Abstract
To reduce toxicity associated with external γ-beam radiation, we investigated radioimmunotherapy with an anti-CD45 mAb labeled with the α-emitter, astatine-211 (211At), as a conditioning regimen in dog leukocyte antigen-identical hematopoietic cell transplantation (HCT). Dose-finding studies in 6 dogs treated with 100 to 618 μCi/kg 211At-labeled anti-CD45 mAb (0.5 mg/kg) without HCT rescue demonstrated dose-dependent myelosuppression with subsequent autologous recovery, and transient liver toxicity in dogs treated with 211At doses less than or equal to 405 μCi/kg. Higher doses of 211At induced clinical liver failure. Subsequently, 8 dogs were conditioned with 155 to 625 μCi/kg 211At-labeled anti-CD45 mAb (0.5 mg/kg) before HCT with dog leukocyte antigen-identical bone marrow followed by a short course of cyclosporine and mycophenolate mofetil immunosuppression. Neutropenia (1-146 cells/μL), lymphopenia (0-270 cells/μL), and thrombocytopenia (1500-6560 platelets/μL) with prompt recovery was observed. Seven dogs had long-term donor mononuclear cell chimerism (19%-58%), whereas 1 dog treated with the lowest 211At dose (155 μCi/kg) had low donor mononuclear cell chimerism (5%). At the end of follow-up (18-53 weeks), only transient liver toxicity and no renal toxicity had been observed. In conclusion, conditioning with 211At-labeled anti-CD45 mAb is safe and efficacious and provides a platform for future clinical trials of nonmyeloablative transplantation with radioimmunotherapy-based conditioning.
Introduction
With the introduction of nonmyeloablative preparative regimens, allogeneic hematopoietic cell transplantation (HCT) has become a curative treatment option for a variety of malignant hematologic diseases in older and medically infirm patients. However, treatment-related toxicity and relapse are still major causes of morbidity and mortality. In an effort to increase the radiation dose delivered to the target organs while further reducing the late toxic effects of external beam γ-irradiation, strategies using radioimmunotherapy (RIT) targeted toward hematopoietic tissues as a part of the conditioning regimen have been investigated. The efficacy and safety of this approach have been demonstrated in several clinical trials where antibody-coupled β-emitters, such as yttrium-90 (90Y), rhenium-188 (188Re), and iodine-131 (131I), have been used to augment a variety of high-dose and reduced-intensity conditioning regimens.1–4 The long path length of β-emitters, which makes them ideal in the setting of poorly perfused or bulky tumors, also makes them less optimal in situations with small volume tumors, minimal residual disease, or as part of an HCT conditioning regimen.5 It has been estimated that only 1.5% and 17% of the energy from 90Y- and 131I-labeled mAbs, respectively, is deposited in tumors that are 200 μm in diameter, whereas the rest is deposited in surrounding tissue resulting in nonspecific toxicity.6 Alternative sources of radiation are available from the α-particle-emitting radionuclides. α-particles are characterized by short path lengths of 40 to 90 μm in vivo, limiting the off-target toxicity to a few cell diameters. Furthermore, α-particles are more cytotoxic and have superior relative biologic effectiveness than β-particles because of a 400-fold greater linear energy transfer and the limited ability of tumor cells to repair α-particle–induced DNA damage.7–9
Apart from our own preclinical experiences with the αemitter bismuth-213 (213Bi), the use of an α-emitter for RIT alone as conditioning in HCT has not been explored. We have previously demonstrated that 213Bi-labeled mAb targeted toward the pan-hematopoietic antigen, CD45, or the T-cell receptor αβ (TCRαβ) could replace 200 to 300 cGy total body irradiation (TBI) as nonmyeloablative conditioning in dog leukocyte antigen (DLA)–identical or haploidentical bone marrow transplantation.10–13 Although the treatment was successful in allowing sustained engraftment with minimal toxicity, obstacles, including short half-life (45.6 minutes), limited availability, and high cost of 213Bi, made the translation of 213Bi-labeled mAb into clinical studies impractical. Astatine-211 (211At; t1/2 = 7.21 hours) is an alternative α-particle–emitting isotope. The advantage of 211At is that it is available in quantities that can be scaled up for clinical studies at much lower cost than 213Bi. More importantly, murine studies have demonstrated that the in vivo effects and nonhematologic toxicity of 211At-labeled anti-CD45 mAb may be superior to 213Bi.14
In the current study, we show that conditioning with 211At linked to anti-CD45 mAb is minimally toxic and is sufficiently immunosuppressive to allow stable long-term engraftment of DLA-identical marrow grafts when combined with postgrafting immunosuppression consisting of mycophenolate mofetil and cyclosporine.
Methods
Antibodies
For radiolabeling, the anti–canine CD45 mAb CA12.10C12 (immunoglobulin IgG1) was used.10–12,15 For flow cytometry, mAbs against canine CD45 (CA12.10C12, IgG1), CD4 (CA13.1.E4, IgG1), CD8 (CA9.JD3, IgG2a),16 and TCRαβ (CA15.9D5, IgG1)17 were used. The anti-CD3 mAb CA17.6B3 (IgG2b) was kindly provided by Dr Peter Moore (University of California, Davis, CA). In addition, we used antibodies against canine CD44 (S5, IgG1)18 and canine myeloid cells (DM5, IgG1).19 mAb 31A (IgG1) directed at the mouse Thy-1 receptor was a negative isotype-matched control.20 All mAbs were produced and purified at the Biologics Production Facility of the Fred Hutchinson Cancer Research Center. In addition, the commercially available anti–human CD14 (Dako Denmark) cross-reacting with canine CD1421 and goat anti–mouse (Fab′)2-FITC (Biosource International) were used. The mAbs were FITC conjugated according to standard protocols.
Radioactivity
All radioactive materials were handled according to approved protocols at the University of Washington and Fred Hutchinson Cancer Research Center. Standard methods for safely handling radioactive samples were used.22 211At was produced by irradiation of bismuth metal with 29.0 MeV α-particles on a Scanditronix MC-50 cyclotron at the University of Washington using conditions previously described.23 The 211At was isolated from the bismuth target using a “wet chemistry” isolation procedure (D.S.W. et al, manuscript under revision). All handling and processing of the irradiated target were done in a glovebox (Innovative Technologies, radioisotope glovebox), which was vented through a charcoal filter on the glovebox exhaust and a second charcoal-filtered Plexiglas enclosure (12 in × 16 in × 21 in; Biodex 112-038) within a radiochemical fume hood. 211At-labeling reactions were conducted in a charcoal-filtered Plexiglas enclosure (radioiodine fume hood, 20 in × 24 in × 36, in; Biodex Medical Systems) housed within a radiochemical fume hood. Measurement of 211At-labeled mAb was conducted on a Capintec CRC-15R Radioisotope Calibrator using the calibration number 44 (designated by Capintec Technical Services).
Conjugation of the anti-CD45 mAb with closo-decaborate (2-)
The conjugation reaction was conducted as previously described (D.S.W., M.-K. Chyan, H. Nakamae, Y.C., D.K.H., E.B.S., B.K., B.M.S., Reagents for astatination of biomolecules, manuscript under revision). Briefly, CA12.10C12 was added to HEPES buffer (100mM with 150mM NaCl, pH 8.6) followed by addition of 10 equivalents (0.350 mg) of isothiocyantophenethyl-ureido-closo-decaborate(2-) in dimethyl sulfoxide (50 μL). The reaction was run overnight at room temperature with gentle tumbling and then was eluted over 2 size-exclusion (PD-10) columns using PBS as eluant. The protein-containing fractions were combined and concentrated in a Vivaspin 6 centrifugal filter (30-kDa molecular weight cut-off) to yield the CA12.10C12-B10 conjugate.
