Abstract
Human placental development combines elements of tumorigenesis and vasculogenesis. The organ’s specialized epithelial cells, termed cytotrophoblasts, invade the uterus where they reside in the interstitial compartment. They also line uterine arteries and veins. During invasion, ectodermally derived cytotrophoblasts undergo pseudovasculogenesis, switching their adhesion molecule repertoire to mimic that of vascular cells. Failures in this transformation accompany the pregnancy complication preeclampsia. Here, we used a combination of in situ and in vitro analyses to characterize the cell’s expression of vascular endothelial growth factor (VEGF) family ligands and receptors, key regulators of conventional vasculogenesis and angiogenesis. Cytotrophoblast differentiation and invasion during the first and second trimesters of pregnancy were associated with down-regulation of VEGF receptor (VEGFR)-2. Invasive cytotrophoblasts in early gestation expressed VEGF-A, VEGF-C, placental growth factor (PlGF), VEGFR-1, and VEGFR-3 and, at term, VEGF-A, PlGF, and VEGFR-1. In vitro the cells incorporated VEGF-A into the surrounding extracellular matrix; PlGF was secreted. We also found that cytotrophoblasts responded to the VEGF ligands they produced. Blocking ligand binding significantly decreased their expression of integrin α1, an adhesion molecule highly expressed by endovascular cytotrophoblasts, and increased apoptosis. In severe preeclampsia and hemolysis, elevated liver enzymes, and low platelets syndrome, immunolocalization on tissue sections showed that cytotrophoblast VEGF-A and VEGFR-1 staining decreased; staining for PlGF was unaffected. Cytotrophoblast secretion of the soluble form of VEGFR-1 in vitro also increased. Together, the results of this study showed that VEGF family members regulate cytotrophoblast survival and that expression of a subset of family members is dysregulated in severe forms of preeclampsia.
The embryo’s acquisition of a supply of maternal blood is a critical hurdle in pregnancy maintenance. In humans, the mechanics of this process (diagrammed in Figure 1▶ ) are accomplished by the placenta’s specialized epithelial cells, termed “cytotrophoblasts.” In a subset of chorionic villi, these cells form aggregates that attach the conceptus to the uterine wall. Cytotrophoblasts that emanate from these sites invade the interstitium of the decidua, the inner third of the myometrium, and uterine blood vessels. Although there are many circumstances in which normal and abnormal cells carry out interstitial invasion, several aspects of cytotrophoblast vascular invasion are unique. During this process, placental cells of fetal origin intravasate the superficial portions of uterine vessels that lie near the implantation site. Initially these vessels are plugged by cytotrophoblast aggregates. As invasion proceeds, the placental cells form heterotypic interactions with, then replace, the endothelium. They also intercalate within the muscular tunica, a process that substantially increases the vessel diameter while decreasing its resistance. In this manner uterine arterioles, and to a lesser extent veins, are remodeled into unique hybrid vessels composed of fetal and maternal cells. Once the lumina reform, uterine blood flow is diverted to the intervillous space where nutrient, gas, and waste exchange occur.
Figure 1.
Diagram of the histological organization of the human maternal-fetal interface at mid-gestation. In this location cytotrophoblasts, specialized (fetal) epithelial cells of the placenta, differentiate and invade the uterine wall, where they also breach maternal blood vessels. The basic structural unit of the placenta is the chorionic villus, composed of a stromal core with blood vessels, surrounded by a basement membrane, and overlain by cytotrophoblast stem cells. As part of their differentiation program, these stem cells detach from the basement membrane and adopt one of two lineage fates. They either fuse to form the syncytiotrophoblasts that cover floating villi (FV), or join a column of extravillous cytotrophoblasts at the tips of anchoring villi (AV). The syncytial covering of floating villi mediates the nutrient, gas, and waste exchange between fetal and maternal blood. The anchoring villi, through the attachment of cytotrophoblast columns, establish physical connections between the fetus and the mother. Invasive cytotrophoblasts penetrate the uterine wall up to the first third of the myometrium. A portion of the extravillus cytotrophoblasts home to uterine spiral arterioles and remodel these vessels by destroying the muscular wall and replacing the endothelial lining. To a lesser extent they also remodel uterine veins. At term, few cytotrophoblast stem cells remain, the syncytiotrophoblast layer thins and the stromal cores expand. (Diagram modified from Hoang and colleagues.61 )
Although the histological features of this process have been studied for decades, the molecular aspects, which are likely to be complex, have only recently been addressed. Our previous work suggests that invading cytotrophoblasts execute a novel epithelial-to-endothelial phenotypic transformation we termed “pseudovasculogenesis.”1,2 This switch is exemplified by changes in the cell’s adhesion molecule expression that take place during uterine invasion. For example, cytotrophoblast stem cells, which express adhesion molecules typical of many epithelial cells, eg, E-cadherin and α6β4 integrin, dramatically alter this repertoire as they invade the uterus. In essence, they replace their epithelial-like receptors with adhesion molecules typical of endothelial cells, eg, vascular endothelial (VE)-cadherin, vascular cell adhesion molecule-1, platelet-endothelial cell adhesion molecule-1, and αVβ3 integrin. Other aspects of the cell surfaces of invasive cytotrophoblasts also resemble vascular cells. For example, they express urokinase plasminogen activator3 and the thrombin receptor.4
The functional importance of this transformation program is highlighted by the fact that failures in executing portions of it are associated with a subset of pregnancy complications—for example, preeclampsia, the leading cause of maternal mortality in the Western world, which also increases perinatal mortality fivefold.5 The clinical diagnostic criteria of this syndrome include the new onset of hypertension and the appearance of proteinuria and edema during pregnancy, all of which could be explained by functional alterations in the maternal vascular endothelium.6 Preeclampsia and approximately half the cases of intrauterine growth restriction are associated with particular placental pathologies. The extent of interstitial invasion by cytotrophoblasts is variable, but frequently shallow, and endovascular invasion is consistently rudimentary, making it extremely difficult to find any maternal vessels that contain cytotrophoblasts.7,8 These anatomical defects suggested to us that in preeclampsia, cytotrophoblast differentiation along the invasive pathway is abnormal. Biopsies of the uterine wall of women with this syndrome showed that invasive cytotrophoblasts retain expression of adhesion receptors characteristic of stem cells and fail to turn on receptors that promote invasion and/or assumption of an endothelial phenotype.8 The concept that failed cytotrophoblast invasion and pseudovasculogenesis are linked to the maternal vascular pathology seems plausible, but has yet to be proved. Thus, the chain of events that links a defect in placentation to the maternal systemic disorder is under intense investigation.
We are very interested in understanding the molecular pathways that regulate cytotrophoblast pseudovasculogenesis, as well as the possible defects that occur in pregnancy complications such as preeclampsia. A vast array of physiological factors (eg, hypoxia, shear stress) and effector pathways (eg, transcription factors, growth factors, chemokines, cytokines, and protein fragments) governs blood vessel development and growth, either directly or indirectly.9 To focus the proposed investigation, we have been considering cytotrophoblast pseudovasculogenesis, which involves cells derived from the extraembryonic lineages, in the context of master regulatory pathways that govern de novo differentiation and assembly of blood vessels within the embryo. Gene deletion studies in mice have pointed to the particular importance of three families of ligands and their tyrosine kinase receptors—vascular endothelial growth factors and their receptors (VEGF/VEGFRs), angiopoietins and their Tie receptors (Ang/Tie), and ephrins and their Eph receptors (ephrin/Ephs)—in vasculogenesis and angiogenesis.10-13 The discrete phenotypes of the null animals suggest distinct roles for individual families, with VEGF family members and their receptors having important actions during the initial stages of vasculogenesis and angiogenesis, both during development and as a result of pathological processes.14,15
In accord with our hypothesis that cytotrophoblast pseudovasculogenesis co-opts a subset of the ligand-receptor interactions that govern conventional vasculogenesis, we studied the expression of VEGF family members and their receptors during this process in vivo. Immunolocalization experiments performed on tissue sections of the maternal-fetal interface showed that the expression of many of these ligands and receptors is modulated as cytotrophoblasts invade the uterus and its blood vessels in normal pregnancy. We also used an in vitro model of this process to understand the effects of interfering with interactions between VEGF and its receptors on cytotrophoblast differentiation/invasion and apoptosis. The results of these experiments, together with data showing that defects in VEGF and VEGFR expression exist in severe forms of preeclampsia, suggest that VEGF-VEGFR interactions play an important role in differentiation and survival of the unique cytotrophoblast subpopulation that invades the uterine wall, occupies the maternal vessels, and channels maternal blood to the placenta during pregnancy.
Materials and Methods
Antibodies and Enzyme-Linked Immunosorbent Assay (ELISA) Kits
Antibodies That Recognize VEGFs and Placental Growth Factor (PlGF)
A mouse anti-VEGF-A monoclonal antibody (mAb) (M293; R & D Systems, Minneapolis, MN) and a goat anti-VEGF-C polyclonal antibody (pAb) (R&D Systems) were used at a dilution of 1:50 for immunolocalization analyses of paraffin-embedded tissues. A goat anti-PlGF-1, 2 pAb (R&D Systems) was used at a dilution of 1:25 for immunolocalization on tissues that were embedded in optimal cutting temperature (OCT) medium (Miles Scientific, Naperville, IL). A goat anti-VEGF-B pAb and a mouse anti-VEGF-D mAb, both from R&D Systems, were also used for immunolocalization analyses of OCT-embedded tissues at dilutions of 1:10 and 1:50, respectively. VEGF-A and PlGF levels in conditioned medium were measured separately using ELISA kits (R&D Systems). A mouse mAb (A4.6.1) that recognizes all isoforms of human VEGF-A (gift of Dr. N. Ferrara, Genentech, South San Francisco, CA) was used in immunoblotting experiments.
Antibodies That Recognize VEGF Receptors
The 190.11 anti-VEGFR-1 mouse mAb (diluted 1:400)16 and the rabbit N-931 anti-VEGFR-2 antibody (diluted 1:200; Santa Cruz Biotechnology Inc., Santa Cruz, CA) were used for immunostaining. The 9D9 anti-VEGFR-3 mouse mAb (Molecular/Cancer Biology Laboratory, University of Helsinki, Finland) was used for immunostaining and immunoblotting (diluted 1:500). The C-17 rabbit anti-human VEGFR-1 antibody (diluted 1:1000, Santa Cruz Biotechnology Inc.) and the 2-10−1 anti-VEGFR-2 mAb (diluted 1:200; gift of Dr. K. Chwalisz, Scherring AG, Berlin, Germany) were used for immunoblotting. Soluble VEGFR-1 (sVEGFR-1) in conditioned medium was quantified with an ELISA kit that was developed using the 190.11 mAb.17
Antibodies That Recognize Adhesion Receptors
The TS2/7 mAb that specifically recognizes integrin α1/β1 (Endogen, Woburn, MA) was used for immunostaining and immunoprecipitation at a dilution of 1:50. mAbs BV6, BV9, and TEA against VE-cadherin (gifts of Dr. Elisabetta Dejana, Mario Negri Institute, Milan, Italy) were all used at a dilution of 1:10 for immunostaining and immunoblotting.
