Abstract
Epidemiological studies have shown dietary magnesium (Mg) intake and serum Mg levels to be inversely correlated with the development of atherosclerosis. We hypothesized that low levels of Mg would promote atherosclerotic plaque development in rabbits. New Zealand white rabbits (4 months old, n = 22) were fed an atherogenic diet containing 0.12% (−Mg), 0.27% (control), or 0.43% (+Mg) Mg for 8 weeks. Blood samples were obtained at baseline, 2, 4, 6, and 8 weeks and were assayed for total cholesterol, high-density lipoprotein (HDL), non-HDL, triglycerides (TG), C-reactive protein, serum Mg, and erythrocyte Mg. Aortas from −Mg had significantly more plaque, with an intima thickness 42% greater than control and 36% greater than +Mg. Serum cholesterol levels rose over time, and at 8 weeks, −Mg had the highest and +Mg the lowest total and non-HDL cholesterol and TG levels, although these results did not reach significance. Over time, serum Mg levels increased, and erythrocyte Mg levels decreased. C-reactive protein significantly increased in all groups at 4 and 6 weeks but returned to baseline levels by 8 weeks. This study supports the hypothesis that inadequate intake of Mg results in an increase in atherosclerotic plaque development in rabbits.
Keywords: Magnesium, C-reactive protein, Cholesterol, Rabbit, Atherosclerosis
1. Introduction
The mean dietary intake of magnesium (Mg) in the United States has been estimated to be 288 mg/d [1], which is lower than the recommended daily allowance (RDA) (310 mg/d for women, 400 mg/d for men) [2]. This suggests that Mg intake from dietary sources in the United States is inadequate. Data from the 1999–2000 National Health and Nutrition Examination Survey (NHANES) indicated that African Americans, the less educated, and the elderly have the lowest intakes of Mg and 23% of US adults had hypomagnesemia (serum Mg concentration, <0.8 mmol/L) [2].
The American Heart Association reported in 2005 that coronary heart disease (CHD) was the single largest killer of Americans and that atherosclerosis was the cause of most cases of CHD [3]. The development of atherosclerosis involves a multitude of factors including dietary habits, glucose and insulin levels, increasing age, being male, and elevated blood lipid levels [4]. Traditional risk factors such as age, sex, elevated blood pressure, smoking, elevated low-density lipoprotein (LDL), and depressed high-density lipoprotein (HDL) have been clearly demonstrated to correlate with incidence of atherosclerosis [5]. More than any single factor, the “global risk profile” or the sum of all risk factors can provide better prediction of cardiovascular events [5]. Promising new risk factors for CHD include homocysteine, oxidized LDL, insulin levels, and C-reactive protein (CRP).
Several studies have reported significant depression in serum Mg levels of persons with vascular diseases, including the Atherosclerosis Risk in Communities study that showed an inverse relationship between dietary Mg and serum Mg with the development of carotid atherosclerosis [6]. Evaluation of NHANES data revealed that for the highest third and fourth quartiles of serum Mg concentration, the risk of dying from heart disease was 20% to 30% lower [7]. The Mg supplementation improved endothelial function in patients with CHD [8]. Not all studies have found this relationship. Serum Mg levels were found to not be associated with evident cardiovascular disease (CVD) [9,10]. Likewise, risk of CVD was not found to correlate to greater Mg intake, and death from CVD did not decrease with increasing levels of Mg intake [10].
The Mg deficiency has been shown to enhance endothelial injury and trigger vasoconstriction, and low serum levels of Mg appear to accelerate atherogenesis through promotion of inflammation and oxidative modification [11]. The Mg deficiency can promote the peroxidation of lipids and reduce antioxidant capacity in serum and tissues [12]. Dietary Mg restriction has been shown to increase serum TG, stimulate lipid peroxidation, and increase lipid deposition into the vascular wall in rodents [11]. Low serum Mg levels promote endothelial dysfunction, partially due to an up-regulation of inflammatory cytokines [13].
Animal studies have suggested that Mg deficiency can accelerate and intensify lipid deposition and lesions and that Mg supplementation can slow the progression of and prevent atherosclerosis [14]. The Mg deficiency has been reported to enhance endothelial injury [15]. Persons who consume less Mg were more likely to have elevated CRP, and individuals in quartiles lower than the RDA were more likely to have elevated CRP levels [13]. Ways in which Mg status may affect CRP levels are not known at this time, but it has been speculated that it is related to endothelial dysfunction or oxidative stress [13].
For the present study, the atherosclerotic rabbit model was chosen because rabbits rapidly develop high serum cholesterol levels that results in the accelerated development of atherosclerosis. Rabbits are prone to atherosclerosis through cholesterol feeding because they cannot increase the rate of excretion of sterols (as opposed to humans, which can increase rate of excretion), and as a result, the liver produces large quantities of lipoproteins that are atherogenic [16]. Our chosen 1% cholesterol diet level is well above what humans consume but is fairly typical for testing dietary effects on atherosclerosis development in rabbits. Previously, other researchers [12,14,17–19] have studied dietary Mg levels and atherosclerosis using rabbits fed similar dietary cholesterol. The rabbit model is also beneficial compared to rats or mice as we will be able to obtain sufficient blood and tissue for subsequent studies involving the adverse effects of ultrasound and ultrasound contrast agents on atherosclerosis.
