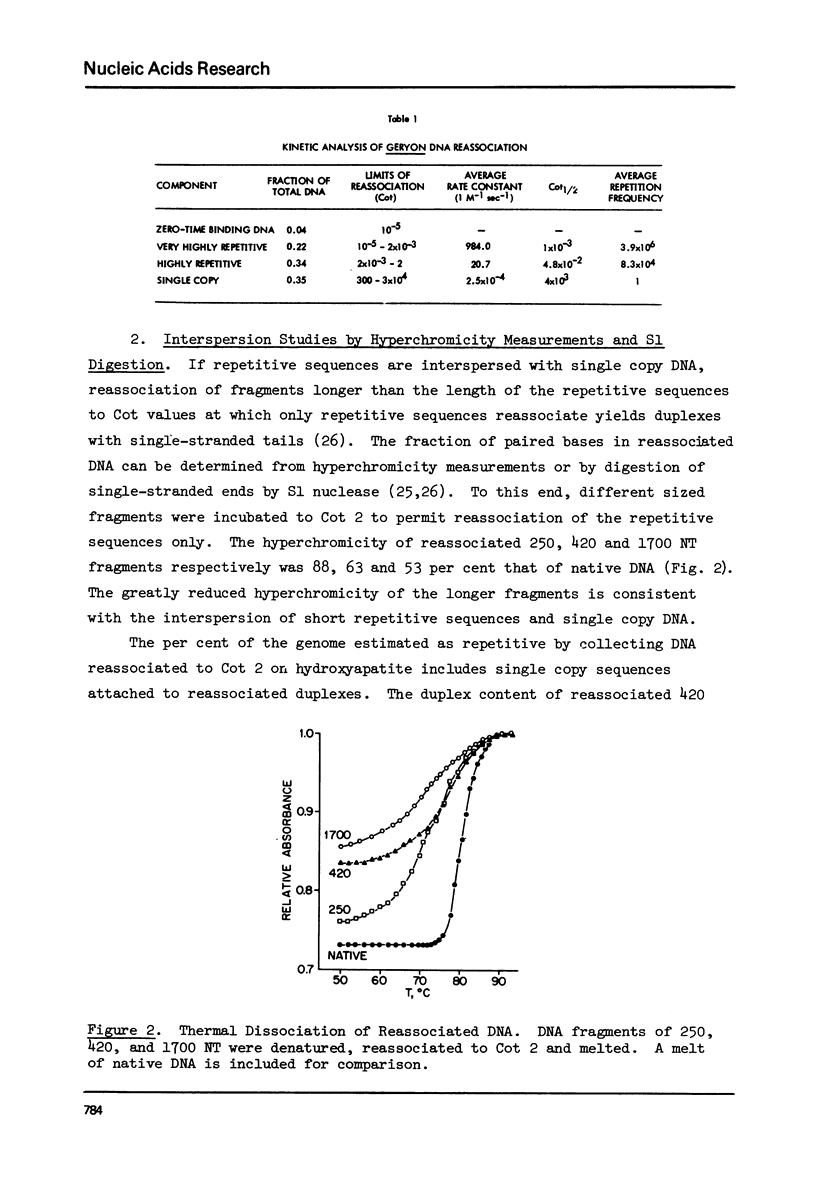
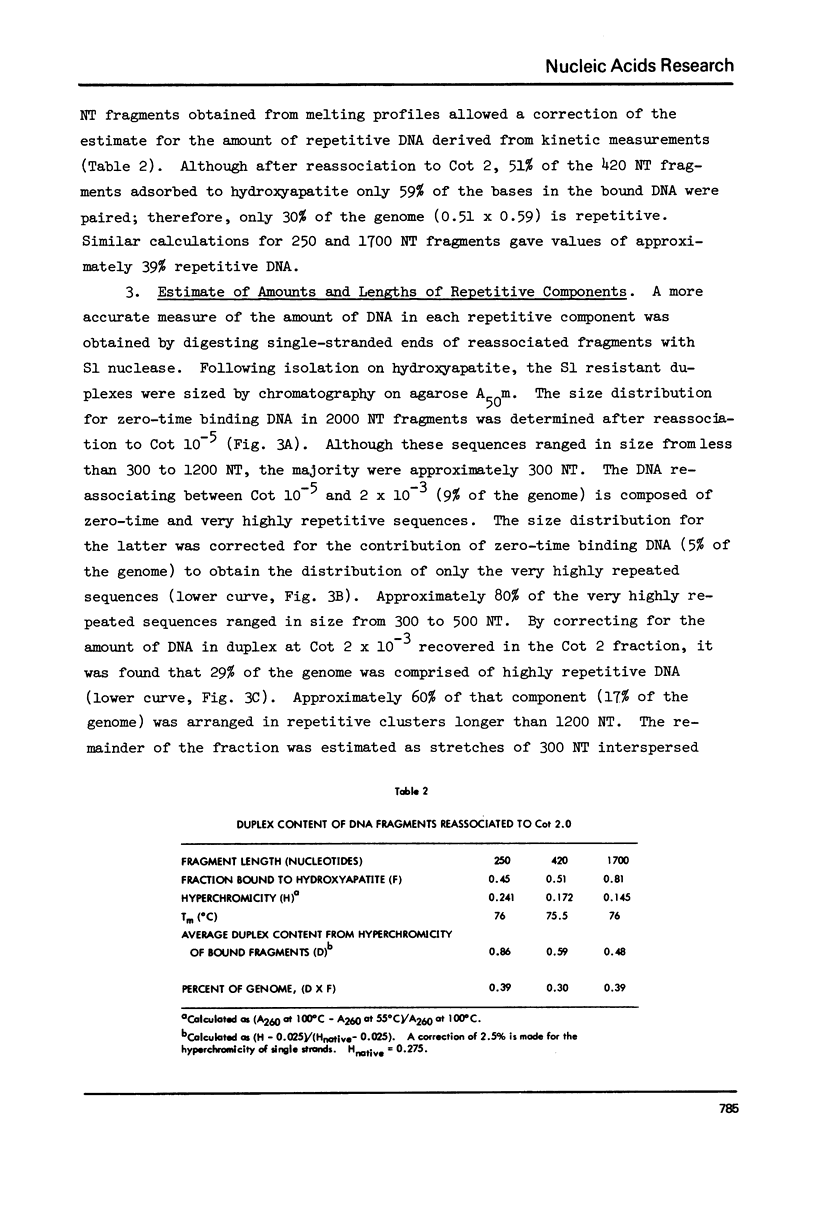
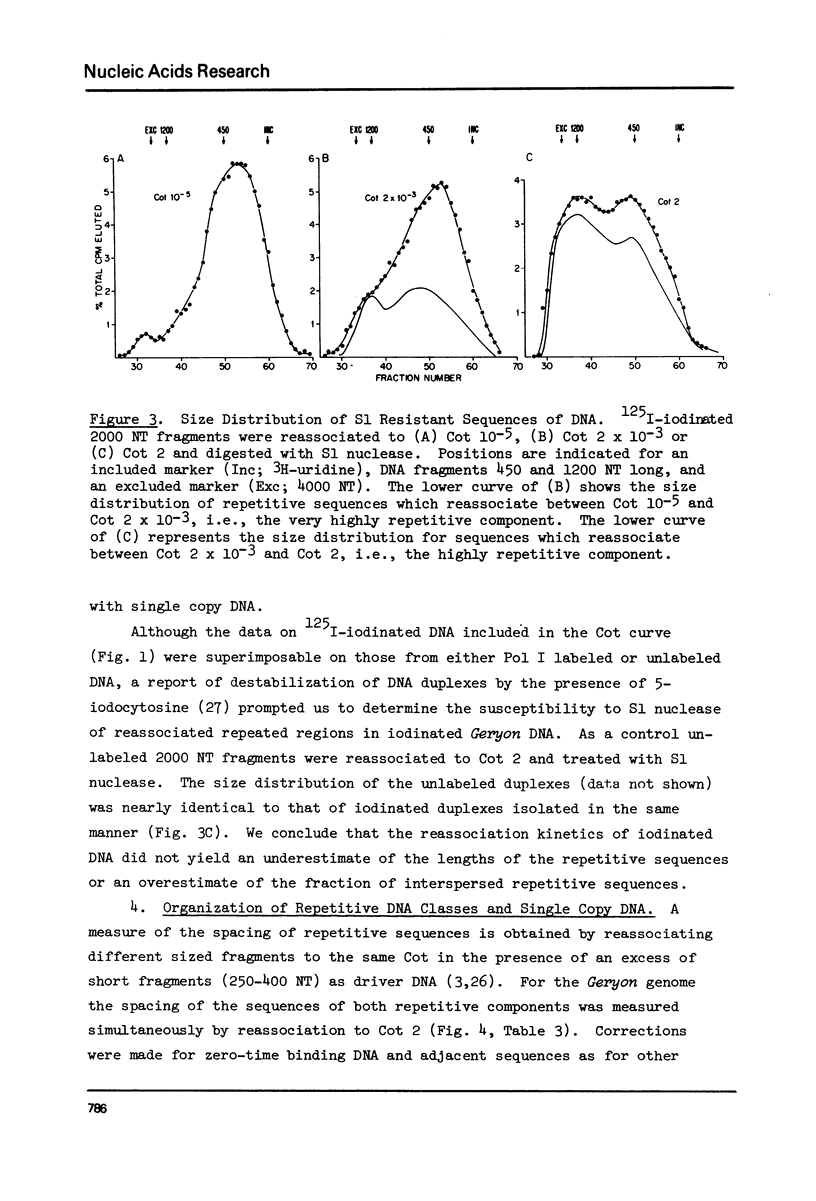
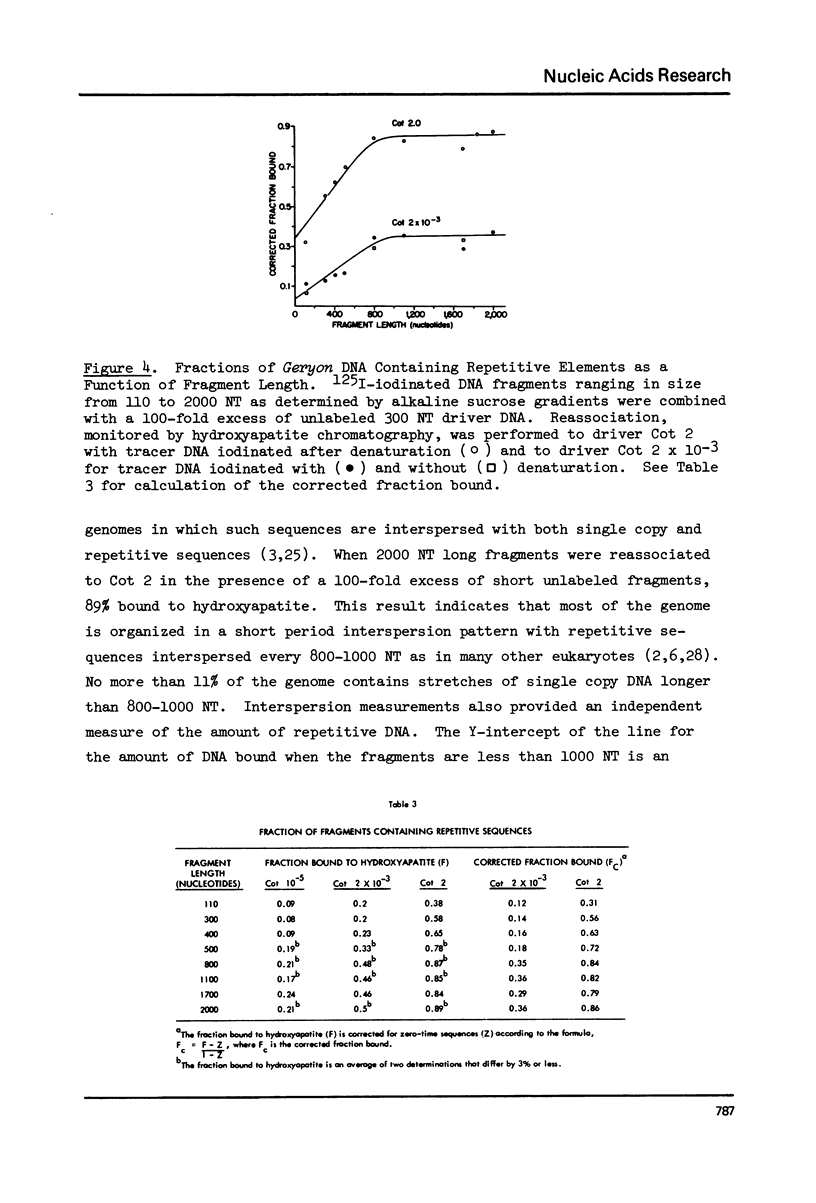
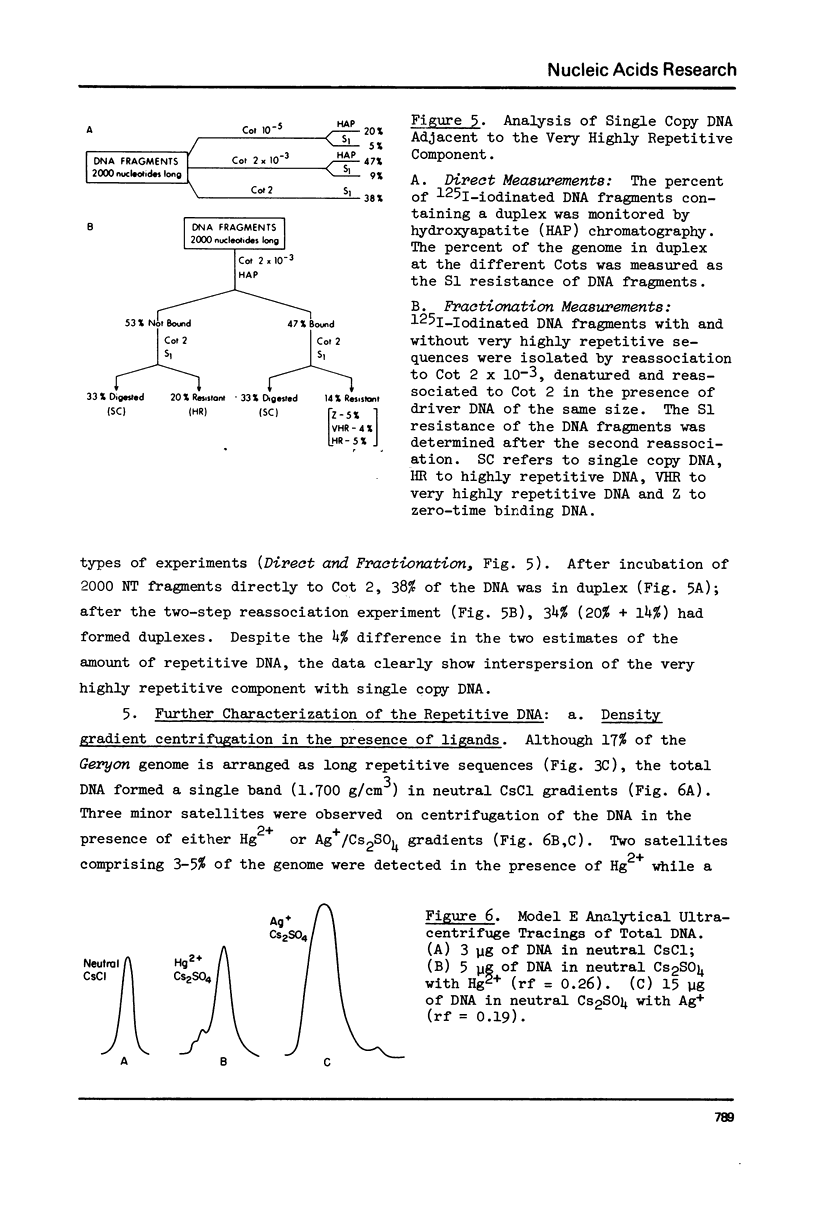
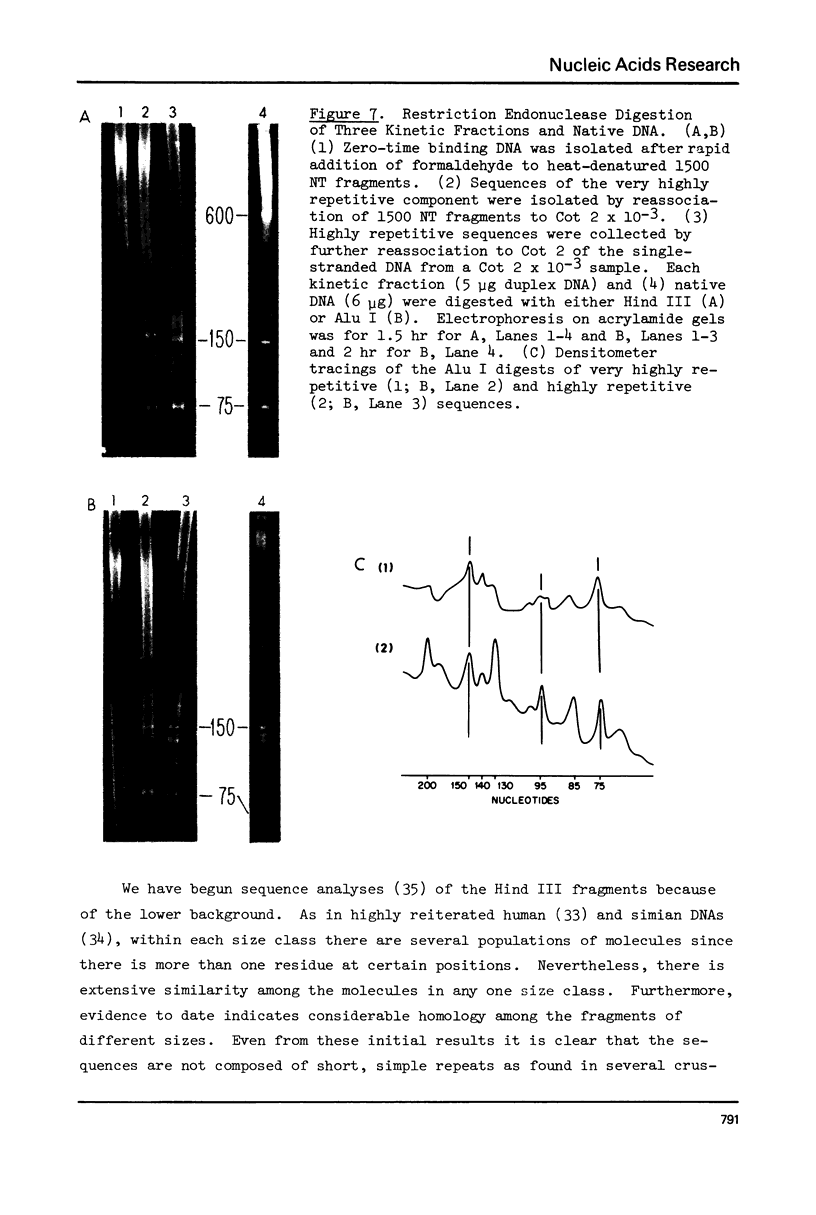
Kinetic analysis of the reassociation of 420 nucleotide (NT) long fragments has shown that essentially all of the repetitive sequences of the DNA of the red crab Geryon quinquedens are highly repetitive. There are negligible amounts of low and intermediate repetitive DNAs. Though atypical of most eukaryotes, this pattern has been observed in all other brachyurans (true crabs) studied (1,2). The major repetitive component is subdivided into short runs of 300 NT and longer runs of greater than 1200 NT while the minor component has an average sequence length of 400 NT. Both components reassociate at rates commonly observed for satellite DNAs. Unique among eukaryotes the organization of the genome includes single copy DNA contiguous to short runs (approximately 300 NT) of both repetitive components. Although patent satellites are not present, subsets of the repetitive DNA have been isolated by either restriction endonuclease digestion or by centrifugation in Ag+ or Hg2+/Cs2SO4 density gradients.