Antibodies That Recognize Cytokeratin
The OV-TL 12/30 mAb against cytokeratin 7 (diluted 1:25; DAKO, Carpinteria, CA) was used for immunolocalization analyses of paraffin-embedded tissues. Rat anti-human cytokeratin mAb 7D3, produced in the Fisher laboratory, was used at a dilution of 1:50 for immunolocalization analyses of OCT-embedded tissues.18
Tissue Sources for Immunolocalization Experiments
Placentas were obtained from normal pregnant women and patients with preeclampsia. We analyzed 19 control samples from patients with no evidence of preeclampsia, gestational hypertension, or a medical history that suggested an increased risk of developing preeclampsia. Where applicable, the labor status is indicated with L denoting women who experienced labor and NL denoting women who did not. The gestational ages at which these samples were collected were as follows: 6 weeks (n = 1), 10 weeks (n = 1), 12 weeks (n = 1), 14 weeks (n = 1), 16 weeks (n = 1), 17 weeks (n = 1), 19 weeks (n = 1), 20 weeks (n = 1), 22 weeks (n = 1), 24 weeks (n = 1; NL), 26 weeks (n = 2; NL), 32 weeks (n = 1; NL), 36 weeks (n = 2; 1 L and 1 NL), 38 weeks (n = 3; L), and 40 weeks (n = 1; NL). We analyzed 13 samples from patients with preeclampsia diagnosed according to the classic criteria originally recommended by Dr. Leon Chesley and modified by the National Institutes of Health:19 no history of hypertension before pregnancy; increase in diastolic pressure of 15 mm Hg or systolic pressure of 30 mm Hg compared with blood pressure obtained before 20 weeks of gestation; proteinuria ≥0.5 g/24 hours or ≥30 mg/dl (or 1+ on urine dipstick) in a catheterized specimen; hyperuricemia >5.5 mg/dl (or 1 SD greater than the normal mean value before term); return to normal blood pressure and resolution of proteinuria by 12 weeks postpartum. Severe preeclampsia (SPE) was diagnosed according to the following criteria:20 systolic blood pressure ≥160 mm Hg and/or diastolic pressure ≥110 mm Hg; proteinuria of ≥5 g in a 24-hour period or 3+ on urine dipstick; presence of cerebral or visual disturbances. The criteria used to diagnose the syndrome of hemolysis, elevated liver enzymes, and low platelets (HELLP) have been published.21 Samples were collected for embedding in OCT from women with the diagnoses indicated at the following weeks of gestation: 26 weeks (n = 3; 1 SPE, 2 HELLP; NL), 28 weeks (n = 1 SPE; NL), 29 weeks (n = 1 SPE; NL), 30 weeks (n = 1 HELLP; L), 31 weeks (n = 1 SPE; L), 32 weeks (n = 1 SPE; L), 33 weeks (n = 1 SPE; L), 35 weeks (n = 1 SPE; L), 36 weeks (n = 1 SPE; L), 38 weeks (n = 2; 1 PE, 1 HELLP; L). Samples were collected for embedding in paraffin from women with the diagnoses indicated at the following weeks of gestation: 25 weeks (n = 1 HELLP; NL), 29 weeks (n = 1 HELLP; NL), 29 weeks (n = 1 SPE; L), 30 weeks (n = 1 HELLP; L), 31 weeks (n = 1 SPE; L), 32 weeks (n = 1 SPE; L), 33 weeks (n = 1 SPE; L), 35 weeks (n = 1 SPE; L), 36 weeks (n = 1 SPE; L), 38 weeks (n = 2; 1 PE, 1 HELLP; L).
Immunolocalization
Placental tissues were processed for double indirect immunolocalization as previously described.22,23 For detection of VEGF-B and VEGF-D and the VEGFRs, tissues were fixed in 3% paraformaldehyde for 30 minutes, washed three times in phosphate-buffered saline (PBS), infiltrated with 5 to 15% sucrose followed by OCT medium, and frozen in liquid nitrogen. Sections (5 μm) were prepared using a cryostat (Slee International, Tiverton, RI) and collected on charged and precleaned microscope slides (Fisher Scientific, Pittsburgh, PA). For detection of PlGF, tissues were directly embedded in OCT and frozen without previous fixation. Sections, prepared as described above, were fixed in cold acetone for 5 minutes before staining as follows. The sections were incubated in a mixture of anti-cytokeratin (to localize trophoblasts) and another primary antibody for 1 hour to overnight. Then the sections were rinsed, incubated with the appropriate species-specific secondary antibodies conjugated to rhodamine or fluorescein, washed three times in PBS for 10 minutes, and mounted with Vectashield medium (Vector, South San Francisco, CA). Samples were examined with a Zeiss Axiophot Epifluorescence microscope (Thornwood, NY) equipped with filters to selectively view the rhodamine and fluorescein fluorescence. For detection of VEGF-A, tissues were fixed in 10% neutral buffered formalin for 24 hours and embedded in paraffin. Sections (5 μm) were cut on a Leica microtome and stained as described by Zhang and colleagues.24 For detection of VEGF-C, sections cut from paraffin blocks were incubated with the primary antibody for 3 hours and washed in PBS (three times for 10 minutes). Bound antibody was detected by incubation with diaminobenzoate (Vector). The sections were examined using a Zeiss microscope. As a control for each experiment, the staining patterns of the primary and secondary antibodies alone were assessed.
For immunolocalization of antigens expressed by cultured cytotrophoblasts, isolated cells were plated on coverslips coated with Matrigel (Collaborative Biomedical Products, Bedford, MA) for various periods of time, then fixed in 3% paraformaldehyde for 5 minutes, and permeabilized with cold acetone or methanol for another 5 minutes. Samples were stained and analyzed as described above.
Cytotrophoblast Isolation and Culture
Cytotrophoblasts were isolated from chorionic villi of 7- to 24-week human placentas by routine procedures established in our laboratory.25 Briefly, the placentas were obtained immediately after elective pregnancy terminations. After a series of collagenase and trypsin digestions, cytotrophoblasts were separated from contaminating cell types on Percoll gradients. Purified cells were used immediately or cultured in serum-free Dulbecco’s modified Eagle’s medium-high glucose, with 2% Nutridoma (Boehringer Mannheim Biochemicals, Indianapolis, IN), on Matrigel-coated or human placental laminin-coated (Life Technologies, Inc., Rockville, MD) substrates for the times indicated.
RNA Isolation and Northern Blot Hybridization
Total RNA was extracted from purified first and second trimester cytotrophoblasts either immediately on isolation or after 12 hours in culture according to published methods.26 Blots were prepared as previously described.27 Before transfer, gels were stained with acridine orange to ensure integrity of the RNA samples and confirm equal loading. The probes were generated by random priming with [32P]CTP and the Klenow fragment of DNA polymerase I28 of the following fragments: bp 57 to 638 of the VEGF165 cDNA (GenBank accession number M32977), bp 1 to 362 of the VEGF-B167 cDNA (GenBank accession number U48801), bp 494 to 1661 of the VEGF-C cDNA (GenBank accession number X94216), the entire coding regions of the VEGF-D (1064 bp) gene (GenBank accession number AJ000185), bp 304 to 944 of the PlGF cDNA (GenBank accession number X54936), bp 706 to 2310 of the Flt1 (VEGFR-1) cDNA (GenBank accession number X51602), bp 6 to 715 of the KDR (VEGFR-2) cDNA (GenBank accession number L04947), and bp 1 to 595 of the Flt4 (VEGFR-3) cDNA (GenBank accession number X68203). Probes had a specific activity of 2 × 109 dpm/pg. The final posthybridization washes were conducted in 0.3× standard saline citrate (150 mmol/L NaCl, 15 mmol/L sodium citrate, pH 7.4) and 0.1% sodium dodecyl sulfate (SDS) at 65°C. Blots were stripped in 0.1× standard saline citrate with 0.5% SDS at 95°C.
ELISAs
Levels of VEGF-A, PlGF, and sVEGFR-1 in first (n = 5), second (n = 5), and third trimester (n = 5) cytotrophoblast-conditioned medium were assessed by ELISA. Patients with preeclampsia whose placentas were used as sources of cells had the diagnoses indicated at the following weeks of gestation: 26 weeks (n = 2; 1 SPE, 1 HELLP), 31 weeks (n = 1 SPE), 32 weeks (n = 1 SPE), and 36 weeks (n = 1 SPE). Equal numbers of cytotrophoblasts (1 × 106/ml) were cultured for 48 hours on Matrigel substrates as described above, and then medium was collected and centrifuged at 12,000 × g for 5 minutes. The ELISAs were performed according to the manufacturer’s instructions (VEGF-A and PlGF) or published methods (sVEGFR-117 ). The results were analyzed on a Vmax kinetic microplate reader (Molecular Devices Corporation, Sunnyvale, CA). The statistical significance of the data was analyzed by using an analysis of variance test.
Cell Extraction and Immunoblotting
Freshly isolated cytotrophoblasts or cytotrophoblasts cultured on either Matrigel-coated or human placental laminin-coated wells were washed twice with PBS and extracted with 200 μl of lysis buffer (50 mmol/L Tris buffer, pH 7.6, containing 1% Nonidet P-40, 0.1% SDS, 120 mmol/L NaCl, 100 μmol/L phenylmethylsulfonyl fluoride, 20 μg/ml aprotinin, 10 μg/ml leupeptin, 10 μg Na2VCO3). Cell extracts were centrifuged at 12,000 × g for 5 minutes to remove insoluble materials. Samples containing equal amounts of protein were mixed with SDS sample buffer and separated by SDS-polyacrylamide gel electrophoresis under nonreducing conditions (VEGF-A and VEGFR-2) or reducing conditions (VEGFR-1 and VEGFR-3 and VE-cadherin). After the proteins were transferred to nitrocellulose, the membranes were incubated first with primary antibody, then with peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch Labs, Inc., West Grove, PA), by using procedures standard in our laboratory.27 Immune complexes were visualized using enhanced chemiluminescence and Hyperfilm (Amersham Life Sciences-USB, Arlington Heights, IL). The entire experiment was done three times using different batches of cytotrophoblasts.
Function-Perturbing Fc Fusion Proteins
Fusion proteins consisting of the first three repeats of VEGFR-1/Flt1 or VEGFR-3/Flt-4 fused to the Fc portion of IgG [VEGFR-1(1-3)-Fc and VEGFR-3(1-3)-Fc] were produced in the Alitalo laboratory.29,30 As a control, nonimmune IgG was added to some of the wells. Initially, the activity of the fusion and control proteins was tested at concentrations ranging from 500 ng/ml to 50 μg/ml. Thereafter, 15 μg/ml, the lowest protein concentration that had maximum effects on cell morphology and integrin α1 expression, was used routinely. The entire experiment was done three times using different batches of cytotrophoblasts.
Cell-Surface Biotin Labeling and Immunoprecipitation
Cytotrophoblasts cultured on Matrigel-coated wells were washed twice with PBS and then incubated for 90 minutes in a 0.1% solution of freshly prepared biotin (Pierce, Rockford, IL) in ice-cold PBS. The cells were washed three times, also in ice-cold PBS, then extracted with 200 μl of the lysis buffer described above. Cell lysates were centrifuged at 12,000 × g for 5 minutes. Samples of equal volume that contained equal amounts of protein were mixed with the TS2/7 mAb (1 μg/ml) and precipitated with protein A-Sepharose CL-4B (Zymed Labs., South San Francisco, CA). The immunoprecipitates were separated by SDS-polyacrylamide gel electrophoresis under reducing conditions and transferred to nitrocellulose. The nitrocellulose membranes were incubated with streptavidin-horseradish peroxidase, and biotin-labeled integrin α1/β1 was visualized by standard enhanced chemiluminescence procedures.
