Abstract
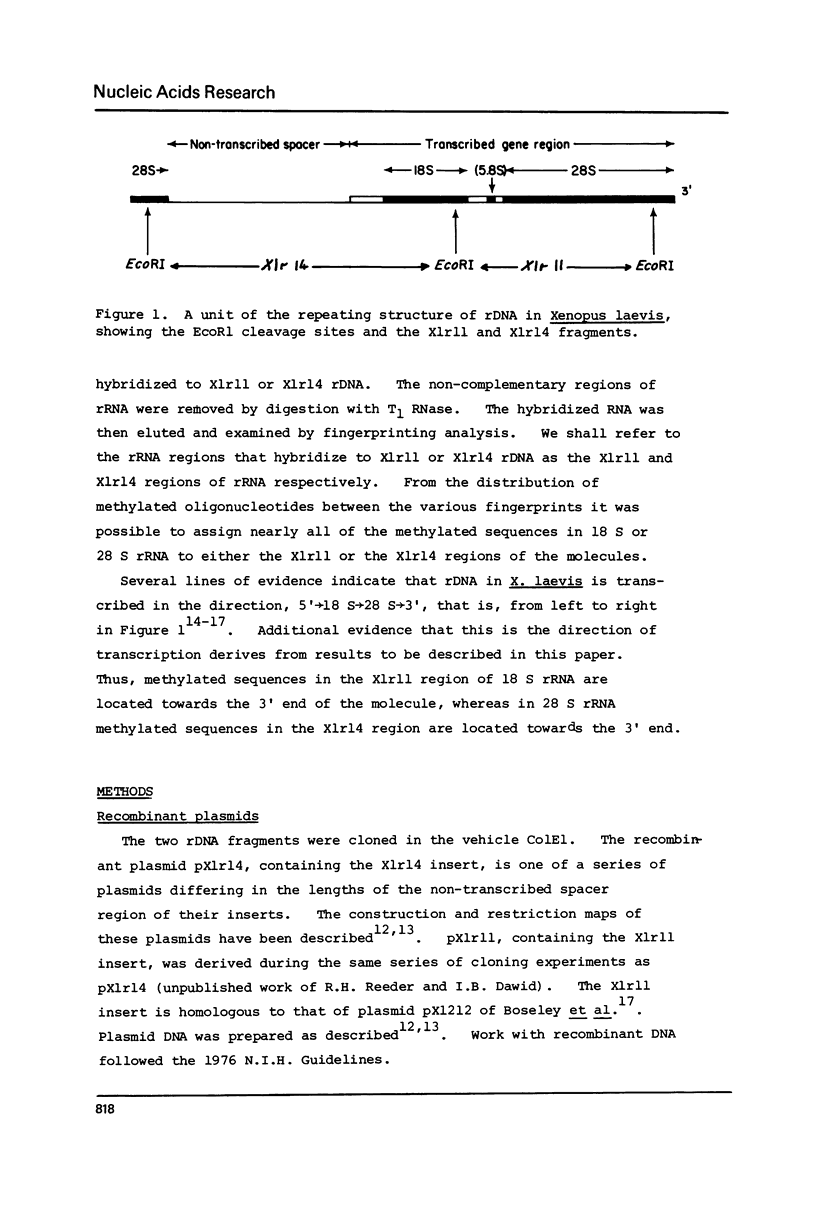
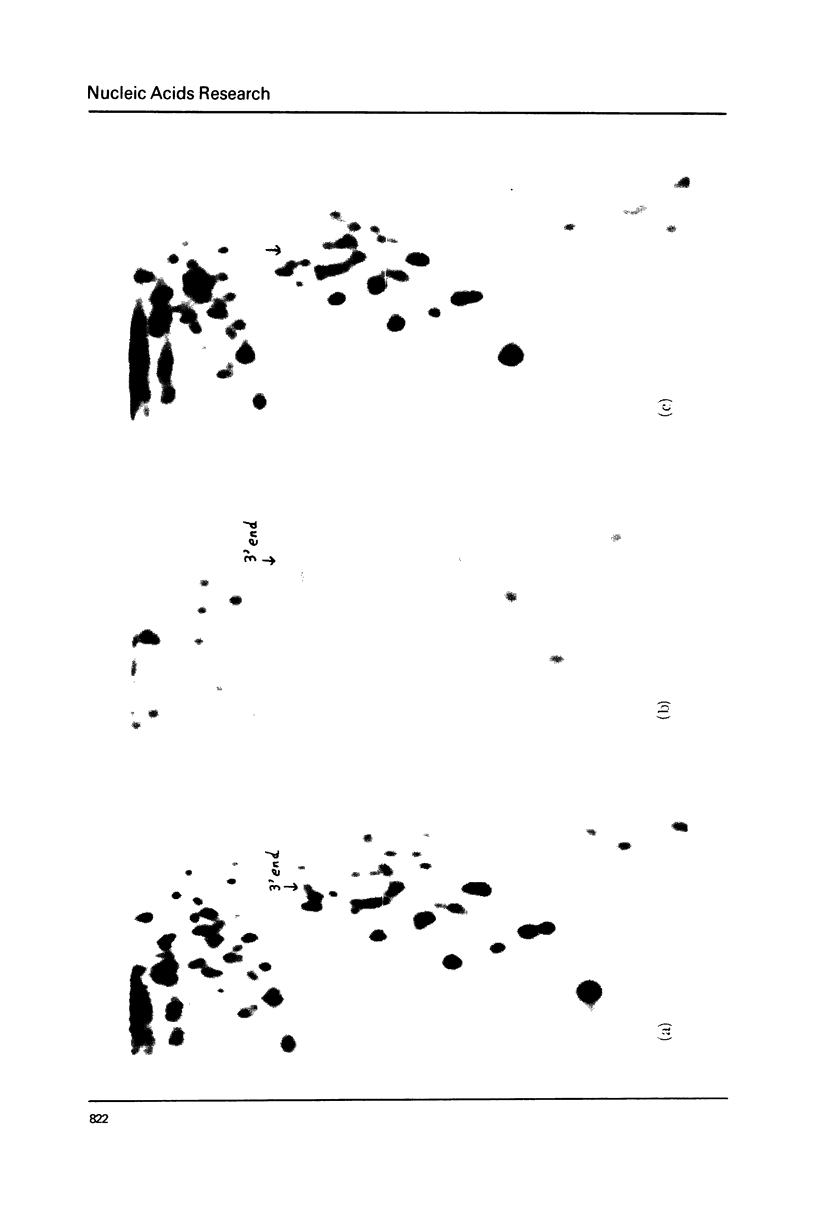
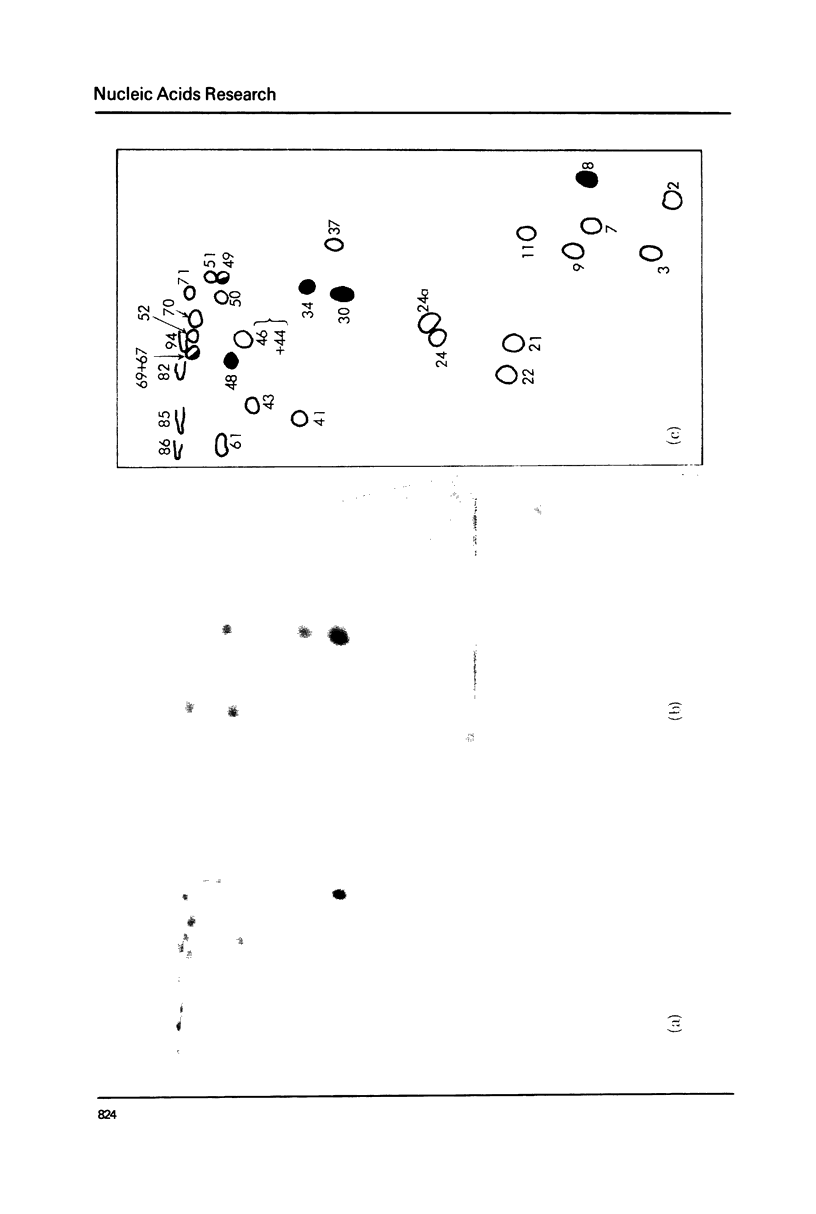
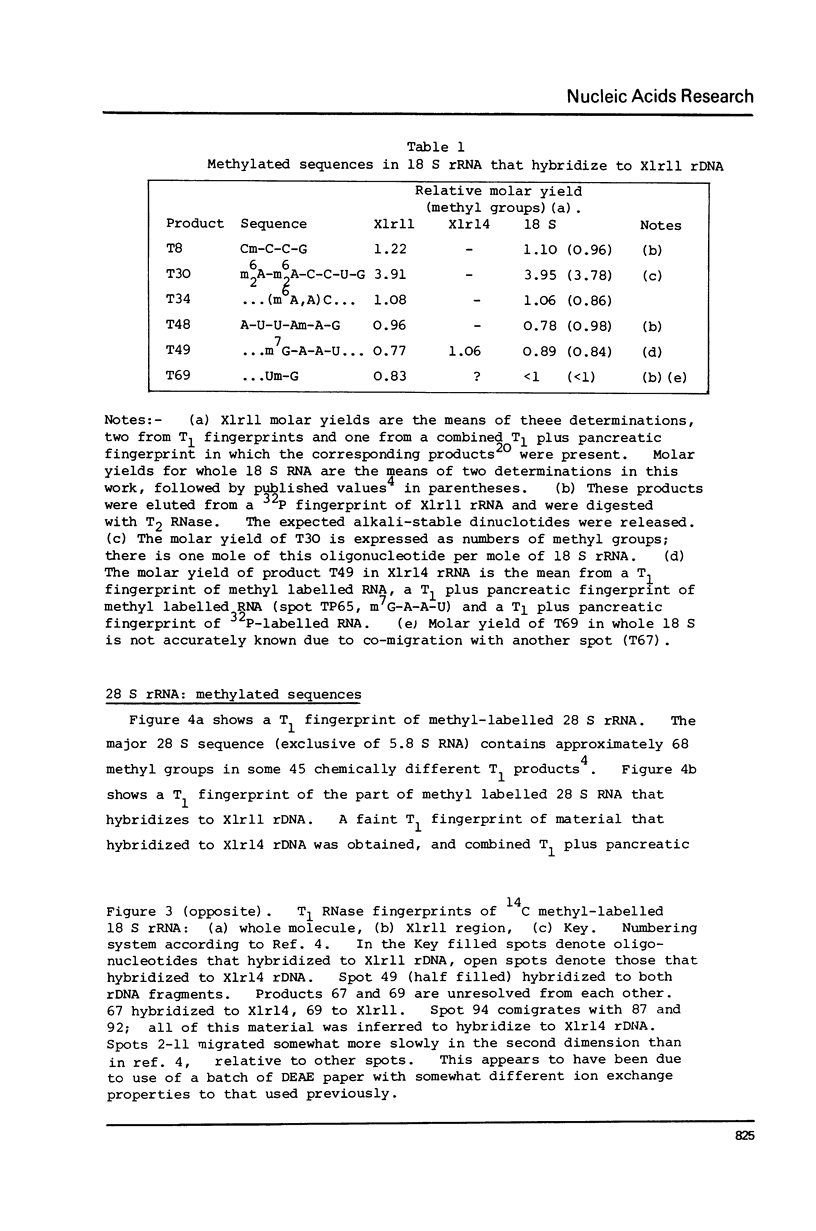
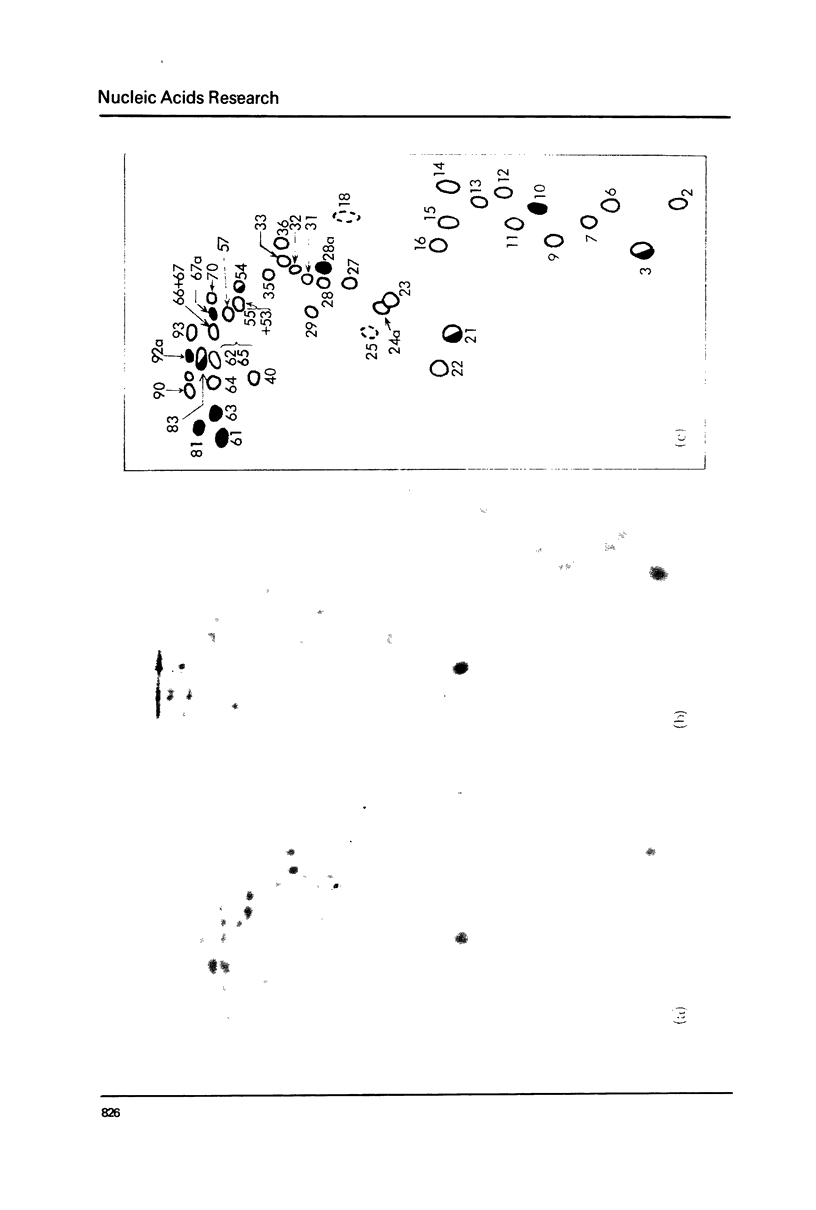
Xenopus laevis rRNA was hybridised to either of two cloned fragments of ribosomal DNA. One fragment, designated X1r11, contains a short region of the 18 S rRNA gene and most of the 28 S rRNA gene. The other fragment, X1r14, contains a short region of the 28 S gene and most of the 18 S gene. After hybridization the non-complementary rRNA was removed by digestion with T1 RNase and the hybridized RNA was then eluted and examined by fingerprinting analysis. The 3' terminal sequence and the dimethyl-A-containing sequence of 18 S rRNA both hybridized to X1r11 rDNA, in agreement with the known direction of transcription of rDNA. The distribution of other methylated oligonucleotides between the various fingerprints permitted assigment of nearly all of the methylated sequences in 18 S and 28 s rRNA to either the short 3' region or the long 5' region of the respective molecules.