Abstract
The OX40 receptor is preferentially expressed by T cells, and its cognate ligand OX40L is primarily expressed by antigen-presenting cells such as dendritic cells following activation by thymic stromal lymphopoietin (TSLP). TSLP is released by the bronchial epithelium, airway smooth muscle, and some inflammatory cells in response to numerous insults such as allergens, viruses, and physical damage. OX40L is a costimulatory molecule that plays a sentinel role in the adaptive immune response by promoting T helper (Th) 2 polarization of naive T cells within the lymph node. These polarized T cells produce Th2 cytokines such as IL-4, IL-5, and IL-13, which have been implicated particularly in allergic eosinophilic asthma. Animal models have positioned both TSLP and OX40/OX40L as critical in the development of airway inflammation and hyperreactivity. In human disease, there is good evidence that TSLP is upregulated in asthma, but there are limited data to demonstrate overexpression of OX40 or OX40L in disease. Targeting the OX40/OX40L axis or TSLP presents a novel therapeutic strategy that has the potential of modifying the disease process and, therefore, impacting on its natural history. Whether this approach can demonstrate efficacy in established disease rather than at disease onset is unknown. Biologic therapies directed toward OX40/OX40L are in early phases of development, and results from these studies are eagerly awaited.
Asthma affects over 300 million people worldwide, and its prevalence is increasing. Asthma is a complex disease characterized by airway hyperresponsiveness, variable airflow obstruction, airway inflammation, varying degrees of subepithelial fibrosis, mucus hyperproduction, and remodeling.1 Atopic asthma has classically been associated with increased expression of T helper (Th) 2 cytokines, which are increased in sputum,2 bronchoalveolar T cells,3 and bronchial biopsies.4 A major effector axis resulting in induction of Th2 polarization is the recognition of allergen presented by dendritic cells in local lymph nodes to CD4+ T cells. This axis is an optimal target for drug development because it orchestrates the inflammation and development of an allergen-specific humoral response and the development of T- and B-cell memory. The differentiation of naive T cells or reactivation of memory T cells depends on various costimulatory molecules primarily expressed on the surface of T cells and their cognate ligands. One of the most promising costimulatory targets is OX40 and its ligand, OX40L. OX40L is directly mediated by thymic stromal lymphopoietin (TSLP), which is produced by epithelial cells,5 mast cells,6 airway smooth muscle,7 and dendritic cells,8 which are all involved in Th2 responses. TSLP was originally identified as a growth-promoting factor found in cultured supernatants of a thymic stromal cell line in 1994 to support the development of murine B cells.9 TSLP plays an important role in many allergic diseases, such as atopic dermatitis and asthma. TSLP is also up-regulated in COPD,10 but its role and relationship to OX40/OX40L signaling in this disease is unclear. TSLP binds to its TSLP receptor and the IL-7 receptor α chain. Dendritic cells play a crucial role in the pathogenesis of allergic disease. TSLP activates immature CD11c+ dendritic cells to express OX40L, and these cells then become mature dendritic cells, which migrate to the draining lymph nodes. There they activate the differentiation of naive CD4+ T cells by binding to the OX40 receptor, where they become inflammatory cells producing IL-4, IL-5, IL-13, tumor necrosis factor-α (TNF-α), and little or no IL-10 (Fig 1).11
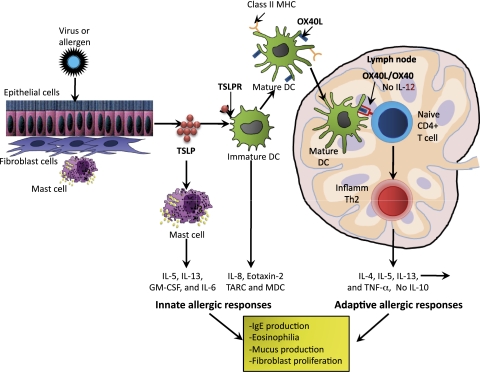
Figure 1.
