Abstract
High-dose melphalan (HDM) plus stem cell transplantation is an effective treatment for light-chain amyloidosis (AL), but is associated with high treatment-related mortality in patients with cardiac involvement. We studied 187 patients with cardiac involvement with AL who underwent HDM between 1996 and 2008. The median age was 57 years and the median time from diagnosis to HDM was 3.6 months. Half of the patients received reduced-dose melphalan (100-160 mg/m2). The median overall survival (OS) was 66 months, 54 months from diagnosis and HDM, respectively, and 91 patients (49%) were alive at the last follow-up 52 months (median) from HDM. Thirty patients (16%) died within 100 days of transplantation; only low serum albumin predicted early deaths. Overall, hematologic response (HR) and cardiac responses were seen in 66% and 41% of patients, respectively. The median OS for patients with and without HR was not reached and 22 months, respectively (P < .01); and for those with any decrease and no decrease in N-terminal-pro-brain natriuretic peptide was not reached and 26 months, respectively (P < .01). In multivariate analysis of baseline factors, only reduced-dose melphalan predicted shorter OS. HDM is feasible in patients with cardiac amyloidosis, and achievement of HR and organ response is associated with improved survival.
Introduction
Amyloidosis is a group of rare diseases characterized by multiorgan deposition of amyloid fibrils. In light-chain (AL) amyloidosis or primary systemic amyloidosis, the fibrils are derived from the immunoglobulin light chains secreted by clonal plasma cells.1 AL amyloidosis represents the most common type of systemic amyloidosis and cardiac involvement is seen in more than half of patients at diagnosis. It affects approximately 10 patients per million per year, including 10%-15% of patients with multiple myeloma (MM).2,3 The treatment approaches used for AL amyloidosis are directed toward the eradication of the abnormal plasma cell clone with the aim of eliminating the supply of the amyloidogenic light chains.4 Compared with myeloma, AL is typically associated with a lower burden of clonal plasma cells.5 High-dose melphalan (HDM) and peripheral blood stem cell transplantation (SCT) have been used since the mid-1990s and remain an effective treatment for AL amyloidosis.6–8 However, the transplantation-related mortality (TRM) in AL amyloidosis (approximately 10%-15%) is significantly higher than that seen in patients with MM and lymphoma (1%-2%) and is often attributed to the presence of underlying cardiac and/or multiorgan involvement.9 The outcome with HDM among patients with cardiac amyloidosis has not been examined systematically. Therefore, we undertook the present study to examine the outcome of patients with AL amyloidosis receiving HDM, with particular focus on transplantation-related complications, hematologic response (HR) and organ response rates, impact on cardiac function, and factors predicting post-HDM survival.
Methods
We selected the patients for the present study from 382 patients with AL amyloidosis who underwent HDM at our institution between May 1996 and September 2008. We used a cutoff of September 2008 to allow for adequate long-term follow-up given the potentially slow rate of organ improvement in this disease. The follow-up status was updated as of December 31, 2010. Among this group, 193 patients had documented cardiac involvement, which was defined by the presence of echocardiographic findings consistent with an infiltrative cardiomyopathy or cardiac biopsy that confirmed the presence of heart involvement.10 Six patients who underwent orthotopic heart transplantation were excluded from the analysis, and the remaining 187 (49%) patients were included. The histological diagnosis of AL amyloidosis was confirmed using Congo red staining of biopsy specimens in all patients. All patients had either a demonstrable monoclonal protein in the serum and/or urine or a clonal plasma cell population in the BM. Patients with secondary, familial, or localized amyloidosis and those with overt MM were excluded. The organ involvement and responses (hematologic and organ) were assessed using the consensus criteria reported in the 10th International Symposium on Amyloid and Amyloidosis.10 A hematologic partial response (PR) requires a 50% reduction in serum M protein, along with a 90% reduction or a reduction to < 200 mg/24 hours in urine M protein. Complete response (CR) requires the disappearance of the M protein by serum and urine immunofixation along with a BM showing < 5% plasma cells. A cardiac response required either a ≥ 2-mm reduction in the interventricular septal thickness by echocardiogram or improvement of ejection fraction (EF) by ≥ 20% (baseline EF must be ≤ 45%).
All patients provided written informed consent for the use of their medical records. Approval from the Mayo Clinic Institutional Review Board was obtained in accordance with federal regulations and the Declaration of Helsinki.
