Abstract
Background
Circulating biomarkers can offer insight into subclinical cardiovascular stress and thus have the potential to aid in risk stratification and tailoring of therapy.
Methods & Results
We measured plasma levels of 4 cardiovascular biomarkers, midregional pro-atrial natriuretic peptide (MR-proANP), midregional pro-adrenomedullin (MR-proADM), C-terminal pro-endothelin-1 (CT-proET-1) and copeptin, in 3717 patients with stable CAD and preserved left ventricular ejection fraction (LVEF) who were randomized to trandolapril or placebo as part of the Prevention of Events with Angiotensin Converting Enzyme (PEACE) trial. After adjustment for clinical cardiovascular risk predictors and LVEF, elevated levels of MR-proANP, MR-proADM, and CT-proET-1 were independently associated with the risk of cardiovascular death or heart failure (HRs per 1-SD of log-transformed biomarker levels of 1.97, 1.48, and 1.47, respectively; P≤0.002 for each biomarker). These three biomarkers also significantly improved metrics of discrimination when added to a clinical model. Trandolapril significantly reduced the risk of cardiovascular death or heart failure in patients who had elevated levels of 2 or more these biomarkers (HR 0.53, 95% CI 0.36–0.80), whereas there was no benefit in patients with elevated levels of 0 or 1 biomarkers (HR 1.09, 95% CI 0.74–1.59) (Pinteraction=0.012).
Conclusions
In patients with stable CAD and preserved LVEF, our results suggest elevated levels of novel biomarkers of cardiovascular stress may help identify patients who are at higher risk of cardiovascular death and heart failure and may be useful to select patients who derive significant benefit from ACE inhibitor therapy.
Keywords: coronary disease, biomarkers, angiotensin converting enzyme inhibitors
Elevated levels of circulating biomarkers related to cardiac volume or pressure overload offer insight into subclinical cardiac stress and thus have the potential to aid in risk stratification.1 Specifically, elevated levels of B-type natriuretic peptide (BNP, either the hormone or the amino-terminal fragment of the prohormone, NT-proBNP) have been shown to be predictive of mortality and/or heart failure events across a broad range of individuals ranging from the general population to patients with overt heart failure.1–7
Development of newer assays that target more stable epitopes of hormones or prohormones whose release is related to cardiomyocyte and/or vascular stress offers the potential for more refined risk assessment. Specifically, atrial natriuretic peptide (ANP) is a vasodilator and natriuretic that is synthesized in the myocardium in response to increased wall tension.8 Adrenomedullin (ADM) is a potent vasodilator synthesized in the adrenal medulla, vascular endothelial cells, the heart, and elsewhere in response to physical stretch and specific cytokines, with levels in the heart elevated in the setting of pressure and volume overload.9, 10 Endothelin-1 (ET-1) is a potent vasoconstrictor and pro-fibrotic hormone that is secreted by vascular endothelial cells, with levels correlating with shear stress and pulmonary artery pressure.11 Copeptin is a stable peptide derived from the precursor to arginine vasopressin, a vasoconstrictor that is secreted from the posterior pituitary in response not only to osmotic stimuli but also to hemodynamic changes detected by cardiac and vascular baroreceptors.12 Higher levels of these biomarkers have been associated with the risk of death and/or heart failure events in patients with established heart failure.13–16 The availability of an assay panel for these four biomarkers of cardiovascular stress that have shown promise in patients with established heart failure created the opportunity to investigate their utility in a broader population.
Angiotensin converting enzyme (ACE) inhibitors substantially reduce the risk of death and heart failure events in patients with heart failure, with the greatest benefit in those patients with the most clinically severe heart failure.17 Among patients with acute myocardial infarction (MI), the benefit of ACE inhibitors is greatest in those with high-risk clinical features such as anterior MI or depressed left ventricular systolic function.18 In contrast, the role of ACE inhibitors in lower risk patients with stable coronary artery disease (CAD) without heart failure is less clear.19–21 We explored the hypotheses that in such patients, elevated levels of midregional pro-ANP (MR-proANP), MR-proADM, C-terminal proET-1 (CT-proET-1) and copeptin would: (1) offer prognostic value for cardiovascular death and heart failure independent of clinical risk factors, and (2) identify patients who derive greater clinical benefit from the use of an ACE inhibitor. We tested these hypotheses by measuring plasma levels of these novel biomarkers of cardiovascular stress in 3717 patients with stable CAD and preserved left ventricular ejection fraction (LVEF) who were randomized to trandolapril or placebo as part of the Prevention of Events with Angiotensin Converting Enzyme (PEACE) trial.
Methods
Patient population
This study involved 3717 patients with documented stable CAD who had been enrolled in the PEACE trial (ClinicalTrials.gov number, NCT00000558) and provided a sample of blood at the time of enrollment. The design and main outcomes of the PEACE trial have been published previously,22 and salient features are detailed in the Supplemental Methods and Supplemental Table 1. In brief, subjects were free of heart failure at baseline and none had been hospitalized with an acute coronary syndrome or undergone coronary revascularization within the 3 months preceding trial entry. Both the parent clinical trial and this substudy were approved by the relevant institutional review boards, and informed consent was obtained from all patients.
Biomarker analyses
Baseline plasma levels of MR-proANP,23 MR-proADM,24 CT-proET-1,25 and copeptin26 (assays from B.R.A.H.M.S. GmbH, Henningsdorf, Germany) were determined in the TIMI Clinical Trials Laboratory (Boston, MA) as detailed in the Supplemental Methods and in Supplemental Table 2. Baseline levels of NT-proBNP and cardiac troponin T (cTnT), as measured with a highly sensitive assay, had been determined in this population, as previously published and summarized in the Supplemental Methods.6, 27 All testing was performed by personnel blinded to clinical outcomes and treatment allocation.
Outcomes
Based on prior data regarding predictive ability of biomarkers of cardiac stress,6 the primary outcome in this analysis was the composite of cardiovascular death or hospitalization for heart failure. Additionally, we also explored other major adverse cardiovascular events that had been recorded in patients in the trial including all-cause death, acute MI, acute stroke, and coronary revascularization (percutaneous or surgical). Event adjudication is detailed in the Supplemental Methods. All clinical events were classified before biomarkers were measured.