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. M., Folk W. R. Iodination of DNA. Studies of the reaction and iodination of papovavirus DNA. Biochemistry. 1976 Mar 9;15(5):1022–1030. doi: 10.1021/bi00650a012. [DOI] [PubMed] [Google Scholar]
- Angerer R. C., Davidson E. H., Britten R. J. DNA sequence organization in the mollusc Aplysia californica. Cell. 1975 Sep;6(1):29–39. doi: 10.1016/0092-8674(75)90070-7. [DOI] [PubMed] [Google Scholar]
- Arthurand R. R., Straus N. A. DNA-sequence organization in the genome of the domestic chicken (Gallus domesticus). Can J Biochem. 1978 Apr;56(4):257–263. doi: 10.1139/o78-040. [DOI] [PubMed] [Google Scholar]
- Arthurand R. R., Straus N. A. DNA-sequence organization in the genome of the domestic chicken (Gallus domesticus). Can J Biochem. 1978 Apr;56(4):257–263. doi: 10.1139/o78-040. [DOI] [PubMed] [Google Scholar]
- Beattie W. G., Skinner D. M. The diversity of satellite DNAs of Crustacea. Biochim Biophys Acta. 1972 Oct 11;281(2):169–178. doi: 10.1016/0005-2787(72)90169-4. [DOI] [PubMed] [Google Scholar]