The current work used a rabbit model fed hypercholesterolemic diets for 8 weeks with 3 differing Mg contents. The National Research Council (NRC) recommends that rabbits have a diet that is 0.3% to 0.4% Mg [20]. The goal of this study was to determine if adequate levels of Mg just below (0.27%; control) and just above (0.43%; +Mg) the NRC recommended range would suppress and if low levels of Mg (0.12%; −Mg) would increase atherosclerotic plaque development in rabbits. We hypothesized that atherosclerotic plaque development, serum cholesterol, and CRP levels would inversely correlate to dietary Mg in a dose-dependent manner.
2. Methods and materials
2.1. Animals and diets
Four-month-old male New Zealand white rabbits (n = 22) were acquired from Myrtle's Rabbitry, Thompson's Station, Tenn. After an acclimation period of 1 week on standard chow diet (catalog no. 2031 Harlan Teklad, Indianapolis, Ind), rabbits were weaned onto a semipurified atherogenic diet for 10 days by substituting 10% daily increments of cholesterol-containing diet for the standard rabbit chow. Diet samples were analyzed by an independent laboratory for Mg, fat, protein, and fiber content (Waters Agricultural Laboratories Inc, Camilla, Ga). The basal diet contained 10% fat, 1% cholesterol, 14% protein, 54% carbohydrates, and 0.12% Mg (see Table 1) (catalog no. 1811279, TestDiet, Richmond, Ind). Magnesium oxide was added to the diet to obtain levels of 0.27% Mg (catalog no. 1811280, TestDiet) and 0.43% Mg (catalog no. 1811281, TestDiet). Rabbits were randomly assigned to 1 of the 3 diets as follows: 0.12% Mg (−Mg, n = 7), 0.27% Mg (control, n = 8), and 0.43% Mg (+Mg, n = 7). Rabbits were provided with deionized water and offered up to 140 g of feed daily for 8 weeks. Feed intake was measured every other day, and rabbits were weighed weekly. Animals were housed individually in standard caging with stainless steel mesh bottom at normal temperature and light cycles. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Illinois Urbana-Champaign.
Table 1.
Diet composition of cholesterol-containing diets with 0.12%, 0.27%, or 0.43% Mga
| Dietary component | Atherosclerotic diet, −Mg | Atherosclerotic diet, Control | Atherosclerotic diet, +Mg |
|---|---|---|---|
| Protein (%) | 14.7 | 14.3 | 14.4 |
| Fat (%) | 10.2 | 9.3 | 10.5 |
| Carbohydrate (%) | 52.9 | 53.2 | 53.0 |
| Cholesterol (%) | 1.0 | 1.0 | 1.0 |
| Fiber (max %) | 21.2 | 21.5 | 21.0 |
| Mg (%) | 0.12 | 0.27 | 0.43 |
| Ca (%) | 0.90 | 0.89 | 0.89 |
| P (%) | 0.70 | 0.69 | 0.70 |
| K (%) | 1.20 | 1.20 | 1.19 |
| S (%) | 0.20 | 0.20 | 0.17 |
| Na (%) | 0.31 | 0.31 | 0.30 |
| Cl (%) | 0.64 | 0.65 | 0.64 |
| Vitamin A (IU/g) | 20 | 20 | 20 |
| Vitamin D (IU/g) | 1 | 1 | 1 |
| Vitamin E (IU/kg) | 134 | 136 | 133 |
| Vitamin K (ppm) | 2 | 2 | 2 |
| Thiamin (ppm) | 2 | 2 | 2 |
| Riboflavin (ppm) | 9 | 9 | 9 |
Diets contained alfalfa meal, cellulose, corn starch, corn, dehulled soybean meal, Crisco, wheat gluten, casein, corn flour, potassium phosphate, soybean oil, cane molasses, oats, calcium carbonate, cholesterol, salt, vitamin/mineral premix, artificial flavors, choline chloride, dl-methionine, l-arginine, l-threonine, and l-tryptophan. Magnesium oxide was added to the 0.27% and 0.43% Mg diets.
2.2. Techniques
Blood samples were obtained at baseline (0), 2, 4, 6, and 8 weeks via the lateral or medial saphenous vein and placed into a noncoated tube (for serum) and a lithium heparin tube (for blood cell collection). Serum samples were allowed to sit for 1 hour at room temperature to promote clotting. They were then centrifuged at 3000 revolutions per minute for 10 minutes, and serum was collected, aliquoted, and frozen at −70°C. Lithium heparin samples were washed with 0.9% saline and centrifuged at 1000 revolutions per minute for 10 minutes, and supernatant was discarded. This was repeated twice (until supernatant was colorless). The cellular fraction left was then frozen at −70°C until analysis.
At 8 weeks, rabbits were anesthetized with a mixture of ketamine hydrochloride (50 mg/kg) and xylazine (10 mg/kg) administered subcutaneously. They were then euthanized with carbon dioxide. Aortas (aortic arch + abdominal aorta) were harvested and fixed in formalin. Liver was harvested and frozen at −70°C until analysis.