Drawing shows the pathophysiologic characteristics of OX40/OX40L and TSLP in allergic inflammation. Cellular damage caused by allergens or viruses triggers mucosal epithelial cells or skin cells (keratinocytes, fibroblasts, and mast cells) to produce TSLP. TSLP initiates the innate phase of allergic immune responses by activating immature DCs by binding to their TSLPR. TSLP/TSLPR-activated DCs produce the chemokines IL-8 and eotaxin-2, TARC, and MDC, and by costimulating mast cells, produce IL-5, IL-13, GM-CSF, and IL-6. The activated immature DCs then mature and produce the OX40L and migrate into the draining lymph nodes, where they trigger the differentiation of allergen-specific naive CD4+ T cells by binding to the receptor OX40 and differentiating the CD4+ T cells into inflammatory Th2 cells, producing IL-4, IL-5, IL-13, and TNF-α, but no IL-10. These Th2 cytokines then initiate inflammation by triggering IgE production, eosinophilia, mucus production, and fibroblast proliferation. DC =dendritic cell; GM-CSF =granulocyte-macrophage colony-stimulated factor; MDC = macrophase-derived chemokine; MHC = major histocompatibility complex; TARC =T-helper 2 attracting chemokines activation-regulated chemokine; Th = T-helper; TNF = tumor necrosis factor; TSLP =thymic stromal lymphopoietin; TSLPR =thymic stromal lymphopoietin receptor.
The sentinel roles of the OX40/OX40L axis in the adaptive immune response and TSLP in both the innate and adaptive responses suggest these molecular targets may present attractive novel therapeutic targets. In this article, we consider the evidence that the OX40/OX40L axis plays a role in asthma, its potential importance as a therapeutic target, and the likely target population.
OX40 and OX40L
OX40 (ACT35, CD134, TNFRSF4) was identified in 1987 and found to be bound to activated T cells.12 Since then, it has been cloned in rat, mouse, and human cells. The OX40 receptor is preferentially expressed on the surface of activated regulatory CD4+ T cells,13 natural killer T cells, natural killer cells, and neutrophils, and more recently, we have found it to be expressed in human airway smooth muscle cells.14 OX40 signaling strongly regulates T-cell division, survival, and cytokine release.15 The OX40 ligand (OX40L, CD252, TNFSF4) was originally identified in 1985 as gp34 (GP34) protein on human T cell leukemic virus-transformed cells16 and is expressed on antigen-expressing cells, for instance, B cells,17 dendritic cells,18 and macrophages19 as well as airway smooth muscle cells.20
Evidence of a Critical Role for OX40/OX40L in the Pathogenesis of Asthma
Animal Models
In murine asthma models, OX40−/− mice challenged with ovalbumin showed significantly reduced Th2 response, lung inflammation, mucus secretion, 80% to 90% reduction in eosinophilia, decreased goblet cell hyperplasia, and significantly attenuated airway hyperreactivity compared with wild-type mice.21 Studies have also demonstrated that OX40L−/− mice sensitized with ovalbumin have significantly reduced total serum IgE, pulmonary eosinophils, cytokines, and pulmonary inflammation compared with wild-type control mice.22,23 Inhibition of OX40-OX40L binding via the administration of anti-OX40L mAb in wild-type mice dramatically reduced airway hyperresponsiveness and associated asthma symptoms, compared with mice challenged with isotype control.23,24 Mouse splenic CD11c+ dendritic cells stimulated for 48 h with TSLP upregulated OX40L expression compared with CD40L or TNF-α and unstimulated cells. A blockade of OX40/OX40L interactions using a specific α-mouse OX40L 4F5 monoclonal antibody significantly inhibited Th2 cytokine production compared with a control antibody. This confirms that OX40L activity on dendritic cells was important for the effects of TSLP in driving Th2 polarization.25 Both in murine and nonhuman primate models of asthma in vivo, a blockade of OX40L inhibited TSLP-mediated Th2 inflammation.25 Studies have demonstrated that OX40 can inhibit the development of adaptive Foxp3+ T regulatory cells that differentiate from naive CD4 T-cell populations in response to TGF-β.26,27 Foxp3, an X chromosome-encoded fork-head transcription factor family member, is critical for the differentiation of regulatory T cells. These cells have an important role in preventing autoimmunity and pathologic changes inflicted by uncontrolled immune responses to infections. Deficiency or mutation in Foxp3 in humans and mice leads to early onset and susceptibility to diseases such as asthma.28
Targeting OX40L may have the potential to improve the efficacy of immunotherapy to promote tolerance. In wild-type mice exposed to intranasal antigen and specific CD4+Foxp3+, regulatory T cells were generated, which outnumbered IL-4 and interferon γ-producing CD4 T cells. Inhaled lipopolysaccharide downregulated the regulatory T cells, but up-regulated IL-4+ and interferon-γ T cells, and it also increased OX40L expression on dendritic cells and B cells. Inhibiting OX40/OX40L interactions with an anti-OX40L antibody upregulated regulatory T cells suppressing lipopolysaccharide stimulation.29
Sensitization to fungi such as Aspergillus fumigatus and Alternaria is associated with poor lung function30 and exacerbations. When bone marrow-derived mouse dendritic cells were stimulated with Alternaria for 48 h, upregulation in OX40L expression was detected using flow cytometry,31 suggesting that Alternaria may play a role in Th2 cytokine release.