Invasion Assay
Invasion assays were conducted as described previously.22,25 Briefly, isolated cytotrophoblasts (0.25 × 106) were plated on Transwell inserts (6.5 mm; Costar, Cambridge, MA) containing polycarbonate filters (pore size, 8 μm) that had been coated with Matrigel. Culture medium containing either a fusion protein [VEGFR-1(1-3)-Fc and VEGFR-3(1-3)-Fc] or control IgG was added. After 48 hours the cultures were stained with the 7D3 antibody, which specifically reacts with human cytokeratin, to visualize the cytotrophoblasts. The filters were cut from the supports and mounted, upper surface facing down, on slides. The number of cytokeratin-positive cells and cell processes on the lower surface of the filter was counted. Each experimental condition was tested in triplicate, and the entire assay was done seven times. Data were expressed as percentage of control. The statistical significance of the data was analyzed by Student’s t-test.
Terminal Deoxynucleotidyl Transferase (TdT)-Mediated dUTP Nick-End Labeling (TUNEL)
Cytotrophoblasts were plated on Matrigel- or human laminin-coated coverslips under the same experimental and control conditions as described above for the invasion assays. After 24 hours, the coverslips were fixed in 3% paraformaldehyde for 30 minutes, washed twice with PBS, and permeabilized with 0.1% Triton X-100 in 0.1% sodium citrate for 4 minutes. Then the cells were incubated in the TUNEL reaction mixture (Boehringer Mannheim Biochemicals, Indianapolis, IN) for 1 hour at 37°C. The results were analyzed using a Zeiss Epifluorescence microscope. Data were expressed as the number of brightly staining cytotrophoblasts/total cytotrophoblasts per ×40 field. Thirty randomly chosen fields were counted on each coverslip. Each experimental condition was tested in triplicate, and the entire experiment was done six times. The statistical significance of the data was analyzed by analysis of variance.
Results
Cytotrophoblast Expression of VEGF Family Ligands and Their Receptors Is Modulated during Uterine Invasion in Vivo
First, we immunolocalized VEGF family members in tissue sections of the maternal-fetal interface (diagrammed in Figure 1▶ ). In first trimester chorionic villi, cells and fetal vessels in villus stromal cores stained with anti-VEGF-A (Figure 2B)▶ , as did a subset of the syncytiotrophoblasts that are in contact with maternal blood at the villous surface (data not shown). The majority of cytotrophoblast stem cells, which are attached to the trophoblast basement membrane of chorionic villi, and cytotrophoblasts in the proximal regions of cell columns, which bridge the gap between the placenta and the uterine wall, did not react with anti-VEGF-A (Figure 2B)▶ . Expression was dramatically up-regulated on cytotrophoblasts in the distal regions of columns. Within the superficial uterine wall, most cytotrophoblasts also stained with anti-VEGF (Figure 2D)▶ , although some cells in the deeper portions of the decidua and the superficial myometrium showed no immunoreactivity. Particularly intense staining was observed in association with the placental cells that occupied the lumina of uterine vessels, although cytotrophoblasts in the wall often lacked immunoreactivity (Figure 2F)▶ . In areas where the maternal endothelium had not yet been replaced, the latter cells also reacted with anti-VEGF-A. Neither the pattern of VEGF-A localization nor the staining intensity changed during the first-to-second-trimester interval analyzed, 6 to 22 weeks of gestation. At term, stromal cells and blood vessels in the villous cores (data not shown) and a subset of cytotrophoblasts in the uterine wall stained with anti-VEGF-A (see Figure 12B▶ ). Very weak anti-VEGF-B staining was detected, such that there was no discernable pattern at any of the gestational ages analyzed, ie, 6 weeks to term (data not shown). In contrast, very strong anti-VEGF-C staining was observed in cytotrophoblast stem cells and in the column region, particularly during the second trimester interval (Figure 3, B and D)▶ . Endovascular cytotrophoblasts also reacted with anti-VEGF-C in a pattern that was similar to staining with anti-VEGF-A (data not shown). This staining pattern did not change from 6 to 22 weeks of gestation, but by term little or no staining was detected (Figure 3F)▶ . We failed to detect expression of VEGF-D (data not shown).
Figure 2.
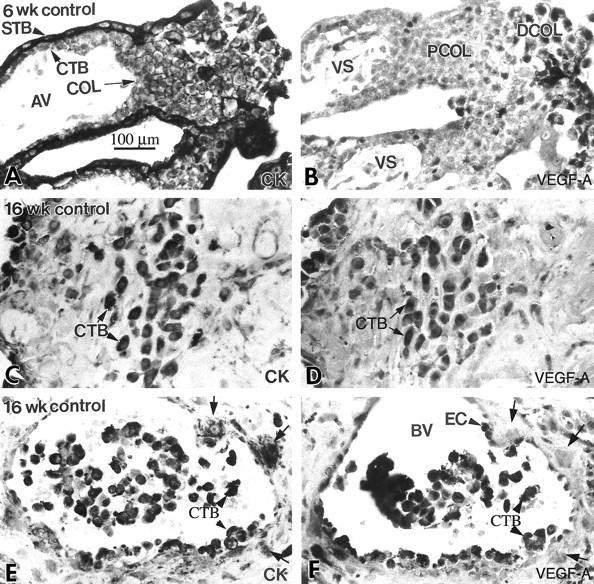
Cytotrophoblast staining for VEGF-A was up-regulated as the cells differentiated and invaded the uterus in situ. Serial paraffin sections of the maternal-fetal interface were stained with anti-cytokeratin (CK) to identify all of the trophoblast populations (A, C, and E), and with anti-VEGF-A (B, D, and F). Essentially the same staining pattern was observed during the first and second trimesters (ie, 6 and 16 weeks of gestation). A few cytotrophoblast (CTB) stem cells and cells in the proximal column (PCOL) region stained with anti-VEGF-A (B), but much more intense staining was observed in association with a majority of cytotrophoblasts in the distal regions of columns (DCOL) and with those that invaded the uterine wall (D). Cytotrophoblasts within the lumina of uterine blood vessels (BV) also exhibited intense staining (arrowheads), as did the maternal endothelial cells (EC; F). In contrast, some cytotrophoblasts in the vessel wall failed to react with anti-VEGF-A (arrows). The cells continued to stain for VEGF-A at term (see Figure 12B▶ ). AV, anchoring villus; STB, syncytiotrophoblast; VS, villous stroma; COL, cytotrophoblast column.
Figure 12.
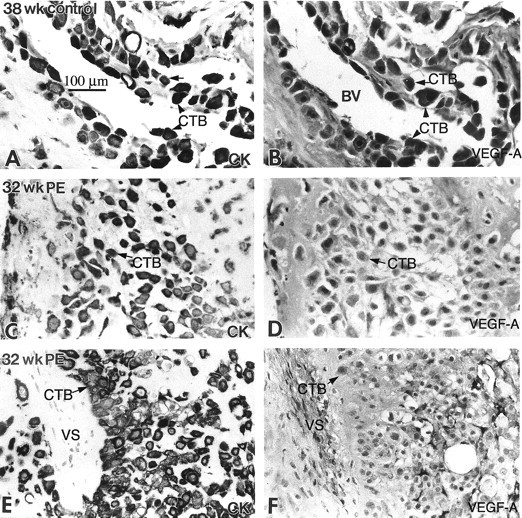
In preeclampsia, staining of invasive cytotrophoblasts with anti-VEGF-A decreased. In the third trimester of normal pregnancy (38 weeks), cytotrophoblasts (CTBs) in the interstitium (arrow) and in the walls of blood vessels (BVs) (arrowheads) stained for VEGF-A (B). In preeclampsia (PE), staining of invasive cytotrophoblasts for VEGF-A was strikingly down-regulated (D and F). CK, cytokeratin; VS, villus stroma.
Figure 3.
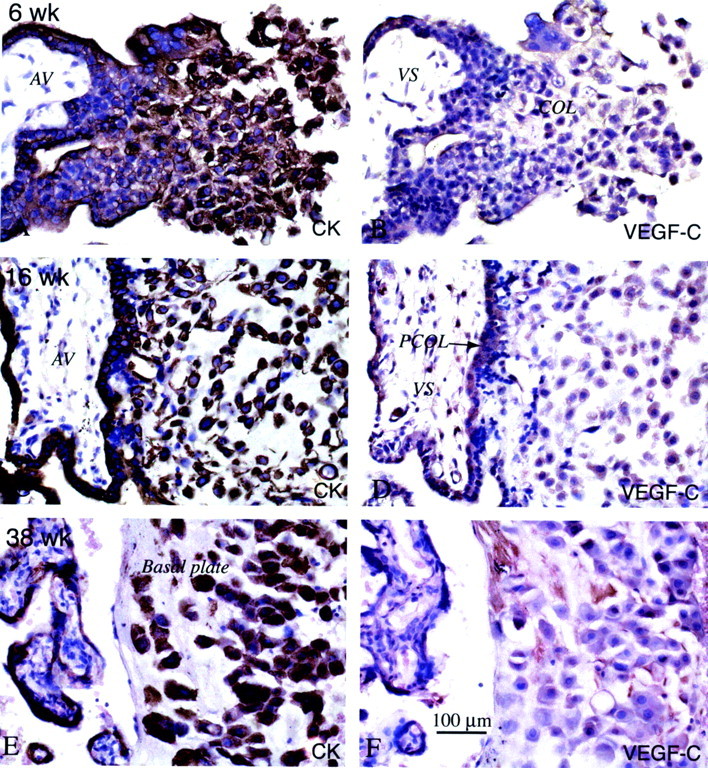
By the second trimester, cytotrophoblasts in all stages of differentiation stained with anti-VEGF-C in situ. Serial paraffin sections of the maternal-fetal interface were stained with anti-cytokeratin (CK) to identify the various trophoblast populations (A, C, and E), and with anti-VEGF-C (B, D, and F). In the first trimester (6 weeks), most of the cytotrophoblast populations stained with anti-VEGF-C, although cells in the distal portions of columns (COL) sometimes exhibited stronger antibody reactivity (B). In the second trimester (16 weeks), staining tended to be stronger than in the first trimester, with cytotrophoblast stem cells, cells in columns, and cytotrophoblasts within the uterine wall exhibiting similar levels of antibody reactivity (D). By term (38 weeks), little or no staining for VEGF-C was observed (F). AV, anchoring villus; VS, villus stroma; PCOL, proximal column.
We also localized the expression of PlGF, another VEGF isoform, at the maternal-fetal interface. In first and second trimester chorionic villi, the fetal vessels, as well as subsets of cytotrophoblast stem cells and syncytiotrophoblasts, stained (data not shown), but the villous stroma and cytotrophoblasts in the proximal column region did not (Figure 4B)▶ . Anti-PlGF reacted with cytotrophoblasts in the distal regions of columns (Figure 4B)▶ and with invasive placental cells in the interstitial (Figure 4D)▶ and endovascular (Figure 4F)▶ uterine compartments. At term, fetal blood vessels stained brightly as compared to cytotrophoblasts in the uterine wall, which in some areas showed weak immunoreactivity and in others failed to stain (data not shown).
Figure 4.
