Abstract
The role of cytokines in the control of tissue parasitism and pathogenesis of experimental Chagas’ disease was investigated. Wild-type and different cytokine as well as inducible nitric oxide synthase (iNOS) knockout mice were infected with the Colombian strain of Trypanosoma cruzi, and the kinetics of tissue parasitism, inflammatory reaction, parasitemia, and mortality were determined. We demonstrate the pivotal role of the interleukin (IL)-12/interferon (IFN)-γ/iNOS axis and the antagonistic effect of IL-4 in controlling heart tissue parasitism, inflammation, and host resistance to acute infection with T. cruzi. Further, the heart and central nervous system were shown the main sites of reactivation of T. cruzi infection in mice lacking functional genes for IFN-γ and IL-12, respectively. Our results also show that in contrast to IFN-γ knockout (KO) mice, splenocytes from IL-12 KO mice infected with T. cruzi produced low levels of IFN-γ upon stimulation with antigen. Consistently, high levels of anti-T. cruzi IgG2a antibodies were detected in the sera from IL-12 KO, but not from IFN-γ KO mice, infected with the Colombian strain of T. cruzi. Thus, our results suggest that the level of IFN-γ deficiency is a major determinant of the site of reactivation of T. cruzi infection in immunocompromised host.
Trypanosoma cruzi is the etiological agent of Chagas’ disease.1,2 In humans, the acute infection with T. cruzi lasts for 2 to 4 months and is characterized by the presence of parasites in the blood stream and different host tissues as well as variable degrees of nonspecific symptoms and myocarditis.3,4 After development of immunity, although somewhat persistent,5 both tissue parasitism and myocarditis are controlled and most symptoms of acute T. cruzi infection are resolved. However, 10 to 20 years after the initial infection, associated with increased tissue parasitism and inflammation,6-8 the digestive and/or cardiac forms of the disease become symptomatic in 10 to 30% of the chronic chagasic patients.9,10 Furthermore, immunodeficiency induced by either treatment with immunosuppressive drugs or infection with HIV-1 leads to reactivation of T. cruzi infection mainly in the central nervous system (CNS).11-13
The symptoms presented by patients with either acute or chronic Chagas’ disease are highly variable, and thought to be dependent on both parasite and host-related factors.3-14 Similarly, the pathology observed in mice infected with T. cruzi is highly dependent on parasite strain,15-17 size of parasite inoculum,18 host genetic background,19,20 and the action of various components of host immune system.10,14 Different studies indicate the crucial role of cytokines, such as interferon (IFN)-γ,21 tumor necrosis factor-α,22 and interleukin (IL)-1223 as well as nitric oxide24-26 in host resistance to infection with T. cruzi. Soon after the first rounds of replication in the vertebrate host tissues, T. cruzi parasites are thought to trigger the synthesis of proinflammatory cytokines that initiate the production of IFN-γ by natural killer (NK) cells,27 CD4+CD8−αβ+ and CD4−CD8+αβ+ T lymphocytes.28 IFN-γ combined with tumor necrosis factor-α will activate macrophages to produce high levels of nitric oxide that are primarily responsible for control of parasite replication during the acute phase of infection.22
However, scanty information is available regarding the role of cytokines controlling parasite replication and pathology in the heart tissue and CNS, the main sites of parasite replication and pathology during Chagas’ disease in immunocompetent and immunodeficient hosts, respectively. The lack of such information is mainly because various studies using the experimental murine model of Chagas’ disease used parasite strains with low tropism to the cardiac tissue and CNS. Secondly, most studies using genetically engineered immunodeficient mice infected with T. cruzi, have limited their evaluation to measurements of parasitemia and mortality rates. The present study was undertaken to determine the role of different cytokines on the kinetics of tissue parasitism and inflammatory cell infiltrates in the heart tissue and CNS of mice infected with the Colombian strain, a T. cruzi strain with high tropism for the cardiomyocytes and previously shown to induce encephalitis in susceptible mice.29-31 Our results show the essential role of IL-12/IFN-γ/inducible nitric oxide synthase (iNOS) axis, and the antagonistic effect of IL-4, in regulating parasite replication in the heart tissue and host resistance to T. cruzi during acute phase of infection. Moreover, we demonstrate that the deficiency of endogenous IL-12 and IFN-γ is determinant of the reactivation of T. cruzi infection in CNS and heart tissues, respectively. Finally, this study provides the first experimental model that resembles the parasite-elicited mass encephalitis, which is often found in chronic chagasic patients with AIDS, and contributes with new understanding of the pathogenesis of T. cruzi infection in immunodeficient hosts.
Materials and Methods
Animals
Female BALB/c and C57BL/6 mice were obtained from the Oswaldo Cruz Foundation-FIOCRUZ (Rio de Janeiro, Brazil). The IL-4-KO in the BALB/c genetic background, IFN-γ-KO, IL-12-KO, and iNOS-KO mice all in the C57BL/6 genetic background were provided by the Laboratory of Gnotobiology, ICB-UFMG (Belo Horizonte, Brazil). Female mice, 6 to 8 weeks of age, were maintained under standard conditions with environmental barriers in the animal house of the Centro de Pesquisas René Rachou, FIOCRUZ (Belo Horizonte, Brazil), and used for the experiments described bellow.
Parasites and Experimental Infection
The Colombian strain of T. cruzi was isolated by Frederici and colleagues31 and maintained by serial passages from mouse to mouse in the Laboratory of Chagas’ Disease, CPqRR-FIOCRUZ (Belo Horizonte, Brazil). BALB/c, C57BL/6, and different KO mice were infected intraperitoneally with 5000 blood trypomastigote forms of the Colombian strain of T. cruzi. In some experiments, the animals were treated with a subcurative dose of benznidazole (100 mg/kg/day) from day 10 to day 17 after infection. The levels of parasitemia were evaluated using 5 μl of blood in an optical microscope as previously described.32 All experiments were repeated three times and conducted according to the institutional guidelines for animal ethics of the Oswaldo Cruz Foundation.
Histological Evaluation
The myocardium and CNS were fixed in neutral 10% formalin, embedded in paraffin, sectioned, stained with hematoxylin and eosin (H&E), and examined by light microscopy. Tissue parasitism was scored by counting the total number of amastigote nests in 25 microscope fields (1 × 200 magnification) per histopathological section. Four sections were counted for each animal and the individual data determined as the mean result of the four sections.
An inflammatory infiltrate was considered when we detected 50 leukocytes or more in each inflammatory area. The inflammatory infiltrate was subdivided into focal and diffuse, depending on how close the inflammatory cells were associated. Most cells from the focal inflammatory infiltrate were in direct contact with each other, forming a continuous site of inflammation. In contrast, the diffuse inflammatory infiltrate was defined as a high density of mononuclear cells scattered throughout the cardiac our nervous tissue or composed of one or more small inflammatory foci containing less than 50 inflammatory cells. Considering that we found a high positive correlation between the intensities of focal and diffuse inflammatory infiltrates, for the sake of simplicity the results are presented as the average of the intensity of focal and diffuse inflammatory infiltrates. For the inflammatory infiltrate score, the total numbers of focal or diffuse inflammatory foci were counted in 25 microscope fields (1 × 200 magnification) per tissue section. Four sections were counted for each animal and individual data determined as the mean result of the four sections.
