Abstract
Similar to hematopoietic stem cells, memory lymphocytes self renew, while their clonally expanded effector progeny differentiate to fight infection and tumors. Recently, Muranski et al. report in Immunity (2011) that a subset of Th17 effector cells functions as memory cells and retain stem cell properties.
A fundamental feature of the adaptive immune system is its capacity for ‘remembering’ previous infections and responding more vigorously upon re-exposure to a pathogen. Upon initial infection, naive T cells are stimulated through their unique T cell receptor to undergo rapid clonal expansion and differentiation. The majority of responding T cells becomes terminally differentiated effector cells that help eradicate the infection and subsequently undergo programmed cell death. However, a small minority of clonal progeny survives this contraction phase and become long-lived, self-renewing memory T cells. Upon re-infection, these persistent memory cells more rapidly acquire effector functions, forming the cellular basis for successful vaccination.
It has long been thought that self-renewal of memory lymphocytes shares features with hematopoietic stem cells (HSCs) (Fearon et al., 2001). Indeed, memory CD8+ T, CD4+ T and B cells are likely the only mature blood cells other than HSCs capable of long-term self-renewal, and a comprehensive comparative analysis established that memory CD8+ T and B cells share at least a partially conserved transcriptional profile with HSCs (Luckey et al., 2006). Recent reports have further suggested that memory T cells are generated by asymmetric cell divisions akin to those that drive stem cells (Chang et al., 2007). Thus, there is mounting descriptive evidence for common strategies employed by memory lymphocytes and HSCs by which they retain the capacity for self-renewal. A new study published by Muranski et al. in Immunity now identify stem-like properties in a subset of effector CD4+ Tcells termed Th17 cells, and through transcriptome profiling identify a molecular pathway associated with their generation (Muranski et al., 2011).
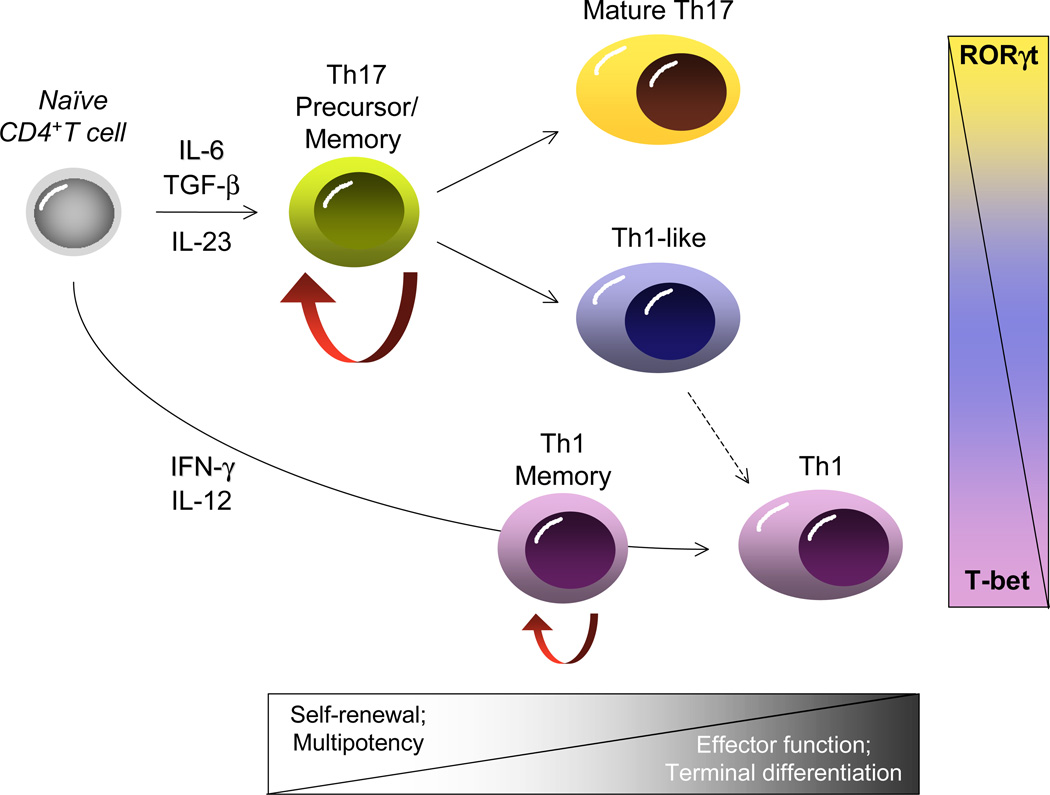
Whereas much of our understanding of T cell memory has been attained through studies of CD8+ T cells, recent strides have been made in defining the features of memory CD4+ T cells (Pepper and Jenkins, 2011). Because naïve CD4+ T cells can differentiate into a diversity of functional effectors contingent upon the type of microbial challenge (e.g., Th1, Th2 and Th17 cells), there is added complexity to understanding CD4+ T cell memory (Figure 1). This is further complicated by the recent demonstration that CD4+ effectors, particularly Th17 cells, have considerable flexibility in their late developmental programming that allows them to acquire at least some features of Th1 cells contingent upon the prevailing cytokine signals they receive during recall responses (Lee et al., 2009). The developmental diversity of CD4+ T cells has therefore posed unique challenges in defining memory in this lineage, and it remains an open question whether the molecular mechanisms that maintain HSC self-renewal are functional in CD4+ T cells, and if so, which ones.