- Botchan M. R. Bovine satellite I DNA consists of repetitive units 1,400 base pairs in length. Nature. 1974 Sep 27;251(5473):288–292. doi: 10.1038/251288a0. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Davidson E. H. Repetitive and non-repetitive DNA sequences and a speculation on the origins of evolutionary novelty. Q Rev Biol. 1971 Jun;46(2):111–138. doi: 10.1086/406830. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Graham D. E., Neufeld B. R. Analysis of repeating DNA sequences by reassociation. Methods Enzymol. 1974;29:363–418. doi: 10.1016/0076-6879(74)29033-5. [DOI] [PubMed] [Google Scholar]
- Chambers C. A., Schell M. P., Skinner D. M. The primary sequence of a crustacean satellite DNA containing a family of repeats. Cell. 1978 Jan;13(1):97–110. doi: 10.1016/0092-8674(78)90141-1. [DOI] [PubMed] [Google Scholar]
- Commerford S. L. Iodination of nucleic acids in vitro. Biochemistry. 1971 May 25;10(11):1993–2000. doi: 10.1021/bi00787a005. [DOI] [PubMed] [Google Scholar]
- Cooke H. J. Evolution of the long range structure of satellite DNAs in the genus Apodemus. J Mol Biol. 1975 May 5;94(1):87–99. doi: 10.1016/0022-2836(75)90406-4. [DOI] [PubMed] [Google Scholar]
- Cordeiro-Stone M., Lee C. S. Studies on the satellite DNAs of Drosophila nasutoides: their buoyant densities, melting temperatures, reassociation rates and localizations in polytene chromosomes. J Mol Biol. 1976 Jun 14;104(1):1–24. doi: 10.1016/0022-2836(76)90002-4. [DOI] [PubMed] [Google Scholar]