The activation of pattern-recognition receptors such as toll-like receptors plays a critical role in Th1 cell differentiation, yet their contribution to the generation of Th2 responses is poorly understood. Interestingly, when mice deficient in either MyD88−/− or TLR4−/− were sensitized intranasally to the common allergen house dust mites and challenged 2 weeks later, they showed diminished Th2 responses as well as fewer OX40Ls presenting dendritic cells in the draining lymph node compared with wild-type mice.32 The activation marker CD30, a member of the TNF receptor family, is expressed on activated T cells. In an acute asthma model, CD30−/− mice developed reduced expression of OX40,33 whereas in contrast, OX40 expression was not downregulated in a chronic murine asthma model.34 These differences in expression may be the result of the fact that in a chronic asthma model, T cells are able to proliferate, leading to chronic airway inflammation. Airway tolerance is vital for protecting the lung from inflammatory disease-driven allergens, but factors that lead to this susceptibility remain elusive. The pattern recognition receptors nucleotide-binding oligomerization domain (Nod)-like receptors Nod1 and Nod2 are both highly expressed by epithelial cells. Intranasal exposure of Nod2, but not Nod1, induced TSLP-promoting OX40L expression. The generation of these ligands also blocked CD4+ fork-head box protein 3+ adaptive regulatory T cells and concomitantly drove IL-4-producing CD4 T cells, leading to allergic disease and asthmatic lung inflammation.35
The animal-model data, therefore, present compelling evidence of a central role for OX40/OX40L in the development of Th2 polarization in response to a number of insults that are considered important in asthma. These data support a role for this axis in the immunopathogenesis of asthma.
Human Models
The role of OX40/OX40L in humans is limited compared with murine-model systems. TSLP was preferentially induced in peripheral blood-isolated myeloid dendritic cells from healthy volunteers to express mRNA for OX40L using microarray analysis.11 Blocking OX40/OX40L interactions using a specific OX40L-neutralizing antibody inhibited the production of Th2 cytokines and TNF-α, but increased the production of IL-10 in CD4+ cells cocultured with TSLP-primed dendritic cells.11 Airway smooth muscle cells are critical in the development of bronchoconstriction in asthma, are the major contributors to airway remodeling and persistent airflow obstruction, and release a number of chemokines/cytokines that bind specifically to activated T cells, resulting in increased cellular proliferation. Studies have reported OX40L to be expressed and released by airway smooth muscle cells of people with and without asthma, but with no significant difference.20 However, following TNF-α stimulation, there was an increase in OX40L, and a decrease after stimulation of TNF-α and interferon-γ combined.36 Cells activated with rOX40:Fc over 24 h released IL-6, which was significantly higher in the patients with asthma compared with people without asthma.36 More recently, our group has reported OX40/OX40L expression to be increased in the bronchial submucosa of patients with mild asthma, but not in those with moderate to severe disease, and this was related to the degree of tissue eosinophilia and IL-4 expression.14
Air pollution, particularly from diesel-exhaust particulates, is associated with worsening in asthma symptoms and control. Bronchial epithelial cells are the first major targets for inhaled pollutants. Bronchial epithelial cells treated with diesel-exhaust particulates express an increase in OX40L expression.37 A recent study also identified TSLP to be highly expressed in isolated nasal epithelial cells from patients with nasal polyposis compared with those without. The TSLP receptor and OX40L receptor were also increased in dendritic cells from the nasal mucosa of patients with nasal polyposis.38
The evidence in humans of a role of OX40/OX40L in asthma is, therefore, circumstantial and the expression data are weak. However, the interaction between OX40/OX40L in Th2 polarization may occur early in the disease pathogenesis and, more importantly, is primarily located in the lymph node rather than the bronchial submucosa. Current strategies to explore this axis have not addressed this compartment, and, therefore, its role has not been fully studied.
