Abstract
Increased immunoglobulin G (IgG) and intrathecally produced oligoclonal bands (OGBs) are characteristic of a limited number of inflammatory central nervous system (CNS) diseases and are often directed against the cause of disease. In subacute sclerosing panencephalitis (SSPE), the cause of disease and the target of the oligoclonal response is measles virus (MV). The authors previously showed that clonally expanded populations of CD38+ plasma cells in SSPE brain, the likely source of OGBs, are directed against MV. In characterizing the breadth of the plasma cell reactivities, the authors found that a large proportion of the less abundant plasma cells are also directed against MV. The intrathecal response may be useful in determining the causes of other inflammatory CNS diseases, such as multiple sclerosis, Behcet’s disease, and neurosarcoidosis.
Keywords: antibodies, antigen specificity, inflammation, plasma cells, SSPE
Increased immunoglobulin G (IgG) and oligoclonal bands (OGBs) are found in the cerebrospinal fluid (CSF) of humans with chronic infectious central nervous system (CNS) diseases such as neurosyphilis, cryptococcal and tuberculous meningitis, Lyme disease, some viral meningitides, varicella-zoster virus vasculopathy, and subacute sclerosing panencephalitis (SSPE). Analysis of the specificity of CSF OGBs has revealed that the oligoclonal IgG in SSPE (Vandvik et al, 1976), neurosyphilis (Vartdal et al, 1981), mumps meningitis (Vandvik et al, 1978), cryptococcal meningitis (Porter et al, 1977), varicellazoster virus vasculopathy (Burgoon et al, 2003), and other disorders is directed against the agent that causes disease (reviewed in Gilden et al, 2001). This led to the hypothesis that the oligoclonal IgG in the brain and CSF of patients with chronic inflammatory CNS disease of unknown etiology such as multiple sclerosis, sarcoidosis, and Behcet’s disease is anti-body directed against the agent that causes disease. Better strategies and techniques to identify disease-relevant antibodies and their cognate antigens may identify the causes of inflammatory diseases of unknown etiologies. We have used SSPE as a model to study the complexity of the intrathecal response to disease-relevant or ancillary antigens.
We previously used laser capture microdissection to isolate individual CD38+ plasma cells from the brain of a patient with SSPE followed by single-cell reverse transcriptase—polymerase chain reaction (RT-PCR) to amplify individual IgG heavy (H) and light (L) chain sequences expressed by each cell (Burgoon et al, 2005). Analysis of a repertoire of the expressed IgGs in brain (Table 1) showed that 55 of the 65 plasma cells were in clonally expanded groups (clones 1 to 11), whereas 10 plasma cells were encountered only once. Analysis of functional recombinant antibodies (rIgGs) constructed from 8 of the clonally expanded Ig sequences, which were most likely to represent the intrathecally synthesized OGBs, showed that most of these rIgGs recognized measles virus (MV), the cause of SSPE (Burgoon et al, 2005).
Table 1.
IgG sequence analysis of CD38+ plasma cells in an SSPE brain
| Clone | CD38+ VH CDR3 | Abundance | Germline | Family | % ID | VK CDR3 | VA CDR3 | Germline | Family | % ID |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ALKKGEGGLRFLELYYFD | 10 | DP47 | VH3 | 92.1 | QTWGSGMGV | Loc4b | Vλ4a | 93.7 | |
| ALKKGEGGLRFLELYYFD | 3 | DP47 | VH3 | 92.1 | ||||||