The baseline evaluation included BM examination with confirmation of plasma cell clonality and Congo red staining for amyloid, echocardiogram, electrocardiogram, 24-hour urine protein measurement, serum and urine protein electrophoresis with immunofixation, and an abdominal fat pad aspirate. Although the inclusion criteria for HDM in patients with AL amyloidosis have changed over the years, patients with an Eastern Cooperative Oncology Group performance status ≤ 2 and a New York Heart Association classification of < III were considered eligible to undergo the procedure.6 A total of 164 patients (88%) had G-CSF alone for stem cell mobilization, and the remaining required cyclophosphamide in addition to G-CSF. Melphalan 200/m2 (n = 84 patients) or melphalan/total body irradiation (n = 10) was used for conditioning in 50% of the patients and the other 50% (n = 93) were conditioned with reduced-dose melphalan (100-160 mg/m2). Patients did not routinely receive growth factor support after SCT.11
Overall survival (OS) from diagnosis was defined as the time between diagnosis and death or last follow-up; OS for HDM was defined as the time between the stem cell infusion and death or last follow-up estimated by the Kaplan-Meier method. Survival curves were compared using the log-rank test. Groups were compared using the Fisher exact test or the t test. Logistic regression was used to identify the best cutoffs for prognostic variables affecting early mortality within 100 days (day-100 mortality). Cox-proportional hazards analysis was done to examine the effect of various baseline characteristics on OS after HDM.
Results
The median age at the time of transplantation was 57 years (range, 31-71) and 122 of the patients were male (64%). The median time to HDM was 3.6 months (range, 1-75) from diagnosis. The median estimated follow-up for the entire cohort from the time of diagnosis and from HDM was 65 months (95% confidence interval [95% CI], 61-74) and 61 months (95% CI, 53-69), respectively, and 59 and 52 months, respectively, for the 91 (49%) patients alive at last follow-up. The baseline clinical and laboratory features are listed in Table 1. The cardiac troponin T/N-terminal-pro-brain natriuretic peptide (NT-ProBNP) stage12 was available for 99 patients (6, 53, and 40 patients in stages 1, 2, and 3, respectively). Isolated involvement of the heart was seen in 32 (17%) patients, whereas the majority of patients (n = 105, 56%) had heart and 1 other organ involved. The remaining 50 (27%) patients had involvement of heart along with 2 or more affected organs. Among the patients with > 1 organ involvement, the kidney was the most commonly affected in 115 (61%) patients. Approximately half of the patients had received at least one treatment before the HDM. Cardiac involvement was confirmed on endomyocardial biopsy in 38 patients. The median septal thickness was 14 mm (range, 9-25) and the median EF was 63% (range, 28%-84%). The baseline cardiac features are included in Table 1.
Table 1.
Patient characteristics
| Baseline characteristics | Total (N = 187) |
|---|---|
| Median age at transplant, y (range) | 57 (31-71) |
| Sex: male | 122 (64%) |
| Median time from diagnosis to transplant, mo (range) | 3.6 (1-75) |
| Number of organs involved | |
| Cardiac only, n (%) | 32 (17%) |
| Cardiac + 1 other organ, n (%) | 105 (56%) |
| Cardiac + ≥ 2 other organs, n (%) | 50 (27%) |
| Baseline cardiac measurements, median (range) | |
| Septal thickness, mm (n = 185) | 14 (9-25) |
| Posterior wall thickness, mm (n = 159) | 14 (9-26) |
| Ejection fraction, % (n = 187) | 63 (28-84) |
| Cardiac output, l/min (n = 164) | 5.8 (2.7-12.8) |
| LV mass index, g/m2 (n = 181) | 124 (60-270) |
| Troponin, ng/ml (n = 169) | 0.02 (< 0.01-0.9) |
| NT-Pro BNP, pg/ml (n = 100) | 2686 (22-35 000) |
| BNP, pg/ml (n = 116) | 330 (18-3050) |
| Other laboratory characteristics, median (range) | |
| Serum creatinine, mg/dl | 1.1 (0.7-12) |
| Serum albumin, gm/dl | 2.9 (0.8-4.4) |
| Bone marrow plasma cell, % | 7% (1-78) |
LV indicates left ventricle; NT-Pro, N-terminal-pro; and BNP, brain natriuetic peptide.