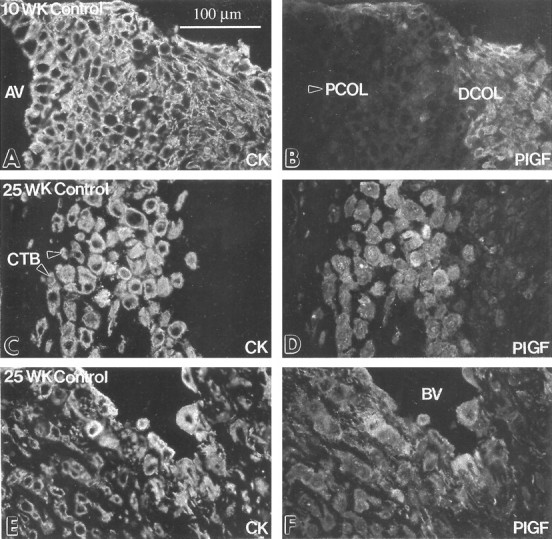
Cytotrophoblast staining for PlGF was up-regulated as the cells differentiated and invaded the uterus in situ. Frozen sections of the maternal-fetal interface in the first trimester (10 weeks) and the second trimester (25 weeks) were double-stained with anti-cytokeratin (CK; A, C, and E) and with anti-PlGF (B, D, and F). Essentially the same staining pattern was observed in the first and second trimesters. Staining for PlGF was first detected on cytotrophoblasts (CTBs) in the distal column (DCOL; B). Anti-PlGF reactivity was also observed in association with cytotrophoblasts that performed both interstitial (D) and endovascular invasion (F). At term cytotrophoblasts within the uterine wall either failed to stain or stained weakly with anti-PlGF (data not shown). AV, anchoring villus; PCOL, proximal column (arrowhead); BV, blood vessel.
To address the possibility that cytotrophoblasts directly respond to the VEGF isoforms they produce, we also localized VEGF receptor expression at the maternal-fetal interface during the first and second trimesters of human pregnancy. In chorionic villi, anti-VEGFR-2 stained cytotrophoblast stem cells and cells in the proximal regions of columns; the outer syncytiotrophoblast layer and cytotrophoblasts in the distal regions of columns did not stain (Figure 5B)▶ . Within the uterine wall, cytotrophoblasts in the decidua and myometrium either failed to react with anti-VEGFR-2 or stained weakly (data not shown). In contrast, placental cells within the lumina of vessels stained brightly as compared to cytotrophoblasts that had invaded the vessel wall and the adjacent decidua (Figure 6B)▶ . Staining for VEGFR-1 showed a different pattern. This antigen was detected in association with syncytiotrophoblasts, but not the villous stroma, cytotrophoblast stem cells, or sites of column initiation (Figure 5D)▶ . Anti-VEGFR-1 reactivity was very specifically up-regulated within the first three to four cell layers of columns, then maintained throughout the rest of the column. Cytotrophoblasts in the decidua and the myometrium stained brightly, but the endovascular subpopulation of placental cells, in either vessel walls or lumina, did not react with anti-VEGFR-1 (Figure 6D)▶ . VEGFR-3 displayed yet a third distinct staining pattern. In chorionic villi, cell columns, decidua, and myometrium the pattern of trophoblast immunoreactivity was nearly identical to that of anti-VEGFR-1, except that syncytiotrophoblast staining was relatively weaker (Figure 5F)▶ . In contrast to VEGFR-1, staining for VEGFR-3 was strong on endovascular cytotrophoblasts (Figure 6F)▶ . At term, staining for VEGFR-1 was comparable to that observed in first and second trimester, whereas anti-VEGFR-2 and anti-VEGFR-3 immunoreactivity was down-regulated in all locations; endovascular expression of the latter receptor was nearly absent (data not shown). Finally, we observed no differences in VEGF ligand and receptor expression between patients who experienced labor and those who did not.
Figure 5.
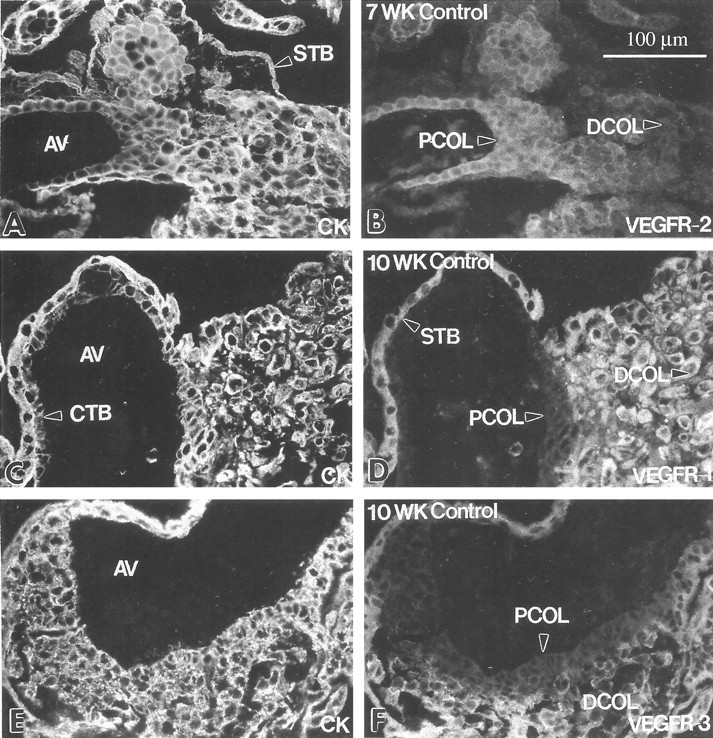
Cytotrophoblast subsets at the maternal-fetal interface differentially stain with antibodies that recognize individual VEGF receptors. Frozen sections of the first trimester maternal-fetal interface were double-stained with anti-cytokeratin (CK; A, C, and E) and antibodies that specifically reacted with one of three VEGF receptors (B, D, and F). Cytotrophoblast (CTB) stem cells and those in the proximal regions of cytotrophoblast columns (PCOL) stained with anti-VEGFR-2; little or no staining was detected in the distal column (DCOL) region and in association with cytotrophoblasts that invaded the uterine wall (B). Staining with anti-VEGFR-1 (D) and anti-VEGFR-3 (F) revealed a different pattern. Cytotrophoblast stem cells and those in the portion of the column immediately adjacent to the anchoring villus (AV) failed to react with either antibody, whereas cells in the rest of the column and within the uterine wall stained brightly with both. Syncytiotrophoblasts (STB) also stained for VEGFR-1 and VEGFR-3 (D and F). Essentially the same pattern was observed in the second trimester (data not shown). At term, only VEGF-R1 staining was detected in association with cytotrophoblasts (see Figure 13B▶ ).
Figure 6.
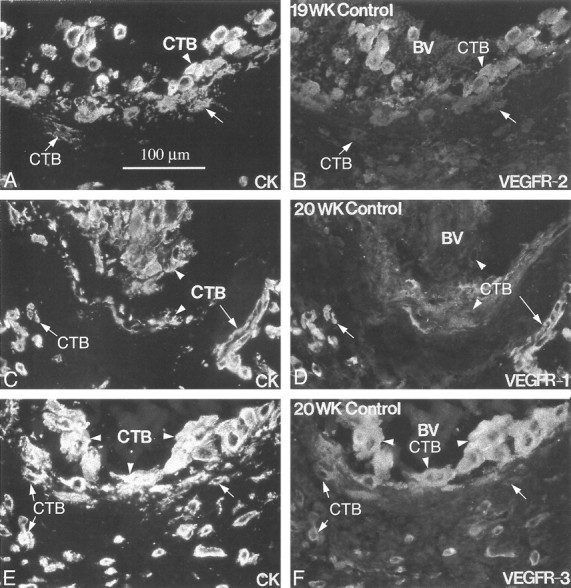
Endovascular cytotrophoblasts differentially stain for individual VEGF receptors. Frozen sections of the uterine wall from samples collected in the second trimester of pregnancy (19 to 20 weeks of gestation) were double-stained with anti-cytokeratin (CK; A, C, and E) and antibodies that specifically reacted with one of three VEGF receptors (B, D, and F). Cytotrophoblasts (CTBs) within the lumina of blood vessels (BV) stained for VEGFR-2 (B; arrowhead) and VEGFR-3 (F; arrowhead), whereas cytotrophoblasts that were embedded in the vessel wall usually stained much less intensely (B and D; arrows). In contrast, endovascular cytotrophoblasts showed little or no staining for VEGFR-1 (D; arrowheads). When staining was detected, reactivity was usually associated with cells embedded in the vessel wall (arrow). Essentially the same pattern was observed in blood vessels that were modified during the first trimester.
Cytotrophoblasts Differentiating in Vitro Express the Same VEGF Ligands and Receptors as Their in Vivo Counterparts
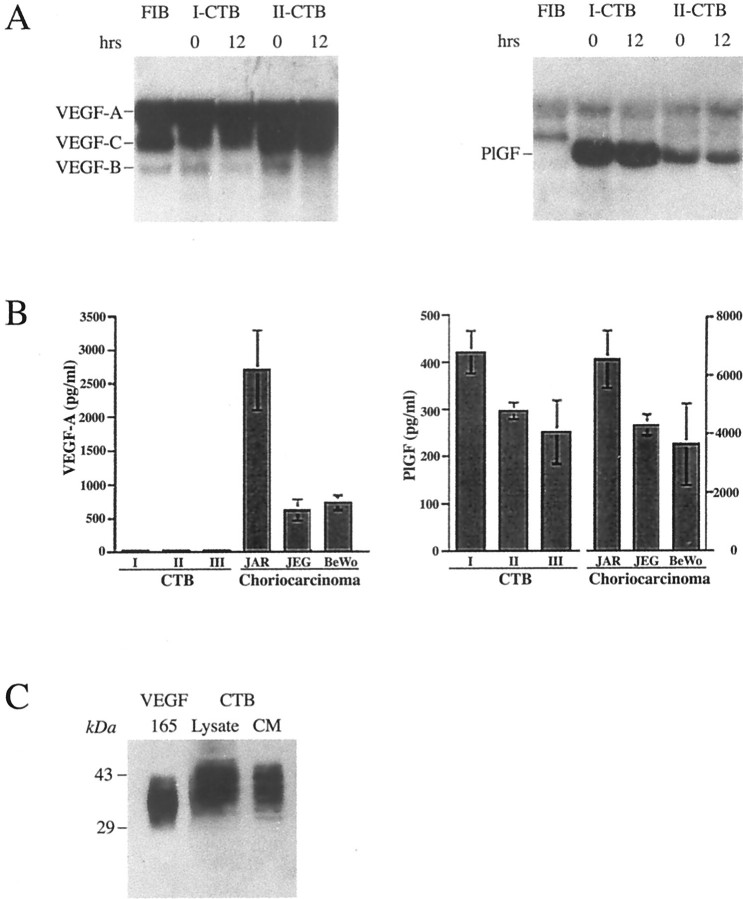
Plating cytotrophoblast stem cells isolated from first and second trimester placentas on complex matrix substrates such as Matrigel promotes their differentiation along the pathway that leads to invasion and pseudovasculogenesis, rather than fusion to form syncytium.2,25 Here we investigated whether these culture conditions supported expression of the same VEGF ligands and receptors that were detected in the immunolocalization experiments performed on tissue sections of the maternal-fetal interface. Northern blot hybridization and immunoblot approaches were used to detect mRNA and protein expression, respectively. Northern blot hybridization (Figure 7A)▶ showed that fibroblasts and cytotrophoblasts isolated from first and second trimester placentas primarily expressed VEGF-A and VEGF-C mRNAs; low levels of VEGF-B mRNA were also detected, whereas VEGF-D was not. In contrast, cytotrophoblasts, but not fibroblasts, expressed PlGF mRNA, and the message levels were higher in the first than in the second trimester. In cytotrophoblasts, VEGF-A, VEGF-C, and PlGF message levels did not change with time in culture. Surprisingly, ELISA failed to detect VEGF-A protein in the culture medium of first and second trimester as well as term cytotrophoblasts plated on Matrigel (Figure 7B▶ , left), a result that was confirmed by immunoblotting (data not shown). In these experiments, VEGF release by the choriocarcinoma cell lines JAR, JEG, and BeWo served as positive controls. If instead cytotrophoblasts were cultured for 12 hours on a defined laminin substrate, substantial amounts of VEGF-A, detected by immunoblotting, were secreted into the medium (Figure 7C)▶ . The immunoreactive bands spanned a higher molecular mass interval than the VEGF165 control, suggesting the presence of higher molecular mass isoforms or glycoforms. In contrast, cytotrophoblasts plated on both Matrigel and laminin substrates released PlGF into the culture medium, as did the choriocarcinoma cell lines (Figure 7B▶ , right).