For both tissue parasitism and inflammation scores, the quantification was performed in four noncontiguous sections (40-μm distance between them) in 25 fields (1 × 200) magnification in a blind manner by three researchers.
Parasite-Specific Immunocytochemistry in the Cardiac Tissue and CNS from Infected Mice
For immunocytochemistry, deparaffinized sections were incubated for 30 minutes at 37°C in 1% bovine serum albumin to reduce nonspecific binding and then incubated in immune anti-T. cruzi mouse serum diluted 1:300 at 4°C overnight. Secondary biotinylated antibodies were goat anti-mouse antibodies. The sensitivity was improved with the avidin-biotin technique (ABC kit, PK-4000; Vector Laboratories, Inc., Burlingame, CA). The reaction was visualized by incubating the section with 3,3′-diaminobenzidine tetrahydrochloride (Amresco, Solon, OH).
Parasite Antigen Preparations
The epimastigote forms of the Colombian strain of T. cruzi were grown at 28°C in cell-free liver infusion tryptose medium supplemented with 10% fetal calf serum. Live parasites were harvested, washed three times in phosphate-buffered saline (PBS), resuspended at a final concentration of 109 parasites/ml and then submitted into three cycles of freeze and thawing at −70°C and 37°C, respectively, followed by five 30-second rounds of sonication. The resulting extract was centrifuged at 10,000 × g for 30 minutes and the supernatant, named soluble T. cruzi antigens (TcAg), frozen at −70°C until use in the stimulation of cytokine synthesis by spleen cells or enzyme-linked immunosorbent assay assays to measure serum levels of anti-T. cruzi antibodies.
Quantification of Parasite-Specific IgG1 and IgG2a
A Maxsorp enzyme-linked immunosorbent assay plates (Nalge Nunc International, Rochester, NY) were covered overnight at 4°C with 10 μg/ml of TcAg in carbonate-bicarbonate buffer (0.1 mol/L, pH 9.6). Serum from individual animals were diluted 1:40 in PBS-0.05% Tween 20 (PBS-Tween) and incubated for 1 hour at 37°C after blocking with 1% albumin in carbonate-bicarbonate buffer for 2 hours at 37°C. One hundred μl of 1:1000 of nonconjugated goat anti-mouse IgG1 or IgG2a (Southern Biotechnology Associates, Birmingham, AL) diluted in PBS-Tween was added to each well and the plate incubated for 1 hour at 37°C. For the detection of anti-IgG1 and IgG2a antibodies, a peroxidase-conjugated anti-goat IgG (1:5000) was incubated for 1 hour at 37°C. The assay was developed using the 2,2′-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) substrate (Sigma Chemical Co., St. Louis, MO), and the reaction was stopped with 20 μl of 20% sulfuric acid solution.
Spleen Cell Preparations and IFN-γ Assay
Spleens were removed aseptically and single-cell suspensions were prepared in complete medium after lysis of red cells. Splenocytes were then cultured in the presence of medium alone, with 20 μg of TcAg, or 5 μg/ml Con-A. After incubation for 72 hours, supernatants were removed and assayed for IFN-γ.29 IFN-γ was assayed in a two-site enzyme-linked immunosorbent assay using a rat anti-IFN-γ mAb R46A2 (ATCC, Rockville, MD) and a polyclonal rabbit serum specific for the cytokine. IFN-γ levels were calculated by reference to a standard curve constructed with recombinant cytokine (Genzyme, Cambridge, MA). Sensitivity of this method was 100 pg/ml.
Statistical Analysis
Arithmetic means (parasitemia, amastigote nests, inflammatory foci, and antibody levels) and standard deviations of the means were calculated. The Student’s t-test was used to determine the statistical significance of the differences observed. Differences were considered statistically significant when P < 0.05. The Kruskal-Wallis test was used to compare the mouse survival rates, and differences were considered statistically significant when P < 0.05.
Results
Comparison of BALB/c and C57BL/6 Mice in Resistance/Susceptibility to Infection with the Colombian Strain of T. cruzi
The tissue parasitism (Figure 1a▶ , and Figure 2, D▶ and E), parasitemia (Figure 1c▶ ), and rate of mortality (Figure 1d▶ ) were all higher in the BALB/c as compared to C57BL/6 mice infected with T. cruzi. In contrast, the intensity and the kinetics of diffuse and focal inflammatory infiltrates were similar in the heart tissue of both isogenic mouse strains infected with T. cruzi parasites (Figure 1b▶ and Figure 2, D▶ and E). Very rare isolated amastigote nests and no or only mild inflammation were found in the CNS of either BALB/c or C57BL/6 mice infected with the Colombian strain of T. cruzi (data not shown). The serum levels of both parasite-specific IgG1 and IgG2a isotypes were higher in BALB/c (Figure 1e▶ ) than in C57BL/6 (Figure 1f▶ ) mice. Further, the IgG1/IgG2a ratio was also higher in BALB/c as compared to C57BL/6 mice infected with the Colombian strain of T. cruzi.
Figure 1.
Course of infection with the Colombian strain of T. cruzi in C57BL/6 and BALB/c mice. a: The number of amastigote nests in 25 histopathological fields (original magnification, 1 × 200) of cardiac sections obtained from either C57BL/6 (▪) or BALB/c (□) mice at different times after infection. b: Number of inflammatory infiltrates present in 25 histopathological fields (original magnification, 1 × 200) of cardiac sections obtained from C57BL/6 (▪) and BALB/c (□) mice at different times after infection. c and e: The parasitemia (c) and survival (d) curves of either C57BL/6 ( ) or BALB/c ( ) mice infected with the Colombian strain of T. cruzi are shown. The serum levels of T. cruzi-specific IgG1 (□) and IgG2a (▪) isotypes are shown for both BALB/c (e) and C57BL/6 (f) mice. Each value is the average and SD of at least four animals. Similar results were obtained in three experiments. One asterisk indicates that differences are statistically significant (P < 0.01), when comparing different parameters from C57BL/6 and BALB/c mice obtained at the same time after infection. The differences between survival rates of C57BL/6 and BALB/c mice were considered statistically significant (P < 0.01).
Figure 2.