Early (day-100) mortality
Overall, 30 (16%) patients died within 100 days of transplantation. Of these, 29 deaths were considered to be directly related to treatment. The causes of death were considered to be due to cardiac-related causes in 15 (52%) patients, multiorgan failure in 7 (24%), bleeding in 4 (14%), and infection in 3 (10%), whereas 1 patient died because of rapidly evolving plasma cell leukemia before day 100. The cardiac stage was known for 15 of these patients; 7 each were stage 2 or 3 and 1 patient was stage 1. Among the various pre-HDM factors examined (age, time from diagnosis to transplantation, serum albumin, serum creatinine, plasma cell percentage, reduced-dose melphalan conditioning, number of organs involved, septal thickness, left ventricular mass index, EF, serum troponin, serum NT-ProBNP, and serum free light chain levels), only serum albumin was found to be significantly related to the risk of death by day 100. Among the 65 patients with a serum albumin < 2.5 gm/dL at the time of transplantation, 17 patients (26%) died by day 100, compared with 11% of the remaining patients (P < .01). In comparison, among the 189 patients with no cardiac involvement receiving transplantations during the same time period, there were only 9 (5%) deaths within 100 days of transplantation (P < .001). We also compared the day-100 mortality rate among the patients with cardiac involvement receiving transplantations between 1996 and 2002 and those receiving transplantations between 2003 and 2008; day-100 mortality was 17% for the initial half of the patients and 15% for the latter half (P = nonsignificant).
Response and survival
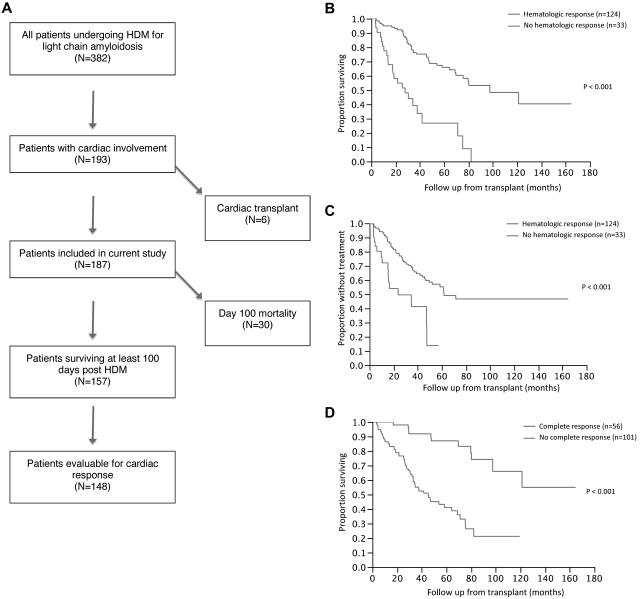
We first examined the hematologic response (HR) after transplantation using an intention-to-treat approach. Overall, an HR (≥ PR) was seen in 124 (66%) patients, including a CR in 56 (30%) patients. We then examined the response rate using a landmark analysis at 100 days, because patients dying early after transplantation are typically not evaluable for response. The median estimated time (Kaplan-Meier estimate, patients censored at last follow-up or initiation of new therapy) to an HR was 3.2 months from HDM (95% CI, 2.9-3.4). Considering only the 157 (84%) patients surviving beyond 100 days, the HR rate (≥ PR) was 79%, including a 36% CR rate. A conventional cardiac response (decrease in septal thickness ≥ 2 mm and/or EF increase ≥ 20%) was observed in 60 (41%) of the 148 patients evaluable for cardiac response. The cardiac response was based on a decrease in septal thickness in 57 of these patients, whereas an increase in EF was the basis of response in only 3 of the patients. The median estimated time (Kaplan-Meier estimate, patients censored at last follow-up or initiation of new therapy) to cardiac response was 51 months from HDM (95% CI, 37-75). At the last follow-up, 65 patients (41%) had received another therapy after HDM because of disease progression or lack of response to HDM with median time to next therapy (TTNT) of 58 months (95% CI, 44, not reached [NR]).
The median estimated OS for the entire cohort (N = 187) was 66 months (95% CI, 42-83) from diagnosis and 54 months (95% CI, 35-76) from HDM. In contrast, the median estimated OS for the 157 patients surviving beyond 100 days from transplantation was 76 months (95% CI, 58-121) from HDM. Among the 66 patients dying after day 100 after transplantation, more than half of the deaths were from cardiac causes and considered to be related to progression of cardiac amyloidosis.