Statistical analyses
Baseline characteristics are reported as means ±SD for normally distributed continuous variables and counts and percentages for categorical variables. Wilcoxon rank-sum and chi-square tests for trend were used to test for differences in continuous and categorical baseline characteristics between quartiles of biomarkers. Spearman’s correlation was used to calculate the association between different biomarkers and categorized based on standard cutpoints.28 The cumulative incidences of clinical outcomes across quartiles of each biomarker were compared using a log-rank test. Cox proportional-hazards models were used to examine the association between biomarker levels and outcome data. In these models, biomarker levels were examined both as a continuous variable (after natural logarithmic transformation) and as a categorical variable by quartiles. Associations were adjusted for age, sex, weight, history of hypertension, history of diabetes mellitus, current tobacco use, prior MI, prior percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG), systolic blood pressure (SBP), estimated glomerular filtration rate (eGFR), ratio of apoB/apoA, LVEF, aspirin use, beta-blocker use, lipid-lowering medication use. Starting with a model containing the aforementioned clinical covariates, a forward selection algorithm (P<0.05 to enter the model) was used to select among the four novel biomarkers as well as NT-proBNP and cTnT. The incremental performance of the biomarkers in addition to clinical predictors was further evaluated by calculating changes in the c-statistic, integrated discrimination improvement (IDI), and the category-free net re-classification improvement (NRI) metrics (see Supplemental Methods for further details).29–31
To examine for heterogeneity in the effect of trandolapril on the risk of cardiovascular death or heart failure, hazard ratios were calculated in patients who were and were not in the highest risk category as defined by being in the top quartile of a biomarker level. To test for statistically significant effect modification, a Cox proportional hazards model was created that included a term for trandolapril, a term for biomarker risk category, and an interaction term.
A P value of less than 0.05 was considered to indicate statistical significance, and all tests were two-sided. No adjustment for multiple comparisons was performed. Although based on previous work with these biomarkers in other populations, all of the analyses we have performed in this biomarker substudy are inherently exploratory. Analyses were performed using STATA/IC (version 10.1, STATA Corp., College Station, TX, USA) and R (version 2.12.1).
Results
Baseline Characteristics of the Patients and Biomarker Levels
Baseline measurements of the four novel biomarkers were available from 3717 patients from the PEACE trial. The clinical characteristics of the patients are displayed in Table 1. By design, all patients had stable coronary artery disease and LVEF was preserved with the mean (±SD) value 58.7±9.6%. Median levels (25th–75th percentile) of MR-proANP, MR-proADM, CT-proET-1, and copeptin at baseline in patients in the PEACE trial were 90.45 (63.68–128.3) pmol/L, 0.53 (0.45–0.64) nmol/L, 47.82 (39.04–57.02) pmol/L, and 6.47 (0–10.67) pmol/L, respectively. The levels tended to be higher than those seen in healthy populations, but, with the exception of MR-proADM, the majority of values were lower than the 97.5 percentile reported in healthy populations, and lower than the values in patients with overt heart failure (Supplemental Table 2). Characteristics of patients according to quartiles of biomarker levels are shown in Supplemental Tables 3–6. In general, levels of biomarkers of cardiovascular stress were positively associated with age and hypertension and negatively associated with estimated glomerular filtration rate. LVEF was negatively associated with MR-proANP and copeptin levels, but differed by only 2.0 and 1.0 absolute percentage points between the top and bottom quartiles for the two biomarkers respectively. Among the novel biomarkers, the only moderately strong correlation was between MR-proADM and CT-proET-1 (r=0.63), the others were moderate to low (r≤0.44) (Supplemental Table 7). As expected, there was a strong positive correlation between levels of MR-proANP and NT-proBNP (r=0.76), but correlations of NT-proBNP and cTnT with other markers were weak (r≤0.38) (Supplemental Table 7).
Table 1.
Baseline Characteristics of Patients
| Baseline Characteristic | All | Placebo | Trandolapril |
|---|---|---|---|
| Number of patients | 3717 | 1868 | 1849 |
| Age, y | 64.1±8.2 | 64.1±8.2 | 64.2±8.1 |
| Female sex | 701 (18.9) | 334 (17.9) | 367 (19.9) |
| Weight, kg | 83.9±15.7 | 83.7±15.7 | 84.2±15.6 |
| Hypertension | 1658 (44.6) | 835 (44.7) | 823 (44.5) |
| Diabetes | 602 (16.2) | 294 (15.7) | 308 (16.7) |
| Current smoker | 564 (15.2) | 290 (15.5) | 274 (14.8) |
| Prior MI | 2087 (56.2) | 1076 (57.6) | 1011 (54.7) |
| Prior PCI or CABG | 2697 (72.6) | 1367 (73.2) | 1330 (72.0) |
| Aspirin | 3389 (91.2) | 1721 (92.2) | 1668 (90.3) |
| Beta-blocker | 2303 (62.0) | 1156 (61.9) | 1147 (62.1) |
| Lipid-lowering therapy | 2667 (71.8) | 1334 (71.5) | 1333 (72.2) |
| SBP, mmHg | 133.4±16.8 | 133.4±16.8 | 133.3±16.8 |
| DBP, mmHg | 78.1±10.0 | 78.2±10.2 | 78.0±9.8 |
| GFR, ml/min/1.73 m2 | 77.9±19.4 | 78.3±19.4 | 77.6±19.3 |
| ApoB, mg/dl | 107.2±23.1 | 107.6±22.9 | 106.8±23.2 |
| ApoA, mg/dl | 138.2±24.6 | 138.6±24.5 | 137.8±24.7 |
| LVEF, % | 58.7±9.6 | 58.7±9.6 | 58.8±9.7 |
Data presented are mean ±SD for normally distributed continuous variables and n (%) for dichotomous variables. CABG = coronary artery bypass grafting; DBP = diastolic blood pressure; GFR = glomerular filtration rate; LVEF = left ventricular ejection fraction; MI = myocardial infarction; PCI = percutaneous coronary intervention; SBP = systolic blood pressure.
Clinical Outcomes
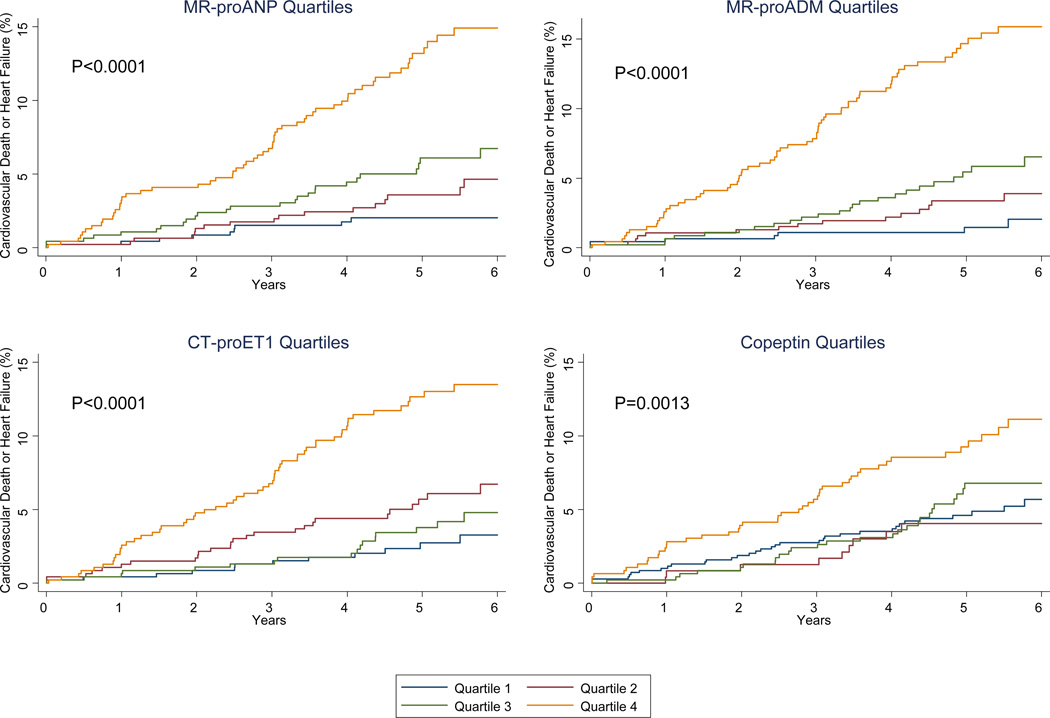
Among patients allocated to the placebo arm of the PEACE trial, higher baseline levels of each of the four novel biomarker of cardiovascular stress were strongly associated with the subsequent risk of cardiovascular death or heart failure (the composite of which occurred in 114 patients), with up to approximately a doubling of the risk per each 1 standard deviation increase in log-transformed biomarker levels (P≤0.002 for each biomarker; Table 2). Risk increased across quartiles, especially the fourth quartile (Figure 1). Similar associations were seen between biomarker levels and the risk of cardiovascular death (which occurred in 67 patients) and of heart failure individually (which occurred in 56 patients) (Supplemental Table 8).