- Crain W. R., Davidson E. H., Britten R. J. Contrasting patterns of DNA sequence arrangement in Apis mellifera (honeybee) and Musca domestica (housefly). Chromosoma. 1976 Dec 6;59(1):1–12. doi: 10.1007/BF00327705. [DOI] [PubMed] [Google Scholar]
- Davidson E. H., Hough B. R., Amenson C. S., Britten R. J. General interspersion of repetitive with non-repetitive sequence elements in the DNA of Xenopus. J Mol Biol. 1973 Jun 15;77(1):1–23. doi: 10.1016/0022-2836(73)90359-8. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A., Crain W. R., Britten R. J., Davidson E. H., Kafatos F. C. DNA sequence organization in the lepidopteran Antheraea pernyi. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2289–2293. doi: 10.1073/pnas.73.7.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getz M. J., Altenburg L. C., Saunders G. F. The use of RNA labeled in vitro with iodine-125 in molecular hybridization experiments. Biochim Biophys Acta. 1972 Dec 22;287(3):485–494. doi: 10.1016/0005-2787(72)90293-6. [DOI] [PubMed] [Google Scholar]
- Gruss P., Sauer G. Repetitive primate DNA containing the recognition sequences for two restriction endonucleases which generate cohesive ends. FEBS Lett. 1975 Dec 1;60(1):85–88. doi: 10.1016/0014-5793(75)80424-8. [DOI] [PubMed] [Google Scholar]
- Hudspeth M. E., Timberlake W. E., Goldberg R. B. DNA sequence organization in the water mold Achlya. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4332–4336. doi: 10.1073/pnas.74.10.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten W., Kersten H., Szybalski W. Physicochemical properties of complexes between deoxyribonucleic acid and antibiotics which affect ribonucleic acid synthesis (actinomycin, daunomycin, cinerubin, nogalamycin, chormomycin, mithramycin, and olivomycin). Biochemistry. 1966 Jan;5(1):236–244. doi: 10.1021/bi00865a031. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Manning J. E., Schmid C. W., Davidson N. Interspersion of repetitive and nonrepetitive DNA sequences in the Drosophila melanogaster genome. Cell. 1975 Feb;4(2):141–155. doi: 10.1016/0092-8674(75)90121-x. [DOI] [PubMed] [Google Scholar]
- Manuelidis L., Wu J. C. Homology between human and simian repeated DNA. Nature. 1978 Nov 2;276(5683):92–94. doi: 10.1038/276092a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowbray S. L., Gerbi S. A., Landy A. Interdigitated repeated sequences in bovine satellite DNA. Nature. 1975 Jan 31;253(5490):367–370. doi: 10.1038/253367a0. [DOI] [PubMed] [Google Scholar]