We then examined the impact of hematologic and organ response on survival and TTNT using a landmark analysis based on survival at 100 days after HDM (N = 157; Figure 1A). The median OS for patients with (n = 124) and without (n = 33) an HR was 98 months (95% CI, 70 [NR]) and 28 months (95% CI, 14-42), respectively (P < .001; Figure 1B). The median TTNT was 62 months for the responders compared with 24 months (95% CI, 15 [NR]) for those with no hematologic PR (P < .001; Figure 1C). The median OS for patients with (n = 56) and without (n = 101) a CR was NR (95% CI, 98 [NR]) and 45 months (95% CI, 33-69), respectively (P < .001; Figure 1D). Similarly, the median TTNT was 35 months for those not achieving a CR and NR for patients with a CR (P < .001).
Figure 1.
Patient disposition and impact of hematological response on overall survival following stem cell transplantation. (A) Number of patients surviving at least 100 days after transplantation and those evaluable for hematologic and cardiac responses. (B) Kaplan-Meier curves comparing OS between patients with HR and those with no HR. The median OS for patients with (n = 124) and without (n = 33) an HR was 98 months (95% CI, 70 [NR]) and 28 months (95% CI, 14-42), respectively (P < .001). (C) Kaplan-Meier curves comparing TTNT between patients with HR and those with no HR. The median TTNT was 62 months for the responders compared with 24 months (95% CI, 15 [NR]) for those with no hematologic PR (P < .001). (D) Kaplan-Meier curves comparing OS between patients with CR and those with no CR. The median OS for patients with (n = 56) and without (n = 101) a CR was NR (95% CI, 98 [NR]) and 45 months (95% CI, 33-69), respectively (P < .001).
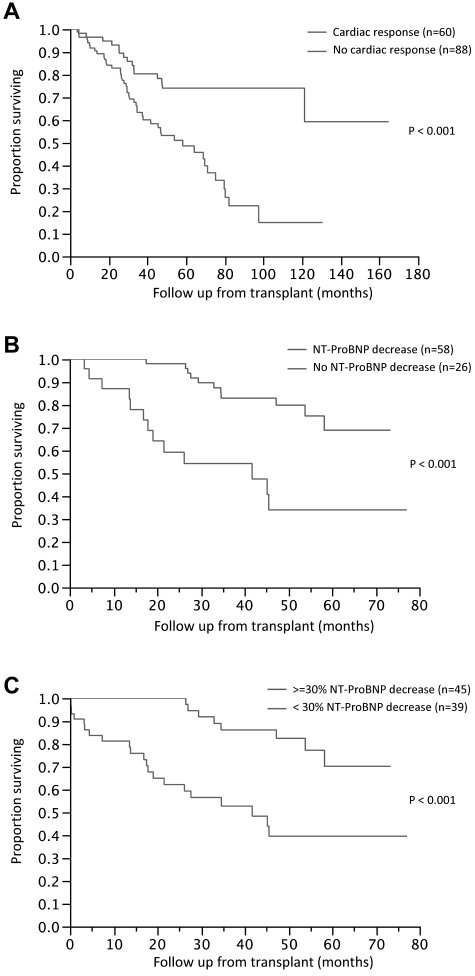
Using a similar landmark analysis at 100 days, we assessed the impact of cardiac response on survival. Among the 148 patients evaluable for a cardiac response, the median OS for patients with (n = 60) and without (n = 88) a cardiac response was NR and 58 months (95% CI, 38-75), respectively (P < .001; Figure 2A). The likelihood of a cardiac response was significantly higher for those with an HR (46%) compared with those with no HR (17%; P = .003). Given the slow onset of cardiac response, we performed additional landmark analyses at 1 and 2 years to examine the impact of cardiac response on survival. The median OS for the cardiac responders (n = 58) was NR compared with 52 months for the nonresponders (n = 74) when landmarked at 1 year (P < .001). Similarly, the median OS for the cardiac responders (n = 55) was NR compared with 58 months for the nonresponders (n = 60) when landmarked at 2 years (P < .001). Changes in NT-ProBNP have been proposed as a marker of cardiac response, so we examined the impact of NT-ProBNP changes on survival. Among the 84 patients with available NT-ProBNP values at baseline and after HDM, the median OS for patients with (n = 58) and without (n = 26) any decrease in NT-ProBNP was NR and 42 months, respectively (P < .001; Figure 2B). Among the 58 patients with any decrease in NT-ProBNP, the median time to the lowest value after HDM was 12 months (range, 3-56). Given the recent proposal to use a 30% decrease in NT-ProBNP as a criteria for response, we examined survival rates of 45 patients (51%) who had at least a 30% decrease in NT-ProBNP. The median OS from HDM was NR for the group with a ≥ 30% decrease compared with 42 months for the rest (Figure 2C)
Figure 2.