Radiolabeling of the CA12.10C12-B10 conjugate with 211At
The 211At-labeling reactions of CA12.10C12-B10 were accomplished generally as follows. To a solution of CA12.10C12-B10 in PBS was added a 200 to 600 μL sodium phosphate solution (0.5M, pH 7.2) followed by 200 to 600 μL of Na[211At]At (2.8-7.7 mCi at pH 7). To the resultant solution was added 10 to 15 μL of a 10 mg/mL solution of chloramine-T in H2O. After 2 minutes at room temperature, the reaction was quenched with 10 to 15 μL of a 10 mg/mL solution of sodium metabisulfite in H2O. The reaction mixture was then eluted over a Sephadex G-25 size-exclusion column (PD-10) using PBS as the eluant. Protein fractions were combined to yield 1.7 to 5.1 mCi (33%-80% radiochemical yields) on 0.9 to 1.7 mg of [211At]CA12.10C12-B10. The [211At]CA12.10C12-B10 was combined with unlabeled CA12.10C12-B10 to prepare an injectate that contained a total 0.5 mg/kg in 4 mL PBS.
Dogs
Littermates of beagles and mini-mongrel-beagle cross-breeds were either raised at Fred Hutchinson Cancer Research Center or purchased from other commercial kennels in the United States. The dogs were quarantined for 1 month and judged to be disease-free before study. They were immunized against distemper, leptospirosis, hepatitis, papilloma virus, and parvovirus. Their median age was 7.9 months (range, 6-11 months), and their median weight was 8.6 kg (range, 5.8-13 kg). One dog (H213) was a pyruvate kinase deficiency carrier. The experimental protocol was approved by the Institutional Animal Care and Use Committee of the Fred Hutchinson Cancer Research Center. The study was performed in accordance with the principles outlined in the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources).24 The kennels were certified by the American Association for Accreditation of Laboratory Animal Care, International. DLA-identical littermates were selected on the basis of identity for highly polymorphic major histocompatibility complex class I and class II microsatellite markers and identity for DLA-DRB1 alleles as determined by direct sequencing.25,26
Flow cytometry
Blood samples were obtained at different time points before and after mAb infusion to quantify peripheral blood leukocyte subsets by flow cytometry. A total of 10 to 30 μg/mL of the respective FITC-conjugated mAb was added to 50 μL of whole blood and incubated at 4°C for 30 minutes. Subsequently, red blood cells were lysed with a hemolytic buffer containing EDTA. The cells were washed first with PBS containing 2% horse serum and 5mM sodium azide, and then with 1× PBS with 5mM sodium azide. After resuspension in 1% paraformaldehyde, the cells were analyzed on a FACSCanto II Flow Cytometer (BD Biosciences).
Pharmacokinetic studies
Saturation of the CD45 antigen by the injected mAb was assessed by flow cytometry on blood samples obtained before and at various intervals after the mAb infusion. A total of 50 μg/mL of nonconjugated anti–CD45-B10 was added to 50 μL whole blood and incubated at 4°C for 30 minutes, and then washed with PBS with 2% horse serum. Subsequently, 10 μg/mL of FITC-conjugated anti-CD45 mAb or 10 μg/mL of FITC-goat-anti–mouse (Fab′)2 was added and reincubated at 4°C for 30 minutes. Cell lysis, washing, and analysis were the same as described in “Flow cytometry.”
Plasma levels of the mAbs were measured with an ELISA.27 Briefly, 96-well polyvinyl plates were coated with 1 μg/mL goat anti–mouse IgG in PBS and incubated with plasma from the infused dogs after blocking with 5% nonfat milk in PBS. Goat anti–mouse IgG HRP was used as the secondary antibody, and 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) was used as the color reagent. Plates were read with a Vmax microtiter plate reader (Molecular Devices) at 405 nm. Plasma obtained from dogs before infusion was used as control, and standard curves with known concentrations of mAbs were established.
NK-cell cytotoxicity assay
To evaluate NK-cell activity before and after transplantation, chromium release assays were performed.28 Effector cells were PBMCs prepared by Ficoll-Hypaque density-gradient centrifugation (density, 1.074), and targets were cells from a canine thyroid adenocarcinoma cell line. Effector-to-target ratios of 50:1, 25:1, 12.5:1, and 6.25:1 in triplicate wells were used. The percentage of cytotoxicity (percent specific lysis) was calculated using the mean value of triplicate cultures as follows: % specific lysis = (experimental release − spontaneous release)/(maximum release − spontaneous release) × 100. Maximum release was determined in wells with target cells and 2% Triton X. Spontaneous release was determined in wells with target cells and medium alone.
MLCs
Mixed leukocyte cultures (MLCs) were used to assess cellular immune function before and after transplantation.29 Dog PBMCs were resuspended in Waymouth medium supplemented with 1% nonessential amino acids, 1% sodium pyruvate, 1% l-glutamine, and 10% heat-inactivated, pooled, normal dog serum. Then, 1 × 105 responder cells per well and 1 × 105 irradiated (2200 cGy) stimulator cells per well were cocultured in triplicate in round-bottom 96-well plates for 7 days at 37°C in a humidified 5% carbon dioxide air atmosphere. Concanavalin A (4 μg) was added on day 3 to responder cell triplicates used as control. On day 6, cultures were pulsed with 1 μCi of 3H-thymidine for 18 hours before harvesting; 3H-thymidine uptake was measured as the mean counts per minute of the 3 replicates using a β-scintillation counter (Packard BioScience).
Dose escalation and toxicity study of [211At]CA12.10C12-B10
To evaluate hematopoietic and nonhematopoietic toxicity of 211At, a dose escalation study was performed on a total of 6 dogs given 211At-labeled anti-CD45 mAb (0.5 mg/kg) at increasing doses of radiation (100-618 μCi/ kg 211At) without subsequent marrow grafts (Table 1). To prevent nonspecific tissue binding of the radiolabeled anti-CD45 mAb, 10% of the total anti-CD45 mAb dose was infused as unlabeled anti-CD45 mAb 1 hour before the labeled anti-CD45, as previously described.10 To assess hematopoietic recovery, hepatic and kidney function, peripheral blood leukocyte subsets, CD45 antigen saturation, and plasma concentration of anti-CD45 mAb, peripheral blood samples were obtained before mAb infusion and at various time points afterward. Changes in peripheral blood counts were compared with 213Bi-labeled anti-CD45 mAb-treated dogs (n = 4)10 and historical controls given external-beam TBI (200 cGy; n = 13),30 both without HCT rescue.
Table 1.
Dogs treated with 211At-anti-CD45 mAb without marrow grafts
| Dog ID | Age, mo | Weight, kg | 211At, μCi/kg | 211At, MBq/kg | Survival, wk | Cause of death | Pathology |
|---|---|---|---|---|---|---|---|
| H313 | 9 | 5.8 | 100 | 3.7 | 53 | End of study | Normal* |
| H297 | 7 | 7.6 | 196 | 7.4 | 54 | End of study | Normal* |
| H248 | 7 | 13.0 | 320 | 11.8 | 54 | End of study | Normal* |
| H213 | 6 | 9.6 | 405 | 15.2 | 49 | Increasing transfusion requirements | Histologic changes consistent with hemolytic anemia† |
| H290 | 7 | 8.0 | 458 | 17.0 | 20 | Liver failure with ascites | Histologic changes in the liver‡ |
| H289 | 11 | 7.2 | 618 | 22.9 | 17 | Liver failure with ascites | Histologic changes in the liver‡ |
The total dose of anti-CD45 mAb was 0.5 mg/kg, whereof 10% was injected as unlabeled anti-CD45 mAb 1 hour before treatment with the radioimmunoconjugate.