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberty H., Raba M., Gross H. J. Isolation from rat liver and sequence of a RNA fragment containing 32 nucleotides from position 5 to 36 from the 3' end of ribosomal 18S RNA. Nucleic Acids Res. 1978 Feb;5(2):425–434. doi: 10.1093/nar/5.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boseley P. G., Tuyns A., Birnstiel M. L. Mapping of the Xenopus laevis 5.8S rDNA by restriction and DNA sequencing. Nucleic Acids Res. 1978 Apr;5(4):1121–1137. doi: 10.1093/nar/5.4.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchan P., Reeder R. H., Dawid I. B. Restriction analysis of the nontranscribed spacers of Xenopus laevis ribosomal DNA. Cell. 1977 Jul;11(3):599–607. doi: 10.1016/0092-8674(77)90077-0. [DOI] [PubMed] [Google Scholar]
- Brand R. C., Klootwijk J., Van Steenbergen T. J., De Kok A. J., Planta R. J. Secondary methylation of yeast ribosomal precursor RNA. Eur J Biochem. 1977 May 2;75(1):311–318. doi: 10.1111/j.1432-1033.1977.tb11531.x. [DOI] [PubMed] [Google Scholar]
- Carbon P., Ehresmann C., Ehresmann B., Ebel J. P. The sequence of Escherichia coli ribosomal 16 S RNA determined by new rapid gel methods. FEBS Lett. 1978 Oct 1;94(1):152–156. doi: 10.1016/0014-5793(78)80926-0. [DOI] [PubMed] [Google Scholar]
- Dawid I. B., Wellauer P. K. A reinvestigation of 5' leads to 3' polarity in 40S ribosomal RNA precursor of Xenopus laevis. Cell. 1976 Jul;8(3):443–448. doi: 10.1016/0092-8674(76)90157-4. [DOI] [PubMed] [Google Scholar]
- De Jonge P., Klootwijk J., Planta R. J. Sequence of the 3'-terminal 21 nucleotides of yeast 17S ribosomal RNA. Nucleic Acids Res. 1977 Oct;4(10):3655–3663. doi: 10.1093/nar/4.10.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eladari M. E., Hampe A., Galibert F. Nucleotide sequence neighbouring a late modified guanylic residue within the 28S ribosomal RNA of several eukaryotic cells. Nucleic Acids Res. 1977 Jun;4(6):1759–1767. doi: 10.1093/nar/4.