Abstract
The deoxycytidine analog decitabine (DAC) can deplete DNA methyl-transferase 1 (DNMT1) and thereby modify cellular epigenetics, gene expression, and differentiation. However, a barrier to efficacious and accessible DNMT1-targeted therapy is cytidine deaminase, an enzyme highly expressed in the intestine and liver that rapidly metabolizes DAC into inactive uridine counterparts, severely limiting exposure time and oral bioavailability. In the present study, the effects of tetrahydrouridine (THU), a competitive inhibitor of cytidine deaminase, on the pharmacokinetics and pharmacodynamics of oral DAC were evaluated in mice and nonhuman primates. Oral administration of THU before oral DAC extended DAC absorption time and widened the concentration-time profile, increasing the exposure time for S-phase–specific depletion of DNMT1 without the high peak DAC levels that can cause DNA damage and cytotoxicity. THU also decreased interindividual variability in pharmacokinetics seen with DAC alone. One potential clinical application of DNMT1-targeted therapy is to increase fetal hemoglobin and treat hemoglobinopathy. Oral THU-DAC at a dose that would produce peak DAC concentrations of less than 0.2μM administered 2×/wk for 8 weeks to nonhuman primates was not myelotoxic, hypomethylated DNA in the γ-globin gene promoter, and produced large cumulative increases in fetal hemoglobin. Combining oral THU with oral DAC changes DAC pharmacology in a manner that may facilitate accessible noncytotoxic DNMT1-targeted therapy.
Introduction
The deoxycytidine analog decitabine (DAC) can deplete DNA methyl-transferase 1 (DNMT1), a key chromatin-modifying enzyme, and thereby modify cellular epigenetics, gene expression, and differentiation.1 Potential clinical applications include increasing erythropoiesis and fetal hemoglobin (HbF) expression to treat hemoglobinopathies,2 inducing terminal differentiation in malignant cells,3–7 and increasing self-renewal of normal hematopoietic stem cells.8–10 Certain aspects of DAC's pharmacology and mechanism of action influence its clinical activity; unlike cytidine analogs such as cytarabine (AraC) or gemcitabine, the sugar moiety of DAC is unmodified. Therefore, at low concentrations, DAC does not terminate DNA chain synthesis11,12 and can deplete DNMT1 without causing significant DNA damage or cytotoxicity both in vitro and in vivo.2,3,11–15 However, at high concentrations, similar to other nucleoside analogs, DAC is cytotoxic. Another important aspect of DAC action is that it is S-phase specific, so exposure timing critically influences efficacy.13,16 Considering these properties of DAC, for the objective of noncytotoxic DNMT1 depletion, the ideal DAC concentration-time profile is low peak drug levels but extended time above minimum concentrations required to deplete DNMT1. Oral administration of DAC could be more likely to produce this concentration-time profile than parenteral administration and would have major logistical advantages. A significant physiologic barrier to DAC oral bioavailability is the enzyme cytidine deaminase (CDA), which is highly expressed in the gut and liver of humans and mice and metabolizes cytidine, deoxycytidine, and analogs thereof into uridine counterparts that cannot deplete DNMT1.17–20 CDA drastically decreases the half-life of DAC to < 20 minutes in vivo21,22 from 5-16 hours at 37°C in vitro.21,22 Furthermore, nonsynonymous single nucleotide polymorphisms in CDA produce person-to-person variability in CDA enzyme activity23–28 and, consequently, clinically significant variation in cytidine analog pharmacokinetics, toxicity, and efficacy that could be amplified with oral administration.29
The uridine analog tetrahydrouridine (THU) is a competitive inhibitor of CDA that has been used as a CDA inhibitor in combination with cytidine analogs both preclinically and clinically for some decades without documentation of toxic side effects.18,19,30–39 Therefore, combination therapy with THU could be used to address the pharmacologic limitations of oral DAC alone. Accordingly, in the present study, the effects of different doses and timing of THU on the pharmacokinetics and pharmacodynamics of oral DAC were evaluated in mice and nonhuman primates to determine whether this combination advances the objective of more accessible and potentially noncytotoxic DNMT1-targeted therapy.
Methods
Drug administration and other procedures in Papio anubis (baboon)
All procedures with baboons (including pharmacokinetic and methylation studies) were approved by the Institutional Animal Care and Use Committee of the University of Illinois at Chicago. All animals were female and their weights are shown in Table 1. Female baboons were chosen for greater ease of handling compared with the more aggressive males. DAC was dissolved in 50mM KH2PO4 buffer at a concentration of 2.5 mg/mL. THU was dissolved in H2O at a concentration of 20 mg/mL. Drugs were administered by IV or subcutaneous (SC) injection or oral gavage through a 9-mm internal diameter endotracheal tube inserted in the esophagus while the animal was anesthetized with ketamine (10 mg/kg)/xylazine (1 mg/kg). BM aspirate for assessment of BM cytotoxicity and DNA methylation were performed during anesthesia. Blood samples (5 mL) for pharmacokinetic analysis were drawn from the femoral vein during anesthesia. Blood was collected at 7 time points, with the most distal time point in the nonhuman primate studies determined by the duration of allowable anesthesia (4 hours) according to the animal care protocols. Therefore, the last sampling point was at 240 minutes for baboons treated with DAC alone and 180 minutes for animals receiving THU 60 minutes before DAC. Blood samples were drawn for < 1 minute) into tubes preloaded with heparin and THU 10 μg/mL to prevent in vitro degradation of DAC and immediately transferred onto ice. These samples were then centrifuged as soon as possible at 600g for 5 minutes at 4°C. After separation, plasma was transferred in 0.2-mL aliquots into prefrozen vials and stored frozen at −80°C until shipment on dry ice for analysis. Experiments in the same animals were conducted after > 2 week washout periods. For repeat-dose administration studies in baboons, phlebotomy was performed 2×/wk to maintain a hematocrit of approximately 25% to model the anemia and reactive BM state of humans with hemoglobinopathy.
Table 1.