No macroscopic or histologic evidence of radioactivity induced organ toxicity.
H213 was a pyruvate kinase deficiency carrier that developed increasing transfusion requirements during follow-up.
Histologic examination of liver tissue revealed clusters of iron-laden macrophages scattered throughout the liver acinus and perivenular area.
DLA-identical marrow grafts
Based on the toxicity studies, 8 dogs were treated on day 3 before marrow grafting (day −3), with anti-CD45 mAb labeled with 211At doses between 155 μCi/kg (5.7 MBq/kg) and 625 μCi/kg (23.1 MBq/kg). On day 0, the dogs were given marrow grafts intravenously (mean, 4.9 × 108 total nucleated cells/kg; range, 3.1-7.9 × 108/kg) from DLA-identical littermates. Total dose of anti-CD45 mAb administered was 0.5 mg/kg with 10% of that amount being infused as unlabeled mAb, as described in the previous section. Mycophenolate mofetil (10 mg/kg given subcutaneously twice daily on day 0 to day +27) and cyclosporine (15 mg/kg given orally twice on day −1 to day +35) were administered for postgrafting immunosuppression.31 Supportive care after antibody infusion and transplantation was given as previously described.32 Dogs that experienced elevations of hepatic enzymes were treated with ursodiol (7.5 mg/kg given orally twice a day) for the remainder of the study period. Engraftment was assessed by the recovery of peripheral blood granulocyte and platelet counts after postirradiation nadirs and marrow cellularities at autopsies. Neutrophil engraftment was defined as the first day of 3 consecutive days with absolute neutrophil counts more than or equal to 500/μL, and platelet engraftment was defined as the first day of 7 consecutive days with platelet counts more than or equal to 20 000/μL without transfusion. At the end of study, dogs were euthanized, and autopsies including histologic examinations were performed to assess marrow engraftment, GVHD, and potential toxicities. Changes in peripheral blood counts were compared with dogs given DLA-identical transplants after 213Bi-labeled anti-CD45 mAb (n = 11) or 200 cGy external beam TBI conditioning (n = 5) and posttransplantation immunosuppression with mycophenolate mofetil and cyclosporine as in the current study.10,31
Chimerism analysis
Donor chimerism was evaluated using a PCR-based assay of polymorphic (CA)n dinucleotide repeats with primers specific for informative microsatellite markers. Genomic DNA from the cells of interest was extracted, and PCR was performed under conditions described previously.33 PCR products were quantified by an electrophoresis-based genotyping system and analyzed using GeneMapper Version 4.0 software (Applied Biosystems). Lineage chimerism analysis was performed on mononuclear cells (MNC), granulocytes, and T cells. Peripheral blood MNC and granulocytes were obtained through Ficoll density-gradient centrifugation (density, 1.074). To assess T-cell chimerism, PBMCs were stained with FITC-conjugated anti-CD3 mAb (CA17.6B3) and purified by flow cytometric sorting (FACSAria II, BD Biosciences). Engraftment was defined as greater than or equal to 5% MNC donor chimerism, and graft rejection was defined as the first day when donor MNC chimerism was less than 5% without subsequent increase. Donor chimerism was assessed weekly until the end of study.
Results
Dose escalation and toxicity study of 211At–anti-CD45 mAb
A total of 6 dogs received anti-CD45 mAb, CA12.10C12-B10, labeled with doses of 211At ranging from 100 to 618 μCi/kg (3.7-22.9 MBq/kg) without hematopoietic cell rescue (Table 1). All dogs, except the one treated with the lowest dose of 211At (100 μCi/kg), experienced increased liver function tests (LFTs). Most marked were the 2 dogs (H289 and H290) that received the highest doses of 211At (618 μCi/kg and 458 μCi/kg). They presented with ascites around day +100 after injection preceded by increases in alkaline phosphatase, aspartate transaminase, and alanine transaminase of up to 10 times the upper normal limit. H289 also experienced pronounced bilirubinemia with jaundice. H289 and H290 were euthanized in poor condition at 17 and 20 weeks after infusion. Pathology sections after necropsy showed collections of iron-laden macrophages scattered throughout the liver sinus and clustered around the perivenular areas, without parenchymal liver injury. The remaining dogs treated with 211At doses between 196 and 405 μCi/kg only showed transient peaks in alkaline phosphatase and liver transaminases 4 to 6 weeks after infusion, which gradually returned to normal levels or within 2 times the upper normal limit. Dogs H248, H297, and H313 were euthanized in good condition after 53 to 54 weeks of follow-up with no macroscopic or histologic evidence of renal, hepatic, or other organ toxicity. Dog H213, which was a pyruvate kinase deficiency carrier, was euthanized week 49 after treatment because of decreasing hematocrit. After recovery of granulocytes, lymphocytes, and platelets, the hematocrit declined below the lower normal limit of 39% to 37% 10 weeks after treatment. During the remaining follow-up period, the hematocrit was a median of 26% and fluctuated between 17% and 26%. Bone marrow biopsy 20 weeks after treatment was consistent with ineffective erythropoiesis; however, the Coombs test was negative and bilirubin levels were normal. Absolute neutrophil count, absolute lymphocyte count, and platelets never dropped again after initial recovery; and on necropsy, histology revealed a normal bone marrow and a spleen with changes consistent with severe and prolonged hemolytic anemia. In the liver, iron-laden macrophages were observed, although less pronounced than in H289 and H290.
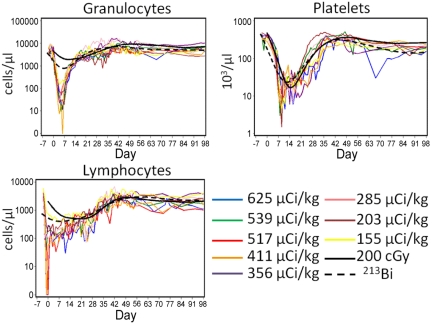
All 6 dogs experienced dose-dependent myelosuppression, lymphopenia, and thrombocytopenia (Figure 1). Median nadir levels for granulocytes, lymphocytes, and platelets were 9 cells/μL (range, 0-298 cells/μL), 60 cells/μL (range, 34-272 cells/μL), and 3000 platelets/μL (range, 1500-22 500 platelets/μL) at a median time of 7 days (range, 6-9 days), 3 days (range, 2-6 days), and 13.5 days (range, 10-24 days), respectively. Hematologic recovery was rapid, and all dogs recovered counts without hematopoietic cell rescue. The first of 3 consecutive days with neutrophil counts more than or equal to 500/μL was at a median of 19 days after infusion (range, 8-25 days after infusion), and the first of 7 consecutive days with platelet counts more than or equal to 20 000/μL was at a median of 40 days (range, 0-49 days; Figure 1). The dogs received a median of 9 transfusions (range, 0-18 transfusions) during the period of platelet counts less than 10 000/μL. Compared with historical controls treated with 200 cGy TBI or 213Bi-labeled anti-CD45 mAb without HCT rescue, the 211At-treated dogs experienced lower neutrophil, lymphocyte, and platelet nadirs earlier after treatment with similar recovery kinetics (Figure 1).10,30
Figure 1.