Impact of organ response on overall survival following stem cell transplantation. (A) Kaplan-Meier curves comparing OS between patients with cardiac response and those with no cardiac response. Among the 148 patients evaluable for a cardiac response, the median OS for patients with (n = 60) and without (n = 88) a cardiac response was NR and 58 months (95% CI, 38-75), respectively (P < .001). (B) Kaplan-Meier curves comparing OS between patients with a decrease in NT-ProBNP and those with no decrease. Among the 84 patients with available NT-ProBNP values at baseline and after HDM, the median OS for patients with (n = 58) and without (n = 26) any decrease in NT-ProBNP was NR and 42 months, respectively (P < .001). (C) Kaplan-Meier curves comparing the OS between patients with a ≥ 30% decrease in NT-ProBNP and those with a < 30% decrease. The median OS from HDM was NR for the group with a ≥ 30% decrease compared with 42 months for the remaining patients.
Baseline characteristics and survival
We then examined the prognostic value of baseline characteristics on OS in this entire cohort. Among the various baseline factors examined (eg, age, time from diagnosis to transplantation, serum albumin, serum creatinine, plasma cell percentage, reduced-dose melphalan conditioning, number of organs involved, septal thickness, left ventricular mass index, EF, serum troponin, serum NT-ProBNP, and serum free light chain levels), univariate analysis revealed elevated troponin, reduced EF, and reduced-dose melphalan to be predictive of shorter OS after HDM. In a multivariate analysis, only reduced-dose melphalan and reduced EF was predictive of poor outcome. When the reduced-dose melphalan was left out of the model, only a reduced EF was predictive of OS. Whereas the cardiac stage itself was not predictive of OS, there was a strong correlation between cardiac stage and reduced dose of melphalan for conditioning, with 75% of stage 3 patients getting reduced-dose melphalan compared with 41% for stage 1 and 2 combined (P < .01). The median OS from HDM was 58 months for stage 3 patients (n = 40) compared with NR for stage 1 and 2 patients (n = 59; P = .3). We also examined the changes in cardiac parameters over time among patients with differing degrees of HRs from among those surviving at least 100 days (Table 2).
Table 2.
Relationship between hematologic response and changes in cardiac parameters
| Patients with a hematologic complete response (N = 56) | Patients with a hematologic response (≥ = PR) (N = 124) | Patients with no hematologic response (N = 33) P | ||||
|---|---|---|---|---|---|---|
| Parameter, N (%) | ||||||
| Conventional cardiac response | 55 | 33 (60) | 122 | 57 (47) | 32 | 5 (16) |
| NT-Pro BNP decrease ≥ 30% | 25 | 21 (84) | 65 | 51 (78) | 19 | 7 (37) |
| Parameter, N, Change from baseline: median (IQR) | ||||||
| Septal thickness, mm | 55 | −2 (−3 to +1) | 122 | −1 (−3 to +0) | 32 | 0 (−1 to +1) |
| Ejection fraction, % | 56 | +4 (−10 to +1) | 124 | +2 (+7 to −2) | 33 | 0 (−4 to +4) |
| LV mass index | 54 | −8 (−36 to +2) | 120 | −11 (−32 to +2) | 32 | 0 (−13 to +15) |
| Troponin T, μg/mL | 42 | −0.01 (−0.04 to +0.01) | 92 | −0.01 (−0.01 to +0.03) | 20 | 0 (−0.01 to +0.01) |
| NT-Pro BNP, pg/mL | 25 | −963 (−1470 to +91) | 65 | −745 (−1979 to +80) | 19 | 0 (−262 to + 1269) |
NT-Pro BNP indicates N-terminal-pro brain natriuretic peptide; and LV, left ventricle.
Discussion
We have reviewed our experience with HDM in a large group of patients with cardiac involvement from AL amyloidosis. Cardiac involvement, seen in nearly half of patients with AL amyloidosis, manifests as syncope or sudden cardiac death because of conduction disturbances and arrhythmias, heart failure, or asymptomatic abnormalities noted only on echocardiogram or electrocardiogram. Nearly half of the patients undergoing HDM at our institution had heart involvement documented by biopsy or echocardiographic abnormalities. However, an isolated involvement of the heart because of AL amyloidosis is uncommon, and the majority (83%) of patients in our study had involvement of at least one other organ.13 The results of the current study highlight several important aspects of this disease.
