Abstract
The P2X1 receptor-channels activated by extracellular ATP contribute to the neurogenic component of smooth muscle contraction in vascular beds and genito-urinary tracts of rodents and humans. In the present study, we investigated the interactions of plasma membrane phosphoinositides with P2X1 ATP receptors and their physiological consequences. In an isolated rat mesenteric artery preparation, we observed a strong inhibition of P2X1-mediated constrictive responses by depletion of PI(4,5)P2 with the PI4-kinase inhibitor wortmannin. Using the Xenopus oocyte expression system, we provided electrophysiological evidence that lowering PI(4,5)P2 levels with wortmannin significantly decreases P2X1 currents amplitude and recovery. Previously reported modulation of recovery of desensitized P2X1 currents by phospholipase C-coupled 5-HT2A metabotropic receptors was also found wortmannin-sensitive. Treatment with wortmannin alters the kinetics of P2X1 activation and inactivation without changing its sensitivity to ATP. The functional impact of wortmannin on P2X1 currents could be reversed by addition of intracellular PI(4,5)P2, but not PI(3,4,5)P3. and direct application of PI(4,5)P2 to excised inside-out macropatches rescued P2X1 currents from rundown. We showed that the proximal region of the intracellular C-terminus of P2X1 subunit directly binds to PI(4,5)P2 and other anionic phospholipids, and we identified the basic residue K364 as a critical determinant for phospholipid binding and sensitivity to wortmannin. Overall, these results indicate that PI(4,5)P2 plays a key role in the expression of full native and heterologous P2X1 function by regulating the amplitude, recovery and kinetics of ionotropic ATP responses through direct receptor-lipid interactions.
P2X receptors are nonselective cation channels gated by extracellular ATP. P2X subunits (P2X1-7) assemble to form either homo- or hetero-trimeric channels, giving rise to a wide range of current phenotypes (North, 2002). Among P2X receptors, the P2X1 subtype shows high sensitivity to the agonist α,β-methylene ATP (α,β-meATP), fast inactivation kinetics and high expression in smooth muscle cells lining the urinary bladder, arteries, and vas deferens, where it regulates contractility (Sneddon, 2000; Burnstock, 2007). The activity of P2X1 receptor-channels can be modulated by phospholipase C-mediated signaling events (Roberts et al., 2006). For instance, recovery of P2X1 responses can be accelerated by activation of α1-adrenergic receptors in the vas deferens of guinea pig (Smith and Burnstock, 2004) or by activation of 5-HT2A serotonin receptors in the rat tail artery (Ase et al., 2005). In recombinant studies, P2X1 receptors currents have been shown to be potentiated by stimulation of co-expressed Gq-coupled mGluR1, P2Y1/2 and 5-HT2A receptors (Vial et al., 2004; Ase et al., 2005). By using cholesterol-depleting agents, Vial and Evans (2005) reported reduced α,β-meATP-induced contractions of the rat tail artery and decreased P2X1-mediated currents in transfected HEK293 cells, suggesting that lipids regulate P2X1 receptor signaling. Phosphoinositides have recently been shown to modulate P2X2 and P2X7 receptors (Fujiwara and Kubo, 2006; Zhao et al., 2007). Phosphoinositides are produced by specific lipid kinases through phosphorylation of phosphatidylinositol (PI) at the 3′, 4′ or 5′ position of the inositol ring. Phosphoinositides exert their regulatory role either indirectly as precursors of the phospholipase C-generated second messengers inositol 1,4,5-trisphosphate and diacylglycerol, or directly by interacting with proteins and thus modulating their localization, conformation, and activity. PI(4,5)P2, one of the most abundant phosphoinositide in the cell membrane, regulates many cellular events such as cytoskeleton remodeling, vesicle transport and ion channels or transporters function (Suh and Hille, 2005; Gamper and Shapiro, 2007).
In the present study, we aimed to explore the interaction of PI(4,5)P2 with native and recombinant P2X1 receptor-channels and its physiological consequences. We report here, using the isolated rat mesenteric artery preparation, a strong modulation of P2X1- mediated vascular reactivity by wortmannin-induced PI(4,5)P2 depletion. Using two-electrode voltage clamp and inside-out patch recordings in the Xenopus oocyte expression system, we also provide functional evidence that PI(4,5)P2 modulates P2X1 current amplitude, recovery, speed of activation and inactivation, and is required for the 5-HT2A-mediated potentiation of P2X1 currents. Finally, using an in vitro lipid binding assay and mutagenesis, we show that the proximal C-terminal region of P2X1 subunit, containing the critical basic residue K364, directly interacts with several anionic phospholipids including PI(4,5)P2.
Materials and Methods
Reactivity of Isolated Rat Mesenteric Arteries
All procedures were as described previously (Elhusseiny and Hamel, 2000; Tong et al., 2005). Briefly, rats were sacrificed by cervical dislocation and the mesenteric arteries isolated and collected in cold Krebs solution (4°C, pH 7.4) containing the following (in mM): 118 NaCl, 4.5 KCl, 2.5 CaCl2, 1 MgSO4, 1 KH2PO4, 25 NaHCO3, and 11 glucose. Arterial segments (about 2 mm long) were transferred to a small superfusion chamber (5 ml, Living System), cannulated on a glass micropipette (80 μm diameter) at one end, sealed to another glass micropipette at the other end, and filled with oxygenated (5% CO2 and 95% O2) Krebs solution (37°C, pH 7.4). Intraluminal pressure was maintained at 60 mmHg, vessels were superfused with Krebs solution and allowed to stabilize and acquire basal tone. On-line measurements of intraluminal diameter were performed using a closed-circuit video system coupled with a video caliper. Direct abluminal application of α,β-meATP (10 μM) was performed on vessels pre-treated or not with the PI3/4-kinases inhibitor wortmannin (100 nM or 1 μM). In the wortmannin experiments, vessels were rapidly washed with fresh Krebs solution before application of α,β-meATP.