Figure 7.
Cytotrophoblasts produce the same repertoire of VEGF family members in vivo and in vitro. Cytotrophoblasts (CTBs) isolated from human placentas were examined for their production of VEGF mRNA (A) and protein (B and C). mRNA, purified from cells before they were plated (0 hours) and after 12 hours of culture on a Matrigel substrate, was analyzed by Northern blot hybridization. First (I) and second (II) trimester samples expressed approximately equal amounts of RNAs encoding VEGF-A and VEGF-C; VEGF-B mRNA levels were much lower and VEGF-D was not detected (A, left). Isolated placental fibroblasts also produced VEGF-A and VEGF-C mRNAs and low levels of VEGF-B mRNA. In contrast, cytotrophoblasts, not fibroblasts, produced PlGF mRNA (A, right). Cytotrophoblast production of VEGF-A and PlGF was assessed by ELISA (B). No VEGF-A was detected in conditioned medium from either first, second, or third (III) trimester cytotrophoblasts that were plated on Matrigel substrates, whereas all of the choriocarcinoma cell lines produced amounts of this growth factor that were easily detected by ELISA (B, left). In contrast, PlGF was secreted by both cytotrophoblasts and choriocarcinoma cells (B, right). The ELISA data in B are the means and standard deviations of three experiments. If the cells were plated on a laminin substrate instead of Matrigel, approximately equal amounts of VEGF-A partitioned in the cell and conditioned medium (CM) fractions as determined by immunoblotting (C). The isoforms produced by cytotrophoblasts migrated more slowly than recombinant VEGF165.
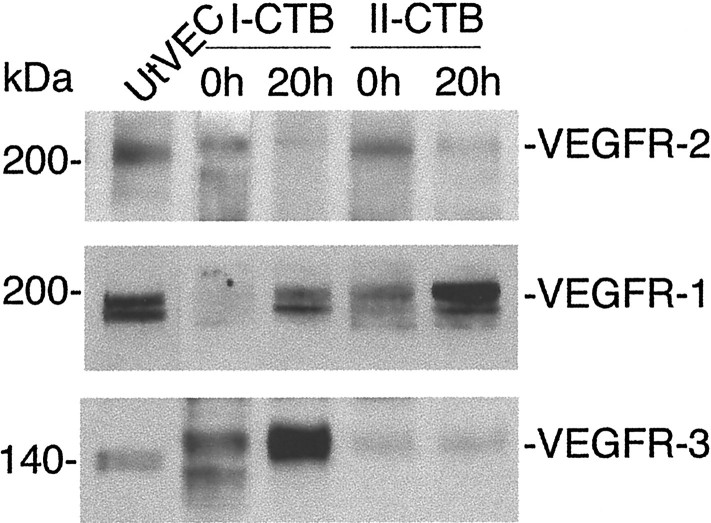
We also studied cytotrophoblast expression of VEGF receptors in vitro. Both first and second trimester cells rapidly down-regulated VEGFR-2 protein expression in culture (Figure 8)▶ . Before plating, lysates prepared from first trimester cytotrophoblasts contained two immunoreactive bands, one that migrated slightly faster and one slightly slower than the endothelial form of the protein. In contrast, in second trimester cell lysates, only the more slowly moving band was detected. We did not further investigate the reason for the differences among the samples in the number and electrophoretic migration of the immunoreactive bands, but the explanation likely includes the unusual polylactosamine-containing carbohydrate structures that cytotrophoblasts add to proteins, a process that is gestationally regulated,31 and/or receptor phosphorylation.14
Figure 8.
Cytotrophoblasts modulate VEGF receptor expression at the protein level as they differentiate in vitro. Cell lysates were prepared from first and second trimester cytotrophoblasts, either immediately after isolation (0 hours) or after they had been plated for 20 hours on Matrigel. Receptor expression was analyzed by immunoblotting. First and second trimester cells down-regulated expression of VEGFR-2 and up-regulated the expression of VEGFR-1. VEGFR-3 expression was higher in first trimester than in second. As a control, uterine vein endothelial cell (UtVEC) expression of the receptors was analyzed in parallel. Multiple bands may be due to receptor phosphorylation in some of the cytotrophoblast samples. Different glycoforms could also be present.
Northern blot hybridization showed that cytotrophoblasts expressed the corresponding VEGFR-2 message immediately after isolation, but no expression was found after the cells were plated on Matrigel for 12 hours (data not shown). In contrast, first trimester and, to a lesser extent, second trimester cytotrophoblasts up-regulated VEGFR-1 protein expression in culture (Figure 8)▶ . VEGFR-3 expression was much higher in the first than in the second trimester, and again variations in the pattern of immunoreactive bands were observed among samples (Figure 8)▶ .
VEGF Family Members Promote Differentiation and Survival of Cytotrophoblasts
To understand the effects of VEGF family members, we plated cytotrophoblast stem cells on Matrigel substrates in the presence of fusion proteins that contained the ligand-binding domains of VEGFR-1 and VEGFR-3, receptors whose expression is up-regulated as cytotrophoblasts differentiate in culture. We examined endpoints indicative of changes in the cell’s adhesive and invasive properties.22 After 12 hours in culture, cytotrophoblasts plated under control versus experimental conditions showed morphological differences. The control cells formed discrete aggregates that were interconnected via cells and cell processes (Figure 9A)▶ . In contrast, cytotrophoblasts plated in the presence of the VEGFR-1(1-3)-Fc fusion protein, which blocks VEGF-A, PlGF, and VEGF-PlGF heterodimer binding to endogenous VEGFR-1, flattened on the matrix substrate, and fewer interconnections were evident (Figure 9D)▶ . The latter effect was enhanced when the cells were plated in the presence of a VEGFR-3(1-3)-Fc fusion protein, which blocks VEGF-C binding to endogenous VEGFR-3 (Figure 9G)▶ .
Figure 9.
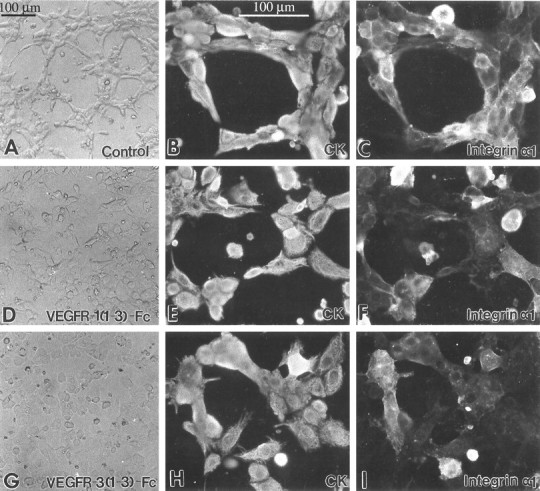
VEGFs regulate cytotrophoblast morphology and some aspects of adhesion molecule expression. Isolated cytotrophoblasts were plated on Matrigel-coated substrates and cultured for 12 hours in the presence of VEGFR-1(1-3)-Fc (15 μg/ml) or VEGFR-3(1-3)-Fc (16 μg/ml) or, as a control, purified preimmune IgG. VEGFR-1(1-3)-Fc blocks VEGF-A, PlGF, and VEGF-PlGF heterodimer binding to endogenous VEGFR-1, and VEGFR-3(1-3)-Fc blocks VEGF-C binding to endogenous VEGFR-3. After 12 hours in culture, control cells that were originally plated as a monolayer formed discrete aggregates that were connected by cellular processes (A). Aggregate formation was impaired by the addition of VEGFR-1(1-3)-Fc (D) and primarily absent in the presence of VEGFR-3(1-3)-Fc (G). The morphological changes suggested that the cell’s adhesion molecule expression might also be affected. Thus, we double-stained cells plated under the same control and experimental conditions with antibodies that recognized the cytotrophoblast marker cytokeratin (CK; B, E, and H) and integrin α1, an adhesion molecule that is crucial for cytotrophoblast adhesion and invasion (C, F, and I). Staining for integrin α1 was reduced in the experimental as compared to the control conditions.
The morphological differences we observed suggested possible effects on cytotrophoblast adhesion moleculeexpression, which under control conditions is extensively modulated as the cells undergo pseudovasculogenesis and invade matrix substrates both in vivo and in vitro.1 To understand the impact of inhibiting either VEGFR-1 or VEGFR-3 ligation on cytotrophoblast adhesion receptor switching, we monitored the cell’s expression of two molecules that are indicative of this process. As expected, by 12 hours of culture most of the control cells began to stain for the laminin and collagen I/IV receptor, integrin α1β1 (Figure 9C)▶ .
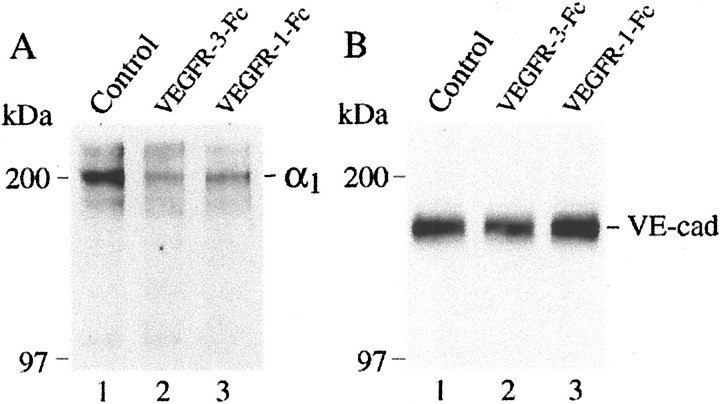
Cytotrophoblasts plated in the presence of the VEGFR-1(1-3)-Fc fusion protein showed wide variations in staining intensities; a few cells stained brightly, many showed much weaker immunoreactivity, and some did not react with the antibody (Figure 9F)▶ . Essentially the same results were obtained when cytotrophoblasts were plated in the presence of the VEGFR-3(1-3)-Fc fusion protein (Figure 9I)▶ . Immunoprecipitation confirmed that control cells expressed higher levels of integrin α1β1 than did cytotrophoblasts cultured in the presence of either fusion protein (Figure 10A)▶ . In contrast, inhibiting ligand binding to VEGFR-1 or VEGFR-3 did not change the staining pattern for VE-cadherin, another cell adhesion molecule whose expression is up-regulated during differentiation (data not shown). In accord with this result, immunoblot analyses also failed to detect any differences in VE-cadherin expression between the control and experimental cells (Figure 10B)▶ .
Figure 10.