It is feasible to perform HDM in a selected group of patients with cardiac disease. Whereas in the earlier period of the study, exclusion was mostly based on the presence of cardiac failure and multiorgan involvement and poor performance status, more recently we have relied on cardiac biomarkers for excluding patients at high risk of early mortality. The troponin T value did not predict for day 100 mortality, as reported in a previous study from our institution that included patients with AL amyloidosis involving not only the heart, but other organs/systems as well.14 However, a low albumin (< 2.4 g/dL) was found to predict for an increased risk of death in the first 100 days after HDM. This highlights the fact that the severity of other organ(s) involvement becomes more relevant when only considering patients with cardiac amyloidosis, and hypoalbuminemia likely serves as a surrogate of amyloidosis affecting the kidney, liver, or GI tract. Although all patients in our study group had cardiac involvement from AL amyloidosis, the TRM rate was comparable to that observed in other studies involving AL amyloidosis patients with involvement of various other organs or systems.9,15–17 This likely reflects the careful selection of patients with cardiac disease for consideration of HDM. The 16% TRM for cardiac AL patients is higher than that seen in noncardiac AL patients, and efforts to further refine patient selection are warranted
When examined across the entire amyloid patient population, the presence and severity of cardiac disease is usually a powerful predictor of OS.13 Other adverse predictors of survival include the involvement of 2 or more organs, BM plasmacytosis greater than 30%, circulating plasma cells in the peripheral blood, elevated BM plasma cell labeling index, increased β2-microglobulin, and time to referral center.9,18 The median OS in patients with AL amyloidosis is approximately 1.1 year from diagnosis of heart involvement and 0.75 years from the onset of heart failure.13 The median OS of patients with cardiac amyloidosis is shorter than that of patients without heart involvement.19 An earlier study from our institution that included patients with amyloidosis affecting all major visceral organs (ie, the heart, kidney, liver, and GI tract) reported the predictive value of troponin, BNP, and NT-proBNP on the OS in these patients.20 However, the cardiac biomarkers failed to discriminate within this group of patients with cardiac AL amyloidosis and were unable to predict for survival in our study population. In the present study, we only included the patients with cardiac involvement, so it is not surprising that variables related to cardiac involvement (such as cardiac biomarkers) do not predict for outcome. Similarly, performance status and cardiac biomarkers have been shown to predict early mortality among all patients with amyloidosis and are not reflected in the current study because it was restricted to patients with cardiac involvement and appropriate performance status to allow them to undergo SCT.21 The single most important predictor of OS in the present study was the intensity of melphalan conditioning. Melphalan dose reduction to minimize dose-related toxicity was instituted in half of the patients to make more of them eligible for HDM. The conditioning intensity has been shown to be an independent predictor of response and survival in prior studies.22 It is likely that the decision to reduce dose reflects multiple factors directly related to the number of organs involved and the severity of the organ involvement, as well as other factors such as overall performance status.
Consistent with previous observations, the most important predictor of organ response was the ability to obtain an HR.15,23,24 In this group of patients with cardiac involvement, those achieving an HR also had a better OS compared with nonresponders. What is striking in the current analysis is the slow rate of cardiac response as assessed by conventional criteria that depend on echocardiographic findings. Whereas patients with a cardiac response had better survival, the slow onset of response makes it difficult to make treatment decisions regarding additional therapy. In this retrospective analysis, no prespecified criteria for additional therapy were initiated for hematologic progression, lack of organ improvement, or worsening of organ function based on treating physician preference, so no definite recommendations can be made. The subjectivity of the measurements and the inability to accurately assess improvement in those with a relatively normal septal thickness or EF underscores the importance of alternate parameters for the assessment of cardiac improvement. The serum level of NT-ProBNP has been suggested as a better marker of cardiac response than echocardiographic changes, which is supported by the finding of a more rapid onset of change seen in the present study. Given that the majority of patients with cardiac involvement have elevated levels of NT-ProBNP, and that data from this and other studies suggest improved survival with improvements in NT-ProBNP, this should be incorporated into future revisions of the response criteria. The use of NT-ProBNP also allows for earlier assessment of response given the shorter time required compared with echocardiographic parameters.
In conclusion, HDM is a feasible approach in selected patients with cardiac AL amyloidosis and is associated with a high rate of hematologic and organ responses that lead to prolonged survival.25 In the present study, improvement in survival was associated with hematologic and organ responses and with NT Pro-BNP reduction after transplantation. Conventional measures of cardiac response are often slow in onset and assessments using cardiac biomarkers may allow earlier determination of therapeutic benefit.