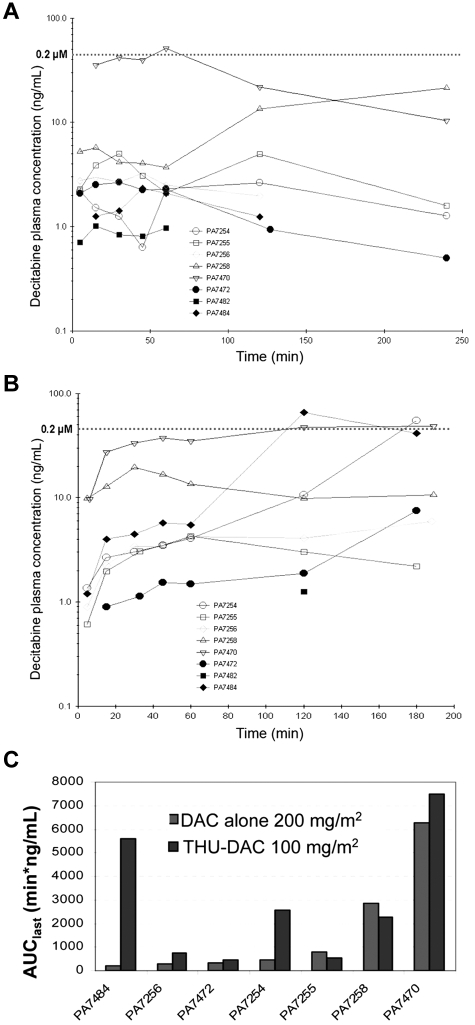
AUClast after oral DAC versus oral THU-DAC in the same baboons
| Weight, kg | AUClast, min/ng/mL* |
|||
|---|---|---|---|---|
| DAC, 200 mg/m2 alone | DAC, 100 mg/m2 alone | DAC 100 mg/m2 60 min after THU 400 mg/m2 | ||
| Baboon no. | ||||
| PA7482† | 12.3 | 49.98 | ND | Not quantifiable |
| PA7484 | 11.5 | 190.35 | 252.73 | 5621.05 |
| PA7256 | 19.8 | 299.02 | ND | 760.27 |
| PA7472 | 10.7 | 327.25 | ND | 444.825 |
| PA7254 | 14.4 | 463 | ND | 2587 |
| PA7255 | 19.9 | 807.8 | ND | 533.47 |
| PA7258 | 12.6 | 2863.6 | ND | 2284.48 |
| PA7470 | 9.6 | 6278.78 | 1219.98 | 7515.32 |
| Mean ± SD | 1604 ± 2262 | 2821 ± 2749 | ||
| Median ± IQR | 463 ± 2565 | 2284 ± 5088 | ||
| Median ± IQR per mg of DAC‡ | 160 ± 226 | 457 ± 1017 | ||
| AUClast variation | ∼ 30-fold | ∼ 14-fold | ||
| AUClast coefficient of variation | 1.41 | 0.97 | ||
ND indicates not done; and IQR, interquartile range.
AUClast calculated over 240 minutes for DAC alone and 180 minutes for THU-DAC.
Data from PA7482 were not included in calculations of mean, median, -fold variation, or coefficients of variation because levels were below the lower limit of quantification at multiple time points.
Significantly different between the DAC-only group and the THU-DAC group (P = .02 by Wilcoxon test).
Drug administration and other procedures in CD-1 mice
Procedures with CD-1 strain mice were approved by the Institutional Animal Care and Use Committee of Avanza. Animals were dosed with DAC or its vehicle 1 hour ± 5 minutes after administration of THU. Administration of both vehicles and test articles was performed via oral gavage at a dose volume of 10 mL/kg (based on the most recent body weight). Blood samples (approximately 0.5 mL or the maximum possible) were collected via intracardiac puncture from nonfasted, anesthetized (70% CO2/30% O2) animals at 15, 30, 60, 90, 120, and 180 minutes after administration of DAC. Sample collection tubes were prepared before each collection day by adding 10 μL/tube of a 10 mg/mL THU solution. This THU solution was prepared by adding sodium phosphate dibasic (1.5 mg/mL), sodium phosphate monobasic (0.4 mg/mL), and THU (10 mg/mL) to sterile water for injection and mixing until visually clear. When DAC was not administered at the target time (1 hour after THU administration), the sample collection time was adjusted accordingly. Samples were collected from the first available 3 animals per time point. All samples were collected within 5 minutes of the target time. To be consistent with the baboon studies, reported results are from female mice only.
Measurement of DAC levels using LC-MS/MS
The LC-MS/MS method has been described previously for the determination of DAC in human and rat plasma.21 The modification of these methods for determination of levels in baboon plasma is described in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Pharmacokinetic analysis
Pharmacokinetic data were derived by noncompartmental analysis or a 2-compartment model with instantaneous input (IV data) using WinNonlin Professional Version 5.2 software (Pharsight). The model-fitting method allowed estimation of terminal half-lives for some datasets. The the area under the curve from the time of dosing to the last measurable concentration (AUClast) was calculated using the linear trapezoidal method. The AUC values for bioavailability estimates were truncated to 60 minutes, which was the time of the last measurable plasma concentration after IV administration.
Body surface area estimation in baboons
Conversion of the milligrams per kilogram dose in baboons into milligrams per square millimeter estimations was based on Michaelis constant (km) values for baboons obtained from US Food and Drug Administration published guidelines. In brief, the baboon dose in milligrams per kilogram was multiplied by the km of 20 to convert the dose to milligrams per square millimeter. The estimated average weight of baboons in the guidelines is 12 kg; the average weight of the baboons used in these studies was 14 kg.
Analysis of γ-globin gene methylation
Erythroid cells were isolated from nucleated BM and fetal liver cells obtained under ketamine/xylazine anesthesia or from euthobarb-euthanized fetuses, by immunomagnetic column separation using baboon anti-RBC mouse mAb (#551299; BD Biosciences) in combination with rat anti–mouse IgG1 magnetic microbeads (#130-047-101; Miltenyi Biotec). After extraction of DNA and bisulfite conversion, quantitative analysis DNA methylation of specific CpG sites within the baboon β-globin gene locus (coordinates based on human β-globin gene locus http://www.ncbi.nlm.nih.gov/nuccore/U01317) was performed by base-specific cleavage and mass spectrometry using the EpiTYPER MassARRAY analysis system (Sequenom). PCR amplification of specific regions of the baboon β-globin gene locus from bisulfite-converted DNA was performed using Hot Star Taq polymerase (QIAGEN) and primers containing the 5′ T7 and 10-mer tags. The tags were selected using Sequenom epidesigner software beta (www.epidesigner.com) based on the baboon DNA sequence generated in our laboratory by PCR amplification of baboon genomic DNA using primers corresponding to regions of the human β-globin gene locus. PCR conditions were optimized to yield single bands by agarose gel analysis in all reactions. Treatment of PCR products with shrimp alkaline phosphatase, in vitro transcription, and RNase treatment were performed in a MassARRAY Liquid Handler using reagents supplied and conditions specified by the manufacturer (Sequenom EpiTYPER Application Guide). DNA methylation of specific CpG residues was quantitated using EpiTYPER software.
Measurement of HbF levels
Analysis of globin chains was performed on a TSP Spectra HPLC system using a LiChristopher 100 RP-8 column and a gradient of acetonitrile-methanol-NaCl.