Expression of Recombinant P2X1 Receptors in Xenopus Oocytes
Ovary lobes were surgically removed from Xenopus laevis frogs deeply anesthetized by immersion in 0.2% Tricaine (Sigma-Aldrich, St. Louis, MO). After treatment with type I collagenase in calcium-free OR2 solution for 2 h at room temperature, stage V–VI oocytes were manually defolliculated. Wild-type or mutant rat P2X1 subunit and rat 5-HT2A receptor cRNAs were transcribed in vitro using the mMessage mMachine kit (Ambion, Austin, TX) from pCDNA3 eukaryotic expression vector (Invitrogen, Carlsbad, CA) and then microinjected into the cytoplasm of oocytes. For expression of P2X1 alone 25 ng of cRNA were injected in each oocyte. In co-expression experiments, 25 ng of P2X1 and 25 ng of 5-HT2A cRNA were injected. Oocytes were kept at 19°C in Barth solution containing 50 μg/ml of gentamycin and 1.8 mM CaCl2 for 48 h before recording.
Electrophysiology
Two-electrode voltage clamp recordings in Xenopus oocytes expressing P2X1 receptors were performed at room temperature using an OC-725C amplifier (Warner Instruments, Hamden, CT) and glass pipettes (1–3 MΩ) filled with 3 M KCl. Oocytes were placed in a 500-μl recording chamber and were perfused at a flow rate of 10–12 ml/min with Ringer solution, pH 7.4, containing 115 mM NaCl, 5 mM NaOH, 2.5 mM KCl, 1.8 mM CaCl2, and 10 mM HEPES. Oocytes were held at −60 mV during recording. Unless specified, stimulations with 10 μM ATP (Sigma-Aldrich) dissolved in Ringer solution were applied consecutively at 5-min intervals and P2X1 responses were stable after the second application of ATP. When wortmannin was used, oocytes were incubated for 2 hours in Barth solution containing 100 nM or 35 μM wortmannin, or vehicle (0.3% DMSO), prior to recording. For experiments involving co-expression of 5-HT2A receptors, 5-HT (1 μM) was applied in the bath for 5 min between the third and fourth ATP application. Potentiation index was defined as the ratio of the fourth over the third ATP-evoked P2X1 peak current. The phospholipids diC8-PI(4,5)P2 or diC8- PI(3,4,5)P3 (Echelon Biosciences) were injected (20 nl, 10 mM) in the cytoplasm of oocytes 30 min prior to final recording. For all the calculations of final drug concentration, we estimated the oocyte cell volume at 1 μl.
Inside-out macropatch recordings in Xenopus oocytes expressing P2X1 channels were performed using an Axopatch 200B amplifier (Axon Instruments, Union City, CA). PClamp 9 was used for data acquisition and analysis. The sampling rate was 2 kHz. A ramp protocol from −100 mV to +100 mV was applied once per second, and current amplitudes were acquired at −80 mV. Electrodes with resistance of 0.5–1.0 MΩ were used. The internal (bath) solution contained (in mM): 96 KCl, 0.5 EGTA, 10 HEPES, pH 7.4. The external (pipet) solution contained also 1.8 mM CaCl2, as well as 20 μM ATP. Solutions were applied through a gravity-driven perfusion system. For each experiment, a minimum of two batches of oocytes were tested.
Lipid Strip Binding Assay
Annealed oligonucleotides coding for a 16 amino acid sequence proximal to the C-terminus of rat P2X1 (I356–A371) flanked by BamHI-EcoRI ends were subcloned into pGEX-2T vector for production of wild-type or mutant GST-P2X1 fusion proteins, before purification on glutathione-sepharose resin. Lipid binding analysis of GST-P2X1 fusion proteins was conducted in vitro using identical amounts of phosphoinositides spotted on nitrocellulose membranes (PIP Strips™, Echelon Biosciences Inc) and the GST-N-terminus of P2X1 or GST alone were used as negative controls. The membranes were blocked with TBS+T solution supplemented with 3% BSA for 1 hour at room temperature, then incubated overnight with 5 μg/ml of GST-fusion proteins in TBS+T with 3% BSA. The membranes were washed with TBS+T six times. Mouse anti-GST antibody (1:1000) was then added in TBS+T, 3% BSA solution for 1 hour at room temperature. Repeated washes with TBS+T were followed by 1 hour incubation with the secondary antibody HRP-labelled goat anti-mouse (1:5000) in TBS+T, 3% BSA at room temperature for detection of GST-P2X1 fusion protein binding with enhanced chemiluminescence.
Statistical Analysis
All the values are expressed as mean ± S.E.M. Data were analyzed using Student’s t test or one-way analysis followed by Newman-Keuls multiple comparison tests, with p < 0.05 considered significant.