Table 2.
Association of biomarker levels and clinical outcomes in the placebo arm
| Risk for CV Death or Heart Failure |
||||||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) across quartiles | P value | |||||||
| Biomarker | HR (95% CI) per 1-SD of log- transformed biomarker values |
P value | Q1 | Q2 | Q3 | Q4 | Multiple partial |
Trend |
| MR-proANP | 2.25 (1.89–2.42) |
<0.001 | Referent | 1.92 (0.85–4.30) |
3.10 (1.46–6.59) |
7.30 (3.62–14.70) |
<0.0001 | <0.0001 |
| MR-proADM | 1.69 (1.52–1.88) |
<0.001 | Referent | 2.15 (0.88–5.28) |
3.65 (1.58–8.45) |
10.25 (4.71–22.33) |
<0.0001 | <0.0001 |
| CT-proET-1 | 1.96 (1.57–2.44) |
<0.001 | Referent | 2.25 (1.14–4.45) |
1.42 (0.68–2.98) |
5.07 (2.72–9.44) |
<0.0001 | <0.0001 |
| Copeptin | 1.30 (1.10–1.55) |
0.002 | Referent | 0.88 (0.43–1.79) |
1.23 (0.74–2.05) |
2.09 (1.32–3.28) |
0.0072 | 0.0013 |
A total of 114 of the 1868 patients allocated to placebo experienced cardiovascular death or heart failure. Each biomarker was analyzed separately. In quartile analyses, “multiple partial” refers to a 3 degree of freedom test for the addition of all quartiles, “trend” refers to a 1 degree of freedom test for linear trend across quartiles.
Figure 1.
Cumulative incidence curves for the composite of cardiovascular death or heart failure among patients in the placebo arm of the PEACE trial (n=1868), categorized by quartiles of MR-proANP (Panel A), MR-proADM (Panel B), CT-proET-1 (Panel C), or Copeptin (Panel D). P values are for log-rank test for trend across quartiles.
After adjusting for traditional clinical risk predictors, estimated glomerular filtration rate, and LVEF (see Methods for a detailed list of covariates), elevated levels of MR-proANP, MR-proADM, and CT-proET-1 each remained significantly associated with an increased risk of cardiovascular death or heart failure, ranging from 47% higher risk to a near doubling of the risk per each 1 standard deviation increase in log-transformed biomarker levels (P≤0.002 for each biomarker); similarly, in terms of quartile analysis, the risk was most pronounced for those patients in the top quartile, who had almost 3 times to more than 5 times the risk seen compared with patients in the lowest quartile. In contrast, after multivariable adjustment, the association with copeptin was no longer significant (Table 3). As was the case for the unadjusted analyses, similar associations were seen between biomarker levels and the risk of cardiovascular death and of heart failure individually (Supplemental Table 8). Compared with cardiovascular death, the associations with the less cardiovascular-specific endpoint of all-cause death were significant but weaker (Supplemental Table 9). As expected based on prior work,6, 27 there were non-significant adjusted associations between levels of novel biomarkers of cardiovascular stress and the risk of acute MI, stroke, or coronary revascularization, with the exception of MR-proANP and stroke (P=0.043) (Supplemental Table 9).
Table 3.
Multivariable-adjusted association of biomarker levels and clinical outcomes in the placebo arm adjusted for clinical factors
| Adjusted Risk for CV Death or Heart Failure |
||||||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) across quartiles | P value | |||||||
| Biomarker | HR (95% CI) per 1-SD of log- transformed biomarker values |
P value | Q1 | Q2 | Q3 | Q4 | Multiple partial |
Trend |
| MR-proANP | 1.97 (1.58–2.46) |
<0.001 | Referent | 1.60 (0.70–3.66) |
2.72 (1.24–5.96) |
4.35 (1.96–9.62) |
<0.0001 | <0.0001 |
| MR-proADM | 1.48 (1.27–1.73) |
<0.001 | Referent | 1.90 (0.77–4.69) |
2.45 (1.03–5.82) |
5.51 (2.38–12.75) |
<0.0001 | <0.0001 |
| CT-proET-1 | 1.47 (1.15–1.88) |
0.002 | Referent | 2.03 (1.03–4.04) |
0.99 (0.46–2.11) |
2.73 (1.41–5.27) |
<0.001 | 0.01 |
| Copeptin | 1.10 (0.91–1.33) |
0.32 | Referent | 0.77 (0.37–1.57) |
1.11 (0.66–1.86) |
1.41 (0.87–2.28) |
0.30 | 0.11 |
Covariates in model include standard clinical factors: age, sex, weight, history of hypertension, history of diabetes mellitus, current tobacco use, prior MI, prior PCI or CABG, systolic blood pressure, estimated GFR, ratio of apoB/apoA, LVEF, aspirin use, beta-blocker use, lipid-lowering medication use. Each biomarker analyzed separately in the placebo arm. In quartile analyses, “multiple partial” refers to a 3 degree of freedom test for the addition of all quartiles, “trend” refers to a 1 degree of freedom test for linear trend across quartiles.
We have previously measured NT-proBNP and cTnT in this population, and the association of those biomarkers with cardiovascular death or heart failure in a model adjusted for the aforementioned clinical covariates is shown in Table 3 and Supplemental Table 10. Ranking each biomarker individually based on the magnitude of risk (HR) per 1 standard deviation, the order was: MR-proANP (1.97), NT-proBNP (1.73), MR-proADM (1.48), CT-proET-1 (1.47) and cTnT (1.37). Given the correlation between the biomarkers and that none are established for routine use in this population, we used an unbiased forward selection algorithm to create a multimarker model. The only 2 biomarkers to enter and remain in a model already containing clinical covariates were MR-proANP (adjusted HR 1.79, 95% CI 1.41–2.26, P<0.001) and MR-proADM (adjusted HR 1.27, 95% CI 1.07–1.51, P=0.007).