Control serum (catalog no. 410-00100, Wako Chemicals USA Inc, Richmond, Va) was used in all cholesterol assays for quality control. Total serum cholesterol was measured using an enzymatic colorimetric kit (catalog no. 439-17501, Wako Chemicals USA Inc). Triglycerides were measured using an enzymatic colorimetric kit (catalog no. 997-37492 and no. 993-37592, Wako Chemicals USA Inc). High-density lipoprotein level was measured using a precipitation reaction followed by an enzymatic colorimetric kit (catalog no. 431-52501, Wako Chemicals USA Inc). Non-HDL cholesterol was then calculated using the following equation: non-HDL cholesterol = total cholesterol − HDL.
Serum CRP was measured using an enzymatic colorimetric kit (catalog no. 999-27001, Wako Chemicals USA Inc). Serum Mg was measured using a clinical diagnostic machine (Clinical Diagnostic Laboratory, College of Veterinary Medicine, University of Illinois at Urbana-Champaign). Erythrocyte Mg levels were measured using atomic absorption spectophotometry (Experiment Station Chemical Laboratories, University of Missouri, Columbia, Mo).
Liver lipids were extracted using a modification of the Folch method [21]. The sample was placed in chloroform-methanol mixture (1:1), homogenized, and filtered via gravity filtration. Sodium chloride solution (0.29%) was added, vortexed briefly, and then centrifuged. The top layer was discarded, and the interface was washed with 0.29% sodium chloride solution. The remaining solution was placed in a weighed test tube, evaporated, placed in a dessicator for at least 48 hours, and weighed to determine total lipids.
Aortas were scored blindly by a pathologist at the Department of Pathobiology, College of Veterinary Medicine, University of Illinois at Urbana-Champaign. A score of 0 to 5 was assigned to each aorta, with 0 being no plaque and 5 being the most plaque seen. After fixation in 10% buffered formalin/sucrose for a minimum of 24 hours, a 0.5-cm parasagittal section of the aortic arch was trimmed, embedded in paraffin, sectioned at 3 µm, stained with hematoxylin and eosin, and mounted on glass slides for histologic evaluation. Intima thickness was then measured in triplicate at the thickest point. All sections were taken from the same area of the aortic arch.
2.3. Statistical analyses
Analysis of variance statistics were used using the SAS computer program version 9.1 (SAS Institute Inc, Cary, NC), and Fisher LSD (least significant difference) method was used to determine statistical differences (P < .05) between diet groups and between time-points for all assays and measurements. All values are given as means ± SEM.
3. Results
Aortas from animals in the −Mg group had significantly more plaque in the aorta and aortic arch (Table 2) than the control or +Mg groups (P < .05). Amount of plaque present in the control and +Mg groups were not significantly different from each other. Histologic examination of the aorta confirmed these results. The −Mg group had a significantly greater intimal thickness than the control and +Mg groups, but the control and +Mg groups were not statistically different from each other.
Table 2.
Aortic intimal thickness and atherosclerotic plaque severity score in rabbits after 8 weeks of cholesterol-containing diets with 0.12%, 0.27%, or 0.43% Mg (means ± SEM)*
| Diet | Intima thickness (µm) | Plaque severity score (0 = no plaque; 5 = most plaque) |
|---|---|---|
| −Mg | 474±58a | 3.4 ± 0.4a |
| Control | 274±49b | 1.6 ± 0.2b |
| +Mg | 303±58b | 2.0 ± 0.3b |
Letters indicate significant difference between treatments (P < .05).
There were no significant differences in body weights between groups at any time-point (data not shown). When average daily feed intake was calculated, the +Mg group had a significantly greater feed intake (77g/d) than the control group (59 g/d), whereas the −Mg group (64 g/d) was not statistically different from either control or +Mg group. This difference in feed intake led to the +Mg group ingesting higher levels of cholesterol than the control group. Liver weight (% of body weight) and liver lipids (% of liver) did not significantly differ according to dietary treatment (Table 3).
Table 3.
Hepatic weight and lipids in rabbits after 8 weeks of cholesterol-containing diets with 0.12%, 0.27%, or 0.43% Mg (means ± SEM)
| Liver weight (% of body weight) | Liver lipids (% of liver) | ||||
|---|---|---|---|---|---|
| −Mg | Control | +Mg | −Mg | Control | +Mg |
| 3.5 ± 0.3 | 3.9 ± 0.1 | 4.1 ± 0.3 | 15.2 ± 1.7 | 14.0 ± 1.1 | 13.8 ± 1.4 |
Total serum cholesterol significantly increased over time in all treatment groups and reached approximately 41 to 48 mmol/L by 8 weeks (Table 4). Total serum cholesterol did not differ by dietary treatment at 2, 4, 6, and 8 weeks, although baseline values were slightly higher in the +Mg diet group. There was a steady increase in non-HDL cholesterol values for all groups, reaching approximately 40 to 47 mmol/L by 8 weeks. Non-HDL values differed significantly between treatment groups at 2 weeks only. The +Mg group had the highest level of non-HDL, the control group had the lowest, and the −Mg group did not differ from either the +Mg or control group at 2 weeks. The HDL levels were significantly different between the −Mg and control groups at 2 and 6 weeks. There was an effect of dietary treatment on serum TG levels at the 6 week time-point with the −Mg and control groups differing significantly. The +Mg group did not significantly differ from either the −Mg or the Control group at any time-point.