| ALKKGEGGLRFLELYYLT | 1 | DP47 | VH3 | 92.1 | QQNYSSPQT | DPK24 | Vκ4 | (t) | ||
| 2 | LPAAGPRSFFETYNWGMD | 8 | DP79 | VH4 | 93.6 | AAWDDSLNAVWV | DPL2 | Vλ1 | 97 | |
| LPADGPRSFFETYNNGMD | 1 | DP79 | VH4 | 93.6 | AAWDDSLNGWV | DPL2 | Vλ1 | 97 | ||
| LPAAGPRSFFETYNWGMD | 1 | DP79 | VH4 | 93.6 | ||||||
| 3a | IRAGAFD | 2 | DP31 | VH3 | 95.2 | MQALQTFTF | DPK15 | Vκ2 | 99.7 | |
| 3b | IRAGAFD | 4 | DP31 | VH3 | 95.2 | MQATQSWTF | DPK16 | Vκ2 | 98.2 | |
| IRAGAFD | 1 | DP31 | VH3 | 95.2 | ||||||
| IRAGAFD | 1 | DP31 | VH3 | 95.2 | (m) | |||||
| 4 | DFTSDSRGPLGWFD | 4 | DP79 | VH4 | 93.9 | YSTDSSGDHRV | Loc3p | Vλ3 | 98.1 | |
| DFTSDSRGPLGWFD | 1 | DP79 | VH4 | 93.9 | ||||||
| 5 | GGLAARARLVLARMD | 3 | DP63 | VH4 | 93.8 | QQSYNTPITF | DPK9 | Vκ1 | 95.1 | |
| GGLAARARLVLARMD | 1 | DP63 | VH4 | 93.8 | ||||||
| 6 | VRATVLTGTSMD | 2 | DP58 | VH3 | 91.8 | GADHGSGSNFVWV | DPL22 | Vλ9 | 97.6 | |
| VRATVLTGTSMD | 1 | DP58 | VH3 | 91.8 | ||||||
| 7 | DTGGSGSNYYHYGMD | 2 | DP10 | VH1 | 93.2 | |||||
| DTGGSGSNYYHYGMD | 1 | DP10 | VH1 | 93.2 | QQYNAWPPALT | DPK21 | Vκ3 | (t) | ||
| 8 | DRGGESDYDVGRGYSDHYGMD | 2 | DP71 | VH4 | 86.9 | QQCGFSPKT | DPK22 | Vκ3 | 92.5 | |
| 9 | DQERGTILTYSDMD | 2 | DP47 | VH3 | 95.9 | LQHNSYPHFRRR* | DPK3 | Vκ1 | 95.5 | |
| 10 | DQVPVNNWFD | 2 | DP14 | VH1 | 95.2 | (m) | ||||
| 11 | SLTMIRGVMAFFD | 2 | DP25 | VH1 | 87.6 | QQTYSSPSTF | DPK9 | Vκ1 | 90.9 | |
| D3 | DQVIYTGWSD | 1 | DP47 | VH3 | 91.2 | CLYAGSTTWV | DPL10 | Vλ2 | 96.3 | |
| B11 | GYYDSTGYKSAND | 1 | DP14 | VH1 | 94.0 | QQTYSSPSTF | DPK9 | Vκ1 | 90.9 | |
| D10 | LKSRIARGSYYQYFMD | 1 | DP27 | VH2 | 93.1 | |||||
| F7 | SADTSTAYYGLD | 1 | DP47 | VH3 | 96.6 | LQDYNYPLTF | DPK3 | Vκ1 | 99.2 | |
| G5 | STGTDYYSYYMD | 1 | DP73 | VH5 | 86.6 | YSTDTSGNFRV | Loc3p | Vλ3 | 99.2 | |
| G6 | EGQLALDQYYYYYMD | 1 | DP50 | VH3 | 96.3 | NSYTSISTVV | DPL11 | Vλ2 | 93.6 | |
| G11 | DRTGYTSFLFD | 1 | DP31 | VH3 | 90.0 | SSYAGRNKGYV | DPL12 | Vλ2 | 96 | |
| H11 | DPEEQWLADYFD | 1 | DP47 | VH3 | 97.6 | GTWDSSLSARV | DPL5 | Vλ1 | 98.9 | |
| LS | VEVGPNEDFYMD | 1 | DP88 | VH1 | 90.1 | QQSYSFPWTF | DPK9 | Vκ1 | 89.4 | |
| K6 | EVAGGADIEVVPAAIGVDYHYGI | 1 | DP79 | VH4 | 97.3 | QSADSSGSYKV | (t) |
Note. Each line identifies the CDR3 amino acid sequence and prevalence of each distinct H chain clone, the germline family, most homologous germline segment, percent identity to the closest germline, and the associated L chain amplification for that clone. Less abundant clones are highlighted in italics. rIgGs were constructed from clones in bold. ∗an in-frame stop codon; (m), mixed sequence that could not be analyzed further; (t), truncated sequence.
The question remains whether additional antibody reactivities are present, particularly toward autoantigens that might confound the disease-relevant response. For example, in multiple sclerosis, antibodies directed against various self or novel antigens have been found in both blood and CSF, but have not been shown to be part of the oligoclonal IgG in most patients (reviewed in Burgoon et al, 2004). Furthermore, antibody to components of myelin have been detected in the serum and CSF in SSPE patients, but the contribution of these minor reactivities to the oligoclonal response has not been determined (Panitch et al, 1980; Ruutianen et al, 1981; Gorny et al, 1983; Mathiesen et al, 1989). Thus, we studied the specificity of antibodies produced by less abundant plasma cells in SSPE brain whose sequences were only seen once during repertoire analysis.