Results
PI(4,5)P2 depletion decreases P2X1-mediated constrictive responses in rat mesenteric artery
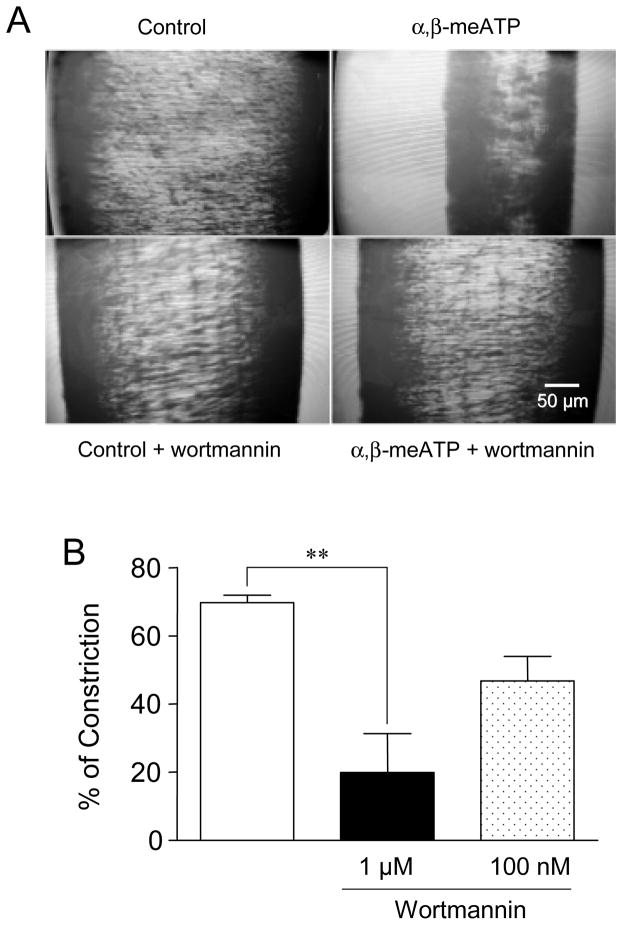
Using the model of isolated rat mesenteric artery, we observed that initial applications of the selective P2X agonist α,β-meATP (10 μM) to vessel segments led to strong muscle constriction responses (69 ± 2% of maximal constriction induced by KCl, n= 6, Fig. 1). Micromolar concentrations of wortmannin, expected to inhibit both PI3-kinases and PI4-kinases, were used to test the impact of lower levels of phosphoinositides on native P2X1-mediated constrictive responses. When the mesenteric vessels were incubated with 1 μM wortmannin for 1 hour, α,β-meATP-mediated constriction was significantly decreased to 28 ± 16% (n= 5) (Fig. 1B). However, no significant changes were measured at a lower (100 nM) concentration of wortmannin that inhibits PI3-kinases only (67 ± 9% of α,β-meATP-mediated constriction, n= 3, Fig. 1B).
Figure 1. P2X1 constrictive responses are sensitive to wortmannin.
(A) Representative video recordings of changes in rat mesenteric artery diameter. Control: vessel diameter measured at baseline tone. α,β-meATP: same vessel one minute after application of α,β-meATP (10 μM). Control + wortmannin: vessel diameter measured at baseline tone 30 min after application of wortmannin (1 μM). α,β-meATP + wortmannin: same vessel, 1 minute after application of α,β-meATP (10 μM). (B) Constriction responses elicited by application of the P2X agonist α,β-meATP (10 μM) on rat mesenteric artery and their partial blockade after treatment with 1 μM wortmannin (n= 3–6, ** p < 0.01).
PI(4,5)P2 depletion reduces P2X1 current amplitude and recovery in Xenopus oocytes without changing its sensitivity to ATP
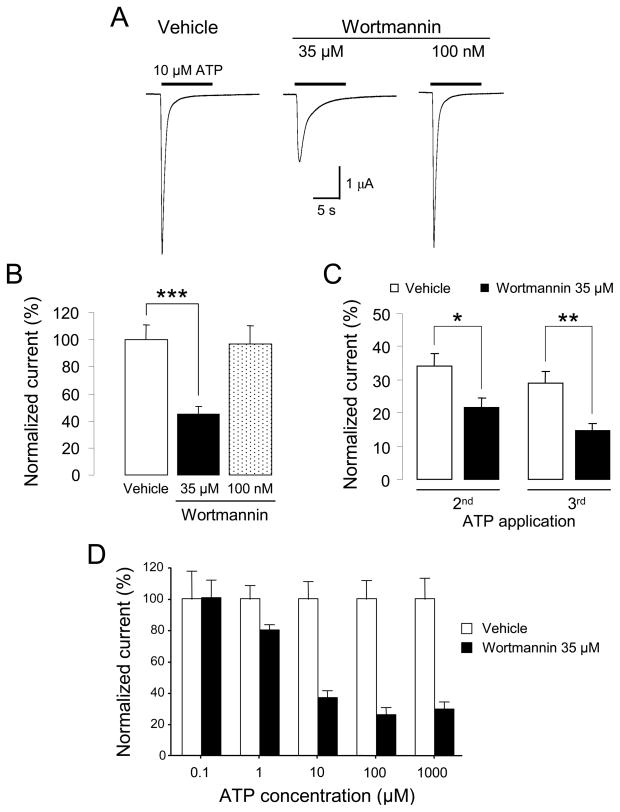
We studied the effects of altering PI(4,5)P2 levels on ATP-evoked currents in Xenopus oocytes expressing recombinant P2X1 receptor channels. The oocytes were pretreated with vehicle or wortmannin at high (35 μM) or low (100 nM) concentration for 2 hours before they were stimulated with 10 μM ATP (EC50= 1 μM) for 10 seconds. We observed that high but not low concentration of wortmannin significantly reduced ATP-induced current amplitudes (45.1 ± 5.5% of control, n= 23) as illustrated by the representative traces and quantitative results in Figure 2A and 2B respectively. Desensitization of P2X1 responses following repeated applications of ATP was significantly stronger in oocytes incubated with 35 μM wortmannin: 2nd response= 21 ± 3%, of initial response, 3rd response= 14 ± 2% (n= 14) compared with 2nd response= 34 ± 4%, 3rd response= 29 ± 3% (n= 18) for vehicle-treated oocytes (Fig. 2C). The inhibitory effect of wortmannin was found to depend on the concentration of ATP (Fig. 2D). When ATP (EC50= 1 μM) was applied at 0.1 μM, the treatment with 35 μM wortmannin had no effect on the current amplitude (3.03 ± 0.5 μA in vehicle-treated oocytes, n= 6, compared to 3.05 ± 0.3 μA in wortmannin-treated oocytes, n= 9). A maximal inhibitory effect was reached at 100 μM ATP (27 ± 5% of control, n= 9). These results indicated that depletion of phosphoinositides with 35 μM wortmannin blunted both the maximal response and the recovery of P2X1 receptors without decreasing their sensitivity to ATP.
Figure 2. Inhibitory effect of wortmannin on recombinant P2X1 current responses.
(A) Representative ATP-evoked inward currents in Xenopus oocytes expressing P2X1 receptors. High (35 μM) but not low (100 nM) concentration of wortmannin decreases P2X1 peak current. (B) Quantitative results (n= 9–17, *** p < 0.001). (C) Treatment with 35 μM wortmannin decreases recovery of P2X1 currents following consecutive applications of ATP (n= 14–18, * p < 0.05; ** p < 0.01). (D) The inhibitory effect of wortmannin is agonist concentration-dependent (n= 6–17).