Hematologic changes after infusions of 211At–anti-CD45 mAb without bone marrow transplantation. Granulocyte, lymphocyte, and platelet counts in 6 dogs treated with 100 to 618 μCi/kg 211At-labeled anti-CD45 on day 0. The total anti-CD45 dose was 0.5 mg/kg, whereof 10% was administered as unlabeled mAb 1 hour before the radioimmunoconjugate. The solid and dashed curves represent median values from historical controls treated with 200 cGy (n = 13)30 or 213Bi-anti-CD45 (n = 4)10 without HCT rescue, respectively.
DLA-identical marrow grafts
Based on experience from the dose finding study, the first dog in the transplantation arm of the study was conditioned with an intermediate dose of 211At. In the subsequent dogs, 211At doses were de-escalated and escalated to determine minimal dose required for engraftment and maximal dose tolerated. A total of 8 dogs were conditioned with 211At doses between 155 and 625 μCi/kg 3 days before receiving the DLA identical marrow graft (Table 2). The total dose of anti-CD45 was 0.5 mg/kg, whereof 10% was infused as unlabeled mAb 1 hour before the radioimmunoconjugate. Antigen saturation was not reached as indicated by flow cytometry, and assessed by ELISA. 211At-labeled anti-CD45 mAb was cleared from plasma by 24 hours after infusion (data not shown).
Table 2.
Dogs given 211At-anti-CD45 mAb and DLA-identical marrow grafts
| Dog ID | Weight, kg | 211At, μCi/kg | 211At, MBq/kg | Marrow TNC, × 108/kg | Donor chimerism, % (maximum to final) |
Survival, wk | Cause of death | Pathology | ||
|---|---|---|---|---|---|---|---|---|---|---|
| MNC | Granulocytes | CD3+ | ||||||||
| H349 | 8.7 | 155 | 5.9 | 2.2 | 21-5 | 34-2 | 6-4 | 18 | Low donor chimerism* | Normal† |
| H308 | 7.4 | 203 | 7.4 | 3.1 | 58-38 | 85-57 | 41-41 | 52 | End of study | Normal† |
| H232 | 8.9 | 285 | 10.7 | 5.5 | 68-42 | 100-63 | 39-35 | 53 | End of study | Normal† |
| H267 | 8.9 | 356 | 13.3 | 7.9 | 55-33 | 85-62 | 42-32 | 52 | End of study | Histologic changes in the liver‡ and thyroid§ |
| H251 | 9.6 | 411 | 15.2 | 4.9 | 60-48 | 95-59 | 69-46 | 52 | End of study | Decreased bone marrow cellularity‖ |
| H379 | 5.7 | 517 | 19.2 | 4.6 | 100-49 | 98-79 | 41-41 | 40 | End of study | Normal† |
| H388 | 6.7 | 539 | 20.0 | 7.8 | 84-58 | 100-66 | 46-46 | 49 | End of study | Normal† |
| H271 | 8.4 | 625 | 22.9 | 3.0 | 86-19 | 97-41 | 57-13 | 48 | End of study | Normal† |
Dogs were treated with 211At-labeled anti-CD45 mAb 3 days before DLA-identical bone marrow transplantation. The total dose of anti-CD45 mAb was 0.5 mg/kg, whereof 10% was injected as unlabeled anti-CD45 mAb 1 hour before treatment with the radioimmunoconjugate. Posttransplantation immunosuppression consisted of cyclosporine 15 mg/kg twice daily from day −1 to day 35 and mycophenolate mofetil 10 mg/kg twice daily from day 0 to day 27.
TNC indicates total nucleated cell.
H349 was euthanized 18 weeks after transplantation because of low-level donor chimerism.
No macroscopic or histologic evidence of radioactivity induced organ toxicity.
Histologic examination of liver tissue revealed mild nodular regenerating hyperplasia.
Thyroid hypercellularity with atrophic follicles.
Slightly decreased to 75% of a normal dog.
All dogs tolerated both antibody infusion and the transplantation well with no immediate side effects or evidence of clinical GVHD. All dogs were euthanized in good condition after a median follow-up of 50.5 weeks (range, 18-53 weeks).
All dogs experienced granulocytopenia, lymphocytopenia, and thrombocytopenia with median nadir levels of 21 cells/μL (range, 1-146 cells/μL), 55 cells/μL (range, 0-270 cells/μL), and 4500 platelets/μL (range, 1500-6560 platelets/μL) at a median time of 5 days (range, 4-7 days), −0.5 days (range, −2 days to 26 days), and 9 days (range, 8-16 days), respectively. All dogs engrafted rapidly with neutrophil and platelet engraftment occurring at a median of 10 days after transplantation (range, 9-11 days after transplantation) and 16 days after transplantation (range, 10-26 days after transplantation; Figure 2). Compared with the composite curves of dogs treated with DLA-identical HCT after 200 cGy external beam TBI (n = 5)31 or 213Bi-labeled anti-CD45 (n = 11)10,11 shown in Figure 2, 211At treated dogs experienced more profound nadirs with similar recovery kinetics. Transfusion requirements were minimal. A median of 1 transfusion per dog (range, 1 or 2) was given because of thrombocytopenia.
Figure 2.
Peripheral blood granulocyte, lymphocyte, and platelet counts in dogs treated with 211At–anti-CD45 mAb-conditioned nonmyeloablative DLA identical bone marrow transplantation. Eight dogs received 155 to 625 μCi/kg 211At-labeled anti-CD45 mAb on day −3 followed by marrow grafts from a DLA- identical donor on day 0. The total anti-CD45 mAb dose was 0.5 mg/kg, whereof 10% was administered as unlabeled mAb 1 hour before the radioimmunoconjugate. Posttransplant immunosuppression consisted of cyclosporine 15 mg/kg twice daily from day −1 to day 35 and mycophenolate mofetil 10 mg/kg twice daily from day 0 to day 27. The solid and dashed curves represent median values from historical controls conditioned with 200 cGy (n = 5)31 or 213Bi-anti-CD45 (n = 11)10,11 before receiving bone marrow from DLA-identical donors, respectively.