DNA damage measurement by γH2AX staining
Phosphorylation of the histone H2A family member H2AX at Ser139 (γH2AX) was measured by flow cytometry. Buffy coat–nucleated cells were fixed with 4% paraformaldehyde and then permeabilized with ice-cold 70% ethanol. Cells were then incubated in blocking solution (0.5% BSA) containing saturating concentration of Alexa Fluor 488–conjugated γH2AX Ab (clone N1-431; BD Biosciences). The percentage of γH2AX–positive cells is analyzed using a Coulter Epics XL-MCL flow cytometer equipped with CXP software.
SDS-PAGE and Western blotting
Approximately 100 μg of cytoplasmic and nuclear protein extracts from cells, together with molecular weight markers, were subjected to SDS-PAGE on 4%-12% gradient gels (Invitrogen), followed by transfer to PVDF membranes (Invitrogen). Blots were probed using Abs for DNMT1 (Abcam ab16632) and anti–β-actin peroxidase (#A3854; Sigma-Aldrich).
Results
In vitro studies to identify a DAC concentration that depletes DNMT1 without causing significant DNA damage or apoptosis in normal hematopoietic precursors
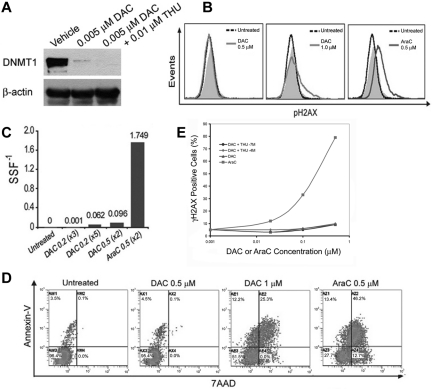
The effects of DAC on DNA damage, apoptosis, and DNMT1 levels in normal CD34+ hematopoietic precursors isolated from cord blood was examined to identify a concentration range that depletes DNMT1 without cytotoxicity. AraC, a cytidine analog that terminates DNA chain synthesis, was used as a positive control for DNA damage and apoptosis induction. DAC 0.005μM alone and in combination with THU 0.01μM substantially depleted DNMT1 (Figure 1A). Concentrations of DAC up to 0.5μM did not cause measurable DNA damage, as measured by levels of phospho-H2AX (γH2AX) and the Fast Micromethod for DNA scission (Figure 1B-C), or apoptosis, as measured by annexin staining (Figure 1D). DAC at 1μM caused measurable DNA damage and apoptosis (Figure 1B-D), although not to the same extent as AraC 0.5μM (Figure 1B-D). In the presence of THU 0.1 or 100μM, DAC up to 0.5μM did not significantly increase DNA damage, as measured by γH2AX (Figure 1E).
Figure 1.
Effects of DAC on DNMT1 depletion, DNA damage, and apoptosis in normal hematopoietic precursors. (A) DAC 0.005μM depletes DNMT1 in normal hematopoietic precursors. Normal CD34+ cells were isolated from cord blood. DAC 0.005μM was added once daily on days 1-4 and DNMT1 was quantified by Western blot on day 5. (B-C) DAC > 0.5μM was required to induce measurable DNA damage. Twenty-four hours after DAC or AraC exposure, DNA damage was measured by flow-cytometric assessment for phosphorylation of histone H2AX (γH2AX; B) or the Fast Micromethod for DNA scission (C). Equimolar levels of AraC were used as positive controls. Gray histogram is the isotype control. (D) DAC > 0.5μM was required to induce apoptosis. Twenty-four hours after DAC or AraC exposure, apoptosis was measured by flow-cytometric assessment for annexin staining. Double annexin/7-amino-actinomycin D (7AAD)–positive cells represent late apoptosis/necrosis. (E) DAC up to 0.5μM in combination with THU did not cause significant DNA damage, as measured by flow-cytometric assessment of γH2AX levels 24 hours after addition of the drug to normal hematopoietic precursors. Results are expressed as a percentage of vehicle-treated controls.
DAC concentration-time profile after IV, SC, and oral administration to nonhuman primates
In vitro studies have suggested that high peak DAC concentrations are unnecessary for DNMT1 depletion and may increase the risk for cytotoxicity. To compare the effect of different routes of administration on the DAC concentration-time profile, plasma DAC levels were compared after IV or SC administration versus oral administration to the same animals (washout period of ≥ 2 weeks between drug administrations to the same animal). In baboon number PA7472, IV DAC 10 mg/m2 produced a peak drug level of > 1.3μM (300 ng/mL) and α half-life < 5 minutes. In contrast, the peak drug level with oral DAC 200 mg/m2 was < 0.015μM and half-life > 100 minutes (supplemental Figure 1A). A lower peak drug level but longer half-life with oral compared with IV administration was also observed in baboon number PA7482 (supplemental Figure 1B). In baboon number PA7254, SC DAC 10 mg/m2 produced a peak drug level of 0.36μM and a half-life of < 50 minutes. In contrast, the peak drug level with oral DAC 200 mg/m2 was < 0.015μM and the half-life was > 150 minutes (supplemental Figure 1C). A lower peak drug level but a longer half-life with oral compared with SC administration was also observed in baboon number PA7258 (supplemental Figure 1D).
Identification of dose and timing of oral THU to increase oral bioavailability of DAC in nonhuman primates
In 2 female baboons, PA7470 and PA7484 (selected for high and low bioavailability of oral DAC alone, respectively), THU 400 mg/m2 (20 mg/kg) 60 minutes before DAC 100 mg/m2 (5 mg/kg) produced higher DAC concentrations than THU 40 mg/m2 (2 mg/kg; supplemental Figure 2A-B). In these same baboons after a washout period, THU 400 mg/m2 60 minutes before DAC produced higher DAC concentrations than THU 400 mg/m2 administered simultaneously or 30 minutes before DAC (supplemental Figure 2A-B).
Effect of THU on DAC oral bioavailability and interindividual variability in nonhuman primates
Eight female baboons were treated with oral DAC 200 mg/m2 (10 mg/kg). The median AUClast with oral DAC alone was 463 min/ng/mL, with a range of 190-6279 min/ng/mL, an approximately 33-fold variation, and a coefficient of variation of 1.41 (Table 1 and Figure 2A). After a washout period of > 2 weeks, the same animals were treated with DAC 100 mg/m2 (5 mg/kg; half the dose used for the DAC alone experiments) 60 minutes after oral THU 400 mg/m2. The median AUClast with THU-DAC was 2284 min/ng/mL, with a range of 534-7515 min/ng/mL, an approximately 14-fold variation, and a coefficient of variation of 0.97 (Table 1 and Figure 2B). The average DAC Cmax was 0.05μM (10.85 ng/mL) for DAC alone and 0.12μM (26.98 ng/mL) for THU-DAC (DAC at half the dose; Figure 2A-B). The decrease in the coefficient of variation in the THU-DAC group was because the largest AUClast increases occurred in animals that had the poorest bioavailability with DAC alone (Table 1 and Figure 2C). The AUClast difference between DAC alone and THU-DAC was statistically significant (P = .02 by Wilcoxon test; Table 1), even though AUClast was calculated at 25% more time for DAC alone (240 minutes vs 180 minutes for THU-DAC). The last measured DAC plasma level was the highest level observed in 4 of 7 THU-DAC–treated animals with DAC levels measurable at more than one time point, and half-life was not reached at 180 minutes in any of these 7 animals (Figure 2B). Therefore, AUClast values for THU-DAC are likely to be underestimates.