Table 4.
Serum lipid values (in millimoles per liter) at each time-point in rabbits after 8 weeks of cholesterol-containing diets with 0.12%, 0.27%, or 0.43% Mg (means ± SEM) *
| 0 wk | 2 wk | 4 wk | 6 wk | 8 wk | ||
|---|---|---|---|---|---|---|
| Total cholesterol | −Mg | 2.6 ± 0.1b | 16.9 ± 0.6† | 22.7 ± 1.8† | 27.6 ± 3.1† | 47.9 ± 3.5† |
| Control | 2.5 ± 0.0b | 14.7 ± 1.0† | 20.2 ± 1.1† | 29.6 ± 4.0† | 43.3 ± 2.5† | |
| +Mg | 2.8 ± 0.1a | 17.3 ± 1.2† | 23.7 ± 1.6† | 26.5 ± 0.8† | 41.7 ± 2.9† | |
| Non-HDL cholesterol | −Mg | 1.2 ± 0.1 | 15.7 ± 0.6†,a,b | 20.9 ± 1.7† | 26.3 ± 3.1† | 46.6 ± 3.5† |
| Control | 1.2 ± 0.1 | 13.1 ± 1.0†,a | 18.2 ± 1.0† | 27.9 ± 4.0† | 42.1 ± 2.8† | |
| +Mg | 1.3 ± 0.1 | 15.9 ± 1.1†,b | 21.9 ± 1.6† | 25.1 ± 0.8† | 40.4 ± 3.1† | |
| HDL cholesterol | −Mg | 1.4 ± 0.1 | 1.2 ± 0.1b | 1.7 ± 0.2 | 1.3 ± 0.1b | 1.2 ± 0.3 |
| Control | 1.4 ± 0.1 | 1.6 ± 0.1a | 2.0 ± 0.1 | 1.7 ± 0.1a | 1.4 ± 0.5 | |
| +Mg | 1.4 ± 0.1 | 1.4 ± 0.1a,b | 1.8 ± 0.1 | 1.5 ± 0.1a,b | 1.4 ± 0.3 | |
| TG | −Mg | 0.3 ± 0.0 | 1.2 ± 0.4† | 1.6 ± 0.2† | 3.2 ± 0.4†,a | 2.5 ± 0.6† |
| Control | 0.3 ± 0.0 | 0.9 ± 0.2 | 1.2 ± 0.1 | 1.5 ± 0.4b | 1.7 ± 0.6 | |
| +Mg | 0.2 ± 0.0 | 1.1 ± 0.2† | 1.2 ± 0.3 | 2.2 ± 0.6†,a,b | 1.2 ± 0.5 |
Letters indicate significant difference (P < .05) between dietary treatment, and
indicates significant difference (P < .05) from baseline.
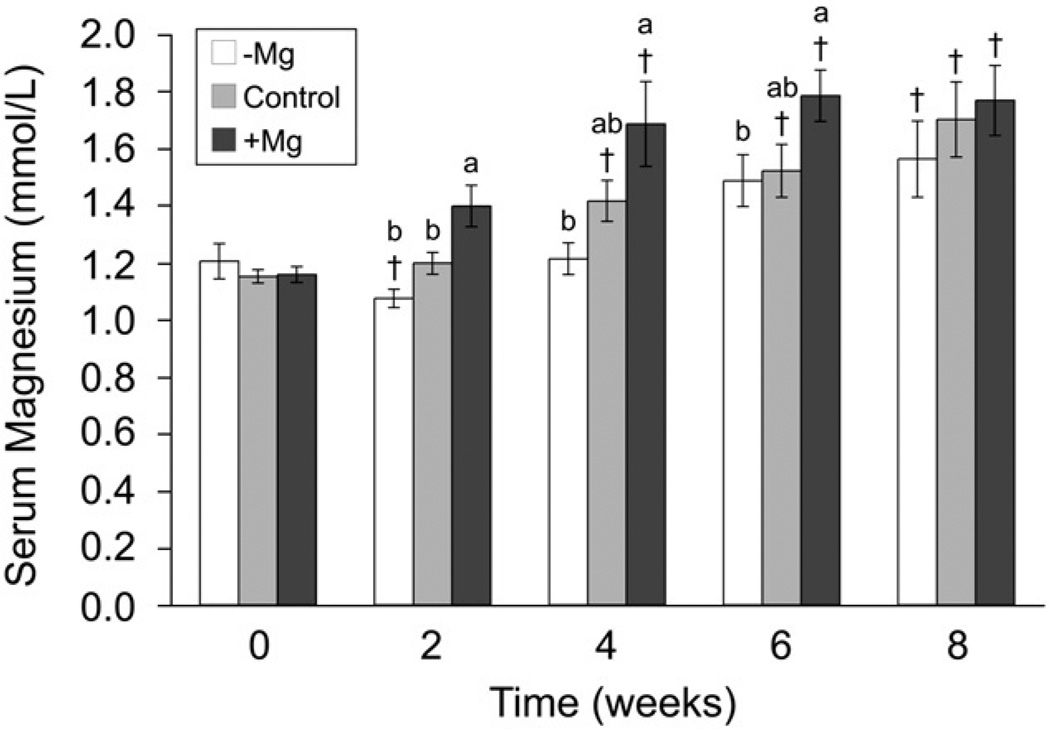
Serum Mg levels increased over time in all groups (Fig. 1). At 2, 4, and 6 weeks, the +Mg group had significantly the highest serum Mg, and the −Mg group had the lowest, with the control group not differing from other groups. By 8 weeks, serum Mg levels stabilized and were not significantly different between any dietary treatments. Erythrocyte Mg decreased over time, and at 6 and 8 weeks, erythrocyte Mg content in all diet groups was significantly lower than baseline (data not shown). Diet group had no significant effect on erythrocyte Mg at any time point. In all treatment groups, CRP increased significantly at 4 and 6 weeks but returned to baseline levels by 8 weeks (Table 5). The CRP levels did not differ between diet groups at any time-point.