Immunoprecipitation confirmed that blocking ligand binding to VEGFR-3 or VEGFR-1 down-regulated cytotrophoblast integrin α1 expression. Cells cultured under the control and experimental conditions for 12 hours were labeled with biotin. Cell lysates were prepared and subjected to immunoprecipitation with anti-integrin α1 as described in Materials and Methods. Control cells expressed higher levels of integrin α1β1 than did cytotrophoblasts cultured in the presence of either fusion protein (A). In contrast, immunoblotting showed that inhibiting ligand binding to VEGFR-1 or VEGFR-3 did not change the staining pattern for VE-cadherin (B).
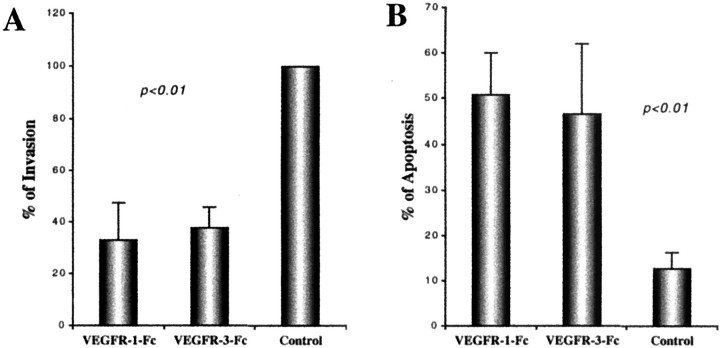
Our previous work suggested that down-regulation of integrin α1 expression is highly correlated with a reduction in cytotrophoblast invasiveness. We looked for the same correlation under the experimental conditions tested here. As compared to control cultures, invasion was reduced by ∼50% when cytotrophoblasts maintained in medium that contained either VEGFR-1(1-3)-Fc or VEGFR-3(1-3)-Fc reached the undersides of the filters (Figure 11A)▶ . During the course of these experiments, we also evaluated the morphology of the cytotrophoblasts that remained on the upper surfaces of the filters. The sharp increase in the number of cells with a shrunken appearance suggested that exposure to either VEGFR-1(1-3)-Fc or VEGFR-3(1-3)-Fc for 48 hours induced apoptosis, an idea that was confirmed by using the TUNEL method to visualize cells undergoing programmed cell death. Typically, 40 to 50% of the cells cultured under the experimental conditions were labeled. This translated into an approximately fivefold increase in cytotrophoblast apoptosis as compared to control cells (Figure 11B)▶ .
Figure 11.
Blocking ligand binding to VEGFR-1 or VEGFR-3 decreased cytotrophoblast invasion and increased cytotrophoblast apoptosis. Cytotrophoblasts from first and second trimester chorionic villi were plated on Matrigel-coated filters in Transwell inserts and cultured for 48 hours in the presence of VEGFR-1(1-3)-Fc, VEGFR-3(1-3)-Fc, or control IgG. Invasion was quantified by counting the number of cell processes and cells that reached the undersides of the filter. Addition of the fusion proteins, but not the control IgG, significantly inhibited invasion (A). Data are means and standard deviations of seven experiments. The mechanism includes a significant increase in apoptosis as detected by the TUNEL method (B). Data are means and standard deviations of six experiments.
Cytotrophoblast Expression of VEGF Family Members Is Down-Regulated in Severe Preeclampsia and HELLP Syndrome; sVEGFR-1 Secretion Increases
In the final set of experiments, we investigated whether the expression of VEGF family members is dysregulated in severe forms of preeclampsia, serious complications that appear during the second half of pregnancy. The associated pathological changes at the maternal-fetal interface include shallow cytotrophoblast invasion and deficits in pseudovasculogenesis.8 Here we compared, in control pregnancies and those complicated by SPE and HELLP syndrome, cytotrophoblast staining for the subset of VEGF ligands and receptors that are expressed until term: VEGF-A and VEGFR-1/Flt-1. In control pregnancy at 38 weeks of gestation, strong staining for VEGF-A was detected on cytotrophoblasts that invaded both the interstitial and vascular compartments of the uterine wall (Figure 12B)▶ . When the pregnancy was complicated by severe forms of preeclampsia, cytotrophoblasts either stained weakly with anti-VEGF-A (Figure 12D)▶ or, more often, failed to demonstrate immunoreactivity (Figure 12F)▶ . As compared to control tissue (Figure 13B)▶ , cytotrophoblast VEGFR-1 expression was also diminished in preeclampsia, although the range of staining intensities was greater than that observed for VEGF-A. In some areas no antibody reactivity was observed (Figure 13D)▶ , whereas in others weak staining was evident (Figure 13F)▶ . Finally, recent reports from other investigators suggest that cytotrophoblasts also secrete the soluble form of VEGFR-1 (sVEGFR-1), a potential angiogenesis inhibitor.32,33 Accordingly, we measured sVEGFR-1 levels in cytotrophoblast-conditioned medium. As compared to control third trimester cells, those isolated from the placentas of women with severe forms of preeclampsia released approximately twice the amount of the soluble receptor (Figure 14)▶ ; levels were also higher than those detected in medium of either first or second trimester control cytotrophoblasts.
Figure 13.
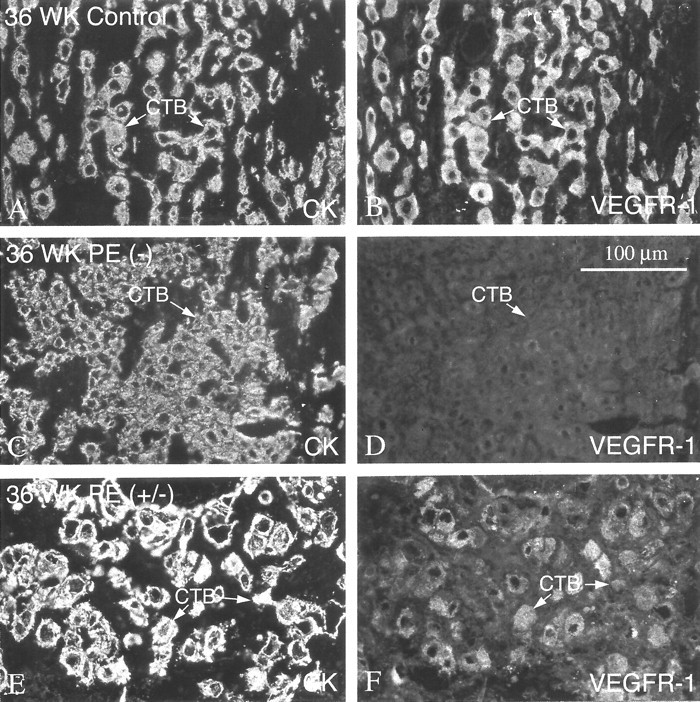
In preeclampsia, staining of invasive cytotrophoblasts with anti-VEGFR-1 decreased. In the third trimester of normal pregnancy (36 weeks), cytotrophoblasts (CTBs) in the interstitium (arrows) exhibited strong staining with anti-VEGFR-1 (B). In preeclampsia (PE), staining of invasive cytotrophoblasts for VEGFR-1 was either absent or weak. In some areas of biopsies, no staining was detected (−; D). In other areas of the same biopsy, weak antibody reactivity was evident (+/−; F).
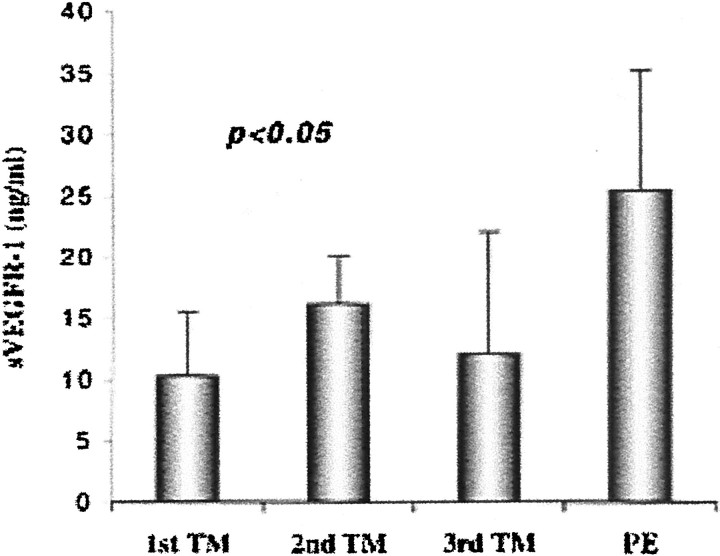
Figure 14.
In preeclampsia, cytotrophoblasts produce higher levels of soluble VEGFR-1 (sVEGFR-1) in vitro as compared to control cells. Cytotrophoblasts were isolated from control first, second, or third trimester (TM) placentas or from the placentas of women whose pregnancies were complicated by preeclampsia (PE). Then the cells were cultured on Matrigel substrates for 48 hours. Quantification of sVEGFR-1 in the conditioned medium by ELISA showed that preeclampsia is associated with a significant elevation of cytotrophoblast sVEGFR-1 secretion as compared to control cells. Data are means and standard deviations of five experiments.
Discussion
Little is known about factors that enable invading placental cytotrophoblasts to undergo pseudovasculogenesis. We are testing the hypothesis that the cell’s ability to mimic certain aspects first of vasculogenesis, and later of angiogenesis, is influenced by the same receptor-ligand pairs that govern conventional blood vessel development, growth, and repair. In this regard, VEGF ligands and their tyrosine kinase receptors are attractive candidates. Our immunolocalization studies on tissue sections of the maternal-fetal interface suggest that this unusual subpopulation of cytotrophoblasts expresses numerous VEGF family members and that their repertoire of these molecules changes as they invade the uterine wall. Gestational age and the pregnancy complication preeclampsia, factors that alter the capacity of placental cells to differentiate, also influence cytotrophoblast expression of a subset of VEGF ligands and receptors. Finally, the importance of these observations was demonstrated by function-perturbation studies that used an in vitro model of cytotrophoblast invasion and pseudovasculogenesis.