Figure 2.
Prior administration of oral THU increases oral bioavailability and decreases interindividual variability in pharmacokinetics of DAC. (A) DAC concentration-time profiles in 8 baboons administered oral DAC 200 mg/m2 (10 mg/kg). (B) DAC concentration-time profiles in the same 8 baboons administered DAC at half the dose (100 mg/m2 [5 mg/kg]) 60 minutes after THU 400 mg/m2 (20 mg/kg; THU-DAC). PK measurements went to 180 instead of 240 minutes because of the allowable duration of anesthesia. (C) The largest increases in AUClast with coadministration of THU were seen in animals with lower intrinsic oral bioavailability of DAC. Histograms show the distribution of AUClast in 7 animals administered DAC alone versus the same 7 animals receiving DAC at half the dose after THU. AUC measurements went to 180 instead of 240 minutes in the THU-DAC group because of the allowable duration of anesthesia.
Effect of THU on oral DAC pharmacokinetics in mice
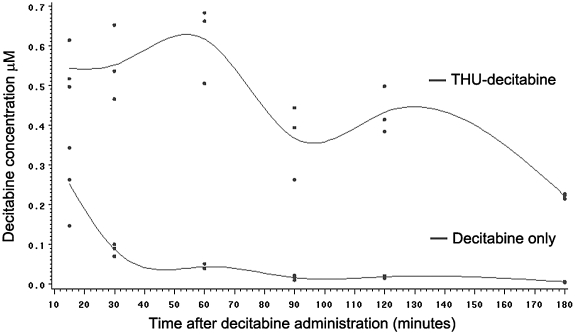
To more completely evaluate the effect of THU on DAC pharmacokinetics to an extent not possible in nonhuman primate studies, studies were conducted in mice. Using oral gavage, female CD-1 mice were administered THU 400 mg/m2 (167 mg/kg) 60 minutes before DAC 0.3, 0.6, or 1.2 mg/m2 (0.1, 0.2, or 0.4 mg/kg, respectively) or DAC 1.2 mg/m2 alone (vehicle was administered instead of THU) twice a week for 3 weeks, and pharmacokinetics were measured after administration of the last dose (day 16). THU extended the period of DAC absorption: the concentration-time curve was widened by early and late absorption (2-parallel first-order absorption; Figure 3). This effect of THU on the shape of the DAC concentration-time profile was reflected in a 9-fold increase in AUCtotal (from 8.45 min/μM with DAC alone to 76.24 min/μM with THU-DAC), compared with a 2.5-fold increase in Cmax (from 0.251 to 0.617μM; Table 2). There was a linear relationship between oral THU-DAC dose and DAC Cmax and AUCtotal (Table 2). The coefficients of variation for AUCtotal were substantially lower than in baboons: 0.24 for DAC 1.2 mg/m2 alone and 0.15 for THU-DAC 1.2 mg/m2 (Table 2).
Figure 3.
The DAC concentration-time profile in mice administered DAC alone or DAC 60 minutes after THU. Drugs were administered by oral gavage. Dots show values from 3 mice for each time point in each treatment group. THU-DAC indicates DAC 0.4 mg/kg 60 minutes after THU 167 mg/kg. DAC indicates DAC 0.4 mg/kg 60 minutes after vehicle.
Table 2.
Pharmacokinetic parameters after oral DAC versus oral THU-DAC in female mice (mean values from 3 mice at each time point in each group)
| Group | THU-DAC 0.1 | THU-DAC 0.2 | THU-DAC 0.4 | DAC 0.4 only |
|---|---|---|---|---|
| DAC dose, mg/kg | 0.1 | 0.2 | 0.4 | 0.4 |
| THU dose, mg/kg | 167 | 167 | 167 | 0 |
| AUCt, min/μM | 17.90 | 35.64 | 76.24 | 8.45 |
| Cmax, μM | 0.138 | 0.338 | 0.617 | 0.251 |
| AUCt coefficient of variation | 0.15 | 0.24 |
Drugs were administered 2 times per week for 3 weeks and blood for pharmacokinetic analysis was collected on the last day of dosing (day 16).
Pharmacodynamic effects in nonhuman primates of repeat-dose oral THU-DAC
To evaluate pharmacodynamic effects with repeat-dose administration, oral THU-DAC 2×/wk for 8 weeks was administered to 4 baboons. Two animals, one each with relatively low and high oral THU-DAC bioavailability (baboon numbers PA7472 and PA7470, respectively) per the pharmacokinetic studies (Table 1), received oral DAC 5 mg/m2 after oral THU 400 mg/m2. Similarly, a pair of animals from the low and high end of the oral THU-DAC pharmacokinetic range (baboon numbers PA7482 and PA7484, respectively) received oral DAC 10 mg/m2 after oral THU. Oral DAC 5 and 10 mg/m2 after THU was expected to produce a Cmax of approximately 0.006 and 0.012μM, respectively, because there is a linear relationship between THU-DAC dose and pharmacokinetic parameters (supplemental Figures 2-3), and oral DAC 100 mg/m2 after THU produced an average Cmax of 0.12μM.