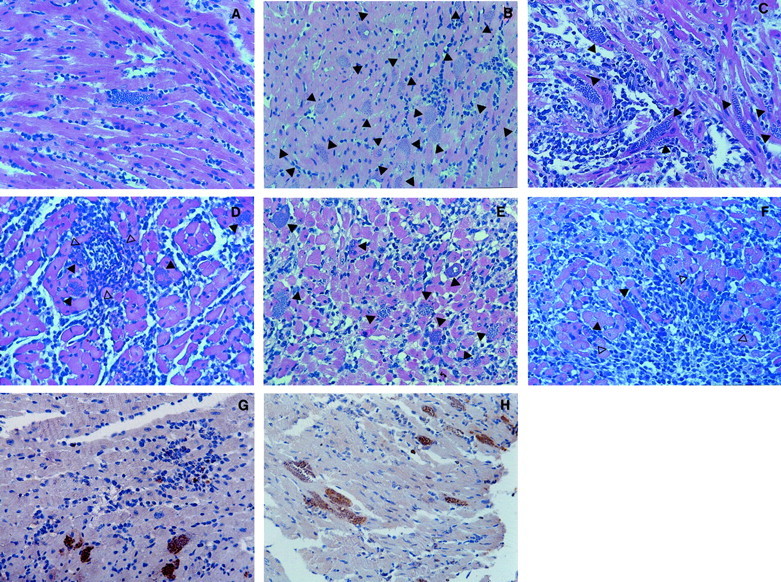
Illustration of T. cruzi parasitism and inflammation in cardiac tissue from WT and different KO mice infected with the Colombian strain of T. cruzi. A: One amastigote nest and mild inflammation is shown in one field of cardiac tissue from a C57BL/6 mouse at 15 days after infection (H&E; original magnification, 1 × 285). B: Twenty amastigote nests (closed arrow) and mild inflammation are shown in one field of cardiac tissue from an IFN-γ KO mouse at 15 days after infection (H&E; original magnification, 1 × 565). C: Seven amastigote nests (closed arrow) and intense diffuse inflammatory reaction are shown in one field of cardiac tissue from an iNOS KO mouse at 15 days after infection (H&E; original magnification, 1 × 285). D: Four amastigote nests (closed arrow) and intense diffuse as well as focal inflammatory reactions (open arrows) are shown in one field of cardiac tissue from a C57BL/6 mouse at 30 days after infection (H&E; original magnification, 1 × 285). E: Nine amastigote nests (closed arrow) and intense diffuse inflammatory reaction are shown in one field of cardiac tissue from a BALB/c mouse at 30 days after infection (H&E; original magnification, 1 × 285). F: Two amastigote nests (closed arrow) and intense diffuse as well as focal inflammatory (open arrows) reactions are shown in one field of cardiac tissue from an IL-4 KO mouse at 30 days after infection (H&E; original magnification, 1 × 285). Parasite-specific immunocytochemistry (brown staining) in cardiac tissue from IL-12 KO mouse at 50 days after infection (G) and IFN-γ KO mouse at 40 days after infection (H) with the Colombian strain of T. cruzi and treated with subcurative dose of benznidazole.
IL-4 Knockout (KO) Mice Are More Resistant to Infection with the Colombian Strain of T. cruzi
Because the BALB/c mice are normally high producers of IL-4, which has been shown to antagonize various IFN-γ-mediated protective immune responses in various systems,33 we evaluated the course of infection with the Colombian strain of T. cruzi in IL-4-KO BALB/c mice. Accordingly, IL-4 KO mice were more resistant to T. cruzi infection as indicated by heart tissue parasitism (Figure 2, E▶ and F, and Figure 3a▶ ), parasitemia (Figure 3c▶ ), and lower mortality ratio (Figure 3d▶ ). No major differences were observed in the intensity of diffuse and focal inflammation in the heart tissue when comparing IL-4 KO and wild-type (WT) animals (Figure 2, E▶ and F, and Figure 3b▶ ). The histopathology sections presented in Figure 2, E▶ and F, show an intense inflammatory reaction in the heart tissue of a BALB/c and an IL-4 KO mouse at 30 days after infection containing various and few amastigote nests, respectively. Figure 3e▶ shows that the levels of anti-T. cruzi-specific IgG1 antibodies were reduced in sera of IL-4 KO mice infected with T. cruzi. In contrast, no alteration in the serum levels of parasite-specific IgG2a antibodies was observed when comparing IL-4 KO (Figure 3e▶ ) and WT BALB/c (Figure 3f▶ ) mice infected with T. cruzi.
Figure 3.
Course of infection with Colombian strain of T. cruzi in BALB/c and IL-4 KO mice. a: The number of amastigote nests in 25 histopathological fields (original magnification, 1 × 200) of cardiac sections obtained from either BALB/c (□) or IL-4 KO (▪) mice at different times after infection. b: The number of inflammatory infiltrates present in 25 histopathological fields (original magnification, 1 × 200) of cardiac sections obtained from BALB/c (□) or IL-4 KO (▪) mice at different times after infection. c and d: Shown are the parasitemia (c) and survival (d) curves in either BALB/c ( ) or IL-4 KO ( ) mice infected with the Colombian strain of T. cruzi. The serum levels of T. cruzi-specific IgG1 (□) and IgG2a (▪) isotypes are shown for both IL-4 KO (e) and BALB/c (f) mice. Each value is the average and SD of at least four animals. Similar results were obtained in three experiments. One asterisk indicates that differences are statistically significant (P < 0.01), when comparing different parameters from IL-4 KO and BALB/c mice obtained at the same time after infection. The differences between survival rates of IL-4 KO and BALB/c mice were considered statistically significant (P < 0.01).
IL-12 KO, IFN-γ KO, or iNOS KO Are Highly Susceptible to Infection with the Colombian Strain of T. cruzi
We also infected IL-12 and IFN-γ C57BL/6 KO mice with the Colombian strain of T. cruzi. Our results show that both IFN-γ KO and IL-12 KO were highly susceptible to infection displaying a high number of amastigote nests (Figure 4a▶ ), parasitemia (Figure 4c▶ ), and 100% of mortality by 20 days after infection (Figure 4d▶ ). In contrast to the intensity of tissue parasitism, we observed limited inflammatory reaction in the heart tissue from IFN-γ KO and IL-12 KO, at 15 days after infection (Figure 4b▶ ). No enhanced tissue parasitism and/or inflammation in the CNS was observed, when comparing IFN-γ KO and IL-12 KO to WT mice acutely infected with the Colombian strain of T. cruzi (data not shown).
Figure 4.
Course of infection with the Colombian strain of T. cruzi in C57BL/6, IFN-γ KO, IL-12 KO, and iNOS KO mice. a: The number of amastigote nests in 25 histopathological fields (original magnification, 1 × 200) of cardiac sections obtained from either C57BL/6 (▪), IFN-γ KO (□), or IL-12 KO ( ) mice at different times after infection. b: The number of inflammatory infiltrates present in 25 histopathological fields (original magnification, 1 × 200) of cardiac sections obtained from C57BL/6 (▪), IFN-γ KO (□), or IL-12 KO ( ) mice at different times after infection. Bottom left: Shown are the parasitemia (c) and survival (d) curves in either C57BL/6 ( ), IFN-γ KO ( ), or IL-12 KO (— —) infected with the Colombian strain of T. cruzi. e: The number of amastigote nests in 25 histopathological fields (original magnification, 1 × 200) of cardiac sections obtained from either C57BL/6 (▪) or iNOS KO ( ) mice at different times after infection. f: The number of inflammatory infiltrates present in 24 histopathological fields (original magnification, 1 × 200) in cardiac sections obtained from either C57BL/6 (▪) or iNOS KO ( ) mice at different times after infection. Bottom right: Shown are the parasitemia (g) and survival (h) curves in either C57BL/6 ( ) or iNOS KO ( ) infected with Colombian strain of T. cruzi. Each value is the average and SD of at least four animals. Similar results were obtained in three experiments. One asterisk indicate that differences are statistically significant at P < 0.01 level, respectively, when comparing different parameters from C57BL/6 to IFN-γ KO, IL-12 KO (left), or to iNOS KO (right) mice obtained at the same time after infection. The differences between survival rates of C57BL/6 and IFN-γ KO, IL-12 KO, or iNOS KO mice infected with T. cruzi were considered statistically significant (P < 0.01).