Interpreting these data in the context of existing information about the function of particular VEGF ligands and receptors gives some insight into the role they play in the unique differentiation pathway that forms the human maternal-fetal interface.14,34 With regard to receptors, deletion of the gene encoding VEGFR-2 has the severest effects; the mice die at embryonic day (E) 8.535 because differentiation of endothelial and hematopoietic cells is blocked.36,37 VEGFR-1 appears to act downstream of VEGFR-2. Null embryos, which also die at E 8.5, have an excess of endothelial cells that do not assemble into functional vessels.38 Unexpectedly, deletion of the receptor’s kinase domain does not interfere with angiogenesis—possible evidence of cooperative interactions with VEGFR-2.39 Deletion of VEGFR-3, which also stimulates growth of lymphatic vessels, causes death at E 9.5. In this case, vasculogenesis and angiogenesis appear to proceed normally, but large vessels are organized abnormally.40 Overexpression of soluble VEGFR-3 produces lymphedema.41
With regard to receptor ligands, threshold levels of VEGF interactions with its receptors (VEGFR-1 and VEGFR-2) are important, because deletion of only one copy of the VEGF gene causes death at E 11 to 12 because of abnormalities in both angiogenesis and blood-island formation.42,43 Recently, additional family members (VEGF-B, VEGF-C, VEGF-D, and PlGF) were discovered. Mice that lack VEGF-B, which binds to VEGFR-1, have impaired cardiac function.44 VEGF-C, a ligand for VEGFR-2 and VEGFR-3, has multiple actions. Mice engineered to overexpress this ligand in the skin have selective hyperplasia of the superficial lymphatic vasculature.45 But VEGF-C also induces angiogenesis.46 Human VEGF-D binds and activates VEGFR-2 and VEGFR-3, suggesting roles in angiogenesis and lymphangiogenesis.47 Finally, PlGF interactions with VEGFR-1 potentiates angiogenic responses to VEGF.48
Other investigators have studied the expression of VEGF ligands and receptors in the murine47 and human placentas.49 In the latter organ, Northern blot hybridization analyses of ligand expression shows that floating villi encode primarily VEGF-B and VEGF-C mRNA.50 In contrast, Clark and colleagues33 found, by in situ hybridization, mRNA for VEGF-A, but not its VEGF-B and VEGF-C forms, in the stroma of floating villi. The latter investigators also showed that mRNA for the VEGF family member PlGF was abundant in villous cytotrophoblasts, syncytium, and invasive cytotrophoblasts. Immunostaining localized both VEGF-A and PlGF protein to the endothelium of fetal blood vessels found within the cores of floating villi.50 VEGF-A and VEGF-C protein expression has also been reported in association with cytotrophoblasts within the uterine wall.51-53 With regard to VEGF receptors, invasive cytotrophoblasts appear to express VEGFR-1 mRNA and protein,51,52,54 whereas VEGFR-2 expression was detected in some studies53 but not others.52 Investigators in the latter study also suggested that the placenta produces and releases a soluble form of Flt-1 that could act as an antagonist.33 Finally, trophoblast expression of VEGFR-3 was recently described.53 The results of the work described here support some of these conclusions, eg, placental production of sVEGFR-1.32,33 Discrepancies are likely explained by the fact that we focused on a unique placental compartment—anchoring villi and the associated invasive cytotrophoblast population that invades the uterine wall, as well as blood vessels that traverse this region. Additionally, we studied this compartment throughout gestation, which revealed that cytotrophoblast expression of VEGF family members changes as pregnancy advances.
Our analyses of cytotrophoblast VEGF ligands and receptors suggested that their expression is precisely regulated as these unusual cells exit the mitotic cycle and undergo pseudovasculogenesis in vivo. In turn, these intricate expression patterns allowed us to generate hypotheses about the autocrine effects of VEGF on cytotrophoblast differentiation and invasion. These processes, which are key to placental development, mainly occur during the first half of pregnancy. Accordingly, we were particularly interested in ligand-receptor pairs that could function during this time period. For example, the coordinated expression of VEGF-C and VEGFR-2 by early gestation cytotrophoblast stem cells suggested that signals transmitted through the latter receptor could affect important functions, eg, proliferation. However, we were unable to test this hypothesis because early gestation cytotrophoblasts rapidly down-regulated VEGFR-2 expression as they differentiated in vitro. The latter observation is in accord with the results of our previous studies that show cytotrophoblast differentiation, both in situ and in culture, is associated with permanent withdrawal from the cell cycle.55,56 Our previous studies also suggest that invading cytotrophoblasts up-regulate the expression of molecules that play functional roles in uterine invasion and pseudovasculogenesis.1 Thus, the immunolocalization data that result from the present study led us to theorize that PlGF, VEGF-A, and VEGF-C interactions with VEGFR-1 are critical for invasion and pseudovasculogenesis, as are VEGF-C signals transduced through VEGFR-3. Our in vitro experiments showed this to be the case, as the addition of fusion proteins that specifically interfered with ligand binding to either receptor altered the cell’s ability to express integrin α1, an adhesion molecule that is an accurate barometer of the cell’s invasive capacity,22,23 a correlation also demonstrated in the experiments described here. Finally, signals transmitted through both receptors also play important functions in cytotrophoblast survival, a finding that is in accord with the central role played by VEGF signals in assembly and maintenance of the vasculature. Thus, the reduced invasion we observed is almost certainly a secondary phenomenon related to increased apoptosis.
Cytotrophoblast-derived VEGF family ligands are also likely to have paracrine effects on other populations of both fetal and maternal cells. In this regard it is interesting to note that PlGF and VEGF-C are more likely to play a role, because cytotrophoblast VEGF-A remained tightly associated with the extracellular matrix, a property of certain isoforms of the molecule (VEGF189, VEGF165, VEGF145) but not others (VEGF121).57 Although we did not study receptor expression by potential target cells, the intrinsic placental vasculature that forms within the stromal cores of chorionic villi could be influenced by factors produced in the trophoblast layers. This arrangement would coordinate trophoblast differentiation with that of the extraembryonic fetal vasculature, an important component of normal placental development. On the opposite side of the placenta, the maternal vasculature is another likely target. In this location cytotrophoblast-derived angiogenesis inhibitors, such as sVEGFR-1, could help cytotrophoblasts gain entrance to and occupy uterine vessels without triggering the cascade of events that normally leads to angiogenesis of the same population of vessels during the normal menstrual cycle.
The effects of severe forms of preeclampsia, conditions associated with failed pseudovasculogenesis and shallow invasion, on cytotrophoblast expression of VEGF family ligands and receptors also suggest the importance of particular molecules. In these cases the cells down-regulated expression of VEGF-A and VEGFR-1; sVEGFR-1 release increased. We speculate that the end result is an imbalance in the production of angiogenic factors at the maternal-fetal interface. Evidence that shows altered VEGF and PlGF levels in the blood of women with preeclampsia supports this hypothesis. For example, some investigators report that VEGF-A levels are elevated.58,59 In vitro studies suggest that this phenomenon could contribute to the maternal vascular defects associated with this syndrome.60 Whether the sources of enhanced levels of circulating VEGF-A include the maternal-fetal interface, where we found that cell-associated staining decreased, remains to be determined.
Since the origin of the preeclampsia syndrome is unknown, we have no way of knowing how near our observations lie to the root causes of these pregnancy complications. Studies from the laboratories of many investigators summarized above suggest that an absence of the normal repertoire of VEGF family members at the maternal-fetal interface could result in the deficits in cytotrophoblast differentiation we observe in preeclampsia. Data presented here offer further support for this link as we were able to replicate important aspects of the phenotype of cytotrophoblasts in preeclampsia by removing endogenous VEGF ligands from the culture medium as the cells differentiated in vitro: decreased integrin α1 expression8 (Figures 9 and 10)▶ ▶ and increased apoptosis62 (Figure 11)▶ .
Finally, VEGF family ligands and receptors are only a portion of the molecules with angiogenic and/or vasculogenic effects that are expressed by trophoblast cells. Thus we predict that other trophoblast-derived vasculogenic/angiogenic factors, such as angiopoietin ligands and their TIE receptors, as well as a broad array of fibroblast growth factor family members, will play important roles in cytotrophoblast pseudovasculogenesis and occupation of the maternal vessels. However, it is interesting to note that the placenta, which often co-opts pathways used in the development of other organs and tissues, can also use novel methods. Thus, we also suspect that these unusual processes could involve cytotrophoblast production of novel molecules with unique functions that are yet to be discovered.
Acknowledgments
We thank Ms. Evangeline Leash for editing the manuscript.
Footnotes
Address reprint requests to Susan Fisher, University of California San Francisco, 513 Parnassus Ave., HSW-604, San Francisco, CA 94143-0512. E-mail: sfisher@cgl.ucsf.
Supported by National Institutes of Health grants HL 64597 and HD 30367.
References
- 1.Damsky CH, Fisher SJ: Trophoblast pseudo-vasculogenesis: faking it with endothelial adhesion receptors. Curr Opin Cell Biol 1998, 10:660-666 [DOI] [PubMed] [Google Scholar]
- 2.Zhou Y, Fisher SJ, Janatpour M, Genbacev O, Dejana E, Wheelock M, Damsky CH: Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion? J Clin Invest 1997, 99:2139-2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Queenan Jr JT, Kao LC, Arboleda CE, Ulloa-Aguirre A, Golos TG, Cines DB, Strauss JFD: Regulation of urokinase-type plasminogen activator production by cultured human cytotrophoblasts. J Biol Chem 1987, 262:10903–10906 [PubMed]
- 4.Even-Ram S, Uziely B, Cohen P, Grisaru-Granovsky S, Maoz M, Ginzburg Y, Reich R, Vlodavsky I, Bar-Shavit R: Thrombin receptor overexpression in malignant and physiological invasion processes. Nat Med 1998, 4:909-914 [DOI] [PubMed] [Google Scholar]
- 5.Walker JJ: Pre-eclampsia. Lancet 2000, 356:1260-1265 [DOI] [PubMed] [Google Scholar]
- 6.Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK: Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol 1989, 161:1200-1204 [DOI] [PubMed] [Google Scholar]
- 7.Brosens IA, Robertson WB, Dixon HG: The role of the spiral arteries in the pathogenesis of preeclampsia. Obstet Gynecol Annu 1972, 1:177-191 [PubMed] [Google Scholar]
- 8.Zhou Y, Damsky CH, Fisher SJ: Preeclampsia is associated with failure of human cytotrophoblasts to mimic a vascular adhesion phenotype. One cause of defective endovascular invasion in this syndrome? J Clin Invest 1997, 99:2152-2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bussolino F, Mantovani A, Persico G: Molecular mechanisms of blood vessel formation. Trends Biochem Sci 1997, 22:251-256 [DOI] [PubMed] [Google Scholar]