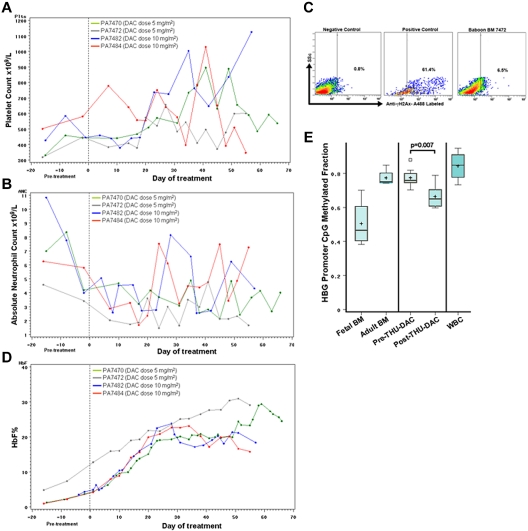
Noncytotoxic modification of hematopoietic differentiation by DAC is expected to produce increases in platelets and decreases in neutrophil counts, as suggested by previous in vitro and clinical studies.2,8,15 In contrast, cytotoxic therapy is expected to produce concurrent decreases in platelets and neutrophils. In the 4 baboons, platelet counts increased during weeks 1-4 of drug administration (Table 3 and Figure 4A). Although this upward trend reversed in 2 baboons during weeks 6-8 of drug administration, platelet counts did not decrease below the lower limit of normal (Figure 4A). Neutrophil counts decreased during weeks 1-3 of drug administration (Table 3 and Figure 4B), but then increased again or remained stable during weeks 4-8 of drug administration (Figure 4B). In baboon number PA7472, a BM aspirate 96 hours after THU-DAC administration provided sufficient cells for analysis of DNA damage/repair by γH2AX. There was a small increase in γH2AX compared with negative control that was not suggestive of major DNA damage, although early DNA damage would have been missed (Figure 4C). In additional experiments, oral THU-DAC was administered 3×/wk with 50% of the various DAC doses, again with concurrent increases in platelet and decreases in neutrophil counts (supplemental Figure 3).
Table 3.
Repeat-dose administration of oral THU-DAC in 4 baboons: effects on HbF percentage and neutrophil and platelet counts
| Baboon no. | DAC dose, mg/m2* | Schedule | Pretreatment HbF, % | Peak HbF, % | ΔHbF | ANC nadir, ×109/L | Platelet count maximum, ×109/L |
|---|---|---|---|---|---|---|---|
| 7470 | 5 | 2×/wk for 8 wks | 9.9 | 29.3 | 29.3 | 2.13 | 895 |
| 7472 | 5 | 2×/wk for 8 wks | 12.8 | 30.1 | 30.1 | 1.46 | 600 |
| 7482 | 10 | 3×/wk for 3 wks 2×/wk for 5 wks | 3.5 | 23.8 | 20.3 | 2.58 | 1004 |
| 7484 | 10 | 2×/wk for 8 wks | 4.2 | 23.1 | 23.1 | 1.68 | 1029 |
Two of these animals had relatively low bioavailability (baboons PA7472 and PA7482) and 2 had relatively high oral THU-DAC bioavailability (baboons PA7484 and PA7470) according to the pharmacokinetic studies.
ANC indicates absolute neutrophil count.
Administered after THU 400 mg/m2.
Figure 4.
Pharmacodynamic effects of repeat-dose oral THU-DAC in nonhuman primates. A baboon with relatively low and a baboon with relatively high oral THU-DAC bioavailability in the pharmacokinetic (PK) studies (baboon numbers PA7472 and PA7470, respectively) received DAC 5 mg/m2, and another pair from each end of the PK range (baboon numbers PA7482 and PA7484, respectively) received DAC 10 mg/m2. DAC was administered 60 minutes after THU 400 mg/m2 2×/wk for 8 weeks. (A) Platelet counts during drug administration. (B) Absolute neutrophil counts during drug administration. (C) Phospho-H2AX (γH2AX) labeling of BM cells 96 hours after THU-DAC administration in week 8 in baboon number PA7472. Positive control HeLa cells treated with camptothecin 10μM. Negative control vehicle treated HeLa cells. (D) HbF expression during treatment. (E) Decrease in methylation of developmentally responsive CpG in the γ-globin gene (HBG) promoter after drug administration in baboon numbers PA7472 and PA7484. Based on the human β-globin gene locus (http://www.ncbi.nlm.nih.gov/nuccore/U01317), the coordinates of these CpGs were 33105, 33221, 34425, and 34483. Mass spectrometry was used to measure methylation levels in DNA extracted from erythroid precursors isolated from fetal BM (FBM), adult BM (ABM), before THU-DAC (pre-THU-DAC), and after 8 weeks of 2×/wk oral THU-DAC (post-THU-DAC) in baboon numbers PA7472 and PA7478, and from WBCs. Box-plot boundaries: interquartile range; horizontal line, median; +, mean; small boxes, outlying values; whiskers, range of values. P values are by Wilcoxon test.
One potential application of long-term DNMT1-depleting therapy is to increase HbF (α2γ2) expression to treat hemoglobinopathies such as sickle cell disease and β-thalassemia. In all 4 animals, HbF levels increased progressively during weeks 1-4 of drug administration (Table 3 and Figure 4D). From weeks 5-8, there was a small decrease from these peak HbF levels in the 2 animals receiving the higher dose of DAC (10 mg/m2), whereas levels continued to increase in the 2 animals receiving DAC 5 mg/m2 (Figure 4D), producing higher peak HbF levels (Table 3). Progressive HbF increases were also noted with 50% of the DAC doses administered 3×/wk after THU (supplemental Figure 3). One intended molecular effect of therapy is to decrease methylation at promoter CpGs that regulate the expression of target genes (eg, the γ-globin gene [HBG] promoter CpG). Methylation levels of 4 CpG sites in the HBG promoter was measured by mass spectrometric analysis of DNA extracted from BM erythroid precursors (from baboon numbers PA7472 and PA7484). After treatment with oral THU-DAC, the methylation levels of these HBG promoter CpGs decreased by approximately 20% compared with pretreatment methylation levels (P = .007; Figure 4E). The relevance of these CpG sites to physiologic regulation of HBG expression was suggested by significant hypomethylation in DNA isolated from fetal BM erythroid precursors versus adult BM erythroid precursors and by significant hypermethylation in DNA isolated from peripheral WBCs (Figure 4E).
Discussion
In both baboons and mice, oral administration of THU to inhibit CDA before oral administration of DAC extended DAC absorption time and widened the DAC concentration-time profile, as reflected in mice by a 9-fold increase in DAC AUCtotal compared with a 2.5-fold increase in DAC Cmax. Because DNMT1 depletion by DAC can occur at very low drug levels but depends on exposure timing,1,16,40,41 the wider concentration-time profile achieved with oral THU-DAC could have efficacy advantages with regard to DNMT1 depletion without the high peak DAC levels that can cause DNA damage and cytotoxicity.