Enhanced susceptibility was also observed in iNOS KO mice that presented higher tissue parasitism (Figure 4e▶ ), parasitemia (Figure 4g▶ ), and accelerated mortality (Figure 4h▶ ) as compared to C57BL/6 mice. These results differed from those obtained from IL-12 KO and IFN-γ KO, in that the iNOS KO survived more than 30 to 40 days of infection presenting the peak of heart tissue parasitism (Figure 4e▶ ) and parasitemia (Figure 4g▶ ) at day 30 after infection. Differences were observed in the intensity of diffuse and focal inflammation in the cardiac tissue, when comparing iNOS KO and WT other KO mice at 15 days after infection, but not at the latter time point.
The histopathology sections show one amastigote nest in the cardiac tissue from C57BL/6 mice (Figure 2A)▶ contrasting with multiple amastigote nests in the heart from IFN-γ KO (Figure 2B)▶ and iNOS KO (Figure 2C)▶ mice, all at 15 days after infection. Note mild inflammation observed in IFN-γ KO mice, contrasting with more intense inflammation in iNOS KO mice (Figure 2C)▶ .
Heart Tissue and CNS as the Major Sites of Reactivation of T. cruzi Infection in IFN-γ KO and IL-12 KO Mice
We next tested the role of endogenous IFN-γ, IL-12, and iNOS in the control of tissue parasitism, parasitemia, and mortality in mice that have latent infection with the Colombian strain of T. cruzi. WT and the various KO mice were infected and submitted to treatment with benznidazole, beginning at 10 days after infection, soon after parasitemia become patent. The mice received 100 mg/kg/day of benznidazole from days 10 to 17 after infection. This therapeutic protocol, although highly efficient in controlling tissue parasitism and parasitemia, is not curative for mice infected with the Colombian strain of T. cruzi. After cessation of chemotherapy, the mice were followed for tissue parasitism, parasitemia, and mortality.
Benznidazole therapy resulted in 100% and 80% of survival (and no measurable parasitemia) in WT and iNOS KO mice, respectively. In contrast, greater parasitemia (Figure 5a▶ ) and accelerated mortality (Figure 5b▶ ) became apparent in the IFN-γ KO and IL-12 KO mice were after cessation of chemotherapy. Although increased, the parasitemia (Figure 5a▶ ) and heart tissue parasitism (Figure 5c▶ and Figure 2G and 2▶ hours) of IL-12 KO was relatively small when compared to that observed in the IFN-γ KO mice. One hundred percent mortality was observed at 40 and 55 days after infection of IFN-γ KO and IL-12 KO mice, respectively (Figure 5b▶ ). The small increase in tissue parasitism observed in IL-12 KO mice was accompanied by a dramatic increase in heart tissue inflammation at day 40 after infection and after (Figure 5e▶ ). In contrast, a weak inflammatory process was observed in the heavily parasitized heart tissue from IFN-γ KO mice at 40 days after infection or earlier (Figure 5e▶ ). The WT and iNOS KO mice were relatively resistant to reactivation of infection up to 100 days after infection (Figure 5, a▶ and b). These findings are in agreement with those published by Saeftel and colleagues34 showing that mouse treatment with iNOS inhibitors does not change the course of late acute and chronic infection with T. cruzi.
Figure 5.
Reactivation of infection with the Colombian strain of T. cruzi in C57BL/6, IFN-γ KO, IL-12 KO, and iNOS KO mice. Top left: Shown are the parasitemia (a) and survival (b) curves in either C57BL/6 ( ), IFN-γ KO ( ), or IL-12 KO (— —) or iNOS KO ( ) mice infected with the Colombian strain of T. cruzi and treated with a subcurative dose of benznidazole. The number of amastigote nests in 25 histopathological fields (original magnification, 1 × 200) of cardiac (c) or brain (d) sections obtained from either C57BL/6 (▪), IFN-γ KO (□), or IL-12 KO ( ) mice at different times after infection and treatment with a subcurative dose of benznidazole. e: The number of inflammatory infiltrates present in 25 histopathological fields (original magnification 1 × 200) of cardiac sections obtained from C57BL/6 (▪), IFN-γ KO (□), or IL-12 KO ( ) mice at different times after infection and treatment with a subcurative dose of benznidazole. f: The number of inflammatory infiltrates present in 25 histopathological fields (original magnification, 1 × 200) of brain sections obtained from C57BL/6 (▪), IFN-γ KO (□), or IL-12 KO ( ) mice at different times after infection and treatment with a subcurative dose of benznidazole. The serum levels of T. cruzi-specific IgG1 (g) and IgG2a (h) isotypes are shown for C57BL/6 (▪), IFN-γ KO (□), and IL-12 KO ( ) mice at different times after infection and treatment with a subcurative dose of benznidazole. i: IFN-γ levels produced by splenocytes cultured in the presence of medium alone (□), TcAg ( ), or Con A (▪) derived from C57BL/6, IFN-γ KO, or IL-12 KO mice at 35 days after infection and treatment with a subcurative dose of benznidazole. Each value is the average and SD of at least four animals. Similar results were obtained in three experiments. One and two asterisks indicate that differences are statistically significant at P < 0.05 and P < 0.01 levels, respectively, when comparing different parameters from C57BL/6 to IFN-γ KO and IL-12 KO mice obtained at the same time after infection. The differences between survival rates of C57BL/6 and iNOS KO to IFN-γ KO and IL-12 KO mice were considered statistically significant (P < 0.01).