The addition individually of MR-proANP, MR-proADM, and CT-proET-1 to the clinical model significantly improved metrics of discrimination (Table 4). In contrast, the addition of copeptin did not improve these metrics. The addition of all 3 biomarkers to the clinical model improved the c-statistic from 0.768 to 0.809, and yielded an IDI of 4.6%, and an NRI of 0.435 (all P≤0.0005). Adding MR-proANP, MR-proADM, and CT-proET-1 uniformly and significantly improved the c-statistic of multivariable models already containing clinical covariates, regardless of whether NT-proBNP, cTnT, or both were also in the model; conversely, adding NT-proBNP and cTnT to a model containing clinical covariates as well as MR-proANP, MR-proADM, and CT-proET-1 did not improve the c-statistic (Supplemental Table 11).
Table 4.
Impact of biomarker levels and metrics of discrimination and reclassification in the placebo arm
| C statistic | Integrated Discrimination Index (IDI) |
Net Reclassification Improvement (NRI) |
||||
|---|---|---|---|---|---|---|
| Model | Value | P value | Value | P value | Value | P value |
| Clinical model alone | 0.768 | n/a | n/a | n/a | n/a | n/a |
| Clinical model + MR-proANP | 0.804 | 0.0018 | 3.8% | <0.0001 | 0.412 | <0.0001 |
| Clinical model + MR-proADM | 0.788 | 0.0064 | 1.9% | 0.0027 | 0.362 | 0.0003 |
| Clinical model + CT-proET-1 | 0.779 | 0.23 | 1.2% | 0.047 | 0.205 | 0.039 |
| Clinical model + Copeptin | 0.769 | 0.85 | 0.2% | 0.14 | 0.061 | 0.54 |
Terms in clinical model include age, sex, weight, history of hypertension, history of diabetes mellitus, current tobacco use, prior MI, prior PCI or CABG, systolic blood pressure, estimated GFR, ratio of apoB/apoA, LVEF, aspirin use, beta-blocker use, lipid-lowering medication use. Each biomarker analyzed separately in the placebo arm. P values are for comparison to clinical model alone.
Interaction with Trandolapril Therapy
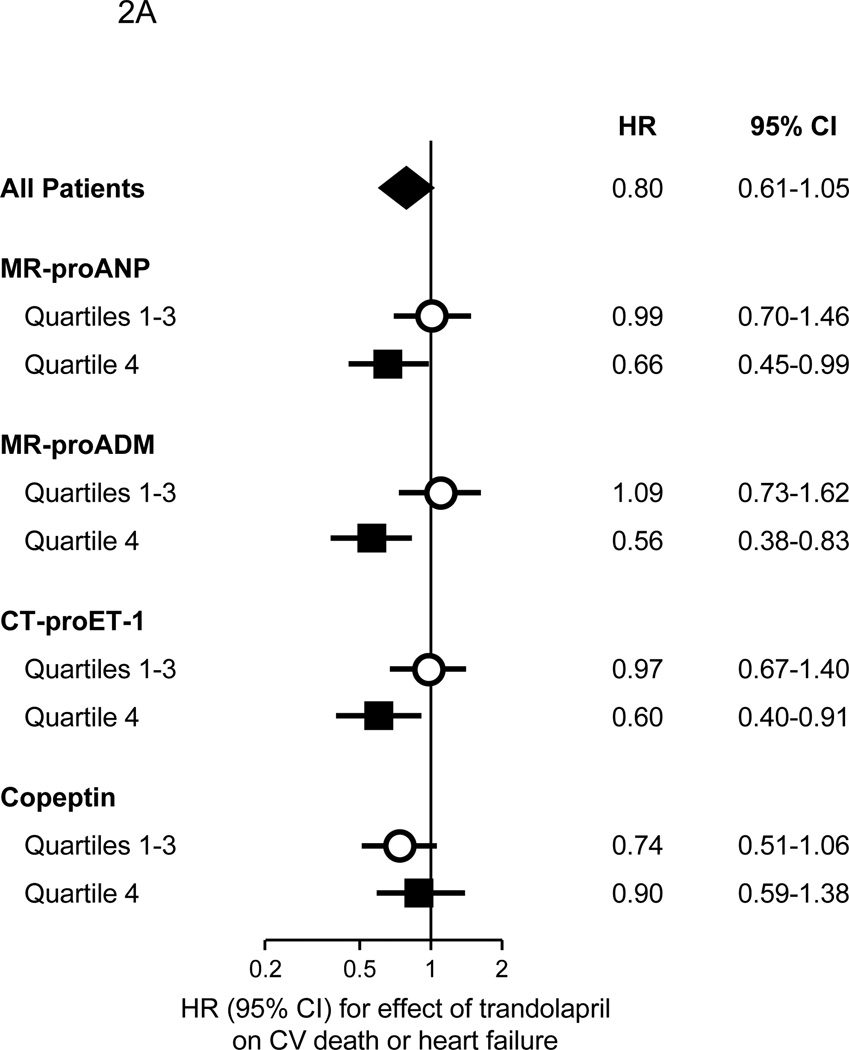
In the overall biomarker cohort, treatment with trandolapril resulted in a HR of 0.80 (95% CI 0.61–1.05) for cardiovascular death or heart failure. Notably, though, among patients having an MR-proANP, MR-proADM, or CT-proET-1 level in the top quartile, and thus at the highest risk of cardiovascular death or heart failure based on these biomarkers, trandolapril significantly reduced the risk of cardiovascular death or heart failure by 34–44%, whereas no benefit was observed among those with lower levels (Figure 2A). In contrast, there was no significant benefit from treatment with trandolapril among patients in the highest quartiles of either NT-proBNP or cTnT (Supplemental Figure 1).