Fig. 1.
Serum Mg levels (in millimole per liter) at each time-point in rabbits after 8 weeks of cholesterol-containing diets with 0.12%, 0.27%, or 0.43% Mg (means ± SEM). [Letters indicate significant difference (P < .05) between dietary treatment, and † indicates significant difference (P < .05) from baseline].
Table 5.
Serum CRP (nanogram per milliliter) at each time-point in rabbits after 8 weeks of cholesterol-containing diets with 0.12%, 0.27%, or 0.43% Mg (means ± SEM)a
| 0 wk | 2 wk | 4 wk | 6 wk | 8 wk | ||
|---|---|---|---|---|---|---|
| CRP | −Mg | 1.06 ± 0.05 | 1.03 ± 0.09 | 1.28 ± 0.04† | 1.24 ± 0.06† | 1.12 ± 0.04 |
| Control | 1.01 ± 0.08 | 1.14 ± 0.06 | 1.28 ± 0.02† | 1.32 ± 0.03† | 1.11 ± 0.02 | |
| +Mg | 1.02 ± 0.12 | 1.04 ± 0.12 | 1.26 ± 0.02† | 1.26 ± 0.04† | 1.10 ± 0.02 |
Significant difference (P<.05) from baseline indicated by †.
4. Discussion
We had hypothesized that atherosclerotic plaque, serum cholesterol, and a marker of inflammation (CRP) would all be inversely correlated to Mg intake in a dose-dependent manner. The major finding of this work was that provision of dietary levels of Mg just below (0.27%) or just above (0.43%) the NRC recommended intake for rabbits (0.3%–0.4%) attenuated the plaque accumulation seen with a Mg-deficient diet (0.12%). This was determined by blinded evaluation of the aortas by a pathologist and was confirmed by histologic analysis of aorta intimal thickness. The control and +Mg groups did not differ from each other in either gross examination of plaque or in histologic analysis of intimal thickness. However, the −Mg group had statistically higher observed plaque and intima thickness (P < .05).
In a review by Maier [17], it was shown that dietary restriction of Mg, comparable to daily intakes by Americans, exacerbated atherosclerosis in rabbits. Two previous rabbit trials evaluated the effect of various levels of Mg on atherosclerosis in rabbits [18,19]. Ouchi et al [19] fed 1% cholesterol-containing diets to rabbits and provided the high end of the range of NRC recommendation level of Mg for rabbits (0.4%) plus diets that were supplemented to reach levels of 0.7%, 1.0%, or 1.3% Mg in the diet. Thus, the study by Ouchi [19] focused upon the potential benefits of high-dose supplementation of Mg. The higher levels of Mg led to a slight, but significant, decrease in atherosclerotic lesions in the aortic intima and in the total cholesterol content of the aorta. The Mg supplementation in that study resulted in a decrease of levels of cholesterol and TG and an increase in HDL cholesterol in the serum, and cholesterol content of aortic lesions and of the aorta were both decreased [19].
Altura et al [18] fed dietary levels of 0.08% Mg (compared to 0.23% Mg) to rabbits consuming a 1% or 2% cholesterol diet for 8 week. These dietary Mg levels (both below the NRC recommendation) resulted in increased lipid deposition in the intima, leading to thickening of the intima. They also provided a very high Mg level (2.3%) by supplementing drinking water with Mg and found that this level of Mg intake further decreased intimal thickness.
The current study advances the work of Ouchi [19] and Altura [18] and confirms that feeding low Mg (0.12%) to cholesterol-fed rabbits results in more severe atherosclerosis than when feeding 0.27% Mg, which is just below the NRC lower range level. Interestingly, increasing the dietary level of Mg to 0.43%, just above the NRC requirement, resulted in no further protection. This work, if translatable to humans, suggests that persons with very low Mg intake are at increased risk of CVD development.