- Orosz J. M., Wetmur J. G. In vitro iodination of DNA. Maximizing iodination while minimizing degradation; use of buoyant density shifts for DNA-DNA hybrid isolation. Biochemistry. 1974 Dec 31;13(27):5467–5473. doi: 10.1021/bi00724a003. [DOI] [PubMed] [Google Scholar]
- Roberts R. J., Myers P. A., Morrison A., Murray K. A specific endonuclease from Arthrobacter luteus. J Mol Biol. 1976 Mar 25;102(1):157–165. doi: 10.1016/0022-2836(76)90079-6. [DOI] [PubMed] [Google Scholar]
- Rosenberg H., Singer M., Rosenberg M. Highly reiterated sequences of SIMIANSIMIANSIMIANSIMIANSIMIAN. Science. 1978 Apr 28;200(4340):394–402. doi: 10.1126/science.205944. [DOI] [PubMed] [Google Scholar]
- Skinner D. M., Beattie W. G., Blattner F. R., Stark B. P., Dahlberg J. E. The repeat sequence of a hermit crab satellite deoxyribonucleic acid is (-T-A-G-G-)n-(-A-T-C-C-)n. Biochemistry. 1974 Sep 10;13(19):3930–3937. doi: 10.1021/bi00716a018. [DOI] [PubMed] [Google Scholar]