Figure 1. Divergent potential for self-renewal and plasticity within CD4+ T cell developmental pathways.
Upon initial antigen recognition, naïve T cells diverge along distinct effector pathways contingent upon on the prevailing cytokine milieu and the key transcription factors they induce (RORγt, Th17; T-bet, Th1). The majority of cells will be driven towards terminally differentiated effectors that direct the immune response and are short-lived. A small minority of effectors enter a long-lived, self-renewing memory pool dependent on cytokines such as IL-7 for their homeostasis (red arrows). Unlike Th1 cells, Th17 cells appear to transit through a progenitor like stage (Th17 precursor), with both enhanced self-renewal characteristics and potential for divergent late developmental fates guided by the balance of key transcription factors induced upon antigen re-encounter.
In their previous work, Restifo and colleagues identified a subpopulation of CD8+ T cells, termed ‘T memory stem cells’ (TSCM), that were derived by ex vivo stimulation of a clonal population of naïve CD8+ T cells in the presence of a GSK3-β inhibitor (Gattinoni et al., 2009). These TSCM cells accumulated high levels of β-catenin, had superior self-renewal capabilities, expressed memory transcriptional profiles, and displayed enhanced anti-tumor activity in comparison to other memory subsets, including T central memory (TCM) or T effector memory (TEM) cells. It was proposed that the GSK3-β inhibitor mimicked activation of the Wnt–β-catenin axis and conferred increased ‘stemness’ properties, providing a renewable reservoir from which anti-tumor effector T cells differentiated. In the current Immunity paper, they find a similar stem cell-like molecular signature in Th17, but not Th1, effector cells generated ex vivo. Interestingly, Th17 cells accumulated β-catenin and were more transcriptionally similar to memory CD8+ T cells than their Th1 counterparts, consistent with a less differentiated state. Further, like TSCM cells, Th17 cells underwent long-term self-renewal and more effectively eradicated established melanoma tumors when transferred into recipient mice, suggesting a common link between the capacity for self-renewal and sustained anti-tumor responses. Interestingly, as previously described in autoimmune models of chronic Th17 responses (Murphy and Stockinger, 2010), effective tumor clearance and autoimmune destruction of melanocytes required the development of Th1-like progeny from Th17 precursors, such that this effect was dependant not only on the Th17 cytokine IL-17A, but also on the Th1 cytokine IFN-γ and the Th1 master transcription factor T-bet.
While providing important new insights into the origins of CD4+ memory cells, and, at least in the case of Th17 cells, establishing new links between stem cell characteristics and late developmental multipotency, this report also raises a number of questions that will require further investigation. The relatively poor capacity of Th1 cells derived ex vivo to survive long term and contribute to the memory pool in the current study would appear to be at odds with several reports that have documented the potential of Th1 effector cells to contribute to long-lived memory in the setting of infection. Insofar as Th17 cells appear to have higher self-renewal capacity than Th1 cells, and when induced to differentiate into Th1-like cells demonstrate restricted developmental flexibility, it is likely that Th17 cells are not unique among effector CD4+ T cell subsets in their potential for long-lived memory but are just better at it due to a less fully committed differentiative state. Alternatively, it is possible that ex vivo conditions used for T cell programming in this study might not mimic physiologic conditions induced by in vivo infections. Though adoptive transfer of lymphocytes expanded ex vivo into sublethally irradiated hosts adheres closely to recent therapeutic protocols for tumor immunotherapy in cancer patients, it is unclear whether signaling pathways are similar to those activated during memory generation induced by infections in lympho-replete hosts. For instance, it is well known that T cells transferred into sublethally irradiated hosts undergo extensive homeostatic proliferation and maturation of T cells driven by excess, non-physiologic levels of IL-7 and IL-15, two key cytokines that support memory T cell homeostatic self-renewal. Future studies that perform fate mapping and transcriptional profiling of effector CD4+ T cells populations derived in the course of natural infections should help to resolve this issue.