Potentiation of P2X1 responses via 5-HT2A activation is blocked by wortmannin induced PI(4,5)P2 depletion
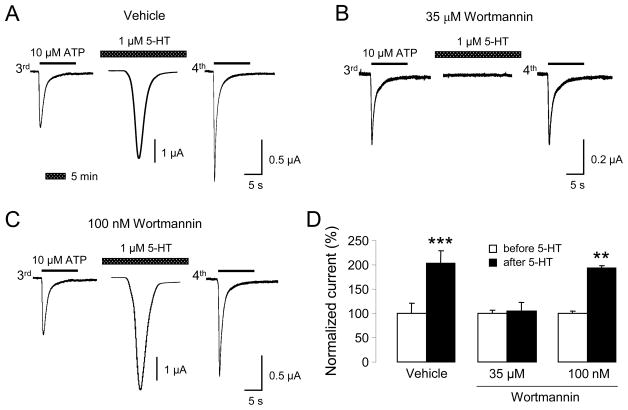
Oocytes coexpressing P2X1 and Gq-coupled 5-HT2A receptors were exposed to Ringer solution or 1 μM 5-HT for 5 min between the third and fourth ATP applications. Under these conditions, 5-HT transiently elicited inward currents (Fig. 3A,C), sometimes oscillatory in nature, indicating secondary activation of endogenous Ca2+-dependent chloride currents. Application of 5-HT significantly potentiated P2X1 current responses, as previously reported (Ase et al., 2005) and illustrated in Figure 3A (see also Fig. 3D for quantitative results). However, 2 hours of preincubation with 35 μM wortmannin blocked the accelerated recovery of P2X1 currents following 5-HT2A activation (Fig. 3B,D). Lower concentration of wortmannin (100 nM) did not have any significant effect on 5-HT-induced modulation of P2X1 currents (Fig. 3C,D).
Figure 3. Wortmannin blocks 5-HT2A-mediated recovery of P2X1 currents.
(A) Representative ATP-evoked (3rd and 4th application) and 5-HT-evoked inward currents from oocytes coexpressing P2X1 and 5-HT2A receptors. A 5 min application of 5-HT accelerates recovery of P2X1 current amplitudes. (B) Traces show blockade of accelerated recovery of P2X1 current responses by preincubation with 35 μM wortmannin (inhibition of PI3- and PI4-kinases). Note that high wortmannin concentration also suppresses 5-HT-evoked Ca2+-activated chloride currents. (C) Traces show absence of effect when low concentration of wortmannin (PI3-kinases inhibition only) is used. (D) Quantification of recovery of P2X1 current responses, normalized to the responses before 5-HT application (n= 5–7, ** p < 0.01; *** p < 0.001).
Rescue of P2X1 current responses and kinetics by PI(4,5)P2
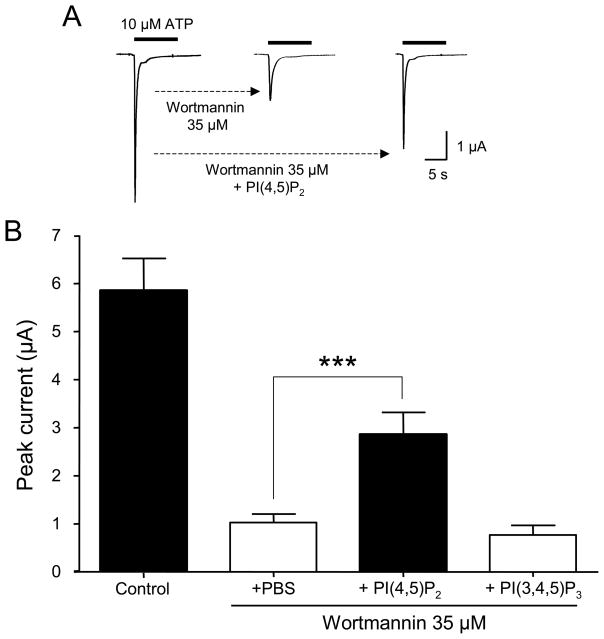
To study the effect of PI(4,5)P2 on P2X1 receptor-channels expressed in oocytes, we injected diC8-PI(4,5)P2 (200 μM) in the cytoplasm 30 min before recording. Under basal conditions, ATP-evoked P2X1 current amplitudes and kinetics were not affected by the addition of diC8-PI(4,5)P2 (data not shown). However, in oocytes preincubated with 35 μM wortmannin, diC8- PI(4,5)P2 addition led to a significant rescue of P2X1 current amplitudes: 2.8 ± 0.5 μA (n= 8) compared to 1.0 ± 0.2 μA in PBS-injected oocytes (n= 14) (Fig. 4A,B). Thus, whereas addition of diC8-PI(4,5)P2 did not seem to increase ATP-evoked P2X1 currents under basal conditions, it did so when endogenous levels of PI(4,5)P2 were previously depleted by wortmannin treatment. In contrast, diC8-PI(3,4,5)P3 (200 μM) injections did not have any rescuing effect on the current amplitudes of P2X1 receptors following wortmannin treatment (Fig. 4B).
Figure 4. PI(4,5)P2 specifically rescues P2X1 current amplitude following wortmannin-mediated blockade.
(A) Representative traces showing ATP-evoked inward current (left), its partial blockade by wortmannin (middle), and its rescue (right) by injection of diC8-PI(4,5)P2 into oocytes expressing P2X1 receptors. (B) Quantitative summary of the results, showing the lack of rescue when diC8-PI(3,4,5)P3 is injected (n= 9, *** p < 0.001).