TSLP Asthma
TSLP is both necessary and sufficient for the development of Th2 cytokine-associated inflammation of the airways in rodents. Mice expressing a TSLP transgene in the airway epithelium develop a spontaneous, progressive inflammatory disease with all the characteristics of human asthma,39 whereas direct intranasal delivery of TSLP (in the presence of antigen) leads to rapid onset of features similar to severe disease.40 Studies have also reported increased TSLP mRNA in bronchial epithelium in asthma in response to allergen, viruses, and other environmental stimuli.41 In human disease, genetic analysis has shown an association of polymorphisms in TSLP with asthma and airway hyperresponsiveness, IgE concentrations, and eosinophilia.42-44 In addition, patients with asthma have higher concentrations of TSLP in their lungs.45,46 The role of TSLP in both the innate and adaptive immune response may suggest that its potential efficacy is broader than targeting the OX40/OX40L axis alone.
Targeting the TSLP and OX40/OX40L Axis in Asthma
There is an increasing recognition that asthma is a heterogeneous condition.47 Complex gene-environment interactions activate several biologic pathways that consequently result in the disordered airway physiologic aspects and symptoms that characterize asthma. The view that asthma is primarily an allergic disease mediated by Th2 cytokines has been challenged because asthma can develop in the absence of atopy. Indeed, allergic sensitization is likely to be more important in early-onset disease and particularly in children, whereas this feature of disease is less prominent in late-onset asthma.48 The application of noninvasive measures of airway inflammation has also led us to observe different inflammatory phenotypes, including eosinophilic- and neutrophilic-predominant asthma.49 The OX40/OX40L axis is particularly important in allergic sensitization and Th2 polarization. Therefore, predictably, patients with Th2-mediated eosinophilic inflammation are likely to be the most appropriate target population. However, the timing of the intervention is important and may be most effective prior to the onset of allergic sensitization and disease presentation, which obviously is unpredictable, and hence this is not a practical strategy. Ongoing activation of the OX40/OX40L axis may be important is some patients with asthma, but to date, this is uncertain and biomarkers to identify this group are unknown. At present, one clinical trial has been completed using a huMAb OX40L in the prevention of allergen-induced airway in adults with mild asthma. This study was funded by Genentech and completed in January 2011. However, the report from this study is still awaited.50
TSLP is likely to be effective in the same population as for OX40/OX40L, but also is critical in the innate immune response, suggesting its potential efficacy may target a broader group of patients with asthma. Critically, the potential efficacy of either approach will need to outweigh the potential side effects. Importantly, to date, early safety studies suggest that the safety profile of anti-OX40 therapy is good.
Conclusion
Current asthma therapies improve symptoms, improve disease control, and reduce exacerbations.1 None are disease modifying whereby they alter the natural history of the underlying disease. Data from animal models present a compelling argument that targeting the TSLP or OX40/OX40L axis will alter allergic sensitization and T-cell polarization. This presents a tantalizing possibility that this therapeutic approach in asthma may be disease modifying. Patients with asthma and researchers share the ambition to achieve a cure for asthma that can only be achieved by disease modification. Therefore, targeting TSLP or the OX40/OX40L axis presents an exciting opportunity that may provide a step-change in the treatment of asthma. Its fate will become apparent over the forthcoming couple of years as the eagerly awaited clinical trials are reported.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Brightling has received consultancy fees and research funding from AstraZeneca, MedImmune, GlaxoSmithKline, Chiesi, Roche, and Novartis. Dr Kaur has reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript.
Abbreviations
- Nod
nucleotide-binding oligomerization domain
- Th
T helper
- TNF
tumor necrosis factor
- TSLP
thymic stromal lymphopoietin
Footnotes
Funding/Support: Dr Brightling is supported by a Wellcome Senior Clinical Fellowship.