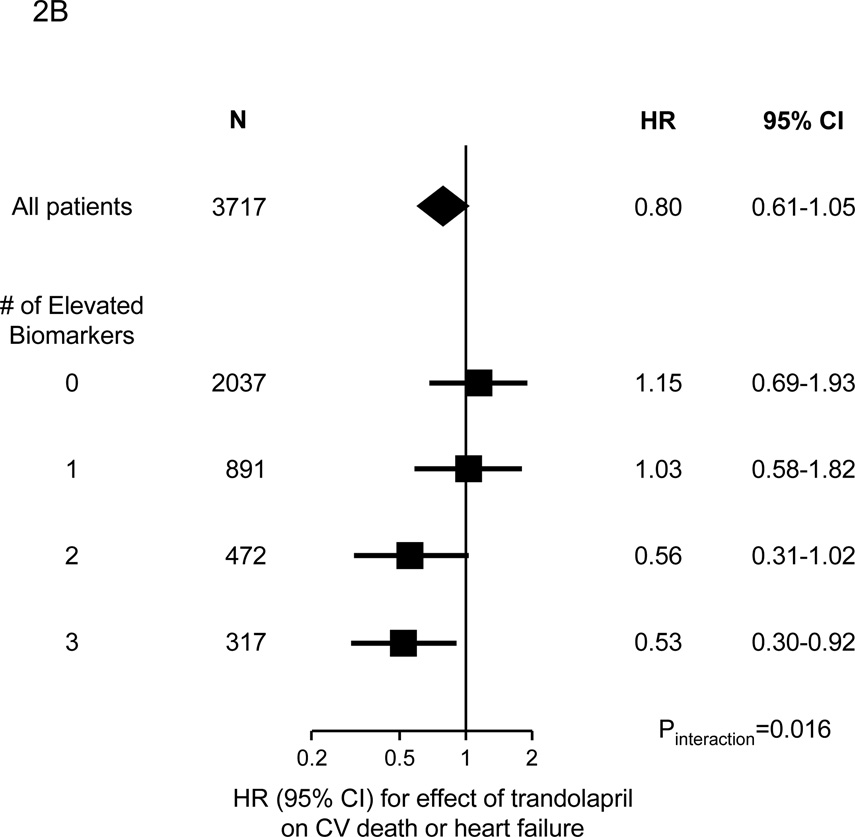
Figure 2.
Benefit of trandolapril on the risk of the composite of cardiovascular death or heart failure in 3717 patients from the PEACE trial, categorized as to their levels of biomarkers of cardiovascular stress. Panel A. Patients are categorized as to whether their level of each biomarker of cardiovascular stress was in the top quartile (quartile 4) or not (quartiles 1–3). The P values for interaction were 0.16, 0.02, 0.09, and 0.72 for MR-proANP, MR-proADM, CT-proET-1, and copeptin, respectively. Panel B. Patients are categorized as to the number of biomarkers (MR-proANP, MR-proADM, and CT-proET-1) in the top quartile; the P value for interaction is 0.016. In both panels, the diamonds indicate the effect in the entire biomarker cohort, with the center indicating the point estimate and the left and right ends indicating the 95% CI. The squares and circles indicate the point estimate and the horizontal lines indicate the 95% CIs for the effect in each subgroup.
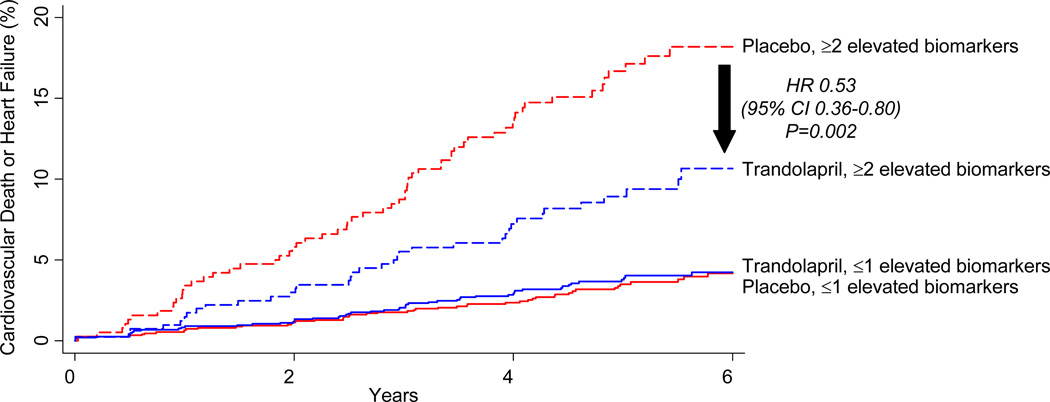
A gradient of benefit (Pinteraction=0.016) with trandolapril therapy was observed in patients categorized as to whether they had elevated levels of 0 (n=2037), 1 (n=891), 2 (n=472), or all 3 (n=317) of the novel biomarkers that we found to be associated with cardiovascular death or heart failure in adjusted analyses (Figure 2B). Dichotomizing the results, among the 2928 patients (79% of the biomarker cohort) with ≤1 elevated biomarker, there was no benefit of trandolapril therapy on the risk of cardiovascular death or heart failure (HR 1.09, 95% CI 0.74–1.59), whereas among the 789 patients (21% of the biomarker cohort) with ≥2 elevated biomarkers, trandolapril significantly reduced the rate of cardiovascular death or heart failure (HR 0.53, 95% CI 0.36–0.80, P=0.002, Pinteraction=0.012; Figure 3). The absolute risk reduction over 6 years in this latter group was 7.5%; thus, in this subset, 14 patients would need to be treated with trandolapril for 6 years to prevent a cardiovascular death or hospitalization for heart failure.
Figure 3.
Cumulative incidence curves for the composite of cardiovascular death or heart failure in 3717 patients from the PEACE trial, categorized as to whether they had ≤1 elevated biomarkers (solid lines; red=1487 patients treated with placebo; blue=1441 patients treated with trandolapril) or ≥2 elevated biomarkers (dashed lines; red=381 patients treated with placebo; blue=408 patients treated with trandolapril).
Discussion
In an exploratory analysis among a large cohort of patients with stable CAD and preserved LVEF, we have demonstrated that elevated levels of 3 novel biomarkers of cardiovascular stress are independently associated with the subsequent risk of cardiovascular death and heart failure. Specifically, MR-proANP, MR-proADM, and CT-proET-1 were associated with cardiovascular death or heart failure independent of clinical factors, renal function, and LVEF, ranging from 47% higher risk to a near doubling of the risk per each 1 standard deviation increase in log-transformed biomarker levels and almost 3 times to more than 5 times the risk when comparing patients in the highest versus the lowest quartile. In contrast, a fourth biomarker, copeptin, was not independently associated with the risk of cardiovascular events. Moreover, and in contrast to previous results with other biomarkers including NT-proBNP and cTnT,6, 27, 32 elevated levels of these 3 biomarkers identified patients in whom, despite appearing to be at low risk clinically, therapy with an ACE inhibitor resulted in a significant reduction in the risk of cardiovascular death or heart failure.
We used assays for the prohormones of ANP, ADM, and ET-1 as the prohormones are released in equimolar ratio to the vasoactive hormones, but have a longer half-life. When possible, we also used assays for a midregional fragment, as these fragments are more stable in vivo and ex vivo than the amino- or carboxy-terminal part of the prohormone, thereby minimizing the risk of underestimation of levels due to early degradation of crucial epitopes at the extreme ends of the molecule.33 In studies of patients with established heart failure, elevated levels of MR-proANP, MR-proADM, and CT-proET-1 have each been shown to be associated with mortality independent of clinical variables, and the biomarkers have displayed prognostic and discriminatory value that has compared favorably to BNP and/or NT-proBNP.13–15
Concordant with those observations, in our dataset we found that when creating a multimarker model adjusted for clinical factors, MR-proANP and MR-proADM proved to be the strongest 2 biomarkers, superior to NT-proBNP and cTnT as measured using a highly sensitive assay. As this was a clinical rather than a mechanistic study, we can only speculate as to the reasons for the superior performance, which could be related to subtle differences in the respective pathobiology underlying elevation of each of the biomarkers or could stem from more favorable analytic properties that translate into a better reflection of subclinical cardiovascular pathology. Regardless, our data are supported by and extend previous findings regarding these biomarkers and atherosclerosis reported by Schnabel and colleagues7 in several ways, including studying patients who were free of heart failure at baseline and whose LVEF was known and incorporated into all multivariable models, using patients enrolled from a much broader number of clinical centers, and examining the specific clinical events biomarkers of cardiac stress are best-suited to predict, namely, cardiovascular death and heart failure, rather than a composite of death or MI.