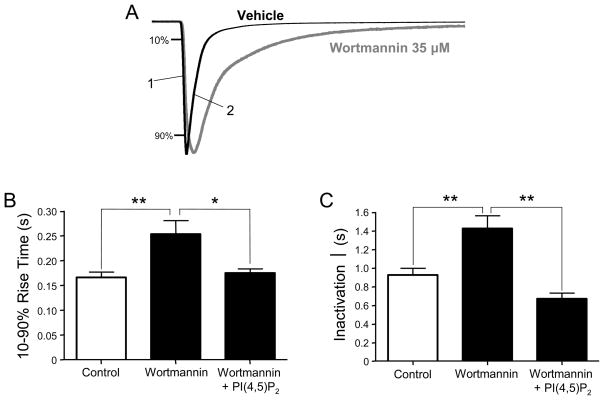
In addition, we found that PI(4,5)P2 levels have a significant impact on the kinetics of P2X1 current responses. Thus, as illustrated by the current traces in Figure 5A, treatment with high concentration of wortmannin clearly slowed down both the 10–90% rise time of the activation phase and the inactivation time (one-exponential fit) of P2X1 current responses. The 10–90% rise time was 0.17 ± 0.01 s (n= 9) in control conditions and 0.25 ± 0.03 s (n= 9) after wortmannin. The time constant of inactivation was 0.92 ± 0.07 s (n= 9) in control conditions and 1.42 ± 0.14 s (n= 9) after wortmannin. We observed that injection of PI(4,5)P2 completely restored the kinetic parameters of P2X1 responses to their control levels (Fig. 5B,C).
Figure 5. PI(4,5)P2 depletion slows down activation and inactivation of P2X1 currents.
(A) Representative traces showing slower activation (1) and inactivation (2) of ATP-evoked P2X1 inward current after wortmannin treatment. (B) Quantitative results show the complete rescue of kinetic parameters of P2X1 currents to normal levels by PI(4,5)P2 delivery into oocytes (n= 9, * p < 0.05; ** p < 0.01).
PI(4,5)P2 activates P2X1 current responses in excised macropatches
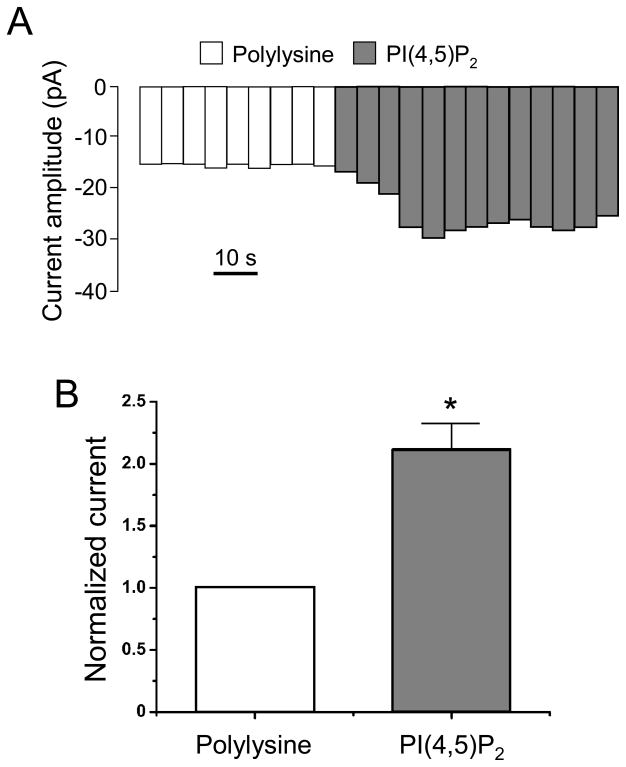
To test direct effects of phosphoinositides on heterologously expressed P2X1 receptors, we applied diC8-PI(4,5)P2 to inside-out macropatch membranes from Xenopus oocytes. Upon patch excision, a fast rundown of the current was observed. Application of 100 μM polylysine, which sequesters PI(4,5)P2, stabilized the P2X1 currents to basal levels (Fig. 6A). Subsequent application of 5 μM diC8-PI(4,5)P2 strongly activated P2X1 currents (Fig. 6A). A quantitative analysis shows significantly larger amplitude (~ 2-fold) for P2X1 currents in the presence of diC8-PI(4,5)P2, compared to rundown baseline levels (Fig. 6B).
Figure 6. PI(4,5)P2-activated P2X currents in excised macropatches from Xenopus oocytes.
(A) Averaged current amplitudes during 5 s intervals at −80 mV. Currents were evoked by ramps from −100 mV to +100 mV. The internal pipette solution contained 20 μM ATP. Polylysine is applied on the intracellular side at 100 μM. (B) Quantitative results show rescue of P2X1 currents from ATP-induced rundown by 5 μM diC8- PI(4,5)P2 application (n= 4–9, * p < 0.05).