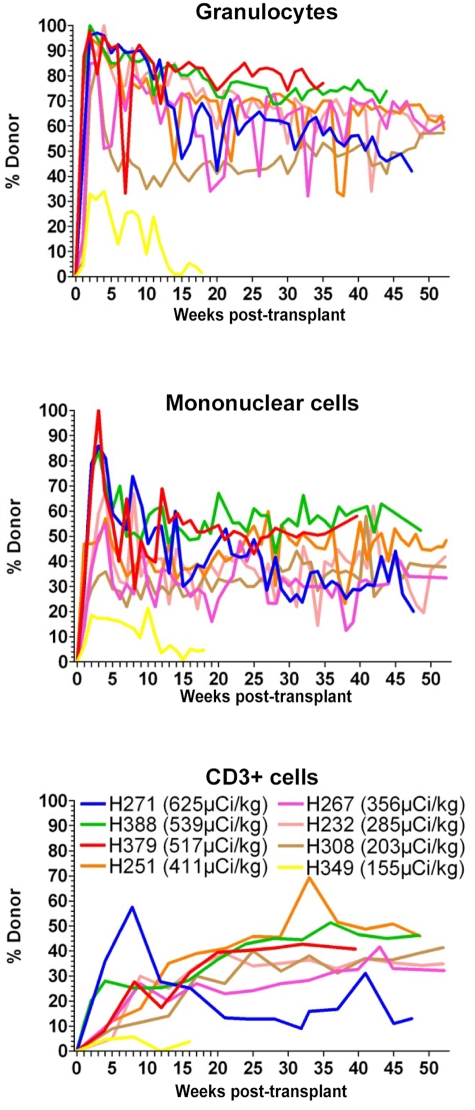
Mixed chimerism in peripheral blood was observed as early as 1 week after transplantation in all dogs (Figure 3). In H349, which received the lowest dose of 211At (155 μCi/kg), 21% donor chimerism was observed in the MNC early after transplantation. However, maximal donor chimerism in the CD3+ cells never increased beyond 6%, and by day 83 MNC donor chimerism declined to 4%. In the following weeks, the MNC donor chimerism varied between 1% and 7%. H349 recovered autologous hematopoiesis and was euthanized 18 weeks after transplantation to get early pathologic and histologic data. At time of death, donor chimerism in all subsets was 5%. At a median time of follow-up of 52 weeks (range, 40-53 weeks), all 7 dogs treated with 211At doses between 203 and 625 μCi/kg achieved long-term mixed chimerism ranging between 19% to 58%, 41% to 79%, and 13% to 46% in MNC, granulocytes, and CD3+ cells, respectively. Microscopic examination of the marrow in euthanized dogs was normal, except for H251, which showed evidence of slightly decreased cellularity with 50% to 60% cells and 40% fat, corresponding to approximately 75% of the normal cellularity for a dog.
Figure 3.
Percentage of donor chimerism in granulocytes, mononuclear cells, and CD3+ in dogs treated with 211At–anti-CD45 mAb-conditioned nonmyeloablative DLA-identical bone marrow transplantation. Eight dogs received 155 to 625 μCi/kg 211At-labeled anti-CD45 mAb on day −3 followed by marrow grafts from a DLA-identical donor on day 0. The total anti-CD45 mAb dose was 0.5 mg/kg, whereof 10% was administered as unlabeled mAb 1 hour before the radioimmunoconjugate.
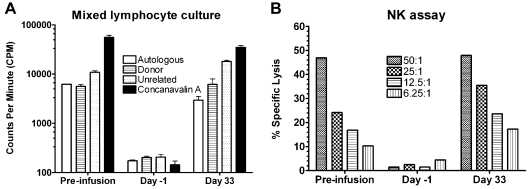
Immune reconstitution was rapid in all dogs, with normal T-cell and NK-cell function as measured by MLC and NK assays within 1 month of transplantation (Figure 4). Quantification of T-cell, B-cell, granulocyte, and monocyte recoveries by flow cytometry showed a rapid return to pretransplantation levels by day 35 (data not shown).
Figure 4.
MLCs and NK assays from 1 dog (H388) treated with 211At–anti-CD45 mAb-conditioned nonmyeloablative DLA-identical bone marrow transplantation. In vitro lymphocyte function from 1 representative dog (H388) treated with 539 μCi/kg 211At-labeled anti-CD45 mAb on day −3 followed by a marrow graft from a DLA-identical donor on day 0. The total anti-CD45 dose was 0.5 mg/kg, whereof 10% was administered as unlabeled mAb 1 hour before the radioimmunoconjugate. Data were obtained before radiolabeled antibody infusion, day −1 before transplantation, and 33 days after transplantation. MLC data are expressed as mean counts per minute from triplicate wells of recipient response to autologous cells, donor cells, cells from an unrelated dog, and to concanavalin A as internal assay control. Error bars represent SEM from 3 wells. In the NK assay, cytotoxicity is expressed as a percentage of specific lysis of target cells (canine thyroid adenocarcinoma cells), calculated using the mean value of triplicate cultures. Effector-to-target ratios were 50:1, 25:1, 12.5:1, and 6.25:1, respectively.
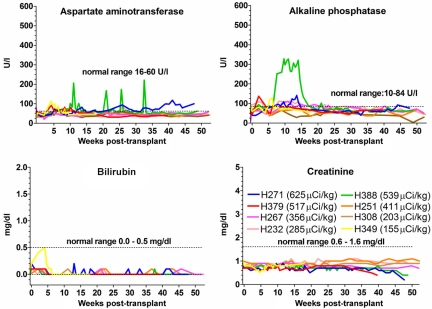
Assessed by blood urea nitrogen and creatinine, no renal toxicity was observed (Figure 5). Transient elevations of alkaline phosphatase and the amino transferases were most common within the first 15 to 20 weeks after transplantation and were most pronounced in dogs receiving 211At doses more than 356 μCi/kg. H388 experienced peaks 3 to 5 times above the upper normal limit throughout the posttransplantation period, whereas the other dogs stayed within 2 times the upper normal limit (Figure 5). Histopathologic changes in the liver were only observed in H267, which showed mild nodular regenerating hyperplasia, without clinical evidence of liver failure.
Figure 5.
Hepatic and renal function of 8 dogs treated with 211At–anti-CD45 mAb-conditioned nonmyeloablative DLA-identical bone marrow transplantation. Eight dogs received 155 to 625 μCi/kg 211At-labeled anti–CD45 mAb on day −3 followed by marrow grafts from a DLA-identical donor on day 0. Dashed lines in graphs indicate the range of values found in normal dogs.
Thyroid-stimulating hormone (TSH) and free T4 were analyzed in a total of 5 dogs at end of study (H271, H388, H379, H251, and H267). TSH, free T4, and thyroid histopathology were normal in 3 dogs, whereas evidence of subclinical hypothyroidism was observed in H267 (TSH: at 0.67 ng/mL; free T4: 3 ng/mL) and H388 (TSH: at 0.54 ng/mL; free T4: 2.1 ng/mL), which showed evidence of subclinical hypothyroidism (TSH, upper normal limit 0.5 ng/mL; free T4, normal range 0.7-3.1 ng/mL). On histopathologic examination, thyroid hypercellularity with atrophic follicles was observed in H267, whereas thyroid histology was normal in H388. No further macroscopic or histologic evidence of organ toxicity or GVHD was observed.
Discussion
The aim of the current study was to assess whether external beam TBI could be replaced by RIT with 211At-labeled anti-CD45 mAb in a canine model of DLA-identical nonmyeloablative HCT. The safety and efficacy of radioimmunotherapy for conditioning in HCT have previously been explored in several clinical studies, where β-emitter based RIT successfully has been used to augment conditioning regimens.1–4 Less explored are the α-emitters, which could be more suitable for conditioning in HCT as their short path length and high linear energy transfer enable delivery of highly localized cytotoxic radiation with minimal nonspecific irradiation of surrounding tissue.5,8,34,35
In relation to HCT, α-emitter–labeled mAbs alone as conditioning have only been investigated by our group. We have previously demonstrated the safety and efficacy of replacing external beam irradiation with an anti-CD45 or anti-TCR mAb labeled with 213Bi in DLA-identical and haploidentical transplantation.10–13 However, very short half-life, high cost, and limited availability of 213Bi made translating 213Bi-labeled mAb into clinical studies impractical. In the current study, we used 211At as an alternative α-particle-emitting isotope, which in addition to being available in quantities ready for scale-up into clinical trials at lower cost, also has properties in vivo that may be superior to 213Bi. Before the transition from 213Bi-labeled radioimmunoconjugates to 211At, the 2 isotopes were compared in a murine biodistribution study.14 213Bi and 211At localized to the same organs, with higher concentrations of 211At in the spleen, because of its longer half-life (211At, 7.21 hours; 213Bi, 45.6 minutes) permitting better localization before decay. Furthermore, at equivalent doses of radioactivity, increased myelosuppression with less toxicity in other organs, such as liver, was observed for 211At-labeled anti-CD45 mAb compared with 213Bi-labeled anti-CD45 mAb. The longer half-life of 211At also allowed sufficient radioactivity to be administered in a single infusion instead of the 6 infusions required for 213Bi treatments, making logistics considerably less challenging.