Functional rIgGs were constructed from 8 of the 10 less abundant plasma cell IgG sequences (bold in Table 1). H chain variable regions were cloned into the modified expression vector pIgG Flag, which contains the remaining constant domains to express a full-length IgG1 H chain (Yu et al, 2006). The entire L chains from plasma cells (kappa or lambda) were cloned into the expression vector pCEP4. The H/L chain constructs representing each plasma cell were cotransfected into HEK293 cells, and the culture supernatants containing secreted rIgG were collected for analysis. After confirmation of size and H/L chain conformation for the rIgGs by electrophoresis in nonreducing gels and immunodetection by antihuman IgG antibody (H+L), the rIgG concentration in the supernatants was determined by capture enzyme-linked immunosorbent assay (ELISA) as described (Burgoon et al, 2005). All rIgGs were used at 3 to 7 μg/ml.
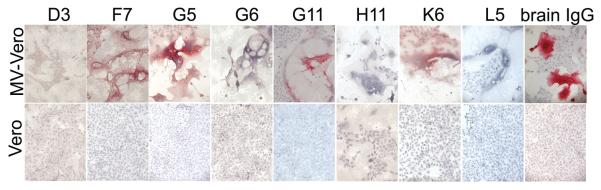
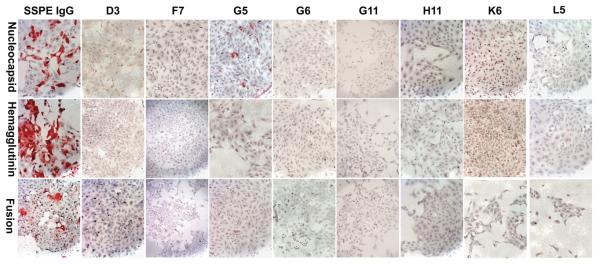
Immunostaining assays revealed that four of the eight rIgGs specifically stained MV-infected cells, but not uninfected Vero cells (Figure 1). rIgGs were also tested on Vero cells transfected with cDNAs encoding each of six major MV proteins. Although native IgG eluted from SSPE brain stained cells trans-fected with the MV nucleocapsid, hemagglutinin, or fusion proteins, only rIgG G5 specifically stained nucleocapsid-transfected cells, but nothing else (Figure 2). None of the antibodies detected the MV phosphoprotein, DNA polymerase, or matrix proteins (data not shown), consistent with the low immunogenicity of these proteins in acute measles infection and in SSPE (Hall et al, 1979; Graves et al, 1984; Dhib-Jalbut et al, 1988). By comparison, the rIgGs prepared from five clonally expanded plasma cell sequences in the same SSPE brain stained MV-infected cells, and all five recognized the MV nucleocapsid (Burgoon et al, 2005).
Figure 1.
rIgGs immunostain measles virus. Recombinant IgGs from eight of the less abundant CD38 plasma cells (7 μg/ml) and IgG eluted from SSPE brain (5 μg/ml) were used to immunostain MV-infected or uninfected Vero cells. Four of the eight rIgGs (F7, G5, G11, K6) and the brain-eluted IgG specifically stained MV-infected cells. Positive staining (red) was visualized by New Fuschin and counterstaining with hematoxylin.
Figure 2.
Immunostaining of individual MV components. The four rIgGs that reacted with MV-infected cells, or IgG eluted from SSPE brain, were used to immunostain Vero cells transfected with the MV nucleocapsid, hemagglutinin, or fusion protein. IgG eluted from SSPE brain recognized each MV component. One rIgG (G5) stained the MV nucleocapsid protein.
In Western blot analysis of MV-infected and uninfected cell lysates, none of the less abundant rIgGs recognized MV-specific proteins (Figure 3a). Similarly, none of the rIgGs immunoprecipitated MV-specific proteins in infected cell lysates (Figure 3b). These results contrast with our previous findings that all five rIgGs prepared from clonally expanded plasma cell sequences that reacted with MV-infected cells recognized a 60-kDa species in MV-infected cells corresponding to the nucleocapsid protein, and that three of these rIgGs precipitated the nucleocapsid protein (Burgoon et al, 2005). The less abundant rIgGs that failed to recognize antigens in the present study were also unsuccessful in immunoblotting any antigens in SSPE brain, and none of the rIgGs or native IgG eluted from SSPE brain recognized specific antigens in normal brain (data not shown).
Figure 3.