The single lysine K364 in the C-terminus of P2X1 subunit is a critical determinant for binding phosphoinositides
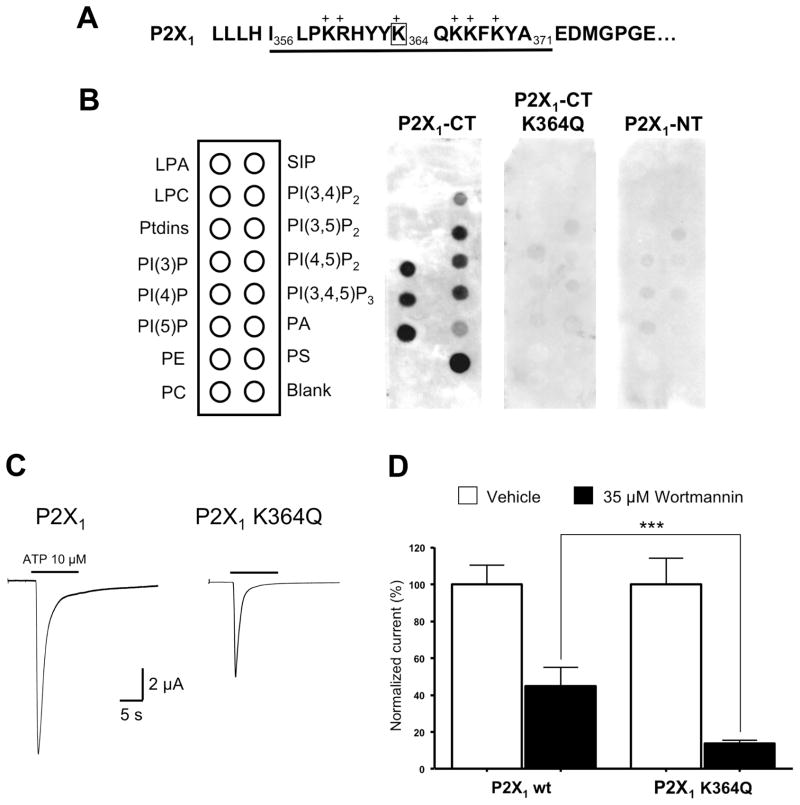
Because phosphoinositides are anionic, we tested for potential interactions of these lipids with positively charged residues on the intracellular domains of the P2X1 subunits by performing a lipid strip assay. The proximal region of the C-terminal domain displays a cluster of basic amino acids (Fig. 7A) that are potentially involved in the interaction with phosphoinositides, as recently reported for the P2X2 receptor (Fujiwara and Kubo, 2006; Zhao et al., 2007). No specific binding was observed when a GST-P2X1 fusion protein expressing the N-terminal peptide F12-V29 was tested (Fig. 7B). However the GST-P2X1 fusion protein containing the C-terminal peptide I356–A371 selectively bound to several phospholipids including PI(3)P, PI(4)P, PI(5)P, PI(3,5)P2, PI(4,5)P2, PI(3,4,5)P3, PS, and to a lesser extent, PI(3,4)P2 and PA, as illustrated in Fig. 7B. We investigated the contribution of each lysine or arginine residue in the C-terminal peptide by replacing them with the neutral residue glutamine. Only one substitution, K364Q, led to the complete suppression of binding (Fig. 7B) therefore the lysine K364 is a critical determinant for interaction with phosphoinositides. As shown in Figure 7C, ATP-evoked current amplitude was smaller in oocytes expressing the mutant receptor P2X1 K364Q (4.94 ± 0.69 μA, n= 10) compared to wild-type receptor (8.27 ± 0.99 μA, n= 10). Moreover, P2X1 K364Q receptors showed higher sensitivity to depletion of PI(4,5)P2 induced by 35 μM wortmannin (13.70 ± 1.80 % of control response, n= 19) than wild-type receptors (44.88 ± 10.13 % of control response, n= 9), confirming a decrease in PI(4,5)P2 binding affinity (Fig. 7D).
Figure 7. Lysine K364 in the proximal C-terminal domain of P2X1 subunit is a critical determinant for phosphoinositides binding and sensitivity to wortmannin.
(A) Sequence of the proximal C-terminal domain of rat P2X1 subunit. (B) The GST construct containing the proximal C-terminal domain I356-A371 of the P2X1 subunit binds to several anionic phosphoinositides, including PI(4,5)P2. Binding is suppressed when the single lysine K364 is replaced with glutamine. No binding is detected when using the GST construct containing the N-terminal domain of P2X1 (P2X1-NT) as negative control. LPA: Lysophosphatidic Acid, LPC: Lysophosphocholine, PE: Phosphatidylethanolamine, PC: Phosphatidylcholine, S1P: Sphingosine-1-phosphate, PA: Phosphatidic Acid, PS: Phosphatidylserine. (C) Representative traces showing that the mutant receptor P2X1 K364Q carries smaller ATP-evoked currents than the wild-type P2X1 receptor (n= 10, * p < 0.05). (D) The mutant receptor P2X1 K364Q is also more sensitive than the wild-type receptor to depletion of PI(4,5)P2 induced by 35 μM wortmannin (n= 9–19, *** p < 0.001).
Discussion
The homomeric P2X1 purinoceptor is an ATP-gated receptor-channel mainly expressed in smooth muscles of the genito-urinary tract and vessels, where it mediates neurogenic contraction (Valera et al., 1994; Hansen et al., 1998). This purinergic component of smooth muscle contraction is present in rodents (Mulryan et al., 2000) as well as in humans (Banks et al., 2006). In the present study, we demonstrated the modulation of P2X1 responses by phosphoinositides and its physiological relevance since α,β-meATP-evoked P2X1-mediated constrictions of the rat mesenteric artery are sensitive to the pharmacological depletion of PI(4,5)P2. We were able to reconstitute in the Xenopus oocyte expression system the modulation of P2X1-mediated responses by blocking the critical lipid kinases involved in phosphoinositides synthesis, thus indicating that ubiquitous intracellular mechanisms are involved. Two-electrode voltage-clamp recordings showed decreased ATP-evoked P2X1 current responses with high (μM) concentration of wortmannin, required to block both PI4-kinases and PI3-kinases (Nakanishi et al., 1995), whereas low (nM) concentrations of wortmannin, known to block PI3-kinases only, did not have any effect. The inhibitory effect of wortmannin is not due to a decrease in sensitivity of P2X1 receptors to ATP because 1) the inhibition was unsurmountable at supramaximal concentrations of ATP, 2) maximal current amplitude was reached at 10 μM ATP after wortmannin treatment as in control conditions, and 3) wortmannin had no effect on P2X1 currents induced by 0.1 μM ATP. We can conclude that ATP becomes a partial agonist for P2X1 when PI(4,5)P2 levels are depleted. In other words, minimal PI(4,5)P2 levels are required for the expression of a full P2X1 response to ATP. Recovery of P2X1 responses was also found sensitive to treatment with high concentration of wortmannin, suggesting that desensitization of P2X1 receptor-channels is regulated by PI(4,5)P2.