Acknowledgments
The authors thank the patients, their caregivers, and the Mayo Clinic Transplant Center staff.
This work was supported by the Mayo Clinic Hematologic Malignancies Program, a Paul Calabresi K12 award from the National Cancer Institute (CA90628 to S.K.), a Kurtz fellowship (to S.M.), and by the Henry J. Predolin Foundation.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: S.M., S.K.K., and M.A.G. designed the study; and S.M., S.K.K., A.D., M.Q.L., S.R.H., F.K.B., D.D., S.V.R., W.J.H., N.L., M.G., and M.A.G. wrote and revised the manuscript.
Conflict-of-interest disclosure: M.Q.L. received research funding from Celgene. A.D. received research funding and a travel award from Celgene; M.A.G. received honoraria from Celgene, Millenium, Neotope Eisai, Lilly Research Laboratories, Optum Health Education, Research to Practice, Physician's Education Resource, and Amgen. S.K.K. received research funding from Celegene, Genzyme, Millenium, Novartis, Bayer, Merck, and Cephalon and is on the advisory board of Genzyme. The remaining authors declare no competing financial interests.
Correspondence: Shaji Kumar, MD, Division of Hematology, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: kumar.shaji@mayo.edu.
References
- 1.Gertz MA. Immunoglobulin light chain amyloidosis: 2011 update on diagnosis, risk-stratification, and management. Am J Hematol. 2011;86(2):180–186. doi: 10.1002/ajh.21934. [DOI] [PubMed] [Google Scholar]
- 2.Merlini G, Palladini G. Amyloidosis: is a cure possible? Ann Oncol. 2008;19(Suppl 4):iv63–66. doi: 10.1093/annonc/mdn200. [DOI] [PubMed] [Google Scholar]
- 3.Skinner M. AL amyloidosis: the last 30 years. Amyloid. 2000;7(1):13–14. doi: 10.3109/13506120009146816. [DOI] [PubMed] [Google Scholar]
- 4.Kumar SK, Dispenzieri A, Lacy MQ, et al. Changes in serum-free light chain rather than intact monoclonal immunoglobulin levels predicts outcome following therapy in primary amyloidosis. Am J Hematol. 2011;86(3):251–255. doi: 10.1002/ajh.21948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar S, Dispenzieri A, Katzmann JA, et al. Serum immunoglobulin free light-chain measurement in primary amyloidosis: prognostic value and correlations with clinical features. Blood. 2010;116(24):5126–5129. doi: 10.1182/blood-2010-06-290668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gertz MA, Lacy MQ, Dispenzieri A, et al. Autologous stem cell transplant for immunoglobulin light chain amyloidosis: a status report. Leuk Lymphoma. 2010;51(12):2181–2187. doi: 10.3109/10428194.2010.524329. [DOI] [PubMed] [Google Scholar]
- 7.Sanchorawala V, Skinner M, Quillen K, Finn KT, Doros G, Seldin DC. Long-term outcome of patients with AL amyloidosis treated with high-dose melphalan and stem-cell transplantation. Blood. 2007;110(10):3561–3563. doi: 10.1182/blood-2007-07-099481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dispenzieri A, Lacy MQ, Kyle RA, et al. Eligibility for hematopoietic stem-cell transplantation for primary systemic amyloidosis is a favorable prognostic factor for survival. J Clin Oncol. 2001;19(14):3350–3356. doi: 10.1200/JCO.2001.19.14.3350. [DOI] [PubMed] [Google Scholar]
- 9.Gertz MA, Lacy MQ, Dispenzieri A, et al. Stem cell transplantation for the management of primary systemic amyloidosis. Am J Med. 2002;113(7):549–555. doi: 10.1016/s0002-9343(02)01208-1. [DOI] [PubMed] [Google Scholar]
- 10.Gertz MA, Comenzo R, Falk RH, et al. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18-22 April 2004. Am J Hematol. 2005;79(4):319–328. doi: 10.1002/ajh.20381. [DOI] [PubMed] [Google Scholar]
- 11.Gertz MA, Lacy MQ, Dispenzieri A, et al. Transplantation without growth factor: engraftment kinetics after stem cell transplantation for primary systemic amyloidosis (AL). Bone Marrow Transplant. 2007;40(10):989–993. doi: 10.1038/sj.bmt.1705848. [DOI] [PubMed] [Google Scholar]