Further, while Restifo and colleagues clearly establish expression parallels between CD8 and CD4 T cell memory subsets that include downstream targets of the Wnt–β-catenin signaling pathway, its precise contribution to memory cell development and function remains to be defined. Although it is tempting to speculate that nuclear β-catenin signaling contributes to the stem cell-like phenotype of memory T cells as it does in several adult stem cell populations, recent reports provide direct evidence that, at least for CD8+ T cells, β-catenin is entirely dispensable for induction of memory (Driessens et al., 2010; Prlic and Bevan, 2011). This suggests that alternate pathways will need to be investigated. Whether or not the increased β-catenin observed in Th17 cells contributes to the increased self-renewal also remains to be determined. Finally, given the diversity of signaling pathways known to impact PI3K/AKT/GSK3-β activity in lymphocytes, the physiologic drivers that modulate activity of this pathway in developing memory cells in vivo remain be determined.
Collectively these studies represent an important advance in the field of CD4+ T cell memory, and provide critical clues regarding the potential signaling mechanisms shared with HSCs that drive memory T cell generation. Although the overall differentiation potential of memory lymphocytes is more restricted than that of HSCs, these studies reinforce the notion that the molecular challenges faced by memory T cells are similar to those of HSCs, albeit on a smaller scale. Both cell types must rely on external self-renewing signals to maintain homeostatic proliferation without terminal differentiation. Both must maintain “open” epigenetic states at multiple genetic loci that are converted to more terminal and fixed states upon induction via external differentiation cues. Thus, these new data support the general hypothesis that memory lymphocytes rely on a subset of stem cell self-renewal programs that are reactivated during their development (Luckey et al., 2006), and set the stage for identification of the pathway(s) and conserved targets that are functionally active in both memory T cells and HSCs. Ultimately, these findings could have considerable impact on novel approaches to enhance vaccine strategies and anti-tumor immunity, as well as approaches to curb autoimmunity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chang JT, Palanivel VR, Kinjyo I, Schambach F, Intlekofer AM, Banerjee A, Longworth SA, Vinup KE, Mrass P, Oliaro J, et al. Science. 2007;315:1687–1691. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- Driessens G, Zheng Y, Gajewski TF. Nature Medicine. 2010;16:513–514. doi: 10.1038/nm0510-513. [DOI] [PubMed] [Google Scholar]
- Fearon DT, Manders P, Wagner SD. Science. 2001;293:248–250. doi: 10.1126/science.1062589. [DOI] [PubMed] [Google Scholar]
- Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, Almeida JR, Gostick E, Yu Z, Carpenito C, et al. Nat Med. 2011;17:1290–1297. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YK, Mukasa R, Hatton RD, Weaver CT. Curr Opin Immunol. 2009;21:274–280. doi: 10.1016/j.coi.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Luckey CJ, Bhattacharya D, Goldrath AW, Weissman IL, Benoist C, Mathis D. Proc Natl Acad Sci U S A. 2006;103:3304–3309. doi: 10.1073/pnas.0511137103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muranski P, Borman ZA, Kerkar SP, Klebanoff CA, Ji Y, Sanchez-Perez L, Sukumar M, Reger RN, Yu Z, Kern SJ, et al. Immunity. 2011;35:972–985. doi: 10.1016/j.immuni.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KM, Stockinger B. Nature Immunology. 2010;11:674–680. doi: 10.1038/ni.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper M, Jenkins MK. Nature Immunology. 2011;131:467–471. doi: 10.1038/ni.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prlic M, Bevan MJ. J Immunol. 2011;187:1542–1546. doi: 10.4049/jimmunol.1100907. [DOI] [PMC free article] [PubMed] [Google Scholar]