This is an Open Access article distributed under the terms of the Creative Commons Attribution-Noncommercial License (http://creativecommons.org/licenses/by-nc/3.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Information for commercial entities is available online (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Bousquet J, Mantzouranis E, Cruz AA, et al. Uniform definition of asthma severity, control, and exacerbations: Document presented for the World Health Organization Consultation on Severe Asthma. J Allergy Clin Immunol. 2010;126(5):926–938. doi: 10.1016/j.jaci.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 2.Berry MA, Parker D, Neale N, et al. Sputum and bronchial submucosal IL-13 expression in asthma and eosinophilic bronchitis. J Allergy Clin Immunol. 2004;114(5):1106–1109. doi: 10.1016/j.jaci.2004.08.032. [DOI] [PubMed] [Google Scholar]
- 3.Brightling CE, Symon FA, Birring SS, Bradding P, Pavord ID, Wardlaw AJ. TH2 cytokine expression in bronchoalveolar lavage fluid T lymphocytes and bronchial submucosa is a feature of asthma and eosinophilic bronchitis. J Allergy Clin Immunol. 2002;110(6):899–905. doi: 10.1067/mai.2002.129698. [DOI] [PubMed] [Google Scholar]
- 4.Saha SK, Berry MA, Parker D, et al. Increased sputum and bronchial biopsy IL-13 expression in severe asthma. J Allergy Clin Immunol. 2008;121(3):685–691. doi: 10.1016/j.jaci.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Semlali A, Jacques E, Koussih L, Gounni AS, Chakir J. Thymic stromal lymphopoietin-induced human asthmatic airway epithelial cell proliferation through an IL-13-dependent pathway. J Allergy Clin Immunol. 2010;125(4):844–850. doi: 10.1016/j.jaci.2010.01.044. [DOI] [PubMed] [Google Scholar]
- 6.Okayama Y, Okumura S, Sagara H, et al. FcepsilonRI-mediated thymic stromal lymphopoietin production by interleukin-4-primed human mast cells. Eur Respir J. 2009;34(2):425–435. doi: 10.1183/09031936.00121008. [DOI] [PubMed] [Google Scholar]
- 7.Zhang K, Shan L, Rahman MS, Unruh H, Halayko AJ, Gounni AS. Constitutive and inducible thymic stromal lymphopoietin expression in human airway smooth muscle cells: Role in chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol. 2007;293(2):L375–L382. doi: 10.1152/ajplung.00045.2007. [DOI] [PubMed] [Google Scholar]
- 8.Kashyap M, Rochman Y, Spolski R, Samsel L, Leonard WJ. Thymic stromal lymphopoietin is produced by dendritic cells. J Immunol. 2011;187(3):1207–1211. doi: 10.4049/jimmunol.1100355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friend SL, Hosier S, Nelson A, Foxworthe D, Williams DE, Farr A. A thymic stromal cell line supports in vitro development of surface IgM+ B cells and produces a novel growth factor affecting B and T lineage cells. Exp Hematol. 1994;22(3):321–328. [PubMed] [Google Scholar]
- 10.Ziegler SF. The role of thymic stromal lymphopoietin (TSLP) in allergic disorders. Curr Opin Immunol. 2010;22(6):795–799. doi: 10.1016/j.coi.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito T, Wang YH, Duramad O, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202(9):1213–1223. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paterson DJ, Jefferies WA, Green JR, et al. Antigens of activated rat T lymphocytes including a molecule of 50,000 Mr detected only on CD4 positive T blasts. Mol Immunol. 1987;24(12):1281–1290. doi: 10.1016/0161-5890(87)90122-2. [DOI] [PubMed] [Google Scholar]
- 13.So T, Lee SW, Croft M. Immune regulation and control of regulatory T cells by OX40 and 4-1BB. Cytokine Growth Factor Rev. 2008;19(3-4):253–262. doi: 10.1016/j.cytogfr.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siddiqui S, Mistry V, Doe C, Stinson S, Foster M, Brightling C. Airway wall expression of OX40/OX40L and interleukin-4 in asthma. Chest. 2010;137(4):797–804. doi: 10.1378/chest.09-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Croft M, So T, Duan W, Soroosh P. The significance of OX40 and OX40L to T-cell biology and immune disease. Immunol Rev. 2009;229(1):173–191. doi: 10.1111/j.1600-065X.2009.00766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanaka Y, Inoi T, Tozawa H, Yamamoto N, Hinuma Y. A glycoprotein antigen detected with new monoclonal antibodies on the surface of human lymphocytes infected with human T-cell leukemia virus type-I (HTLV-I) Int J Cancer. 1985;36(5):549–555. doi: 10.1002/ijc.2910360506. [DOI] [PubMed] [Google Scholar]