The baboon model has been accurate and useful in predicting by body surface area scaling a safe human equivalent dose for SC DAC treatment and for combination oral THU and 5-azacytidine therapy.2,18,19,33,42–46 Therefore, the THU dose (400 mg/m2) and timing (60 minutes before DAC) that are likely to be useful for human translation were identified by studies in baboons. In the pharmacokinetic studies in baboons, AUClast estimates for DAC alone were calculated over 240 minutes, whereas estimates for THU-DAC were calculated over 180 minutes (the permissible total duration of anesthesia in the nonhuman primate studies was 240 minutes; therefore, the administration of DAC 60 minutes after THU decreased the duration of sampling in THU-DAC–administered animals). Furthermore, the concentration-time profiles suggested that DAC levels may have continued to increase beyond the last sampling time in many THU-DAC–treated animals. Therefore, the presented values underestimate the increase in DAC bioavailability produced by preceding THU administration in baboons. Although the murine studies enabled more comprehensive analyses of the effects of THU on DAC pharmacokinetics, dose-exposure extrapolation by body surface area scaling from mice to humans is not useful, because dose for dose, there is a more than 100-fold greater DAC exposure in rodents versus humans.21 Similarly, there is more than 100-fold greater exposure of the cytidine analog AraC in mice versus monkeys dose for dose.47 The reasons for this log-scale increase in cytidine analog exposure in rodents compared with primates are unknown.
An important limitation of DAC and other cytidine analogs is interindividual variations in pharmacokinetics, toxicity, and efficacy that are associated with single nucleotide polymorphisms in CDA.23–29,48,49 THU, by inhibiting CDA, might attenuate the role of these pharmacogenetic variations in cytidine analog pharmacokinetics and pharmacodynamics, because in the baboons, THU decreased substantial interindividual variability in DAC pharmacokinetics compared with oral DAC alone. Similarly, THU decreased substantial interindividual variability in HbF elevations (peak levels ranged between 23% and 30%) compared with previous experience with oral DAC alone (peak levels ranged from 10%-60%50). One caveat to these studies, in addition to the small number of baboons analyzed, is that the basis for the interindividual variability in baboons has not been characterized, and baboon interindividual variability may be substantially greater than that in humans receiving oral cytidine analog.29 Consistent with a genetic contribution to interindividual variability, variability in mice of the same gender was substantially less than in baboons. Combination therapy with THU might offer other advantages, because CDA up-regulation is a putative mechanism of cancer cell resistance to cytidine analogs, and cancer cells may find sanctuary from cytidine analogs in tissues expressing high levels of CDA (see supplemental References). These possible advantages of combining THU with DAC will require evaluation in further preclinical and clinical studies.
One potential clinical application of DNMT1-targeted therapy is to increase HbF expression as a treatment for sickle cell disease and β-thalassemia.2,15 In baboons, repeat administration of oral THU-DAC using a DAC dose that would produce peak DAC concentrations less than 0.2μM was not myelotoxic, hypomethylated HBG promoter CpG, and produced large cumulative increases in HbF expression in RBCs. The potential of this approach has also been suggested by a clinical trial combining oral THU with oral 5-azacytidine to treat 2 patients with sickle cell disease (5-azacytidine is similar to DAC but with a ribose instead of deoxyribose sugar moiety)33: the oral THU dose was 1.5-2.0 mg/kg (∼ 75 mg/m2) in divided doses 60 minutes before and then again simultaneously with oral 5-azacytidine 0.2 mg/kg (∼ 7.5 mg/m2) 3×/wk for 28-72 weeks. No decreases in platelet, WBC, or reticulocyte counts were observed with this regimen, and the intended pharmacodynamic effect, an increase in HbF, occurred and was accompanied by clinical improvement.33 In the present studies, DAC has been used instead of 5-azacytidine because for the purposes of targeting DNMT1, the RNA incorporation that occurs with 5-azacytidine is an off-target effect. A phase 1 evaluation of oral THU-DAC in patients with sickle cell disease has been initiated. Although the present data, the structure of DAC, and previous preclinical and clinical studies indicate that noncytotoxic DNMT1 depletion with DAC is possible,2,3,11–15 DNMT1 depletion occurs after DNA incorporation of DAC and postreplicative immobilization of DNMT1, and therefore genotoxicity remains a possible side effect that will have to be evaluated in clinical studies with oral THU-DAC.
In conclusion, in baboons and mice, preceding administration of oral THU substantially increases oral bioavailability of DAC, creates a concentration-time profile that suits the purpose of DNMT1 depletion with less cytotoxicity, and decreases interindividual variability. Therefore, combination oral THU-DAC might facilitate more accessible, safe, and efficacious DNMT1-targeted therapy.
Supplementary Material
Acknowledgments
The authors acknowledge the assistance of and comments from the National Institutes of Health Rapid Access to Interventional Development (NIH-RAID) program, in particular, Jim Cradock.
This work was supported by the National Heart, Lung, and Blood Institute (U54HL090513), the National Institute of Diabetes and Digestive and Kidney Diseases, Congressionally Directed Medical Research Programs (PR081404), and the National Cancer Institute (contracts N01-CM-52205 and N01-CM-42204 under the NIH-RAID Pilot Program).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: D.L., K.V., Y.L., M.A.R., R.M., K.P.N., S.N., and K.J.E. performed the experiments; D.L., N.S., P.T., K.J.E., J.C., K.K.C., J.D., and Y.S. designed the experiments and analyzed the data; J.D. and Y.S. obtained the funding; and Y.S. wrote the manuscript with editorial input from D.L., N.S., P.T., K.J.E., J.C., K.K.C., and J.D.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yogen Saunthararajah, MD, Taussig Cancer Institute, 9500 Euclid Ave R40, Cleveland, OH 44195; e-mail: saunthy@ccf.org.