The CD45 antigen was selected as the target because it is selectively expressed on all nonmalignant hematopoietic cells during all stages of maturation and on the majority of hematologic malignancies.36,37 The density of the antigen is very high, averaging 200 000 copies on the surface of circulating lymphocytes, which permits short-lived α-emitters to deliver sufficient irradiation in readily achievable specific activities.
In the current study, the anti-CD45 mAb CA12.10C12 was conjugated with a closo-decaborate (2-)–containing reagent and subsequently radiolabeled. The conjugation did not alter the properties of the mAb, and the 211At was stable to in vivo de-astatination (D.S.W., M.-K. Chyan, H. Nokamae, Y.C., D.K.H., E.B.S., B.K., B.M.S., Reagents for astatination of biomolecules, manuscript under revision). Consistent with other studies using anti-CD45 mAb for RIT,38,39 including our own with 213Bi-labeled anti-CD45 mAb,10 the highest uptake of the radioimmunoconjugate was observed in blood, lymph nodes, bone marrow, and spleen. Among nontarget organs, the liver, gallbladder, and thyroid had the highest uptake, whereas no appreciable uptake was observed in the kidney (D.S.W., M.-K. Chyan, H. Nokamae, Y.C., D.K.H., E.B.S., B.K., B.M.S., Reagents for astatination of biomolecules, manuscript under revision). Clearance of the antibody from plasma was fast, with anti-CD45 mAb levels declining below detection level within 24 hours, presumably because of the rapid binding of the mAb to cellular CD45. As previously observed, the used mAb doses did not result in saturation of the target antigen, hereby preventing circulation of unbound radiolabeled mAb.10–12
To assess the in vivo effects of escalating doses of 211At-labeled anti-CD45 mAb, 6 dogs were treated without marrow grafting. The treatments were well tolerated; and as expected, declines in peripheral blood counts with subsequent autologous recovery were observed. Analogous to the 213Bi study,10 off-target toxicity was only observed in the liver, where clinically relevant changes were present in dogs treated with more than 405 μCi/kg. Although histologic findings, LFT changes, and clinical presentation were not consistent with the classic presentation of radiation-induced liver injury, it is probable that the hepatotoxicity observed was caused by the RIT as approximately 18% of the cells in the liver are of hematopoietic origin.40–42 In addition, circulating immunoglobulins are nonspecifically taken up in the liver by Kupffer cells and endothelial cells, which also could lead to increased radiation damage.43,44 On more recent review of the histology of the livers from dogs treated with 213Bi-anti–CD45 mAb, similar iron-laden macrophages in the liver were also observed in those dogs that had persistent elevations of LFT.10
To determine whether radioimmunotherapy with the 211At-labeled anti-CD45 mAb conjugate could replace TBI as nonmyeloablative conditioning, we performed DLA-identical marrow transplantation studies in 8 dogs. Combined with short courses of immunosuppression, all dogs treated with 211At doses ranging from 203 to 625 μCi/kg achieved rapid and stable donor engraftment throughout the study period. Even the dog treated with the lowest dose of 211At (155 μCi/kg) engrafted with low but detectable donor chimerism. The immune reconstitution was rapid, returning to baseline levels within 1 to 2 months after transplantation. The infusion of the radioimmunoconjugate and the transplantation was well tolerated without immediate side effects or evidence of clinical or histologic GVHD. The dose range of 211At used in the transplanted dogs and the nontransplanted dogs was similar. However, the impact of the RIT on hepatic function in the transplanted dogs was less severe with lower elevations in LFT and no cases of overt liver failure. How marrow transplantation ameliorates radiation-induced liver damage in the canine transplant model remains to be explored. However, evidence from a rat model suggests that bone marrow–derived progenitor cells have the ability to repair irradiation-induced hepatic injury.45
211At is a halogen-like iodine and is taken up by the thyroid epithelial cells but not incorporated into T3 and T4.46,47 Of 2 dogs presenting with subclinical hypothyroidism, only 1 had histologic changes in the thyroid that could be consistent with radiation damage. Although a strong relationship exists between hypothyroidism and high-dose external or internal 131I irradiation (30-80 Gy and > 185 MBq), the association is less for the low-dose ranges used in the current study.48 Whether the subclinical hypothyroidism in the current animals is a consequence of RIT cannot be ruled out. However, histopathologic changes in the thyroid were not observed in any of the dogs treated with higher doses of 211At; and taking the short half-life of 211At into account, it is unlikely that the thyroid received a high enough dose to induce a state of deficiency.
In conclusion, we demonstrate that RIT with anti-CD45 mAb labeled with the α-emitting radionuclide 211At is safe, efficacious, and associated with minimal toxicity. Granulocyte and lymphocyte nadirs were more profound than what has previously been observed with 200 cGy external beam radiation and 213Bi-labeled anti-CD45 mAb in the same model.10,11,30,31 Donor chimerisms in the current study look similar, if not higher, compared with dogs conditioned with 200 cGy TBI.31 Properties, such as these, could make conditioning with 211At-labeled anti-CD45 mAb more favorable in settings where 200 cGy external beam irradiation is not sufficient for disease control or engraftment. Although the low-level donor chimerism that was observed in the dog conditioned with the lowest dose of 211At (155 μCi/kg) was not sufficient to correct disorders otherwise treatable with allogeneic HCT, a 211At RIT low-dose approach could be applicable to solid organ transplants where low-level or transient donor chimerism is enough to encourage development of donor-specific tolerance.49 Furthermore, the current study provides a platform for further investigation of α-emitter-based RIT conditioning and future clinical trials.
Acknowledgments
The authors thank Drs George McDonald and Howard Shulman for additional review of the liver pathology; Michele Spector, DVM, the investigators who participated in the weekend treatments, and the technicians in the canine facilities; Stacy Zellmer for DLA typing; and Helen Crawford, Bonnie Larson, and Sue Carbonneau for manuscript preparation.
This work was supported by the National Institutes of Health (grants R01CA118940, P01HL036444, and CA15704). Y.C. was supported by the China Scholarship Council and Shandong University. B.K. was supported by the Danish Cancer Society (fellowship DP08135) and Frøken Amalie Jørgensens Mindelegat.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health nor its subsidiary Institutes and Centers.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: Y.C. and B.K. conducted the study, drafted and revised the manuscript, and analyzed and interpreted data; D.K.H. provided vital reagents and designed and conducted the study; G.E.S. provided pathologic review of the histology; E.B.S. designed and conducted the study, revised the manuscript, and analyzed and interpreted data; D.S.W. designed and supervised the study, provided vital reagents, analyzed and interpreted data, and drafted and revised the manuscript; B.E.S. provided statistical review and analyzed the data; R.S. designed the study and revised the manuscript; B.M.S. conceived, designed, conducted, and supervised the study, analyzed and interpreted data, and drafted and revised the manuscript; and all authors read and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for Y.C. is Shandong University, Shandong, Peoples Republic of China. The current affiliation for B.K. is Rigshospitalet, Copenhagen, Denmark.