- 10.Hanahan D: Signaling vascular morphogenesis and maintenance. Science 1997, 277:48-50 [DOI] [PubMed] [Google Scholar]
- 11.Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z: Vascular endothelial growth factor VEGF and its receptors. FASEB J 1999, 13:9-22 [PubMed] [Google Scholar]
- 12.Risau W: Mechanisms of angiogenesis. Nature 1997, 386:671-674 [DOI] [PubMed] [Google Scholar]
- 13.Yancopoulos GD, Klagsbrun M, Folkman J: Vasculogenesis, angiogenesis, and growth factors: ephrins enter the fray at the border. Cell 1998, 93:661-664 [DOI] [PubMed] [Google Scholar]
- 14.Veikkola T, Karkkainen M, Claesson-Welsh L, Alitalo K: Regulation of angiogenesis via vascular endothelial growth factor receptors. Cancer Res 2000, 60:203-212 [PubMed] [Google Scholar]
- 15.Carmeliet P, Jain RK: Angiogenesis in cancer and other diseases. Nature 2000, 407:249-257 [DOI] [PubMed] [Google Scholar]
- 16.Simon M, Rockl W, Hornig C, Grone EF, Theis H, Weich HA, Fuchs E, Yayon A, Grone HJ: Receptors of vascular endothelial growth factor/vascular permeability factor (VEGF/VPF) in fetal and adult human kidney: localization and [125I] VEGF binding sites. J Am Soc Nephrol 1998, 9:1032-1044 [DOI] [PubMed] [Google Scholar]
- 17.Hornig C, Behn T, Bartsch W, Yayon A, Weich HA: Detection and quantification of complexed and free soluble human vascular endothelial growth factor receptor-1 (sVEGFR-1) by ELISA. J Immunol Methods 1999, 226:169-177 [DOI] [PubMed] [Google Scholar]
- 18.Damsky CH, Fitzgerald ML, Fisher SJ: Distribution patterns of extracellular matrix components and adhesion receptors are intricately modulated during first trimester cytotrophoblast differentiation along the invasive pathway, in vivo. J Clin Invest 1992, 89:210-222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gifford Jr RW, August PA, Cunningham G, Green LA, Lindheimer MD, McNellis D, Roberts JM, Roccella EJ, Sibai BM, Taler SJ: , National Institutes of Health, publication no: 00-3029, 2000
- 20.Sibai BM: Treatment of hypertension in pregnancy women. N Engl J Med 1996, 335:257-265 [DOI] [PubMed] [Google Scholar]
- 21.Sibai BM: The HELLP syndrome (hemolysis, elevated liver enzymes, and low platelets): much ado about nothing? Am J Obstet Gynecol 1990, 162:311-316 [DOI] [PubMed] [Google Scholar]
- 22.Damsky CH, Librach C, Lim KH, Fitzgerald ML, McMaster MT, Janatpour M, Zhou Y, Logan SK, Fisher SJ: Integrin switching regulates normal trophoblast invasion. Development 1994, 120:3657-3666 [DOI] [PubMed] [Google Scholar]
- 23.Zhou Y, Damsky CH, Chiu K, Roberts JM, Fisher SJ: Preeclampsia is associated with abnormal expression of adhesion molecules by invasive cytotrophoblasts. J Clin Invest 1993, 91:950-960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L, Scott PA, Turley H, Leek R, Lewis CE, Gatter KC, Harris AL, Mackenzie IZ, Rees MC, Bicknell R: Validation of anti-vascular endothelial growth factor (anti-VEGF) antibodies for immunohistochemical localization of VEGF in tissue sections: expression of VEGF in the human endometrium. J Pathol 1998, 185:402-408 [DOI] [PubMed] [Google Scholar]
- 25.Librach CL, Werb Z, Fitzgerald ML, Chiu K, Corwin NM, Esteves RA, Grobelny D, Galardy R, Damsky CH, Fisher SJ: 92-kD type IV collagenase mediates invasion of human cytotrophoblasts. J Cell Biol 1991, 113:437-449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chomczynski P, Sacchi N: Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987, 162:156-159 [DOI] [PubMed] [Google Scholar]
- 27.McMaster MT, Librach CL, Zhou Y, Lim KH, Janatpour MJ, DeMars R, Kovats S, Damsky C, Fisher SJ: Human placental HLA-G expression is restricted to differentiated cytotrophoblasts. J Immunol 1995, 154:3771-3778 [PubMed] [Google Scholar]
- 28.Tabor S, Sruhl K, Scharf SJ, Gelfand DH: Enzymatic manipulation of DNA and RNA. Current Protocols in Molecular Biology. Edited by FM Ausubel. New York, John Wiley & Sons, 1993, pp 3.0.1-3.19.18
- 29.Pajusola K, Aprelikova O, Armstrong E, Morris S, Alitalo K: Two human FLT4 receptor tyrosine kinase isoforms with distinct carboxy terminal tails are produced by alternative processing of primary transcripts. Oncogene 1993, 8:2931-2937 [PubMed] [Google Scholar]
- 30.Hirashima M, Kataoka H, Nishikawa S, Matsuyoshi N: Maturation of embryonic stem cells into endothelial cells in an in vitro model of vasculogenesis. Blood 1999, 93:1253-1263 [PubMed] [Google Scholar]
- 31.Moss L, Prakobphol A, Wiedmann TW, Fisher SJ, Damsky CH: Glycosylation of human trophoblast integrins is stage and cell-type specific. Glycobiology 1994, 4:567-575 [DOI] [PubMed] [Google Scholar]
- 32.Kendall RL, Wang G, Thomas KA: Identification of a natural soluble form of the vascular endothelial growth factor receptor, FLT-1, and its heterodimerization with KDR. Biochem Biophys Res Commun 1996, 226:324-328 [DOI] [PubMed] [Google Scholar]
- 33.Clark DE, Smith SK, He Y, Day KA, Licence DR, Corps AN, Lammoglia R, Charnock-Jones DS: A vascular endothelial growth factor antagonist is produced by the human placenta and released into the maternal circulation. Biol Reprod 1998, 59:1540-1548 [DOI] [PubMed] [Google Scholar]
- 34.Olofsson B, Jeltsch M, Eriksson U, Alitalo K: Current biology of VEGF-B and VEGF-C. Curr Opin Biotechnol 1999, 10:528-535 [DOI] [PubMed] [Google Scholar]
- 35.Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC: Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature 1995, 376:62-66 [DOI] [PubMed] [Google Scholar]
- 36.Schuh AC, Faloon P, Hu QL, Bhimani M, Choi K: In vitro hematopoietic and endothelial potential of flk-1(−/−) embryonic stem cells and embryos. Proc Natl Acad Sci USA 1999, 96:2159-2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hidaka M, Stanford WL, Bernstein A: Conditional requirement for the Flk-1 receptor in the in vitro generation of early hematopoietic cells. Proc Natl Acad Sci USA 1999, 96:7370-7375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fong GH, Rossant J, Gertsenstein M, Breitman ML: Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature 1995, 376:66-70 [DOI] [PubMed] [Google Scholar]
- 39.Hiratsuka S, Minowa O, Kuno J, Noda T, Shibuya M: Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc Natl Acad Sci USA 1998, 95:9349-9354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dumont DJ, Jussila L, Taipale J, Lymboussaki A, Mustonen T, Pajusola K, Breitman M, Alitalo K: Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science 1998, 282:946-949 [DOI] [PubMed] [Google Scholar]
- 41.Makinen T, Jussila L, Veikkola T, Karpanen T, Kettunen MI, Pulkkanen KJ, Kauppinen R, Jackson DG, Kubo H, Nishikawa S, Yla-Herttuala S, Alitalo K: Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble VEGF receptor-3. Nat Med 2001, 7:199-205 [DOI] [PubMed] [Google Scholar]
- 42.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, Powell-Braxton L, Hillan KJ, Moore MW: Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 1996, 380:439-442 [DOI] [PubMed] [Google Scholar]
- 43.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A: Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 1996, 380:435-439 [DOI] [PubMed] [Google Scholar]
- 44.Bellomo D, Headrick JP, Silins GU, Paterson CA, Thomas PS, Gartside M, Mould A, Cahill MM, Tonks ID, Grimmond SM, Townson S, Wells C, Little M, Cummings MC, Hayward NK, Kay GF: Mice lacking the vascular endothelial growth factor-B gene (VEGFB) have smaller hearts, dysfunctional coronary vasculature, and impaired recovery from cardiac ischemia. Circ Res 2000, 86:E29-E35 [DOI] [PubMed] [Google Scholar]
- 45.Jeltsch M, Kaipainen A, Joukov V, Meng X, Lakso M, Rauvala H, Swartz M, Fukumura D, Jain RK, Alitalo K: Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science 1997, 276:1423-1425 [DOI] [PubMed] [Google Scholar]
- 46.Cao Y, Linden P, Farnebo J, Cao R, Eriksson A, Kumar V, Qi JH, Claesson-Welsh L, Alitalo K: Vascular endothelial growth factor C induces angiogenesis in vivo. Proc Natl Acad Sci USA 1998, 95:14389-14394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Achen MG, Gad JM, Stacker SA, Wilks AF: Placenta growth factor and vascular endothelial growth factor are co-expressed during early embryonic development. Growth Factors 1997, 15:69-80 [DOI] [PubMed] [Google Scholar]
- 48.Carmeliet P, Moons L, Luttun A, Vincenti V, Compernolle V, De Mol M, Wu Y, Bono F, Devy L, Beck H, Scholz D, Acker T, DiPalma T, Dewerchin M, Noel A, Stalmans I, Barra A, Blacher S, Vandendriessche T, Ponten A, Eriksson U, Plate KH, Foidart JM, Schaper W, Charnock-Jones DS, Hicklin DJ, Herbert JM, Collen D, Persico MG: Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med 2001, 7:575-583 [DOI] [PubMed] [Google Scholar]
- 49.He Y, Smith SK, Day KA, Clark DE, Licence DR, Charnock-Jones DS: Alternative splicing of vascular endothelial growth factor (VEGF)-R1 (FLT-1) pre-mRNA is important for the regulation of VEGF activity. Mol Endocrinol 1999, 13:537-545 [DOI] [PubMed] [Google Scholar]
- 50.Vuorela P, Hatva E, Lymboussaki A, Kaipainen A, Joukov V, Persico MG, Alitalo K, Halmesmäki E: Expression of vascular endothelial growth factor and placenta growth factor in human placenta. Biol Reprod 1997, 56:489-494 [DOI] [PubMed] [Google Scholar]
- 51.Ahmed A, Li XF, Dunk C, Whittle MJ, Rushton DI, Rollason T: Colocalisation of vascular endothelial growth factor and its Flt-1 receptor in human placenta. Growth Factors 1995, 12:235-243 [DOI] [PubMed] [Google Scholar]
- 52.Clark DE, Smith SK, Sharkey AM, Charnock-Jones DS: Localization of VEGF and expression of its receptors fit and KDR in human placenta throughout pregnancy. Hum Reprod 1996, 11:1090-1098 [DOI] [PubMed] [Google Scholar]
- 53.Dunk C, Ahmed A: Expression of VEGF-C and activation of its receptors VEGFR-2 and VEGFR-3 in trophoblast. Histol Histopathol 2001, 16:359-375 [DOI] [PubMed] [Google Scholar]
- 54.Charnock-Jones DS, Sharkey AM, Boocock CA, Ahmed A, Plevin R, Ferrara N, Smith SK: Vascular endothelial growth factor receptor localization and activation in human trophoblast and choriocarcinoma cells. Biol Reprod 1994, 51:524-530 [DOI] [PubMed] [Google Scholar]
- 55.Genbacev O, Zhou Y, Ludlow JW, Fisher SJ: Regulation of human placental development by oxygen tension. Science 1997, 277:1669-1672 [DOI] [PubMed] [Google Scholar]
- 56.Genbacev O, McMaster M, Fisher SJ: A repertoire of cell cycle regulators whose expression is coordinated with human cytotrophoblast differentiation. Am J Pathol 2000, 157:1337-1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ortega N, L’Faqihi FE, Plouet J: Control of vascular endothelial growth factor angiogenic activity by the extracellular matrix. Biol Cell 1998, 90:381-390 [DOI] [PubMed] [Google Scholar]
- 58.Baker PN, Krasnow J, Roberts JM, Yeo KT: Elevated serum levels of vascular endothelial growth factor in patients with preeclampsia. Obstet Gynecol 1995, 86:815-821 [DOI] [PubMed] [Google Scholar]
- 59.Sharkey AM, Cooper JC, Balmforth JR, McLaren J, Clark DE, Charnock-Jones DS, Morris NH, Smith SK: Maternal plasma levels of vascular endothelial growth factor in normotensive pregnancies and in pregnancies complicated by pre-eclampsia. Eur J Clin Invest 1996, 26:1182-1185 [DOI] [PubMed] [Google Scholar]
- 60.Brockelsby J, Hayman R, Ahmed A, Warren A, Johnson I, Baker P: VEGF via VEGF receptor-1 (Flt-1) mimics preeclamptic plasma in inhibiting uterine blood vessel relaxation in pregnancy: implications in the pathogenesis of preeclampsia. Lab Invest 1999, 79:1101-1111 [PubMed] [Google Scholar]
- 61.Hoang VM, Foulk R, Clauser K, Burlingame A, Gibson BW, Fisher SJ: Functional proteomics: examining the effects of hypoxia on the cytotrophoblast protein repertoire. Biochemistry 2001, 40:4077-4086 [DOI] [PubMed] [Google Scholar]
- 62.DiFederico E, Genbacev O, Fisher SJ: Preeclampsia is associated with widespread apoptosis of placental cytotrophoblasts within the uterine wall. Am J Pathol 1999, 155:293-301 [DOI] [PMC free article] [PubMed] [Google Scholar]