- Skinner D. M., Beattie W. G. Cs2S04 gradients containing both Hg2+ and Ag+ effect the complete separation of satellite deoxyribonucleic acids having identical densities in neutral CsCl gradients. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3108–3110. doi: 10.1073/pnas.70.11.3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner D. M. SATELLITE DNA'S IN THE CRABS Gecarcinus lateralis AND Cancer pagurus. Proc Natl Acad Sci U S A. 1967 Jul;58(1):103–110. doi: 10.1073/pnas.58.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn J. C. DNA reassociation kinetics and chromosome structure in the crabs Cancer borealis and Libinia emarginata. Chromosoma. 1975;50(3):243–257. doi: 10.1007/BF00283469. [DOI] [PubMed] [Google Scholar]
- Walbot V., Dure L. S., 3rd Developmental biochemistry of cotton seed embryogenesis and germination. VII. Characterization of the cotton genome. J Mol Biol. 1976 Mar 15;101(4):503–536. doi: 10.1016/0022-2836(76)90242-4. [DOI] [PubMed] [Google Scholar]
- Waring M., Britten R. J. Nucleotide sequence repetition: a rapidly reassociating fraction of mouse DNA. Science. 1966 Nov 11;154(3750):791–794. doi: 10.1126/science.154.3750.791. [DOI] [PubMed] [Google Scholar]
- Wilson D. A., Thomas C. A., Jr Palindromes in chromosomes. J Mol Biol. 1974 Mar 25;84(1):115–138. doi: 10.1016/0022-2836(74)90216-2. [DOI] [PubMed] [Google Scholar]