Correspondence: Brenda M. Sandmaier, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D1-100, Seattle, WA 98109-1024; e-mail: bsandmai@fhcrc.org.
References
- 1.Appelbaum FR, Matthews DC, Eary JF, et al. Use of radiolabeled anti-CD33 antibody to augment marrow irradiation prior to marrow transplantation for acute myelogenous leukemia. Transplantation. 1992;54(5):829–833. doi: 10.1097/00007890-199211000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Bunjes D, Buchmann I, Duncker C, et al. Rhenium 188-labeled anti-CD66 (a, b, c, e) monoclonal antibody to intensify the conditioning regimen prior to stem cell transplantation for patients with high-risk acute myeloid leukemia or myelodysplastic syndrome: results of a phase I-II study. Blood. 2001;98(3):565–572. doi: 10.1182/blood.v98.3.565. [DOI] [PubMed] [Google Scholar]
- 3.Pagel JM, Gooley TA, Rajendran J, et al. Allogeneic hematopoietic cell transplantation after conditioning with 131I-anti-CD45 antibody plus fludarabine and low-dose total body irradiation for elderly patients with advanced acute myeloid leukemia or high-risk myelodysplastic syndrome. Blood. 2009;114(27):5444–5453. doi: 10.1182/blood-2009-03-213298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schulz AS, Glatting G, Hönig M, et al. Radio-immunotherapy-based conditioning for hematopoietic cell transplantation in children with malignant and nonmalignant diseases. Blood. 2011;117(17):4642–4650. doi: 10.1182/blood-2010-06-284349. [DOI] [PubMed] [Google Scholar]
- 5.Wilbur DS. Potential use of alpha emitting radionuclides in the treatment of cancer. Antibody Immunoconjugates Radiopharmaceuticals. 1991;4(1):85–97. [Google Scholar]
- 6.O'Donoghue JA, Bardies M, Wheldon TE. Relationships between tumor size and curability for uniformly targeted therapy with beta-emitting radionuclides. J Nucl Med. 1995;36(10):1902–1909. [PubMed] [Google Scholar]
- 7.Raju MR, Eisen Y, Carpenter S, Inkret WC. Radiobiology of alpha particles: III. Cell inactivation by alpha-particle traversals of the cell nucleus. Radiat Res. 1991;128(2):204–209. [PubMed] [Google Scholar]
- 8.Dadachova E. Cancer therapy with alpha-emitters labeled peptides [review]. [Erratum appears in Semin Nucl Med. 2010;40(4):219.] Semin Nucl Med. 2010;40(3):204–208. doi: 10.1053/j.semnuclmed.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Kennel SJ, Stabin M, Roeske JC, et al. Radiotoxicity of bismuth-213 bound to membranes of monolayer and spheroid cultures of tumor cells. Radiat Res. 1999;151(3):244–256. [PubMed] [Google Scholar]
- 10.Sandmaier BM, Bethge WA, Wilbur DS, et al. Bismuth 213-labeled anti-CD45 radioimmunoconjugate to condition dogs for nonmyeloablative allogeneic marrow grafts. Blood. 2002;100(1):318–326. doi: 10.1182/blood-2001-12-0322. [DOI] [PubMed] [Google Scholar]
- 11.Bethge WA, Wilbur DS, Storb R, et al. Radioimmunotherapy with Bismuth-213 as conditioning for nonmyeloablative allogeneic hematopoietic cell transplantation in dogs: a dose deescalation study. Transplantation. 2004;78(3):352–359. doi: 10.1097/01.tp.0000128853.62545.b2. [DOI] [PubMed] [Google Scholar]
- 12.Nakamae H, Kerbauy FR, Wilbur DS, et al. Pilot study of a 213bismuth-labeled anti-CD45 mAb as a novel nonmyeloablative conditioning for DLA-haploidentical littermate hematopoietic transplantation. Transplantation. 2010;89(11):1336–1340. doi: 10.1097/TP.0b013e3181d98c3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bethge WA, Wilbur DS, Storb R, et al. Selective T-cell ablation with bismuth-213-labeled anti-TCRab as nonmyeloablative conditioning for allogeneic canine marrow transplantation. Blood. 2003;101(12):5068–5075. doi: 10.1182/blood-2002-12-3867. [DOI] [PubMed] [Google Scholar]
- 14.Nakamae H, Wilbur DS, Hamlin DK, et al. Biodistribution, myelosuppression, and toxicities in mice treated with an anti-CD45 antibody labeled with the alpha-emitting radionuclides bismuth-213 or astatine-211. Cancer Res. 2009;69(6):2408–2415. doi: 10.1158/0008-5472.CAN-08-4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caniatti M, Roccabianca P, Scanziani E, Paltrinieri S, Moore PF. Canine lymphoma: immunocytochemical analysis of fine-needle aspiration biopsy. Vet Pathol. 1996;33(2):204–212. doi: 10.1177/030098589603300210. [DOI] [PubMed] [Google Scholar]
- 16.Moore PF, Rossitto PV, Danilenko DM, Wielenga JJ, Raff RF, Severns E. Monoclonal antibodies specific for canine CD4 and CD8 define functional T-lymphocyte subsets and high density expression of CD4 by canine neutrophils. Tissue Antigens. 1992;40(2):75–85. doi: 10.1111/j.1399-0039.1992.tb01963.x. [DOI] [PubMed] [Google Scholar]
- 17.Barsoukov AA, Moore PF, Storb R, Santos EB, Sandmaier BM. The use of an anti-TCRab monoclonal antibody to control host-versus-graft reactions in canine marrow allograft recipients conditioned with low dose total body irradiation. Transplantation. 1999;67(10):1329–1335. doi: 10.1097/00007890-199905270-00007. [DOI] [PubMed] [Google Scholar]
- 18.Sandmaier BM, Storb R, Appelbaum FR, Gallatin WM. An antibody that facilitates hematopoietic engraftment recognizes CD44. Blood. 1990;76(3):630–635. [PubMed] [Google Scholar]
- 19.Sandmaier BM, Schuening FG, Bianco JA, et al. Biochemical characterization of a unique canine myeloid antigen. Leukemia. 1991;5(2):125–130. [PubMed] [Google Scholar]
- 20.Denkers E, Badger CC, Ledbetter JA, Bernstein ID. Influence of antibody isotype on passive serotherapy of lymphoma. J Immunol. 1985;135(3):2183–2186. [PubMed] [Google Scholar]
- 21.Jacobsen CN, Aasted B, Broe MK, Petersen JL. Reactivities of 20 anti-human monoclonal antibodies with leucocytes from ten different animal species. Vet Immunol Immunopathol. 1993;39(4):461–466. doi: 10.1016/0165-2427(93)90075-f. [DOI] [PubMed] [Google Scholar]
- 22.Wilbur DS, Hamlin DK, Vessella RL, et al. Antibody fragments in tumor pretargeting: evaluation of biotinylated Fab′ colocalization with recombinant streptavidin and avidin. Bioconjug Chem. 1996;7(6):689–702. doi: 10.1021/bc9600628. [DOI] [PubMed] [Google Scholar]
- 23.Wilbur DS, Vessella RL, Stray JE, Goffe DK, Blouke KA, Atcher RW. Preparation and evaluation of para-[211At]astatobenzoyl labeled anti-renal cell carcinoma antibody A6H F(ab′)2: in vivo distribution comparison with para-[125I]iodobenzoyl labeled A6H F(ab′)2. Nucl Med Biol. 1993;20(8):917–927. doi: 10.1016/0969-8051(93)90092-9. [DOI] [PubMed] [Google Scholar]