The extensions of CNS tissue damage, probably the main cause of death in IL-12 KO mice infected with the Colombian strain of T. cruzi and treated with benznidazole, were analyzed by histopathology and immunocytochemistry. From day 45 after infection IL-12 KO mice start showing signs of neurological disturbances, marked by complete or partial hind limb paralysis as well as unbalanced movements. Consistently, the tissue parasitism (Figure 5d▶ and Figure 6, E▶ and F), inflammation (Figures 5f▶ and 6E), and lesions in the brain (Figures 6, E▶ and G, and 6 hours) of IL-12 KO progressively increased from day 40 to 55 after infection, as indicated by analysis at days 40, 45 (not shown), 50, and 55 (not shown) days after infection. The inflammatory reaction in the IL-12 KO mice was characterized by glial nodules and necrotic lesions (Figure 6E▶ ), vascular cuffing by lymphocytes (Figure 6G▶ ), and meningitis (Figure 6H▶ ). An intense tissue parasitism accompanying the inflammatory reaction in the brain of IL-12 KO mice at 50 to 55 days after infection, can be observed in either histopathological section (Figure 6E▶ ) or immunocytochemistry (Figure 6F▶ ). Although less intense then in IL-12 KO mice, a gradual increased tissue parasitism and inflammation in the brain was also observed during reactivation of infection in IFN-γ KO (Figure 5d▶ and Figure 6, C▶ and D) mice, at 30, 35 (not shown), and 40 days after infection. Inflammation (Figures 5e▶ and 6A) and amastigote nests (Figures 5d▶ and 6B) were a rare finding in the brain of WT mice infected with Colombian strain of T. cruzi. It is noteworthy that under a similar protocol, infection with Y strain of T. cruzi resulted in reactivation of infection in the IFN-γ KO but not in the IL-12 KO mice (data not shown), indicating a parasite strain dependency for the reactivation of T. cruzi in the CNS.
Figure 6.

Illustration of T. cruzi parasitism and inflammation in the brain from WT, IFN-γ KO, and IL-12 KO mice infected with the Colombian strain of T. cruzi and treated with benznidazole. Brain section from C57BL/6 at 50 days after infection with no inflammation (H&E; original magnification, 1 × 285) (A) and a rare amastigote nest (B) as detected by immunocytochemistry (brown staining). Brain section from IFN-γ KO at 40 days after infection with focal inflammations/glial nodules (open arrows) and three amastigote nests (closed arrow) (H&E; original magnification, 1 × 285) (C). Several amastigote nests (D) are evidenced in the CNS from IFN-γ KO at 40 days after infection by immunocytochemistry (brown staining). Brain section from IL-12 KO at 50 days after infection with an intense focal inflammation (glial nodule) and 10 amastigote nests (closed arrow) containing various number of parasites (H&E; original magnification, 1 × 285) (E). Various amastigote nests (F) in the CNS from IL-12 KO at 50 days after infection are stained with a parasite-specific antibody (brown staining). G (H&E; 1 × 285) and H (H&E; original magnification, 1 × 1000) show, respectively, the intense cuffing of brain arterioles (open arrows) and meningitis (open arrows) observed in IL-12 KO mouse at 50 days after infection with the Colombian strain of T. cruzi.
We then determined the composition of inflammatory infiltrate both in the heart and brain from IL-12 KO mice infected with T. cruzi and submitted to early treatment with benznidazole. Heart and brain were harvested after cessation of chemotherapy at 50 days after infection. Our immunocytochemistry results show that the inflammatory infiltrate in the heart of IL-12 KO presented a ratio of 0.1:1.0:3.4 for macrophages: CD4+ T cells: CD8+ T cells. In contrast, in the brain the inflammatory infiltrate was mostly devoid of CD8+T cells presenting the ratio of 3.7:3.7:1.0 for macrophages: CD4+T cells: CD8+ T cells. It is noteworthy that in advanced lesions the inflammatory infiltrates in the brain, but not in the heart, from IL-12 KO were also very rich in polymorphic nuclear cells, mainly neutrophils, also reflected by the higher expression of MAC-1 (granulocytes and macrophage markers) relative to F4/80 (macrophage marker). Our immunocytochemistry results also demonstrate the presence of cells producing IFN-γ in the in the inflammatory processes found in the heart from IL-12 KO mice at 45 to 55 days after infection. IFN-γ-producing cells were a rare finding in the CNS of IL-12 KO mice at 45 to 55 days after infection with the Colombian strain of T. cruzi (data not shown).
We also measured the levels of anti-T. cruzi IgG1 and IgG2a antibodies in the sera of mice infected with Colombian strain and treated with benznidazole. Our results show that the parasite-specific IgG1 (Figure 5g▶ ) and IgG2a (Figure 5h▶ ) levels were higher in IFN-γ KO and IL-12 KO, respectively. In agreement with these results, we found that when stimulated with TcAg, spleen cells from IL-12 KO mice produced ∼20% of the total IFN-γ produced by splenocytes from WT animals (Figure 5i▶ ). Together these results indicate that an IL-12-independent pathway for IFN-γ synthesis is operating during T. cruzi infection.
Discussion
The precise mechanisms involved in the pathogenesis of Chagas’ disease are primarily unknown. Nevertheless, follow-up studies of chagasic patients9 and in experimental models15,18 suggest a positive correlation between severity of disease and/or heart alterations during acute disease and chronic cardiac pathology. Moreover, when compared to asymptomatic individuals, tissue parasitism is higher both in the heart and in esophagus of patients displaying the severe forms of chronic Chagas’ disease.6-8 Additional evidences for the role of parasite in the pathogenesis of Chagas’ disease are the different tissue tropism and disease outcome in experimental models15-17 infected with distinct T. cruzi strains. Finally, various components of the host immune system have been shown to primarily contribute to host resistance to T. cruzi infection and suggested to play a crucial role in the immunopathogenesis of Chagas’ disease.10,14
In the present study, we used the Colombian strain of T. cruzi that causes an intense cardiomyopathy29,31,35 as well as encephalitis30 during experimental infection in mice. Our previous study showed that inflammation, elicited by the infection with Colombian strain in mice, is accompanied by enhanced expression of a mixed profile of type 1 (ie, IL-12 and IFN-γ) as well as type 2 cytokine (ie, IL-4 and IL-10) mRNAs in the heart.29 Herein, by using isogenic as well as cytokine and iNOS KO mice, we investigated the role of different cytokines on control of tissue parasitism and inflammation during infection with the Colombian strain of T. cruzi in mice.
Initially, we used the C57BL/6 and BALB/c isogenic mice strains that, on various stimuli or microbial infections, have been shown to develop a dominant type 1 and type 2 immune response, respectively.36,37 Consistent, with studies performed with other T. cruzi strains,19,20 BALB/c mice were shown to be more susceptible than C57BL/6 when infected with the Colombian strain. The enhanced susceptibility of BALB/c mice to T. cruzi infection was associated with enhanced production of IL-4 (data not shown) as also indicated by the higher parasite-specific IgG1/IgG2a ratios, when compared to C57BL/6 mice. More importantly, we could identify IL-4 as a major immunological factor mediating susceptibility of BALB/c mice, as suggested by the lower number of amastigote nests, parasitemia, and lower rate of mortality of IL-4 KO BALB/c mice acutely infected with the Colombian strain of T. cruzi. In contrast to our results, early reports demonstrate no differences of resistance in IL-4 KO or STAT6 KO as compared to WT mice infected with other T. cruzi strains (ie, Y and Brazil), that present low tropism for the cardiac tissue.38,39 Indeed, we have previously observed that in contrast to the Colombian strain, the Y strain of T. cruzi induces lower levels of IL-4 synthesis and a more dominant IL-12 and IFN-γ immune response during infection in the murine model.29,32
In the conditions used here, the levels of cardiac inflammation were similar when comparing BALB/c and C57BL/6 or IL-4 BALB/c KO mice. Other studies have demonstrated the important anti-inflammatory role of IL-440 and IL-1041,42 in preventing excessive inflammation in animals infected with T. cruzi. The anti-inflammatory effect of IL-10 during infection with T. cruzi also seems to be dependent on parasite strain, because IL-10 does not influence the course of infection with the Y strain of T. cruzi.43 Nevertheless, these studies suggest that IL-4 and IL-10 may act in synergy to control myocarditis elicited during infection with T. cruzi. However, as shown here, if the immunoregulatory role of IL-4 is dominant it will favor parasite replication leading to enhanced host susceptibility to infection. It is noteworthy that, a study performed by Reis and colleagues44 show a close association of IL-4-producing T cells and the presence of amastigote nests in the myocarditis elicited by T. cruzi parasites in humans.