The low Mg diet (0.12%) in our study with male rabbits provided 30% to 40% of the NRC recommended range of Mg intake for rabbits. This would correspond to an intake of 120 to 160 mg/d for humans. This intake of Mg is very low, but it has been found that median dietary intake of Mg in African American women can be as low as 177 mg/d [1]. Most adult Americans fail to consume adequate Mg and Mg intake decreases with age [1]. Ford and Mokdad [1] analyzed 24-hour dietary recall data from more than 4000 participants of the NHANES 1999–2000 survey and reported that substantial numbers of US adults fail to consume adequate Mg in their diets. For example, white men consumed a median intake of 237 mg/d, which is 59% of the RDA (400 mg/d) for men older than 30 years [1].
Altura et al [18] observed, as we did, that cholesterol feeding produced elevations of serum Mg in all groups over time. They proposed that this increase in serum Mg was due to influx of Mg into the serum from tissue pools. Although we have no explanation for the influx of Mg into the serum, we took this one step further and measured erythrocyte Mg levels. Our study supports the hypothesis by Altura et al [18] by demonstrating that erythrocyte Mg decreases in all diet groups over time while serum Mg levels concurrently rose. Our observed levels of serum Mg at baseline for all groups was at the low end, whereas at 8 weeks, they were at the high end of the reference range for rabbits of 0.9 to 1.7 mmol/L [22]. Serum Mg in our −Mg group was within normal ranges at all times, yet plaque development was increased in the −Mg group, supporting the notion that serum Mg measurement alone is not adequate to determine risk for CVD.
We have built upon the studies done by Altura et al [18] and Ouchi et al [19] by not only measuring erythrocyte Mg but also by measuring a circulating marker of inflammation (CRP). C-reactive protein is a serum protein that has been associated with inflammation and increased risk of CVD events [23]. C-reactive protein seems to be an independent risk factor for cardiovascular events and probably contributes to the disease process [24]. C-reactive protein can directly affect endothelial cells and smooth muscle cells and has been shown to stimulate uptake of LDL into macrophages [25]. Dietary Mg intake has been inversely correlated to CRP levels and decreased serum Mg levels have been documented in obese patients with elevated CRP [13]. Those who consume less Mg were more likely to have elevated CRP, and individuals in any quartile below the RDA were significantly more likely to have elevated CRP levels [13]. Ways in which Mg status may affect CRP levels is not known at this time, but it has been speculated that it is related to oxidative stress or endothelial dysfunction [13]. Although in humans serum levels of CRP have been seen to inversely correlate with serum Mg [13], our study found no relationship between dietary Mg intake and serum Mg with CRP in this model. C-reactive protein increased from baseline levels in all groups at 4 and 6 weeks and was not significantly different from baseline by 8 weeks, and dietary treatment did not affect CRP levels. The increased CRP in all groups supports previous literature that reported that feeding rabbits high levels of fat and cholesterol causes an inflammatory reaction [16].
It has been reported that serum Mg levels are positively correlated to cholesterol levels in humans [26]. Although total cholesterol, LDL, HDL, and TG levels were not significantly different in our study, it is possible that the more atherogenic fractions (including oxidized-LDL and β-very-low-density lipoprotein) of cholesterol were different among treatment groups. These lipoproteins are important in the development of atherosclerosis. Magnesium is a cofactor in numerous lipid metabolic reactions including lecithin cholesterol acyltransferase (LCAT), lipoprotein lipase (LPL), Mg2+-ATP [27], and peroxisome proliferator-activated receptors (PPARs) [28]. Lecithin cholesterol acyltransferase was found to be positively correlated to ionized Mg in humans [29], and LPL was found to help decrease remnants of LDL and very-low-density lipoprotein [30]. The PPAR dysfunction has been associated with hyperlidemia and inflammation [28]. Measuring LCAT, LPL, or PPAR would have been advantageous for this study.
Magnesium has been proposed to act as a long-term regulator of cell functions, opposed to calcium (Ca) that is responsible for acute events [17]. It has been found that Ca entry into myocytes may be a part of the cardiac pathologic condition that occurs after ischemia [31]. Magnesium directly antagonizes Ca [17], and Mg levels influence the synthesis of nitric oxide and intracellular Ca release [32]. Calcium channel blockers have been found to affect atherogenic cellular changes [33], and Mg is known to be a calcium channel blocker [17].
Limitations of this study include significant differences in feed intake among groups, the small number of inflammatory and lipid metabolism assays performed, and the difficulty in extrapolating cholesterol levels in rabbits to human levels of cholesterol in the blood. Because we only measured one short-term phase inflammatory marker, enhanced inflammation as the causative factor of increased plaque development in the −Mg group cannot be ruled out. There are many inflammatory markers in the serum that could have been measured including interleukin-6, interleukin-1, and tissue necrosis factor-α. There are also serum markers that directly relate to vascular endothelial injury that were not evaluated. These include P-selectin, E-selectin, von Willebrand factor, intercellular adhesion molecule-1, and vascular cell adhesion molecule-1 [34]. Likewise, markers of lipid metabolism such as LCAT and LPL could have been measured. We are currently pursuing the influence of Mg status on additional inflammatory and endothelial injury markers.