References
- 1.Santi DV, Garrett CE, Barr PJ. On the mechanism of inhibition of DNA-cytosine methyltransferases by cytosine analogs. Cell. 1983;33(1):9–10. doi: 10.1016/0092-8674(83)90327-6. [DOI] [PubMed] [Google Scholar]
- 2.Saunthararajah Y, Hillery CA, Lavelle D, et al. Effects of 5-aza-2′-deoxycytidine on fetal hemoglobin levels, red cell adhesion, and hematopoietic differentiation in patients with sickle cell disease. Blood. 2003;102(12):3865–3870. doi: 10.1182/blood-2003-05-1738. [DOI] [PubMed] [Google Scholar]
- 3.Jones PA, Taylor SM. Cellular differentiation, cytidine analogs and DNA methylation. Cell. 1980;20(1):85–93. doi: 10.1016/0092-8674(80)90237-8. [DOI] [PubMed] [Google Scholar]
- 4.Pinto A, Attadia V, Fusco A, et al. 5-Aza-2′-deoxycytidine induces terminal differentiation of leukemic blasts from patients with acute myeloid leukemias. Blood. 1984;64(4):922–929. [PubMed] [Google Scholar]
- 5.Negrotto S, Hu Z, Alcazar O, et al. Noncytotoxic differentiation treatment of renal cell cancer. Cancer Res. 2011;71(4):1431–1441. doi: 10.1158/0008-5472.CAN-10-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ng KP, Ebrahem Q, Negrotto S, et al. p53 Independent epigenetic-differentiation treatment in xenotransplant models of acute myeloid leukemia. Leukemia. 2011;25(11):1739–50. doi: 10.1038/leu.2011.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alcazar O, Achberger S, Aldrich W, et al. Epigenetic regulation by decitabine of melanoma differentiation in vitro and in vivo [published online ahead of print July 27, 2011]. Int J Cancer. doi: 10.1002/ijc.26320. doi: 10.1002/ijc.26320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milhem M, Mahmud N, Lavelle D, et al. Modification of hematopoietic stem cell fate by 5aza 2′deoxycytidine and trichostatin A. Blood. 2004;103(11):4102–4110. doi: 10.1182/blood-2003-07-2431. [DOI] [PubMed] [Google Scholar]
- 9.Hu Z, Negrotto S, Gu X, et al. Decitabine maintains hematopoietic precursor self-renewal by preventing repression of stem cell genes by a differentiation-inducing stimulus. Mol Cancer Ther. 2010;9(6):1536–1543. doi: 10.1158/1535-7163.MCT-10-0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Negrotto S, Ng KP, Jankowska AM, et al. CpG methylation patterns and decitabine treatment response in acute myeloid leukemia cells and normal hematopoietic precursors [published online ahead of print August 12, 2011]. Leukemia. doi: 10.1038/leu.2011.207. doi: 10.1038/leu.2011.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Covey JM, D'Incalci M, Tilchen EJ, Zaharko DS, Kohn KW. Differences in DNA damage produced by incorporation of 5-aza-2′-deoxycytidine or 5,6-dihydro-5-azacytidine into DNA of mammalian cells. Cancer Res. 1986;46(11):5511–5517. [PubMed] [Google Scholar]
- 12.Schermelleh L, Haemmer A, Spada F, et al. Dynamics of Dnmt1 interaction with the replication machinery and its role in postreplicative maintenance of DNA methylation. Nucleic Acids Res. 2007;35(13):4301–4312. doi: 10.1093/nar/gkm432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Momparler RL, Goodman J. In vitro cytotoxic and biochemical effects of 5-aza-2′-deoxycytidine. Cancer Res. 1977;37(6):1636–1639. [PubMed] [Google Scholar]
- 14.Halaban R, Krauthammer M, Pelizzola M, et al. Integrative analysis of epigenetic modulation in melanoma cell response to decitabine: clinical implications. PLoS One. 2009;4(2):e4563. doi: 10.1371/journal.pone.0004563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olivieri NF, Saunthararajah Y, Thayalasuthan V, et al. A pilot study of subcutaneous decitabine in {beta}-thalassemia intermedia. Blood. 2011;118(10):2708–2711. doi: 10.1182/blood-2011-03-341909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel K, Dickson J, Din S, et al. Targeting of 5-aza-2′-deoxycytidine residues by chromatin-associated DNMT1 induces proteasomal degradation of the free enzyme. Nucleic Acids Res. 2010;38(13):4313–4324. doi: 10.1093/nar/gkq187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Camiener GW, Smith CG. Studies of the enzymatic deamination of cytosine arabinoside. I. Enzyme distribution and species specificity. Biochem Pharmacol. 1965;14(10):1405–1416. doi: 10.1016/0006-2952(65)90175-9. [DOI] [PubMed] [Google Scholar]
- 18.Neil GL, Moxley TE, Kuentzel SL, Manak RC, Hanka LJ. Enhancement by tetrahydrouridine (NSC-112907) of the oral activity of 5-azacytidine (NSC-102816) in L1210 leukemic mice. Cancer Chemother Rep. 1975;59(3):459–465. [PubMed] [Google Scholar]
- 19.DeSimone J, Heller P, Molokie RE, Hall L, Zwiers D. Tetrahydrouridine, cytidine analogues, and hemoglobin F. Am J Hematol. 1985;18(3):283–288. doi: 10.1002/ajh.2830180310. [DOI] [PubMed] [Google Scholar]
- 20.Beumer JH, Eiseman JL, Parise RA, et al. Modulation of gemcitabine (2′,2′-difluoro-2′-deoxycytidine) pharmacokinetics, metabolism, and bioavailability in mice by 3,4,5,6-tetrahydrouridine. Clin Cancer Res. 2008;14(11):3529–3535. doi: 10.1158/1078-0432.CCR-07-4885. [DOI] [PubMed] [Google Scholar]
- 21.Liu Z, Marcucci G, Byrd JC, et al. Characterization of decomposition products and preclinical and low dose clinical pharmacokinetics of decitabine (5-aza-2′-deoxycytidine) by a new liquid chromatography/tandem mass spectrometry quantification method. Rapid Commun Mass Spectrom. 2006;20(7):1117–1126. doi: 10.1002/rcm.2423. [DOI] [PubMed] [Google Scholar]
- 22.Liu Z, Liu S, Xie Z, et al. Characterization of in vitro and in vivo hypomethylating effects of decitabine in acute myeloid leukemia by a rapid, specific and sensitive LC-MS/MS method. Nucleic Acids Res. 2007;35(5):e31. doi: 10.1093/nar/gkl1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirch HC, Schroder J, Hoppe H, et al. Recombinant gene products of two natural variants of the human cytidine deaminase gene confer different deamination rates of cytarabine in vitro. Exp Hematol. 1998;26(5):421–425. [PubMed] [Google Scholar]
- 24.Yue L, Saikawa Y, Ota K, et al. A functional single-nucleotide polymorphism in the human cytidine deaminase gene contributing to ara-C sensitivity. Pharmacogenetics. 2003;13(1):29–38. doi: 10.1097/00008571-200301000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Gilbert JA, Salavaggione OE, Ji Y, et al. Gemcitabine pharmacogenomics: cytidine deaminase and deoxycytidylate deaminase gene resequencing and functional genomics. Clin Cancer Res. 2006;12(6):1794–1803. doi: 10.1158/1078-0432.CCR-05-1969. [DOI] [PubMed] [Google Scholar]