Different studies have demonstrated the importance of IL-12/IFN-γ/iNOS axis in resistance to T. cruzi infection.21-26 Therefore, our next set of experiments evaluated the role of endogenous IL-12, IFN-γ, and iNOS on host resistance to infection with the Colombian strain of T. cruzi. Our results indicate that either IL-12 or IFN-γ are essential for host resistance to acute infection with this parasite. According to a previous study,26 our experiments show that iNOS is also essential to control parasite replication in the cardiac tissue and host resistance to infection with T. cruzi. It is noteworthy that the iNOS KO mice were somewhat more resistant than either IL-12 KO or IFN-γ KO mice, indicating that an additional IFN-γ-dependent mechanism, but iNOS-independent may also contribute to the control of parasite replication in heart tissue and host resistance to the acute infection with the Colombian strain of T. cruzi.
Regarding the reactivation of T. cruzi infection in IFN-γ KO mice was characterized by extremely high levels of parasitemia and cardiac tissue parasitism, and relatively low levels of inflammation. In contrast, a small increase in parasitemia and cardiac tissue parasitism was associated to a dramatic increase in inflammation in cardiac tissue of the IL-12 KO mice after cessation of chemotherapy. Interestingly, similar findings are observed in heart tissue of AIDS patients during reactivation of chronic T. cruzi infection.45 More importantly, reactivation of T. cruzi infection in IL-12 KO mice was associated with signs of neurological disturbances, encephalitis, and areas of the CNS with intense parasite replication, inflammation, and tissue damage. The essential role of endogenous IL-12 on control of Toxoplasma gondii and Cryptococcus neoformans infection in the CNS from the murine model has also been reported.46,47
Based on parasite-elicited IFN-γ production by spleen cells and parasite-specific IgG isotype measurements in sera from WT, IL-12 KO, and IFN-γ KO mice, we favor the hypothesis that the level of endogenous IFN-γ is a major determinant of the site of parasite replication during reactivation of T. cruzi infection. Indeed, previous studies have suggested that the induction of IFN-γ during T. cruzi infection may also occur via an IL-12-independent manner.23,48 Thus, in the IFN-γ KO mice, where virtually no IFN-γ is produced, the reactivation of infection occurred in peripheral organs mainly in the heart tissue. In contrast, in the IL-12 KO mice, the levels of IFN-γ and IFN-γ-dependent effector mechanisms, although lower than in the WT mice, are sufficient to control parasite replication in the peripheral organs. However, the IL-12-independent IFN-γ-based immunity seems not to be sufficient to control parasite replication in the CNS. Our studies with iNOS KO mice indicate that the main mechanism responsible for the control of reactivation of T. cruzi infection is dependent on IFN-γ, but relatively independent of iNOS.
Importantly, during reactivation of infection the inflammatory processes in the CNS from IL-12 KO mice were rich in polymorphic nuclear cells and mostly devoid of CD8+ T cells. Further, the CD4:CD8 ratio in the CNS and heart from benznidazole-treated IL-12 KO mice, at 50 days after infection, were 3.7: 1 and 1:3.4, respectively. Consistent with the hypothesis that CD8+ T lymphocytes are major sources of IFN-γ during T. cruzi infection, we found IFN-γ-producing cells in the heart, but not in the CNS from these animals. Thus, considering the important role of CD8+ T lymphocytes in resistance to T. cruzi,28,49 the increased susceptibility to parasite replication and mass encephalitis could be explained by the lack of CD8+ T lymphocyte migration and local production of IFN-γ in the CNS from IL-12 KO mice. The parasite-specific IgG2a antibodies could be an alternative IFN-γ-inducible effector mechanism responsible for controlling parasite replication and preventing reactivation of T. cruzi infection. However, we found that in contrast to IFN-γ KO, the IL-12 KO mice produce high levels of parasite-specific IgG2a when infected with T. cruzi. Thus, it is possible that parasite-specific antibodies from IgG2a isotype play an important role in controlling parasitemia and tissue parasitism in the peripheral organs but not in the CNS.
Finally, this study provides for the first time an experimental model and new basis to understand the pathogenesis of the T. cruzi-induced mass encephalitis in immunodeficient hosts. Thus, our results indicate that host resistance to T. cruzi replication in the CNS (an immunologically privileged site) as compared to peripheral organs, requires higher levels of endogenous IFN-γ and/or IFN-γ-mediated effector functions. Considering the gradual decline in IL-12 and IFN-γ production in HIV-1-infected patients,50-52 these findings may explain, in part, why the CNS is the main site of reactivation of infection with T. cruzi12,13 in AIDS patients.
Acknowledgments
We thank Jerry E. Manning, Conceição Machado, and Keith Luhrs for critically reading this manuscript, and Ronilda M. Paula, Antônio M. Vaz, and Maria Helena de Oliveira for maintaining the colonies of KO mice.
Footnotes
Address reprint requests to Dr. Ricardo T. Gazzinelli, Laboratory of Immunopathology, Centro de Pesquisas René Rachou, FIOCRUZ, Av. Augusto de Lima 1715, Barro Preto, 30190-002, Belo Horizonte, MG, Brazil. E-mail: ritoga@cpqrr.fiocruz.br.
Supported in part by the FAPEMIG (CBB 916/96), PAPES-FIOCRUZ (no. 2), and CNPq (522.056/95-4). R. T. G., L. Q. V., and J. L. V. are research fellows from CNPq. V. M. and N. M. S. are graduate students with scholarships from FIOCRUZ and CNPq, respectively.