Critically, whereas other biomarker analyses have been embedded in observational cohorts, we had the benefit of studying these biomarkers in a randomized clinical trial, allowing us to examine the interaction between baseline biomarker levels and the efficacy of the randomized therapy without concern for the inherent bias in examining non-randomly allocated therapies. Using a panel of these novel biomarkers of cardiovascular stress, we were able to identify approximately one fifth of enrolled patients with stable CAD in whom ACE inhibitor therapy nearly halved the risk of cardiovascular death or heart failure. Our findings are conceptually analogous to results from Richards and colleagues who showed that elevated levels of biomarkers of cardiovascular stress identified patients with ischemic left ventricular dysfunction who benefited from beta-blockade.34, 35
Current practice guidelines for the management of patients with stable CAD recommend ACE inhibitor therapy in those patients with an LVEF <40%; in addition, based in part on data from the HOPE trial, ACE inhibitors are recommended for patients who are relatively high-risk and/or have another compelling clinical indication (e.g., hypertension, diabetes, or chronic kidney disease).36 In contrast, for lower-risk patients like those in the PEACE trial, in which the event rate in the placebo arm was lower than the event rate in the ACE inhibitor arm from the HOPE trial, the guidelines note that it is reasonable but not recommended to use ACE inhibitors when cardiovascular risk factors are well controlled and revascularization has been performed. Our data now support the hypothesis that within this very large population of patients who appear to be of lower risk clinically, biomarker of cardiovascular stress levels may be useful to help guide such decision-making. Although additional prospective analyses would need to be done if these biomarkers become available for routine clinical use in the US, targeting long-term drug therapy based on a panel of biomarkers should be cost-effective.
There are several potential limitations of our study that deserve consideration. The PEACE clinical trial population, which was predominantly a white, male population over the age of 50, is not representative of the general population. However, the clinical and laboratory characteristics of patients in this study are typical of patients with stable coronary disease and a high proportion of patients were treated with beta-blockers and lipid-lowering therapy. Blood samples were obtained from only a subgroup of the participants in the overall PEACE trial, but there were no clinically relevant differences between patients who did and did not participate in the biomarker substudy. Banked biosamples were used, but any sample degradation should be random with respect to cardiovascular outcomes and thus any resultant misclassification should only bias toward the null hypothesis. The formation of the multimarker score for interaction with therapy should be considered exploratory, and the optimal combination of biomarkers and their cutpoints merits validation in additional populations. Heart-failure events were not a component of the prespecified primary outcome in the original trial design, but are a well-established outcome predicted by biomarkers of cardiac stress and prevented by ACE inhibitors in other populations.6, 19, 20, 27
In conclusion, in apparently low-risk patients with stable coronary artery disease and preserved left ventricular ejection fraction, elevated levels of novel biomarkers reflecting cardiovascular stress may be useful both to identify patients who are at higher risk of cardiovascular death and heart failure and to select patients who derive a significant benefit from ACE inhibitor therapy.
Clinical Perspective.
The benefit of ACE inhibitors in low risk patients with stable CAD without heart failure remains controversial, and current practice guidelines note that it is reasonable but not recommended to use ACE inhibitors when cardiovascular risk factors are well controlled and revascularization has been performed. We now demonstrate that elevated levels of three novel biomarkers of cardiovascular stress, midregional pro-atrial natriuretic peptide (MR-proANP), midregional pro-adrenomedullin (MR-proADM), and C-terminal pro-endothelin-1 (CT-proET-1), are associated with the subsequent risk of cardiovascular death and heart failure independent of clinical factors (adjusted HRs per 1-SD of 1.97, 1.48, and 1.47, respectively; P≤0.002 for each biomarker). Furthermore, elevated levels of these biomarkers identified patients in whom therapy with an ACE inhibitor resulted in a significant reduction in the risk of cardiovascular death or heart failure. Specifically, trandolapril significantly reduced the risk of cardiovascular death or heart failure in patients who had elevated levels of 2 or more these biomarkers (HR 0.53, 95% CI 0.36–0.80), whereas there was no benefit in patients with elevated levels of 0 or 1 biomarkers (HR 1.09, 95% CI 0.74–1.59) (Pinteraction=0.012). Thus, in patients with stable CAD and preserved LVEF, elevated levels of novel biomarkers of cardiovascular stress identify patients who are at higher risk of cardiovascular death and heart failure and may be useful to select patients who derive significant benefit from ACE inhibitor therapy.