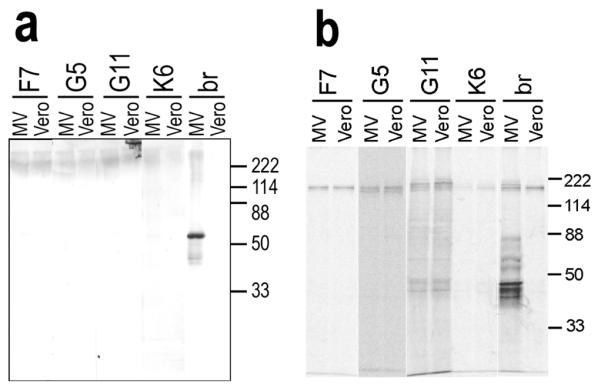
Immunoblotting and immunoprecipitation of MV by rIgG. (a) Each positive rIgG (3 μg/ml) was applied to lysates of MV-infected or uninfected Vero cells. None of the rIgG recognized specific proteins, although IgG (3 μg/ml) extracted from SSPE brain (br) recognized multiple MV proteins in MV-infected cells. (b) Positive rIgGs or IgG extracted from SSPE brain (br) (2 μg) were used to immunoprecipitate 35S-labeled lysates of MV-infected or uninfected Vero cells. The rIgGs did not precipitate MV proteins, but SSPE brain-extracted IgG precipitated multiple bands from the MV-infected lysates. Molecular mass standards are indicated on the right.
These analyses demonstrate that a significant proportion of the rIgGs from the less abundant plasma cell sequences encountered in SSPE brain are still disease relevant. Like those derived from the more abundant, clonally expanded plasma cell sequences, four of the eight rIgGs produced in SSPE brain were directed against MV. However, compared to rIgGs prepared from clonally expanded plasma cells, less abundant rIgGs were generally less successful in identifying specific MV proteins in the assays used here. This could be due to a lower abundance of certain MV proteins in transfected cells compared to productively infected cells in tissue culture. For example, each rIgG prepared from clonally expanded plasma cells recognized the MV nucleocapsid protein (Burgoon et al, 2005), which is the most abundant and immunologically reactive component of MV (Graves et al, 1984; Griffin, 2001). Alternatively, the conformation of MV-specific epitopes may have been changed by the immunoblotting or immunoprecipitation conditions, or some antigens might represent epitopes formed by interactions of MV components with cellular proteins, which fail to be recognized by immunoprecipitation analysis. The MV expressed in these assays may also contain epitopes that differ from those of the MV strain that developed in this SSPE brain. Finally, the less abundant rIgGs reactive to MV-infected cells could be lower-affinity antibodies. Identifying specific reactivities of rIgGs to these MV components will require additional assays.
Our findings were based on an analysis of 65 total plasma cells, 55 of which were clonally expanded. Although the 10 plasma cell sequences characterized here were encountered only once, it is possible that these sequences might be clonally expanded if a larger repertoire were examined. Nevertheless, these plasma cell sequences were less abundant than the large clonally expanded groups, and are less likely to represent the major oligoclonal immune response characteristic of the disease.
It is remarkable that when specific reactivity was detected for these minor clones, they were also disease-relevant, i.e., specific for MV. Although diverse immune specificities have been found in inflammatory CNS disease such as multiple sclerosis and acute disseminated encephalomyelitis, rIgGs prepared from either clonally expanded or less abundant plasma cells resident in chronic SSPE brain did not react with any ancillary antigens. Overall, functional analysis of rIgGs prepared from minor plasma cell sequences in SSPE brain revealed only disease-relevant antigens. In other inflammatory CNS conditions, molecular immunological analysis of plasma cells and B lymphocytes resident in brain may be use-ful to identify their cognate antigens and the inciting agents in disease.
Acknowledgments
This work supported in part by Public Health Service grants NS 41549 and NS 32623 from the National Institutes of Health. The authors thank Marina Hoffman for editorial review and Cathy Allen for preparing the manuscript.