- 17.Stüber E, Neurath M, Calderhead D, Fell HP, Strober W. Cross-linking of OX40 ligand, a member of the TNF/NGF cytokine family, induces proliferation and differentiation in murine splenic B cells. Immunity. 1995;2(5):507–521. doi: 10.1016/1074-7613(95)90031-4. [DOI] [PubMed] [Google Scholar]
- 18.Ohshima Y, Tanaka Y, Tozawa H, Takahashi Y, Maliszewski C, Delespesse G. Expression and function of OX40 ligand on human dendritic cells. J Immunol. 1997;159(8):3838–3848. [PubMed] [Google Scholar]
- 19.Weinberg AD, Wegmann KW, Funatake C, Whitham RH. Blocking OX-40/OX-40 ligand interaction in vitro and in vivo leads to decreased T cell function and amelioration of experimental allergic encephalomyelitis. J Immunol. 1999;162(3):1818–1826. [PubMed] [Google Scholar]
- 20.Burgess JK, Carlin S, Pack RA, et al. Detection and characterization of OX40 ligand expression in human airway smooth muscle cells: A possible role in asthma? J Allergy Clin Immunol. 2004;113(4):683–689. doi: 10.1016/j.jaci.2003.12.311. [DOI] [PubMed] [Google Scholar]
- 21.Jember AG, Zuberi R, Liu FT, Croft M. Development of allergic inflammation in a murine model of asthma is dependent on the costimulatory receptor OX40. J Exp Med. 2001;193(3):387–392. doi: 10.1084/jem.193.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arestides RS, He H, Westlake RM, et al. Costimulatory molecule OX40L is critical for both Th1 and Th2 responses in allergic inflammation. Eur J Immunol. 2002;32(10):2874–2880. doi: 10.1002/1521-4141(2002010)32:10<2874::AID-IMMU2874>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 23.Hoshino A, Tanaka Y, Akiba H, et al. Critical role for OX40 ligand in the development of pathogenic Th2 cells in a murine model of asthma. Eur J Immunol. 2003;33(4):861–869. doi: 10.1002/eji.200323455. [DOI] [PubMed] [Google Scholar]
- 24.Salek-Ardakani S, Song J, Halteman BS, et al. OX40 (CD134) controls memory T helper 2 cells that drive lung inflammation. J Exp Med. 2003;198(2):315–324. doi: 10.1084/jem.20021937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seshasayee D, Lee WP, Zhou M, et al. In vivo blockade of OX40 ligand inhibits thymic stromal lymphopoietin driven atopic inflammation. J Clin Invest. 2007;117(12):3868–3878. doi: 10.1172/JCI33559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.So T, Croft M. Cutting edge: OX40 inhibits TGF-beta- and antigen-driven conversion of naive CD4 T cells into CD25+Foxp3+ T cells. J Immunol. 2007;179(3):1427–1430. doi: 10.4049/jimmunol.179.3.1427. [DOI] [PubMed] [Google Scholar]
- 27.Vu MD, Xiao X, Gao W, et al. OX40 costimulation turns off Foxp3+ Tregs. Blood. 2007;110(7):2501–2510. doi: 10.1182/blood-2007-01-070748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt-Weber CB, Blaser K. The role of the FOXP3 transcription factor in the immune regulation of allergic asthma. Curr Allergy Asthma Rep. 2005;5(5):356–361. doi: 10.1007/s11882-005-0006-z. [DOI] [PubMed] [Google Scholar]
- 29.Duan W, So T, Croft M. Antagonism of airway tolerance by endotoxin/lipopolysaccharide through promoting OX40L and suppressing antigen-specific Foxp3+ T regulatory cells. J Immunol. 2008;181(12):8650–8659. doi: 10.4049/jimmunol.181.12.8650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fairs A, Agbetile J, Hargadon B, et al. IgE sensitization to Aspergillus fumigatus is associated with reduced lung function in asthma. Am J Respir Crit Care Med. 2010;182(11):1362–1368. doi: 10.1164/rccm.201001-0087OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kobayashi T, Iijima K, Radhakrishnan S, et al. Asthma-related environmental fungus, Alternaria, activates dendritic cells and produces potent Th2 adjuvant activity. J Immunol. 2009;182(4):2502–2510. doi: 10.4049/jimmunol.0802773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tarkowski M. CD30 expression on allergen- and non-allergen-specific T cell lines and its role in cytokine production. Arch Immunol Ther Exp (Warsz) 2003;51(5):335–343. [PubMed] [Google Scholar]