Supplementary Material
Acknowledgments
Funding Sources: The PEACE trial was supported by a contract from the National Heart, Lung, and Blood Institute (N01 HC65149) and by Knoll Pharmaceuticals and Abbott Laboratories, which also provided the study medication. Dr. Sabatine was supported in part by grant R01 HL094390 from the National Heart, Lung, and Blood Institute. Reagent for measurement of MR-proANP, MR-proADM, CT-proET-1, and copeptin were provided by B.R.A.H.M.S. GmbH (Henningsdorf, Germany). Neither the NHLBI, Knoll Pharmaceuticals, Abbott Laboratories, nor B.R.A.H.M.S. GmbH had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: Dr. Sabatine, Dr. Morrow, Ms. Sloan, and Dr. Braunwald are members of the TIMI Study Group, which has received research grant support from Accumetrics, Amgen, AstraZeneca, Beckman Coulter, BG Medicine, B.R.A.H.M.S. GmbH, Bristol-Myers Squibb, CV Therapeutics, Daiichi Sankyo Co Ltd, diaDexus, Eli Lilly and Co, Genentech, GlaxoSmithKline, Integrated Therapeutics, Johnson & Johnson, Merck and Co, Nanosphere, Novartis Pharmaceuticals, Nuvelo, Ortho-Clinical Diagnostics, Pfizer, Roche Diagnostics, Sanofi-Aventis, Siemens, and Singulex. Dr. Sabatine also reports receiving honoraria for educational presentations from Bristol-Myers Squibb and diaDexus; remuneration for consulting from AstraZeneca, Bristol-Myers Squibb / Sanofi-Aventis Joint Venture, Daiichi-Sankyo / Lilly Partnership, Sanofi-Aventis, and Singulex. Dr Morrow also reports receiving honoraria for educational presentations from Eli Lilly; receiving remuneration for consulting from Beckman-Coulter, Boehringer Ingelheim, Cardiokinetix, Critical Diagnostics, Gilead, Instrumentation Laboratory, Ikaria, Menarini, Merck, OrthoClinical Diagnostics, Servier, Roche Diagnostics, and Siemens, and remuneration from AstraZeneca for adjudication as a member of a Clinical Events Committee. Dr. de Lemos reports receiving grant support from Roche and Alere, Inc (formerly Biosite), consulting income from Alere, Johnson and Johnson Roche Diagnostics, and Tethys Biomedical. Dr. Omland reports having received speakers’ honoraria from Roche Diagnostics and Abbott Laboratories. Ms. Sloan has no other relevant disclosures. Dr. Jarolim reports receiving research support from Amgen, Beckman-Coulter, Ortho Clinical Diagnostics, Roche Diagnostics, and Siemens Healthcare Diagnostics; honoraria for educational presentations from Ortho Clinical Diagnostics; and consulting fees from T2 Biosystems. Dr. Solomon has no relevant disclosures. Dr. Pfeffer reports receiving grant support from Amgen, Baxter, Celladon, Novartis, and Sanofi-Aventis; consulting fees from Affectis, Anthera, Boehringer Ingelheim, Boston University, Boston Scientific, Bristol-Myers Squibb, Daiichi Sankyo, GlaxoSmithKline, Medtronic, Mirabila, Nicox, Novartis, Roche, Sanofi Aventis, Servier, University of Oxford; and that Brigham and Women’s Hospital has patents for the use of inhibitors of the rennin-angiotensin system in selected survivors of MI. Dr. Pfeffer is a co-inventor. The licensing agreement with Novartis and Boehringer, which are irrevocably transferred to charity, are not linked to sales. Dr. Braunwald also reports receiving remuneration for symposia and/or consulting from Amorcyte, CardioRentis, CVRx, Daiichi Sankyo, Eli Lilly, Genzyme, Medicines Co, and Merck & Co.
References
- 1.Braunwald E. Biomarkers in heart failure. N Engl J Med. 2008;358:2148–2159. doi: 10.1056/NEJMra0800239. [DOI] [PubMed] [Google Scholar]
- 2.Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T, Wolf PA, Vasan RS. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350:655–663. doi: 10.1056/NEJMoa031994. [DOI] [PubMed] [Google Scholar]
- 3.Zethelius B, Berglund L, Sundstrom J, Ingelsson E, Basu S, Larsson A, Venge P, Arnlov J. Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N Engl J Med. 2008;358:2107–2116. doi: 10.1056/NEJMoa0707064. [DOI] [PubMed] [Google Scholar]
- 4.de Lemos JA, Morrow DA, Bentley JH, Omland T, Sabatine MS, McCabe CH, Hall C, Cannon CP, Braunwald E. The prognostic value of B-type natriuretic peptide in patients with acute coronary syndromes. N. Engl. J. Med. 2001;345:1014–1021. doi: 10.1056/NEJMoa011053. [DOI] [PubMed] [Google Scholar]
- 5.Morrow DA, de Lemos JA, Blazing MA, Sabatine MS, Murphy SA, Jarolim P, White HD, Fox KA, Califf RM, Braunwald E. Prognostic value of serial B-type natriuretic peptide testing during follow-up of patients with unstable coronary artery disease. JAMA. 2005;294:2866–2871. doi: 10.1001/jama.294.22.2866. [DOI] [PubMed] [Google Scholar]
- 6.Omland T, Sabatine MS, Jablonski KA, Rice MM, Hsia J, Wergeland R, Landaas S, Rouleau JL, Domanski MJ, Hall C, Pfeffer MA, Braunwald E. Prognostic value of B-Type natriuretic peptides in patients with stable coronary artery disease: the PEACE Trial. J Am Coll Cardiol. 2007;50:205–214. doi: 10.1016/j.jacc.2007.03.038. [DOI] [PubMed] [Google Scholar]
- 7.Schnabel RB, Schulz A, Messow CM, Lubos E, Wild PS, Zeller T, Sinning CR, Rupprecht HJ, Bickel C, Peetz D, Cambien F, Kempf T, Wollert KC, Benjamin EJ, Lackner KJ, Munzel TF, Tiret L, Vasan RS, Blankenberg S. Multiple marker approach to risk stratification in patients with stable coronary artery disease. Eur Heart J. 2010;31:3024–3031. doi: 10.1093/eurheartj/ehq322. [DOI] [PubMed] [Google Scholar]
- 8.Levin ER, Gardner DG, Samson WK. Natriuretic peptides. N. Engl. J. Med. 1998;339:321–328. doi: 10.1056/NEJM199807303390507. [DOI] [PubMed] [Google Scholar]
- 9.Bunton DC, Petrie MC, Hillier C, Johnston F, McMurray JJ. The clinical relevance of adrenomedullin: a promising profile? Pharmacology & therapeutics. 2004;103:179–201. doi: 10.1016/j.pharmthera.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Jougasaki M, Stevens TL, Borgeson DD, Luchner A, Redfield MM, Burnett JC., Jr Adrenomedullin in experimental congestive heart failure: cardiorenal activation. Am J Physiol. 1997;273:R1392–R1399. doi: 10.1152/ajpregu.1997.273.4.R1392. [DOI] [PubMed] [Google Scholar]
- 11.Spieker LE, Noll G, Ruschitzka FT, Luscher TF. Endothelin receptor antagonists in congestive heart failure: a new therapeutic principle for the future? J Am Coll Cardiol. 2001;37:1493–1505. doi: 10.1016/s0735-1097(01)01210-4. [DOI] [PubMed] [Google Scholar]
- 12.Finley JJt, Konstam MA, Udelson JE. Arginine vasopressin antagonists for the treatment of heart failure and hyponatremia. Circulation. 2008;118:410–421. doi: 10.1161/CIRCULATIONAHA.108.765289. [DOI] [PubMed] [Google Scholar]
- 13.Moertl D, Berger R, Struck J, Gleiss A, Hammer A, Morgenthaler NG, Bergmann A, Huelsmann M, Pacher R. Comparison of midregional pro-atrial and B-type natriuretic peptides in chronic heart failure: influencing factors, detection of left ventricular systolic dysfunction, and prediction of death. J Am Coll Cardiol. 2009;53:1783–1790. doi: 10.1016/j.jacc.2009.01.057. [DOI] [PubMed] [Google Scholar]