- 12.Dispenzieri A, Gertz MA, Kyle RA, et al. Serum cardiac troponins and N-terminal pro-brain natriuretic peptide: a staging system for primary systemic amyloidosis. J Clin Oncol. 2004;22(18):3751–3757. doi: 10.1200/JCO.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 13.Dubrey SW, Cha K, Anderson J, et al. The clinical features of immunoglobulin light-chain (AL) amyloidosis with heart involvement. QJM. 1998;91(2):141–157. doi: 10.1093/qjmed/91.2.141. [DOI] [PubMed] [Google Scholar]
- 14.Gertz M, Lacy M, Dispenzieri A, et al. Troponin T level as an exclusion criterion for stem cell transplantation in light-chain amyloidosis. Leuk Lymphoma. 2008;49(1):36–41. doi: 10.1080/10428190701684518. [DOI] [PubMed] [Google Scholar]
- 15.Skinner M, Sanchorawala V, Seldin DC, et al. High-dose melphalan and autologous stem-cell transplantation in patients with AL amyloidosis: an 8-year study. Ann Intern Med. 2004;140(2):85–93. doi: 10.7326/0003-4819-140-2-200401200-00008. [DOI] [PubMed] [Google Scholar]
- 16.Vesole DH, Perez WS, Akasheh M, Boudreau C, Reece DE, Bredeson CN. High-dose therapy and autologous hematopoietic stem cell transplantation for patients with primary systemic amyloidosis: a Center for International Blood and Marrow Transplant Research Study. Mayo Clin Proc. 2006;81(7):880–888. doi: 10.4065/81.7.880. [DOI] [PubMed] [Google Scholar]
- 17.Sanchorawala V, Wright DG, Seldin DC, et al. An overview of the use of high-dose melphalan with autologous stem cell transplantation for the treatment of AL amyloidosis. Bone Marrow Transplant. 2001;28(7):637–642. doi: 10.1038/sj.bmt.1703200. [DOI] [PubMed] [Google Scholar]
- 18.Moreau P, Leblond V, Bourquelot P, et al. Prognostic factors for survival and response after high-dose therapy and autologous stem cell transplantation in systemic AL amyloidosis: a report on 21 patients. Br J Haematol. 1998;101(4):766–769. doi: 10.1046/j.1365-2141.1998.00772.x. [DOI] [PubMed] [Google Scholar]
- 19.Obici L, Perfetti V, Palladini G, Moratti R, Merlini G. Clinical aspects of systemic amyloid diseases. Biochim Biophys Acta. 2005;1753(1):11–22. doi: 10.1016/j.bbapap.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 20.Dispenzieri A, Gertz MA, Kyle RA, et al. Prognostication of survival using cardiac troponins and N-terminal pro-brain natriuretic peptide in patients with primary systemic amyloidosis undergoing peripheral blood stem cell transplantation. Blood. 2004;104(6):1881–1887. doi: 10.1182/blood-2004-01-0390. [DOI] [PubMed] [Google Scholar]
- 21.Kumar SK, Gertz MA, Lacy MQ, et al. Recent improvements in survival in primary systemic amyloidosis and the importance of an early mortality risk score. Mayo Clin Proc. 2011;86(1):12–18. doi: 10.4065/mcp.2010.0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gertz MA, Lacy MQ, Dispenzieri A, et al. Risk-adjusted manipulation of melphalan dose before stem cell transplantation in patients with amyloidosis is associated with a lower response rate. Bone Marrow Transplant. 2004;34(12):1025–1031. doi: 10.1038/sj.bmt.1704691. [DOI] [PubMed] [Google Scholar]
- 23.Gertz MA, Lacy MQ, Dispenzieri A, et al. Effect of hematologic response on outcome of patients undergoing transplantation for primary amyloidosis: importance of achieving a complete response. Haematologica. 2007;92(10):1415–1418. doi: 10.3324/haematol.11413. [DOI] [PubMed] [Google Scholar]
- 24.Perfetti V, Siena S, Palladini G, et al. Long-term results of a risk-adapted approach to melphalan conditioning in autologous peripheral blood stem cell transplantation for primary (AL) amyloidosis. Haematologica. 2006;91(12):1635–1643. [PubMed] [Google Scholar]
- 25.Seldin DC, Anderson JJ, Sanchorawala V, et al. Improvement in quality of life of patients with AL amyloidosis treated with high-dose melphalan and autologous stem cell transplantation. Blood. 2004;104(6):1888–1893. doi: 10.1182/blood-2004-01-0089. [DOI] [PubMed] [Google Scholar]