- 26.Fitzgerald SM, Goyal RK, Osborne WR, et al. Identification of functional single nucleotide polymorphism haplotypes in the cytidine deaminase promoter. Hum Genet. 2006;119(3):276–283. doi: 10.1007/s00439-006-0142-0. [DOI] [PubMed] [Google Scholar]
- 27.Bhatla D, Gerbing RB, Alonzo TA, et al. Cytidine deaminase genotype and toxicity of cytosine arabinoside therapy in children with acute myeloid leukemia. Br J Haematol. 2009;144(3):388–394. doi: 10.1111/j.1365-2141.2008.07461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahlknecht U, Dransfeld CL, Bulut N, et al. SNP analyses in cytarabine metabolizing enzymes in AML patients and their impact on treatment response and patient survival: identification of CDA SNP C-451T as an independent prognostic parameter for survival. Leukemia. 2009;23(10):1929–1932. doi: 10.1038/leu.2009.113. [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Manero G, Stoltz ML, Ward MR, Kantarjian H, Sharma S. A pilot pharmacokinetic study of oral azacitidine. Leukemia. 2008;22(9):1680–1684. doi: 10.1038/leu.2008.145. [DOI] [PubMed] [Google Scholar]
- 30.Riccardi R, Chabner B, Glaubiger DL, Wood J, Poplack DG. Influence of tetrahydrouridine on the pharmacokinetics of intrathecally administered 1-beta-D-arabinofuranosylcytosine. Cancer Res. 1982;42(5):1736–1739. [PubMed] [Google Scholar]
- 31.Kreis W, Chan K, Budman DR, et al. Effect of tetrahydrouridine on the clinical pharmacology of 1-beta-D-arabinofuranosylcytosine when both drugs are coinfused over three hours. Cancer Res. 1988;48(5):1337–1342. [PubMed] [Google Scholar]
- 32.Kreis W, Budman DR, Chan K, et al. Therapy of refractory/relapsed acute leukemia with cytosine arabinoside plus tetrahydrouridine (an inhibitor of cytidine deaminase)–a pilot study. Leukemia. 1991;5(11):991–998. [PubMed] [Google Scholar]
- 33.Dover GJ, Charache S, Boyer SH, Vogelsang G, Moyer M. 5-Azacytidine increases HbF production and reduces anemia in sickle cell disease: dose-response analysis of subcutaneous and oral dosage regimens. Blood. 1985;66(3):527–532. [PubMed] [Google Scholar]
- 34.Wong PP, Currie VE, Mackey RW, et al. Phase I evaluation of tetrahydrouridine combined with cytosine arabinoside. Cancer Treat Rep. 1979;63(8):1245–1249. [PubMed] [Google Scholar]
- 35.Ho DH, Bodey GP, Hall SW, et al. Clinica, pharmacology of tetrahydrouridine. J Clin Pharmacol. 1978;18(5-6):259–265. doi: 10.1002/j.1552-4604.1978.tb02444.x. [DOI] [PubMed] [Google Scholar]
- 36.Kreis W, Woodcock TM, Gordon CS, Krakoff IH. Tetrahydrouridine: Physiologic disposition and effect upon deamination of cytosine arabinoside in man. Cancer Treat Rep. 1977;61(7):1347–1353. [PubMed] [Google Scholar]
- 37.Beumer JH, Parise RA, Newman EM, et al. Concentrations of the DNA methyltransferase inhibitor 5-fluoro-2′-deoxycytidine (FdCyd) and its cytotoxic metabolites in plasma of patients treated with FdCyd and tetrahydrouridine (THU). Cancer Chemother Pharmacol. 2008;62(2):363–368. doi: 10.1007/s00280-007-0603-8. [DOI] [PubMed] [Google Scholar]
- 38.Marsh JH, Kreis W, Barile B, et al. Therapy of refractory/relapsed acute myeloid leukemia and blast crisis of chronic myeloid leukemia with the combination of cytosine arabinoside, tetrahydrouridine, and carboplatin. Cancer Chemother Pharmacol. 1993;31(6):481–484. doi: 10.1007/BF00685039. [DOI] [PubMed] [Google Scholar]
- 39.Goldenthal EI, Cookson KM, Geil RG, Wazeter FX. Preclinical toxicologic evaluation of tetrahydrouridine (NSC-112907) in beagle dogs and rhesus monkeys. Cancer Chemother Rep 3. 1974;5(1):15–16. [PubMed] [Google Scholar]
- 40.Santi DV, Norment A, Garrett CE. Covalent bond formation between a DNA-cytosine methyltransferase and DNA containing 5-azacytosine. Proc Natl Acad Sci U S A. 1984;81(22):6993–6997. doi: 10.1073/pnas.81.22.6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Creusot F, Acs G, Christman JK. Inhibition of DNA methyltransferase and induction of Friend erythroleukemia cell differentiation by 5-azacytidine and 5-aza-2′-deoxycytidine. J Biol Chem. 1982;257(4):2041–2048. [PubMed] [Google Scholar]
- 42.Koshy M, Dorn L, Bressler L, et al. 2-deoxy 5-azacytidine and fetal hemoglobin induction in sickle cell anemia. Blood. 2000;96(7):2379–2384. [PubMed] [Google Scholar]
- 43.Akpan I, Banzon V, Ibanez V, et al. Decitabine increases fetal hemoglobin in Papio anubis by increasing gamma-globin gene transcription. Exp Hematol. 2010;38(11):989–993. doi: 10.1016/j.exphem.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chin J, Singh M, Banzon V, et al. Transcriptional activation of the gamma-globin gene in baboons treated with decitabine and in cultured erythroid progenitor cells involves different mechanisms. Exp Hematol. 2009;37(10):1131–1142. doi: 10.1016/j.exphem.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lavelle D, Vaitkus K, Hankewych M, Singh M, Desimone J. Effect of 5-aza-2′-deoxycytidine (Dacogen) on covalent histone modifications of chromatin associated with the epsilon-, gamma-, and beta-globin promoters in Papio anubis. Exp Hematol. 2006;34(3):339–347. doi: 10.1016/j.exphem.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 46.Lavelle D, DeSimone J, Heller P. Fetal hemoglobin reactivation in baboon and man: a short perspective. Am J Hematol. 1993;42(1):91–95. doi: 10.1002/ajh.2830420118. [DOI] [PubMed] [Google Scholar]
- 47.Dareer SM, Mulligan LT, Jr, White V, et al. Distribution of [3H]cytosine arabinoside and its products in mice, dogs, and monkeys and effect of tetrahydrouridine. Cancer Treat Rep. 1977;61(3):395–407. [PubMed] [Google Scholar]
- 48.Kreis W, Chaudhri F, Chan K, et al. Pharmacokinetics of low-dose 1-beta-D-arabinofuranosylcytosine given by continuous intravenous infusion over twenty-one days. Cancer Res. 1985;45(12 pt 1):6498–6501. [PubMed] [Google Scholar]
- 49.Periclou AP, Avramis VI. NONMEM population pharmacokinetic studies of cytosine arabinoside after high-dose and after loading bolus followed by continuous infusion of the drug in pediatric patients with leukemias. Cancer Chemother Pharmacol. 1996;39(1-2):42–50. doi: 10.1007/s002800050536. [DOI] [PubMed] [Google Scholar]
- 50.Lavelle D, Chin J, Vaitkus K, et al. Oral decitabine reactivates expression of the methylated gamma-globin gene in Papio anubis. Am J Hematol. 2007;82(11):981–985. doi: 10.1002/ajh.21020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.