- 14.von Haehling S, Filippatos GS, Papassotiriou J, Cicoira M, Jankowska EA, Doehner W, Rozentryt P, Vassanelli C, Struck J, Banasiak W, Ponikowski P, Kremastinos D, Bergmann A, Morgenthaler NG, Anker SD. Mid-regional pro-adrenomedullin as a novel predictor of mortality in patients with chronic heart failure. Eur J Heart Fail. 2010;12:484–491. doi: 10.1093/eurjhf/hfq031. [DOI] [PubMed] [Google Scholar]
- 15.Jankowska EA, Filippatos GS, von Haehling S, Papassotiriou J, Morgenthaler NG, Cicoira M, Schefold JC, Rozentryt P, Ponikowska B, Doehner W, Banasiak W, Hartmann O, Struck J, Bergmann A, Anker SD, Ponikowski P. Identification of chronic heart failure patients with a high 12-month mortality risk using biomarkers including plasma C-terminal pro-endothelin-1. PloS one. 2011;6:e14506. doi: 10.1371/journal.pone.0014506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stoiser B, Mortl D, Hulsmann M, Berger R, Struck J, Morgenthaler NG, Bergmann A, Pacher R. Copeptin, a fragment of the vasopressin precursor, as a novel predictor of outcome in heart failure. Eur J Clin Invest. 2006;36:771–778. doi: 10.1111/j.1365-2362.2006.01724.x. [DOI] [PubMed] [Google Scholar]
- 17.Garg R, Yusuf S. Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. Collaborative Group on ACE Inhibitor Trials. JAMA. 1995;273:1450–1456. [PubMed] [Google Scholar]
- 18.ACE Inhibitor Myocardial Infarction Collaborative Group. Indications for ACE inhibitors in the early treatment of acute myocardial infarction: systematic overview of individual data from 100,000 patients in randomized trials. Circulation. 1998;97:2202–2212. doi: 10.1161/01.cir.97.22.2202. [DOI] [PubMed] [Google Scholar]
- 19.The Heart Outcomes Prevention Evaluation Study Investigators. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N. Engl. J. Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 20.The EURopean trial On reduction of cardiac events with Perindopril in stable coronary Artery disease Investigators. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double-blind, placebo-controlled, multicentre trial (the EUROPA study) Lancet. 2003;362:782–788. doi: 10.1016/s0140-6736(03)14286-9. [DOI] [PubMed] [Google Scholar]
- 21.The PEACE Trial Investigators. Angiotensin-converting-enzyme inhibition in stable coronary artery disease. N Engl J Med. 2004;351:2058–2068. doi: 10.1056/NEJMoa042739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Braunwald E, Domanski MJ, Fowler SE, Geller NL, Gersh BJ, Hsia J, Pfeffer MA, Rice MM, Rosenberg YD, Rouleau JL. Angiotensin-converting-enzyme inhibition in stable coronary artery disease. N Engl J Med. 2004;351:2058–2068. doi: 10.1056/NEJMoa042739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morgenthaler NG, Struck J, Thomas B, Bergmann A. Immunoluminometric assay for the midregion of pro-atrial natriuretic peptide in human plasma. Clin Chem. 2004;50:234–236. doi: 10.1373/clinchem.2003.021204. [DOI] [PubMed] [Google Scholar]
- 24.Morgenthaler NG, Struck J, Alonso C, Bergmann A. Measurement of midregional proadrenomedullin in plasma with an immunoluminometric assay. Clin Chem. 2005;51:1823–1829. doi: 10.1373/clinchem.2005.051110. [DOI] [PubMed] [Google Scholar]
- 25.Papassotiriou J, Morgenthaler NG, Struck J, Alonso C, Bergmann A. Immunoluminometric assay for measurement of the C-terminal endothelin-1 precursor fragment in human plasma. Clin Chem. 2006;52:1144–1151. doi: 10.1373/clinchem.2005.065581. [DOI] [PubMed] [Google Scholar]
- 26.Morgenthaler NG, Struck J, Alonso C, Bergmann A. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem. 2006;52:112–119. doi: 10.1373/clinchem.2005.060038. [DOI] [PubMed] [Google Scholar]
- 27.Omland T, de Lemos JA, Sabatine MS, Christophi CA, Rice MM, Jablonski KA, Tjora S, Domanski MJ, Gersh BJ, Rouleau JL, Pfeffer MA, Braunwald E. A sensitive cardiac troponin T assay in stable coronary artery disease. N Engl J Med. 2009;361:2538–2547. doi: 10.1056/NEJMoa0805299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guilford JP. Fundamental Statistics in Psychology and Education. New York: McGraw Hill; 1956. [Google Scholar]
- 29.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. discussion 207-112. [DOI] [PubMed] [Google Scholar]
- 30.Harrell FE. Harrell Miscellaneous. R Graphical Manual. 2009 2008-12-26. Available at: http://biostat.mc.vanderbilt.edu/s/Hmisc,
- 31.Pencina MJ, D'Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sabatine MS, Morrow DA, Jablonski KA, Rice MM, Warnica JW, Domanski MJ, Hsia J, Gersh BJ, Rifai N, Ridker PM, Pfeffer MA, Braunwald E. Prognostic significance of the Centers for Disease Control/American Heart Association high-sensitivity C-reactive protein cut points for cardiovascular and other outcomes in patients with stable coronary artery disease. Circulation. 2007;115:1528–1536. doi: 10.1161/CIRCULATIONAHA.106.649939. [DOI] [PubMed] [Google Scholar]
- 33.Ala-Kopsala M, Magga J, Peuhkurinen K, Leipala J, Ruskoaho H, Leppaluoto J, Vuolteenaho O. Molecular heterogeneity has a major impact on the measurement of circulating N-terminal fragments of A- and B-type natriuretic peptides. Clin Chem. 2004;50:1576–1588. doi: 10.1373/clinchem.2004.032490. [DOI] [PubMed] [Google Scholar]
- 34.Richards AM, Doughty R, Nicholls MG, Macmahon S, Ikram H, Sharpe N, Espiner EA, Frampton C, Yandle TG. Neurohumoral prediction of benefit from carvedilol in ischemic left ventricular dysfunction. Australia-New Zealand Heart Failure Group. Circulation. 1999;99:786–792. doi: 10.1161/01.cir.99.6.786. [DOI] [PubMed] [Google Scholar]
- 35.Richards AM, Doughty R, Nicholls MG, MacMahon S, Sharpe N, Murphy J, Espiner EA, Frampton C, Yandle TG. Plasma N-terminal pro-brain natriuretic peptide and adrenomedullin: prognostic utility and prediction of benefit from carvedilol in chronic ischemic left ventricular dysfunction. Australia-New Zealand Heart Failure Group. J Am Coll Cardiol. 2001;37:1781–1787. doi: 10.1016/s0735-1097(01)01269-4. [DOI] [PubMed] [Google Scholar]
- 36.Fraker TD, Jr, Fihn SD, Gibbons RJ, Abrams J, Chatterjee K, Daley J, Deedwania PC, Douglas JS, Ferguson TB, Jr, Fihn SD, Fraker TD, Jr, Gardin JM, O'Rourke RA, Williams SV, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Halperin JL, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura R, Page RL, Riegel B, Tarkington LG, Yancy CW. 2007 chronic angina focused update of the ACC/AHA 2002 Guidelines for the management of patients with chronic stable angina: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines Writing Group to develop the focused update of the 2002 Guidelines for the management of patients with chronic stable angina. Circulation. 2007;116:2762–2772. doi: 10.1161/CIRCULATIONAHA.107.187930. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.