Our study demonstrates that inadequate intake of Mg results in a marked increase in atherosclerotic plaque development in rabbits fed a hypercholesterolemic diet. The mechanism by which adequate intake of Mg provided protection from atherosclerotic plaque development cannot be discerned from the parameters measured in this study. Our findings support the epidemiological and in vivo observations that adequate Mg intake can help to decrease incidence of atherosclerotic disease and that assessment of adequate Mg status or dietary intake of Mg might be a good addition to therapy and prevention of CVD. Our findings suggest that serum Mg levels do not correlate with degree of atherosclerotic plaque development and that erythrocyte Mg levels might be of interest when assessing Mg status clinically.
Acknowledgment
This study was supported by grant no. NIH R37 EB002641 (National Institutes of Health, Bethesda, MD).
Abbreviations
- Ca
calcium
- CHD
coronary heart disease
- CRP
C-reactive protein
- CVD
cardiovascular disease
- HDL
high-density lipoprotein
- LCAT
lecithin cholesterol acyltransferase
- LDL
low-density lipoprotein
- LPL
lipoprotein lipase
- Mg
magnesium
- NHANES
National Health and Nutrition Examination Survey
- NRC
National Research Council
- PPAR
peroxisome proliferator-activated receptors
- RDA
recommended daily allowance
- TG
triglycerides.
References
- 1.Ford ES, Mokdad AH. Dietary magnesium intake in a national sample of US adults. J Nutr. 2003;133(9):2879–2882. doi: 10.1093/jn/133.9.2879. [DOI] [PubMed] [Google Scholar]
- 2.Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board, Institute of Medicine. Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board, Institute of Medicine, editor. Dietary reference intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. 1997th ed. Washington, DC: National Academy Press; 1997. [Google Scholar]
- 3.American Heart Association. Heart disease and stroke statistics, 2005 update. American Heart Association. 2005 [Google Scholar]
- 4.Kannel B, Larson M. Long-term epidemiologic prediction of coronary disease: the Framingham experience. Cardiology. 1993;82:137–152. doi: 10.1159/000175864. [DOI] [PubMed] [Google Scholar]
- 5.Fruchart JC, Nierman MC, Stroes ES, Kastelein JJ, Duriez P. New risk factors for atherosclerosis and patient risk assessment. Circulation. 2004;109(23 Suppl 1):III15–III19. doi: 10.1161/01.CIR.0000131513.33892.5b. [DOI] [PubMed] [Google Scholar]
- 6.Ma J, Folsom AR, Melnick SL, Eckfedlt JH, Sharrett AR, Nabulsi AA, et al. Association of serum and dietary magnesium with cardiovascular disease, hypertension, diabetes, insulin, and carotid arterial wall thickness: the ARIC study. J Clin Epidemiology. 1995;48:927–940. doi: 10.1016/0895-4356(94)00200-a. [DOI] [PubMed] [Google Scholar]
- 7.Leone N, Courbon D, Ducimetiere P, Zureik M. Zinc, copper, and magnesium and risks for all cause, cancer, and cardiovascular mortality. Epidemiology. 2006;17(3):308–314. doi: 10.1097/01.ede.0000209454.41466.b7. [DOI] [PubMed] [Google Scholar]
- 8.Shechter M, Sharir M, Labrador MJ, Forrester J, Silver B, Bairey Merz CN. Oral magnesium therapy improves endothelial function in patients with coronary artery disease. Circulation. 2000;102(19):2353–2358. doi: 10.1161/01.cir.102.19.2353. [DOI] [PubMed] [Google Scholar]
- 9.Abraham AS, Weinstein M, Eylath U, Czaczkes E. Magnesium levels in patients with chronic ischemic heart disease. Am J Clin Nutr. 1978;31(8):1400–1402. doi: 10.1093/ajcn/31.8.1400. [DOI] [PubMed] [Google Scholar]
- 10.Song Y, Manson J, Cook N, Albert C, Buring J, Liu S. Dietary magnesium intake and risk of cardiovascular disease among women. Am J Cardiol. 2005;96:1135–1141. doi: 10.1016/j.amjcard.2005.06.045. [DOI] [PubMed] [Google Scholar]
- 11.Amighi J, Sabeti S, Schlager O, Mlekusch W, Exner M, Lalouschek W, et al. Low serum magnesium predicts neurological events in patients with advanced atherosclerosis. Stroke. 2004;35(1):22–27. doi: 10.1161/01.STR.0000105928.95124.1F. [DOI] [PubMed] [Google Scholar]
- 12.Mahfouz MM, Zhou Q, Kummerow FA. Cholesterol oxides in plasma and lipoproteins of magnesium-deficient rabbits and effects of their lipoproteins on endothelial barrier function. Magnes Res. 1994;7(3–4):207–222. [PubMed] [Google Scholar]