References
- 1.Burleigh BA, Andrews NW: The mechanisms of Trypanosoma cruzi invasion of mammalian cells. Ann Rev Microbiol 1995, 49:175-200 [DOI] [PubMed] [Google Scholar]
- 2.: World Health Organization: Chagas’ disease. Tropical Disease Research. 1993, :pp 125-135 World Health Organization, Geneva, Switzerland Twelfth Programme of UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR) [Google Scholar]
- 3.Rassi A, Luquetti AO, Rassi A, Jr, Rassi SG, Rassi AG: Chagas disease—clinical features. Wendel S Brener Z Camargo M Rassi A eds. Chagas Disease (American Trypanosomiasis): Its Impact on Transfusion and Clinical Medicine, chap 6. 1992, :pp 81-101 International Society of Blood Transfusion Brazil, São Paulo [Google Scholar]
- 4.Parada H, Carrasco HA, Añez N, Fuenmayor C, Inglessis I: Cardiac involvement is a constant finding in acute Chagas’disease: a clinical parasitological and histopathological study. Int J Cardiol 1997, 60:49-54 [DOI] [PubMed] [Google Scholar]
- 5.Añez N, Carrasco H, Parada H, Crisante G, Rojas A, Fuenmayor C, Gonzales N, Percoco G, Borges R, Guevara P, Ramirez JL: Myocardial parasite persistence in chronic chagasic patients. Am J Trop Med Hyg 1999, 60:726-732 [DOI] [PubMed] [Google Scholar]
- 6.Jones EM, Colley DG, Tostes S, Lopes ER, Vnencak-Jones CL, Mccurley TL: Amplification of a Trypanosoma cruzi DNA sequence from inflammatory lesions in human chagasic cardiomyopathy. Am J Trop Med Hyg 1993, 48:348-357 [DOI] [PubMed] [Google Scholar]
- 7.Higuchi ML, Brito T, Reis MM, Barbosa A, Bellotti G, Pereira-Barreto AC, Pileggi F: Correlation between Trypanosoma cruzi parasitism and myocardial inflammatory infiltrate in human chronic chagasic myocarditis: light microscopy and immunohistochemical findings. Cardiovasc Pathol 1993, 2:101-106 [DOI] [PubMed] [Google Scholar]
- 8.Vago AR, Macedo AM, Adad SJ, Reis DD, Corrêa-Oliveira R: PCR detection of Trypanosoma cruzi in oesophageal tissues of patients with chronic digestive Chagas’ disease. Lancet 1996, 348:891-892 [DOI] [PubMed] [Google Scholar]
- 9.Dias JCP: The indeterminate form of human chronic Chagas’ disease a clinical epidemiological review. Rev Soc Bras Med Trop 1989, 22:147-156 [DOI] [PubMed] [Google Scholar]
- 10.Brener Z, Gazzinelli RT: Immunological control of Trypanosoma cruzi infection and pathogenesis of Chagas’ disease. Int Arch Allergy Immunol 1997, 114:103-110 [DOI] [PubMed] [Google Scholar]
- 11.Jardim E, Takayanagui OM: Chagasic meningoencephalitis with detection of Trypanosoma cruzi in the cerebrospinal fluid of an immunodepressed patient. J Trop Med Hyg 1994, 97:367-370 [PubMed] [Google Scholar]
- 12.Rocha A, de Meneses AC, da Silva AM, Ferreira MS, Nishioka SA, Burgarelli MK, Almeida E, Turcato G, Jr, Metze K, Lopes ER: Pathology of patients with Chagas’ disease and acquired immunodeficiency syndrome. Am J Trop Med Hyg 1994, 50:261-268 [DOI] [PubMed] [Google Scholar]
- 13.Silva N, O’Bryan L, Medeiros E, Holand H, Suleiman J, Mendonça JS, Patronas N, Reed SG, Klein HG, Masur H, Badaro R: Trypanosoma cruzi meningoencephalitis in HIV-infected patients. Acquir Immune Defic Syndr Hum Retrovirol 1999, 20:342-349 [DOI] [PubMed] [Google Scholar]
- 14.Minoprio P, Joskowicz MH: 36th Forum in Immunology, Chagas’disease: Trypanosoma cruzi vs the host immune system. Res Immunol 1991, 142:137-167 [DOI] [PubMed] [Google Scholar]
- 15.Andrade SG: Influence of Trypanosoma cruzi strain on the pathogenesis of chronic myocardiopathy in mice. Mem Inst Oswaldo Cruz 1990, 85:17-27 [DOI] [PubMed] [Google Scholar]
- 16.Andrade LO, Machado CRS, Chiari E, Pena SDJ, Macedo AM: Differential tissue distribution of diverse clones of Trypanosoma cruzi in infected mice. Mol Biochem Parasitol 1999, 100:163-172 [DOI] [PubMed] [Google Scholar]
- 17.Vago AR, Andrade LO, Leite AA, dÁvila Reis D, Macedo AM, Adad SJ, Tostes S, Moreira MC, Filho GB, Pena SD: Genetic characterization of Trypanosoma cruzi directly from tissues of patients with chronic Chagas disease: differential distribution of genetic types into distinct organs. Am J Pathol 2000, 156:1805-1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marinho CRF, D’Ímpério, Lima MR, Grisotto MG, Alvarez JM: Influence of acute-phase on pathology, parasitism, and activation of the immune system at late chronic phase of Chagas’ disease. Infect Immun 1999, 67:308-318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wrightsman R, Krassner S, Watson J: Genetic-control of responses to Trypanosoma cruzi in mice—multiple genes influencing parasitemia and survival. Infect Immun 1982, 36:637-644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trischmann TM, Bloom BR: Genetics of murine resistance to Trypanosoma cruzi. Infect Immun 1982, 35:546-551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torrico F, Heremans H, Rivera MT, Van ME, Billiau A, Carlier Y: Endogenous IFN-gamma is required for resistance to acute Trypanosoma cruzi infection in mice. J Immunol 1991, 146:3626-3632 [PubMed] [Google Scholar]
- 22.Silva JS, Vespa GNR, Cardoso MAG, Aliberti JC, Cunha FQ: Tumor necrosis factor alpha mediates resistance to Trypanosoma cruzi in mice by inducing nitric oxide production in infected IFN-γ-activated macrophages. Infect Immun 1995, 63:4862-4867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aliberti JCS, Cardoso MAG, Martins GA, Gazzinelli RT, Vieira LQ, Silva JS: IL-12 mediates resistance to Trypanosoma cruzi in mice and is produced by murine macrophages in response to live trypomastigotes. Infect Immun 1996, 64:1961-1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gazzinelli RT, Oswald IP, Hieny S, James S, Sher A: The microbicidal activity of interferon-γ treated macrophages against Trypanosoma cruzi involves an L-arginine-dependent, nitrogen oxide-mediated mechanism inhibitable by interleukin-10 and transforming growth factor-β. Eur J Immunol 1992, 22:2501-2506 [DOI] [PubMed] [Google Scholar]