References
- Burgoon MP, Gilden DH, Owens GP. B cells in multiple sclerosis. Front Biosci. 2004;9:786–796. doi: 10.2741/1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoon MP, Hammack BN, Owens GP, Maybach AL, Eikelenboom MJ, Gilden DH. Oligoclonal immunoglobulins in cerebrospinal fluid during varicella zoster virus (VZV) vasculopathy are directed against VZV. Ann Neurol. 2003;54:459–463. doi: 10.1002/ana.10685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoon MP, Keays KM, Owens GP, Ritchie AM, Rai PR, Cool CD, Gilden DH. Laser-capture microdissection of plasma cells from subacute sclerosing panencephalitis brain reveals intrathecal disease-relevant antibodies. Proc Natl Acad Sci U S A. 2005;102:7245–7250. doi: 10.1073/pnas.0502323102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhib-Jalbut S, McFarland HF, Mingioli ES, Sever JL, McFarlin DE. Humoral and cellular immune responses to matrix protein of measles virus in subacute sclerosing panencephalitis. J Virol. 1988;62:2483–2489. doi: 10.1128/jvi.62.7.2483-2489.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilden DH, Burgoon MP, Kleinschmidt-DeMasters BK, Williamson RA, Ghausi O, Burton DR, Owens GP. Molecular immunologic strategies to identify antigens and B-cell responses unique to multiple sclerosis. Arch Neurol. 2001;58:43–48. doi: 10.1001/archneur.58.1.43. [DOI] [PubMed] [Google Scholar]
- Gorny MK, Wroblewska Z, Pleasure D, Miller SL, Wajgt A, Koprowski H. CSF antibodies to myelin basic protein and oligodendrocytes in multiple sclerosis and other neurological diseases. Acta Neurol Scand. 1983;67:338–347. doi: 10.1111/j.1600-0404.1983.tb03151.x. [DOI] [PubMed] [Google Scholar]
- Graves M, Griffin DE, Johnson RT, Hirsch RL, de Soriano IL, Roedenbeck S, Vaisberg A. Development of anti-body to measles virus polypeptides during complicated and uncomplicated measles virus infections. J Virol. 1984;49:409–412. doi: 10.1128/jvi.49.2.409-412.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin DE. Measles virus. In: Knipe DM, Howley PM, editors. Fields virology. Lipincott-Raven; Philadelphia: 2001. pp. 1401–1441. [Google Scholar]
- Hall WW, Lamb RA, Choppin PW. Measles and subacute sclerosing panencephalitis virus protein: lack of antibodies to the M protein in patients with subacute sclerosing panencephalitis. Proc Natl Acad Sci U S A. 1979;76:2047–2051. doi: 10.1073/pnas.76.4.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiesen T, von Holst H, Fredrikson S, Wirsen G, Hederstedt B, Norrby E, Sundqvist VA, Wahren B. Total, anti-viral, and anti-myelin IgG subclass reactivity in inflammatory diseases of the central nervous system. J Neurol. 1989;236:238–242. doi: 10.1007/BF00314506. [DOI] [PubMed] [Google Scholar]
- Panitch HS, Hooper CJ, Johnson KP. CSF anti-body to myelin basic protein. Measurement in patients with multiple sclerosis and subacute sclerosing panencephalitis. Arch Neurol. 1980;37:206–209. doi: 10.1001/archneur.1980.00500530044005. [DOI] [PubMed] [Google Scholar]
- Porter KG, Sinnamon DG, Gillies RR. Cryptococcus neoformans-specific oligoclonal immunoglobulins in cerebrospinal fluid in cryptococcal meningitis. Lancet. 1977;1:1262. doi: 10.1016/s0140-6736(77)92473-4. [DOI] [PubMed] [Google Scholar]
- Ruutiainen J, Arnadottir T, Molnar G, Salmi A, Frey H. Myelin basic protein antibodies in the serum and CSF of multiple sclerosis and subacute sclerosing panencephalitis patients. Acta Neurol Scand. 1981;64:196–206. doi: 10.1111/j.1600-0404.1981.tb04399.x. [DOI] [PubMed] [Google Scholar]
- Vandvik B, Norrby E, Nordal HJ, Degre M. Oligoclonal measles virus-specific IgG antibodies isolated from cerebrospinal fluids, brain extracts, and sera from patients with subacute sclerosing panencephalitis and multiple sclerosis. Scand J Immunol. 1976;5:979–992. doi: 10.1111/j.1365-3083.1976.tb03050.x. [DOI] [PubMed] [Google Scholar]
- Vandvik B, Norrby E, Steen-Johnson J, Sensvold K. Mumps meningitis: Prolonged pleocytosis and occurrence of mumps virus-specific oligoclonal IgG in the cerebrospinal fluid. Eur Neurol. 1978;17:13–22. doi: 10.1159/000114916. [DOI] [PubMed] [Google Scholar]
- Vartdal F, Vandvik B, Michaelsen TE, Loe K, Norrby E. Neurosyphilis: intrathecal synthesis of oligoclonal antibodies to Treponema pallidum. Ann Neurol. 1981;11:35–40. doi: 10.1002/ana.410110107. [DOI] [PubMed] [Google Scholar]
- Yu X, Gilden DH, Ritchie AM, Burgoon MP, Keays KM, Owens GP. Specificity of recombinant antibodies generated from multiple sclerosis cerebrospinal fluid probed with a random peptide library. J Neuroimmunol. 2006;172:121–131. doi: 10.1016/j.jneuroim.2005.11.010. [DOI] [PubMed] [Google Scholar]