- 13.King DE, Mainous AG, III, Geesey ME, Woolson RF. Dietary magnesium and C-reactive protein levels. J Am Coll Nutr. 2005;24(3):166–171. doi: 10.1080/07315724.2005.10719461. [DOI] [PubMed] [Google Scholar]
- 14.Altura BM, Gebrewold A, Altura BT, Brautbar N. Magnesium depletion impairs myocardial carbohydrate and lipid metabolism and cardiac bioenergetics and raises myocardial calcium content in vivo: relationship to etiology of cardiac diseases. Biochem Mol Bio Int. 1996;40:1183–1190. doi: 10.1080/15216549600201823. [DOI] [PubMed] [Google Scholar]
- 15.Shivakumar K. Model of cardiovascular injury in magnesium deficiency. Med Hypotheses. 2001;56:110–113. doi: 10.1054/mehy.2000.1123. [DOI] [PubMed] [Google Scholar]
- 16.Yanni AE. The laboratory rabbit: an animal model of atherosclerosis research. Lab Anim. 2004;38(3):246–256. doi: 10.1258/002367704323133628. [DOI] [PubMed] [Google Scholar]
- 17.Maier JA. Low magnesium and atherosclerosis: an evidence-based link. Mol Aspects Med. 2003;24(1–3):137–146. doi: 10.1016/s0098-2997(02)00095-x. [DOI] [PubMed] [Google Scholar]
- 18.Altura BT, Brust M, Bloom S, Barbour RL, Stempak JG, Altura BM. Magnesium dietary intake modulates blood lipid levels and atherogenesis. Proc Natl Acad Sci U S A. 1990;87(5):1840–1844. doi: 10.1073/pnas.87.5.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ouchi Y, Tabata RE, Stergiopoulos K, Sato F, Hattori A, Orimo H. Effect of dietary magnesium on development of atherosclerosis in cholesterol-fed rabbits. Arteriosclerosis. 1990;10(5):732–737. doi: 10.1161/01.atv.10.5.732. [DOI] [PubMed] [Google Scholar]
- 20.National Research Council. Nutrient requirements of rabbits. 2nd ed. Washington, DC, USA: National Academy of Sciences; 1977. [Google Scholar]
- 21.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226(1):497–509. [PubMed] [Google Scholar]
- 22.Hein J, Hartmann K. Reference ranges for laboratory parameters in rabbits. Tierarztliche Praxis Ausgabe Kleintiere Heimtiere. 2003;31(5):321–328. [Google Scholar]
- 23.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 24.Clifton PM. Diet and C-reactive protein. Curr Atheroscler Rep. 2003;5(6):431–436. doi: 10.1007/s11883-003-0032-z. [DOI] [PubMed] [Google Scholar]
- 25.Nan B, Yang H, Yan S, Lin P, Lumsden A, Yao Q, et al. C-reactive protein decreases expression of thrombomodulin and endothelial protein C receptor in human endothelial cells. Surgery. 2005;138(2):212–222. doi: 10.1016/j.surg.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 26.Randell EW, Mathews M, Gadag V, Zhang H, Sun G. Relationship between serum magnesium values, lipids, and anthropometric risk factors. Atherosclerosis. 2008;196(1):413–419. doi: 10.1016/j.atherosclerosis.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 27.Inoue I. Lipid metabolism and magnesium. Clin Calcium. 2005;15(11):65–76. [PubMed] [Google Scholar]
- 28.Fujii H. Nuclear Receptor PPARs and magnesium. Clin Calcium. 2005;15(11):52–64. [PubMed] [Google Scholar]
- 29.Nozue T, Ide N, Okabe H, Narui K, Kobayashi A. Correlation of serum HDL-cholesterol and LCAT levels with the fraction of ionized magnesium in children. Magnes Res. 1999;12(4):297–301. [PubMed] [Google Scholar]
- 30.Wang X, Greilberger J, Jurgens G. Calcium and lipoprotein lipase synergistically enhance the binding and uptake of native and oxidized LDL in mouse peritoneal macrophages. Atherosclerosis. 2000;150:357–363. doi: 10.1016/s0021-9150(99)00413-x. [DOI] [PubMed] [Google Scholar]
- 31.Zhang W, Xu C. Calcium sensing receptor and heart diseases. Pathophysiology. 2009 doi: 10.1016/j.pathophys.2009.02.013. [Article in press] [DOI] [PubMed] [Google Scholar]
- 32.Maier JA, Malpuech-Brugere C, Zimowska W, Rayssiguier Y, Mazur A. Low magnesium promotes endothelial cell dysfunction: Implications for atherosclerosis, inflammation and thrombosis. Biochim Biophys Acta. 2004;1689(1):13–21. doi: 10.1016/j.bbadis.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 33.Mason R. Atheroprotective effects of long-acting dihydropyridine-type calcium channel blockers: evidence from clinical trials and basic scientific research. Cerebrovasc Dis. 2003;16 suppl 3:11–17. doi: 10.1159/000070272. [DOI] [PubMed] [Google Scholar]
- 34.Constans J, Conri C. Circulation markers of endothelial function in cardiovascular disease. Con Chim Acta. 2006;368(1–2):33–47. doi: 10.1016/j.cca.2005.12.030. [DOI] [PubMed] [Google Scholar]