We and others have reported the potentiation of P2X1 responses by activation of Gq-coupled receptors (Vial et al., 2004; Ase et al., 2005) so we tested the impact of wortmannin on the modulation of P2X1 responses by Gq-coupled 5-HT2A receptors. The lack of potentiation of P2X1 responses after blockade of PI4-kinases by 35 μM wortmannin, as well as the absence of effect of PI3-kinases blockade by 100 nM wortmannin, supported a critical role for PI(4,5)P2 as PLC substrate in the crosstalk between 5-HT2A and P2X1 receptors. The effectiveness of the blockade of PI4-kinases and PI(4,5)P2 synthesis was confirmed by the suppression of IP3-induced Ca2+-dependent chloride currents, triggered by 5-HT, after treatment with 35 μM wortmannin. Stimulation of the PLC-coupled platelet-derived growth-factor tyrosine kinase receptor has been shown to decrease PI(4,5)P2-sensitive P2X7 currents (Zhao et al., 2007) however we did not observe any decrease of P2X1 responses after 5-HT2A stimulation. This suggests that a transient stimulation of Gq-coupled receptors does not significantly deplete the steady-state levels of PI(4,5)P2 available at the plasma membrane, in agreement with what is known about the high turnover rate of this phospholipid (Hilgemann, 2007).
The specific involvement of PI(4,5)P2 in modulating P2X1 channels responses is directly supported by the fact that addition of PI(4,5)P2, and not PI(3,4,5)P3, significantly rescued the amplitude of P2X1 currents after blockade of PI4-kinases with 35 μM wortmannin. Decreased levels of phosphoinositides not only affected the amplitude of P2X1 currents but also their kinetics, as wortmannin clearly slowed down their 10–90% rise time and increased their time constant of inactivation. Addition of intracellular PI(4,5)P2 after treatment with wortmannin normalized the kinetics of the P2X1 currents back to their control values. Under basal conditions however, increasing PI(4,5)P2 levels by direct injection into the cytoplasm did not affect neither basal P2X1 current amplitudes nor activation/inactivation kinetics. Thus, at least in oocytes, endogenous PI(4,5)P2 levels saturate the P2X1 receptor-channels expressed at the cell surface.
Our results using inside/out macropatches excised from P2X1-expressing oocytes provided evidence for the sensitivity of P2X1 receptor-channels to direct application of PI(4,5)P2. P2X1 shows little activity upon patch excision due to a rapid rundown resulting from receptor desensitization and dephosphorylation of PI(4,5)P2 by lipid phophatases (Huang et al., 1998; Zhang et al., 1999). Direct regulation of channel activity by phosphoinositides in excised patches has been reported for several types of ion channels, including the prototypical Kir and KCNQ channels also modulated by PI(4,5)P2 (Lopes et al., 2002; Suh and Hille, 2002, 2005; Zhang et al., 2003). Application of exogenous PI(4,5)P2 on the intracellular side of the patch rescued P2X1 currents from rundown, indicating the requirement of PI(4,5)P2 for full P2X1 function and arguing against trafficking to the surface as the mechanism for PI(4,5)P2-induced potentiation of P2X1 currents.
Searching for phospholipid-binding motifs in the intracellular sequences of P2X1 subunits, we confirmed the direct binding of PI(4,5)P2 to the carboxy-terminal domain I356-A371. This domain proximal to the second transmembrane domain, in a position favorable for interactions with the anionic head groups of phospholipids according to the transmembrane topology of P2X subunits, contains a high density of basic residues (5 lysines and 1 arginine) and we have found that the single lysine K364 plays a critical role in these interactions. The mutant receptor P2X1 K364Q remains functional but with a lower binding affinity for PI(4,5)P2 indicated by a higher sensitivity to wortmannin-induced depletion, analogously to mutants of other PI(4,5)P2-sensitive channels (Rohàcs et al., 2005). The fact that the K364Q mutation in the functional P2X1 receptor-channel did not lead to complete loss of PI(4,5)P2 binding and insensitivity to wortmannin suggests that other determinants contribute to phosphoinositides binding. Interestingly, the C-terminal sequence of P2X1 also showed strong affinity for other anionic phospholipids. PI(4,5)P2 and PI(4)P are by far the most abundant phosphoinositides in the plasma membrane however the dependence of ion channel activity on other phosphoinositides is well documented (Zhainazarov et al., 2004; Pochynyuk et al., 2005; Srivastava et al. 2005). A similar but qualitatively distinct lipid-binding profile was recently reported for the homologous C-terminal sequence of P2X2 (Fujiwara and Kubo, 2006). Therefore, this short domain appears to be of general importance for the regulation of P2X function by phosphoinositides.
In conclusion we have provided strong evidence that the phospholipid PI(4,5)P2 is a physiological ligand of P2X1 receptor-channels, and our results suggest a novel mode of regulation of ionotropic P2X1 responses by the various metabotropic pathways linked to phosphoinositides metabolism.
Acknowledgments
This work has been supported by operating grants from CIHR (PS, EH) and NIH (DEL). LPB holds a studentship from the CIHR training grant “Pain: Molecules to Community”.