- 24.National Research Council, Commitee for the Update of the Guide for the Care and Use of Laboratory Animals, Institute for Laboratory Animal Research. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academics Press; 2011. [Google Scholar]
- 25.Wagner JL, Burnett RC, DeRose SA, Francisco LV, Storb R, Ostrander EA. Histocompatibility testing of dog families with highly polymorphic microsatellite markers. Transplantation. 1996;62(6):876–877. doi: 10.1097/00007890-199609270-00032. [DOI] [PubMed] [Google Scholar]
- 26.Wagner JL, Works JD, Storb R. DLA-DRB1 and DLA-DQB1 histocompatibility typing by PCR-SSCP and sequencing [brief communication]. Tissue Antigens. 1998;52(4):397–401. doi: 10.1111/j.1399-0039.1998.tb03063.x. [DOI] [PubMed] [Google Scholar]
- 27.Sandmaier BM, Storb R, Bennett KL, Appelbaum FR, Santos EB. Epitope specificity of CD44 for monoclonal antibody dependent facilitation of marrow engraftment in a canine model. Blood. 1998;91(9):3494–3502. [PubMed] [Google Scholar]
- 28.Loughran TP, Jr, Deeg HJ, Storb R. Morphologic and phenotypic analysis of canine natural killer cells: evidence for T-cell lineage. Cell Immunol. 1985;95(2):207–217. doi: 10.1016/0008-8749(85)90309-0. [DOI] [PubMed] [Google Scholar]
- 29.Raff RF, Deeg HJ, Farewell VT, DeRose S, Storb R. The canine major histocompatibility complex: population study of DLA-D alleles using a panel of homozygous typing cells. Tissue Antigens. 1983;21(5):360–373. [PubMed] [Google Scholar]
- 30.Storb R, Raff RF, Graham T, et al. Marrow toxicity of fractionated versus single dose total body irradiation is identical in a canine model. Int J Radiat Oncol Biol Phys. 1993;26(2):275–283. doi: 10.1016/0360-3016(93)90207-c. [DOI] [PubMed] [Google Scholar]
- 31.Storb R, Yu C, Wagner JL, et al. Stable mixed hematopoietic chimerism in DLA-identical littermate dogs given sublethal total body irradiation before and pharmacological immunosuppression after marrow transplantation. Blood. 1997;89(8):3048–3054. [PubMed] [Google Scholar]
- 32.Ladiges WC, Storb R, Thomas ED. Canine models of bone marrow transplantation. Lab Anim Sci. 1990;40(1):11–15. [PubMed] [Google Scholar]
- 33.Yu C, Ostrander E, Bryant E, Burnett R, Storb R. Use of (CA)n polymorphisms to determine the origin of blood cells after allogeneic canine marrow grafting. Transplantation. 1994;58(6):701–706. [PubMed] [Google Scholar]
- 34.Macklis RM, Kinsey BM, Kassis AI, et al. Radioimmunotherapy with alpha-particle-emitting immunoconjugates. Science. 1988;240(4855):1024–1026. doi: 10.1126/science.2897133. [DOI] [PubMed] [Google Scholar]
- 35.Claesson K, Magnander K, Kahu H, Lindegren S, Hultborn R, Elmroth K. RBE of alpha-particles from (211)At for complex DNA damage and cell survival in relation to cell cycle position. Int J Radiat Biol. 2011;87(4):372–384. doi: 10.3109/09553002.2011.538127. [DOI] [PubMed] [Google Scholar]
- 36.Omary MB, Trowbridge IS, Battifora HA. Human homologue of murine T200 glycoprotein. J Exp Med. 1980;152(4):842–852. doi: 10.1084/jem.152.4.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andres TL, Kadin ME. Immunologic markers in the differential diagnosis of small round cell tumors from lymphocytic lymphoma and leukemia. Am J Clin Pathol. 1983;79(5):546–552. doi: 10.1093/ajcp/79.5.546. [DOI] [PubMed] [Google Scholar]
- 38.Matthews DC, Appelbaum FR, Eary JF, et al. Radiolabeled anti-CD45 monoclonal antibodies target lymphohematopoietic tissue in the macaque. Blood. 1991;78(7):1864–1874. [PubMed] [Google Scholar]
- 39.Matthews DC, Badger CC, Fisher DR, et al. Selective radiation of hematolymphoid tissue delivered by anti-CD45 antibody. Cancer Res. 1992;52(5):1228–1234. [PubMed] [Google Scholar]
- 40.Mehal WZ, Azzaroli F, Crispe IN. Immunology of the healthy liver: old questions and new insights [review]. Gastroenterology. 2001;120(1):250–260. doi: 10.1053/gast.2001.20947. [DOI] [PubMed] [Google Scholar]
- 41.Prickett TC, McKenzie JL, Hart DN. Characterization of interstitial dendritic cells in human liver. Transplantation. 1988;46(5):754–761. doi: 10.1097/00007890-198811000-00024. [DOI] [PubMed] [Google Scholar]
- 42.Miyata E, Masuya M, Yoshida S, et al. Hematopoietic origin of hepatic stellate cells in the adult liver. Blood. 2008;111(4):2427–2435. doi: 10.1182/blood-2007-07-101261. [DOI] [PubMed] [Google Scholar]
- 43.Johansson AG, Lovdal T, Magnusson KE, Berg T, Skogh T. Liver cell uptake and degradation of soluble immunoglobulin G immune complexes in vivo and in vitro in rats. Hepatology. 1996;24(1):169–175. doi: 10.1002/hep.510240128. [DOI] [PubMed] [Google Scholar]
- 44.Lawrence TS, Robertson JM, Anscher MS, Jirtle RL, Ensminger WD, Fajardo LF. Hepatic toxicity resulting from cancer treatment [review]. Int J Radiat Oncol Biol Phys. 1995;31(5):1237–1248. doi: 10.1016/0360-3016(94)00418-K. [DOI] [PubMed] [Google Scholar]
- 45.Harb R, Xie G, Lutzko C, et al. Bone marrow progenitor cells repair rat hepatic sinusoidal endothelial cells after liver injury. Gastroenterology. 2009;137(2):704–712. doi: 10.1053/j.gastro.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lindencrona U, Nilsson M, Forssell-Aronsson E. Similarities and differences between free 211At and 125I: transport in porcine thyroid epithelial cells cultured in bicameral chambers. Nucl Med Biol. 2001;28(1):41–50. doi: 10.1016/s0969-8051(00)00179-7. [DOI] [PubMed] [Google Scholar]
- 47.Shellabarger CJ, Durbin PW, Parrott MW, Hamilton JG. Effects of thyroxine and KSCN on capacity of rat thyroid gland to accumulate astatine211. Proc Soc Exp Biol Med. 1954;87(3):626–629. doi: 10.3181/00379727-87-21465. [DOI] [PubMed] [Google Scholar]
- 48.Ron E, Brenner A. Non-malignant thyroid diseases after a wide range of radiation exposures [review]. Radiat Res. 2010;174(6):877–888. doi: 10.1667/RR1953.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuhr CS, Allen MD, Junghanss C, et al. Tolerance to vascularized kidney grafts in canine mixed hematopoietic chimeras. Transplantation. 2002;73(9):1487–1493. doi: 10.1097/00007890-200205150-00020. [DOI] [PubMed] [Google Scholar]