- 33.Polte T, Behrendt AK, Hansen G. Direct evidence for a critical role of CD30 in the development of allergic asthma. J Allergy Clin Immunol. 2006;118(4):942–948. doi: 10.1016/j.jaci.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 34.Polte T, Fuchs L, Behrendt AK, Hansen G. Different role of CD30 in the development of acute and chronic airway inflammation in a murine asthma model. Eur J Immunol. 2009;39(7):1736–1742. doi: 10.1002/eji.200839004. [DOI] [PubMed] [Google Scholar]
- 35.Duan W, Mehta AK, Magalhaes JG, et al. Innate signals from Nod2 block respiratory tolerance and program T(H)2-driven allergic inflammation. J Allergy Clin Immunol. 2010;126(6):1284–1293. doi: 10.1016/j.jaci.2010.09.021. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burgess JK, Blake AE, Boustany S, et al. CD40 and OX40 ligand are increased on stimulated asthmatic airway smooth muscle. J Allergy Clin Immunol. 2005;115(2):302–308. doi: 10.1016/j.jaci.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 37.Bleck B, Tse DB, Gordon T, Ahsan MR, Reibman J. Diesel exhaust particle-treated human bronchial epithelial cells upregulate Jagged-1 and OX40 ligand in myeloid dendritic cells via thymic stromal lymphopoietin. J Immunol. 2010;185(11):6636–6645. doi: 10.4049/jimmunol.1000719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu T, Song CH, Liu AM, et al. Forkhead box P3+ T cells express interleukin-17 in nasal mucosa of patients with both allergic rhinitis and polyposis. Clin Exp Immunol. 2011;163(1):59–64. doi: 10.1111/j.1365-2249.2010.04278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou B, Comeau MR, De Smedt T, et al. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol. 2005;6(10):1047–1053. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- 40.Headley MB, Zhou B, Shih WX, Aye T, Comeau MR, Ziegler SF. TSLP conditions the lung immune environment for the generation of pathogenic innate and antigen-specific adaptive immune responses. J Immunol. 2009;182(3):1641–1647. doi: 10.4049/jimmunol.182.3.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Allakhverdi Z, Comeau MR, Jessup HK, et al. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med. 2007;204(2):253–258. doi: 10.1084/jem.20062211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gudbjartsson DF, Bjornsdottir US, Halapi E, et al. Sequence variants affecting eosinophil numbers associate with asthma and myocardial infarction. Nat Genet. 2009;41(3):342–347. doi: 10.1038/ng.323. [DOI] [PubMed] [Google Scholar]
- 43.He JQ, Hallstrand TS, Knight D, et al. A thymic stromal lymphopoietin gene variant is associated with asthma and airway hyperresponsiveness. J Allergy Clin Immunol. 2009;124(2):222–229. doi: 10.1016/j.jaci.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 44.Hunninghake GM, Lasky-Su J, Soto-Quirós ME, et al. Sex-stratified linkage analysis identifies a female-specific locus for IgE to cockroach in Costa Ricans. Am J Respir Crit Care Med. 2008;177(8):830–836. doi: 10.1164/rccm.200711-1697OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harada M, Hirota T, Jodo AI, et al. Functional analysis of the thymic stromal lymphopoietin variants in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2009;40(3):368–374. doi: 10.1165/rcmb.2008-0041OC. [DOI] [PubMed] [Google Scholar]
- 46.Ying S, O’Connor B, Ratoff J, et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol. 2005;174(12):8183–8190. doi: 10.4049/jimmunol.174.12.8183. [DOI] [PubMed] [Google Scholar]
- 47.Haldar P, Pavord ID, Shaw DE, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008;178(3):218–224. doi: 10.1164/rccm.200711-1754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miranda C, Busacker A, Balzar S, Trudeau J, Wenzel SE. Distinguishing severe asthma phenotypes: Role of age at onset and eosinophilic inflammation. J Allergy Clin Immunol. 2004;113(1):101–108. doi: 10.1016/j.jaci.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 49.Brightling CE. Clinical applications of induced sputum. Chest. 2006;129(5):1344–1348. doi: 10.1378/chest.129.5.1344. [DOI] [PubMed] [Google Scholar]
- 50.National Institutes of Health Clinical Center A study of huMAb OX40L in the prevention of allergen-induced airway obstruction in adults with mild allergic asthma. NCT00983658. ClinicalTrials.gov. Bethesda, MD: National Institutes of Health; 2009. http://clinicaltrials.gov/ct2/show/NCT00983658. Updated April 15, 2011.