Abbreviations
- α,β-meATP
α,β-methylene ATP
- PI
phosphatidylinositol
References
- Ase AR, Raouf R, Belanger D, Hamel E, Seguela P. Potentiation of P2X1 ATPgated currents by 5-hydroxytryptamine 2A receptors involves diacylglycerol-dependent kinases and intracellular calcium. J Pharmacol Exp Ther. 2005;315:144–54. doi: 10.1124/jpet.105.089045. [DOI] [PubMed] [Google Scholar]
- Banks F, Knight GE, Calvert RC, Thompson CS, Morgan RJ, Burnstock G. The purinergic component of human vas deferens contraction. Fertility and Sterility. 2006;85:932–939. doi: 10.1016/j.fertnstert.2005.09.024. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- Elhusseiny A, Hamel E. Muscarinic--but not nicotinic--acetylcholine receptors mediate a nitric oxide-dependent dilation in brain cortical arterioles: a possible role for the M5 receptor subtype. J Cereb Blood Flow Metab. 2000;20:298–305. doi: 10.1097/00004647-200002000-00011. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y, Kubo Y. Regulation of the desensitization and ion selectivity of ATP-gated P2X2 channels by phosphoinositides. J Physiol. 2006;576:135–149. doi: 10.1113/jphysiol.2006.115246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamper N, Shapiro MS. Regulation of ion transport proteins by membrane phosphoinositides. Nat Rev Neurosci. 2007;8:921–934. doi: 10.1038/nrn2257. [DOI] [PubMed] [Google Scholar]
- Hansen MA, Balcar VJ, Barden JA, Bennett MR. The distribution of single P2x1-receptor clusters on smooth muscle cells in relation to nerve varicosities in the rat urinary bladder. J Neurocytol. 1998;27:529–39. doi: 10.1023/a:1006908010642. [DOI] [PubMed] [Google Scholar]
- Hilgemann DW. Local PIP(2) signals: when, where, and how? Pflugers Arch. 2007;455:55–67. doi: 10.1007/s00424-007-0280-9. [DOI] [PubMed] [Google Scholar]
- Huang CL, Feng S, Hilgemann DW. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gβγ. Nature. 1998;391:803–806. doi: 10.1038/35882. [DOI] [PubMed] [Google Scholar]
- Lopes CM, Zhang H, Rohacs T, Jin T, Yang J, Logothetis DE. Alterations in conserved Kir channel-PIP2 interactions underlie channelopathies. Neuron. 2002;34:933–944. doi: 10.1016/s0896-6273(02)00725-0. [DOI] [PubMed] [Google Scholar]
- Mulryan K, Gitterman DP, Lewis CJ, Vial C, Leckie BJ, Cobb AL, Brown JE, Conley EC, Buell G, Pritchard CA, Evans RJ. Reduced vas deferens contraction and male infertility in mice lacking P2X1 receptors. Nature. 2000;403:86–89. doi: 10.1038/47495. [DOI] [PubMed] [Google Scholar]
- Nakanishi S, Catt KJ, Balla T. A wortmannin-sensitive phosphatidylinositol 4- kinase that regulates hormone-sensitive pools of inositolphospholipids. Proc Natl Acad Sci USA. 1995;92:5317–5321. doi: 10.1073/pnas.92.12.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Pochynyuk O, Staruschenko A, Tong Q, Medina J, Stockand JD. Identification of a functional phosphatidylinositol 3,4,5-trisphosphate binding site in the epithelial Na+ channel. J Biol Chem. 2005;280:37565–37571. doi: 10.1074/jbc.M509071200. [DOI] [PubMed] [Google Scholar]
- Rohács T, Lopes CM, Michailidis I, Logothetis DE. PI(4,5)P2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nat Neurosci. 2005;8:626–634. doi: 10.1038/nn1451. [DOI] [PubMed] [Google Scholar]
- Smith NC, Burnstock G. Mechanisms underlying postjunctional synergism between responses of the vas deferens to noradrenaline and ATP. Eur J Pharmacol. 2004;498:241–8. doi: 10.1016/j.ejphar.2004.07.055. [DOI] [PubMed] [Google Scholar]
- Sneddon P. Electrophysiology of autonomic neuromuscular transmission involving ATP. J Auton Nerv Syst. 2000;81:218–224. doi: 10.1016/s0165-1838(00)00141-7. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Li Z, Lin L, Liu G, Ko K, Coetzee WA, Skolnik EY. The phosphatidylinositol 3-phosphate phosphatase myotubularin-related protein 6 (MTMR6) is a negative regulator of the Ca2+-activated K+ channel KCa3.1. Mol Cell Biol. 2005;25:3630–3638. doi: 10.1128/MCB.25.9.3630-3638.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh BC, Hille B. Recovery from muscarinic modulation of M current channels requires phosphatidyl 4,5-bisphosphate synthesis. Neuron. 2002;35:507–520. doi: 10.1016/s0896-6273(02)00790-0. [DOI] [PubMed] [Google Scholar]
- Suh BC, Hille B. Regulation of ion channels by phosphatidylinositol 4,5- bisphosphate. Curr Opin Neurobiol. 2005;15:370–378. doi: 10.1016/j.conb.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Tong XK, Nicolakakis N, Kocharyan A, Hamel E. Vascular remodeling versus amyloid beta-induced oxidative stress in the cerebrovascular dysfunctions associated with Alzheimer’s disease. J Neurosci. 2005;25:11165–74. doi: 10.1523/JNEUROSCI.4031-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera S, Hussy N, Evans RJ, Adami N, North RA, Surprenant A, Buell G. A new class of ligand-gated ion channel defined by P2x receptor for extracellular ATP. Nature. 1994;371:516–519. doi: 10.1038/371516a0. [DOI] [PubMed] [Google Scholar]
- Vial C, Tobin AB, Evans RJ. G-protein-coupled receptor regulation of P2X(1) receptors does not involve direct channel phosphorylation. Biochem J. 2004;382:101–110. doi: 10.1042/BJ20031910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vial C, Evans RJ. Disruption of lipid rafts inhibits P2X1 receptor-mediated currents and arterial vasoconstriction. J Biol Chem. 2005;280:30705–30711. doi: 10.1074/jbc.M504256200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhainazarov AB, Spehr M, Wetzel CH, Hatt H, Ache BW. Modulation of the olfactory CNG channel by Ptdlns (3,4,5), P3. J Membr Biol. 2004;201:51–57. doi: 10.1007/s00232-004-0707-4. [DOI] [PubMed] [Google Scholar]
- Zhang H, Cracium LC, Mirshahi T, Rohacs T, Lopes CMB, Jin T, Logothetis DE. PIP2 activated KCNQ channels and its hydrolysis underlies receptor-mediated inhibition of M currents. Neuron. 2003;37:963–975. doi: 10.1016/s0896-6273(03)00125-9. [DOI] [PubMed] [Google Scholar]
- Zhang H, He C, Yan X, Mirshahi T, Logothetis DE. Activation of inwardly rectifying K+ channels by distinct PtdIns(4,5)P2 interactions. Nat Cell Biol. 1999;1:183–8. doi: 10.1038/11103. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Yang M, Ting AT, Logothetis DE. PIP2 regulates the ionic current of P2X receptors and P2X7 receptor-mediated cell death. Channels. 2007;1:46–55. [PubMed] [Google Scholar]