- 25.Vespa GNR, Cunha FQ, Silva JS: Nitric oxide is involved in the control of Trypanosoma cruzi induced parasitemia and directly kills parasite in vitro. Infect Immun 1994, 62:5177-5182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holscher C, Kohler G, Muller U, Mossmann H, Schaub GA, Brombacher F: Defective nitric oxide effector functions lead to extreme susceptibility of Trypanosoma cruzi-infected mice deficient in gamma interferon receptor or inducible nitric oxide synthase. Infect Immun 1998, 66:1208-1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cardillo F, Voltarelli JC, Reed SG, Silva JS: Regulation of Trypanosoma cruzi infection in mice by gamma interferon and interleukin 10: role of NK cells. Infect Immun 1996, 64:128-134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tarleton R, Sun J, Zhang L, Postan M: Depletion of T-cell subpopulations results in exacerbation of myocarditis and parasitism in experimental Chagas’ disease. Infect Immun 1994, 62:1820-1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Talvani A, Ribeiro CS, Aliberti JCS, Michailowsky V, Santos PVA, Murta SMF, Romanha AJ, Almeida IC, Farber J, Lannes-Vieira J, Silva JS, Gazzinelli RT: Kinetics of cytokine genes expression in experimental chagasic cardiomyopathy: tissue parasitism and IFN-γ as determinants of chemokine mRNAs expression during infection with Trypanosoma cruzi. Microbes Infect 2000, 2:851-866 [DOI] [PubMed] [Google Scholar]
- 30.Silva AA, Roffê E, Marino APMP, Santos PVA, Quirico-Santos T, Paiva CN, Lannes-Vieira J: Chagas’ disease encephalitis: intense CD8+ lymphocytic infiltrates is restricted to acute phase, but is not related to the presence of Trypanosoma cruzi antigens. Clin Immunol 1999, 92:56-66 [DOI] [PubMed] [Google Scholar]
- 31.Frederici EE, Albemann WH, Neva FA: Chronic and progressive myocarditis and myositis in C3H mice infected with Trypanosoma cruzi. Am J Trop Med Hyg 1964, 13:272-280 [DOI] [PubMed] [Google Scholar]
- 32.Michailowsky V, Murta SMF, Carvalho-Oliveira L, Pereira MES, Ferreira LRP, Brener Z, Romanha AJ, Gazzinelli RT: Interleukin-12 enhances in vivo parasiticidal effect of benznidazole during acute experimental infection with a naturally drug-resistant strain of Trypanosoma cruzi. Antimicrob Agents Chemother 1997, 42:2549-2556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paludan SR: Interleukin-4 and interferon-γ: the quintessence of a mutual antagonistic relationship. Scand J Immunol 1998, 48:459-468 [DOI] [PubMed] [Google Scholar]
- 34.Saeftel M, Fleischer B, Hoerauf A: Stage-dependent role of nitric oxide in control of Trypanosoma cruzi infection. Infect Immun 2001, 69:2252-2259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.dos Santos RR, Rossi MA, Laus JL, Silva JS, Savino W, Mengel J: Anti-CD4 abrogates rejection and reestablishes long-term tolerance to syngeneic newborn hearts grafted in mice chronically infected with Trypanosoma cruzi. J Exp Med 1992, 175:29-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsieh CS, Macatonia SE, O’Garra A, Murphy KM: T cell genetic background determines default T helper phenotype development in vitro. J Exp Med 1995, 181:713-721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heinzel FP, Sadick MD, Holaday BJ, Coffman RL, Locksley RM: Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med 1989, 169:59-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abrahamsohn IA, da Silva AP, Coffman RL: Effects of interleukin-4 deprivation and treatment on resistance to Trypanosoma cruzi. Infect Immun 2000, 68:1975-1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tarleton RL, Grusby MJ, Zhang L: Increased susceptibility of Stat4-deficient and enhanced resistance in Stat6-deficient mice to infection with Trypanosoma cruzi. J Immunol 2000, 165:1520-1525 [DOI] [PubMed] [Google Scholar]
- 40.Soares MBP, Silva-Mota KN, Lima RS, Bellintani MC, Lain CPC, dos Santos RR: Modulation of chagasic cardiomyopathy by interleukin-4: dissociation between intensity of inflammation and parasitism. Am J Pathol 2001, 159:703-709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hunter CA, Ellis-Neyes LA, Slifer T, Kanaly S, Grunig G, Fort M, Rennick D, Araujo FG: IL-10 is required to prevent immune hyperactivity during infection with Trypanosoma cruzi. J Immunol 1997, 158:3311-3316 [PubMed] [Google Scholar]
- 42.Holscher C, Mohrs M, Daí WJ, Kohler G, Ryffel B, Schaub GA, Mossmann H, Brombacher F: Tumor necrosis factor alpha-mediated toxic shock in Trypanosoma cruzi-infected interleukin 10-deficient mice. Infect Immun 2000, 68:4075-4083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abrahamsohn IA, Coffman RL: Trypanosoma cruzi: IL-10, TNF, IFN-gamma, and IL-12 regulate innate and acquired immunity to infection. Exp Parasitol 1996, 84:231-234 [DOI] [PubMed] [Google Scholar]
- 44.Reis MM, Higuchi ML, Benvenuti LA, Aiello VD, Gutierrez PS, Bellotti G, Pileggi F: An in situ quantitative immunohistochemical study of cytokines and IL-2R+ in chronic human chagasic myocarditis: correlation with the presence of myocardial Trypanosoma cruzi antigens. Clin Immunol Immunopathol 1997, 83:165-172 [DOI] [PubMed] [Google Scholar]
- 45.Sartori AM, Lopes MH, Benvenuti LA, Caramelli B, di Pietro A, Nunes EV, Ramirez LP, Shikanai-Yasuda MA: Reactivation of Chagas’ disease in a human immunodeficiency virus-infected patient leading to severe heart disease with a late positive direct microscopic examination of the blood. Am J Trop Med Hyg 1998, 5:784-786 [DOI] [PubMed] [Google Scholar]
- 46.Yap G, Pesin M, Sher A: IL-12 is required for the maintenance of IFN-gamma production in T cells mediating chronic resistance to the intracellular pathogen, Toxoplasma gondii. J Immunol 2000, 165:628-631 [DOI] [PubMed] [Google Scholar]
- 47.Decken K, Kohler G, Palmer-Lehmann K, Wunderlin A, Mattner F, Magram J, Gately MK, Albert G: IL-12 is essential for protective Th1 response in mice infected with Cryptococcus neoformans. Infect Immun 1998, 66:4994-5000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buschenfelde CMZ, Cramer S, Trumpfheller C, Fleischer B, Frosh S: Trypanosoma cruzi induces strong IL-12 and IL-18 gene expression in vivo: correlation with interferon-gamma (IFN-γ) production. Clin Exp Immunol 1997, 110:378-385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tarleton RL: Depletion of CD8+ cells increases susceptibility and reverses vaccine-induced immunity in mice infected with Trypanosoma cruzi. J Immunol 1990, 15:717-724 [PubMed] [Google Scholar]
- 50.Murray HW, Rubin BY, Masur H, Roberts RB: Impaired production of lymphokines and immune (γ) interferon in acquired immunodeficiency syndrome. N Engl J Med 1984, 310:883-889 [DOI] [PubMed] [Google Scholar]
- 51.Chehimi J, Starr SE, Frank I, D’Andrea A, Ma X, MacGregor RR, Sennelier J, Trinchieri G: Impaired interleukin-12 production in human immunodeficiency virus-infected patients. J Exp Med 1994, 179:1361-1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gazzinelli RT, Bala S, Stevens R, Baseler M, Wahl L, Kovacs J, Sher A: HIV infection suppresses Th1 lymphokine and IL-12 responses to Toxoplasma gondii but fails to inhibit parasite induced monokine synthesis. J Immunol 1995, 155:1565-1574 [PubMed] [Google Scholar]