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Hagenbüchle O., Santer M., Steitz J. A., Mans R. J. Conservation of the primary structure at the 3' end of 18S rRNA from eucaryotic cells. Cell. 1978 Mar;13(3):551–563. doi: 10.1016/0092-8674(78)90328-8. [DOI] [PubMed] [Google Scholar]
- Khan M. S., Maden B. E. Nucleotide sequences within the ribosomal ribonucleic acids of HeLa cells, Xenopus laevis and chick embryo fibroblasts. J Mol Biol. 1976 Feb 25;101(2):235–254. doi: 10.1016/0022-2836(76)90375-2. [DOI] [PubMed] [Google Scholar]
- Khan M. S., Salim M., Maden B. E. Extensive homologies between the methylated nucleotide sequences in several vertebrate ribosomal ribonucleic acids. Biochem J. 1978 Mar 1;169(3):531–542. doi: 10.1042/bj1690531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klootwijk J., Planta R. J. Analysis of the methylation sites in yeast ribosomal RNA. Eur J Biochem. 1973 Nov 15;39(2):325–333. doi: 10.1111/j.1432-1033.1973.tb03130.x. [DOI] [PubMed] [Google Scholar]
- Lau R. Y., Kennedy T. D., Lane B. G. Wheat-embryo ribonucleates. III. Modified nucleotide constituents in each of the 5.8S, 18S and 26S ribonucleates. Can J Biochem. 1974 Dec;52(12):1110–1123. doi: 10.1139/o74-155. [DOI] [PubMed] [Google Scholar]
- Maden B. E., Khan M. S. Methylated nucleotide sequences in HeLa-cell ribosomal ribonucleic acid. Correlation between the results from 'fingerprinting' hydrolysates obtained by digestion with T1 ribonuclease and with T1 plus pancreatic ribonuclease. Biochem J. 1977 Oct 1;167(1):211–221. doi: 10.1042/bj1670211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maden B. E., Salim M. The methylated nucleotide sequences in HELA cell ribosomal RNA and its precursors. J Mol Biol. 1974 Sep 5;88(1):133–152. doi: 10.1016/0022-2836(74)90299-x. [DOI] [PubMed] [Google Scholar]
- Morrow J. F., Cohen S. N., Chang A. C., Boyer H. W., Goodman H. M., Helling R. B. Replication and transcription of eukaryotic DNA in Escherichia coli. Proc Natl Acad Sci U S A. 1974 May;71(5):1743–1747. doi: 10.1073/pnas.71.5.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeder R. H., Higashinakagawa T., Miller O., Jr The 5' leads to 3' polarity of the Xenopus Ribosomal RNA precursor molecule. Cell. 1976 Jul;8(3):449–454. doi: 10.1016/0092-8674(76)90158-6. [DOI] [PubMed] [Google Scholar]
- Reeder R. H., Sollner-Webb B., Wahn H. L. Sites of transcription initiation in vivo on Xenopus laevis ribosomal DNA. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5402–5406. doi: 10.1073/pnas.74.12.5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vass J. K., Maden B. E. Studies on the conformation of the 3' terminus of 18-S rRNA. Eur J Biochem. 1978 Apr;85(1):241–247. doi: 10.1111/j.1432-1033.1978.tb12232.x. [DOI] [PubMed] [Google Scholar]
- Wellauer P. K., Dawid I. B., Brown D. D., Reeder R. H. The molecular basis for length heterogeneity in ribosomal DNA from Xenopus laevis. J Mol Biol. 1976 Aug 25;105(4):461–486. doi: 10.1016/0022-2836(76)90229-1. [DOI] [PubMed] [Google Scholar]
- Wellauer P. K., Reeder R. H., Carroll D., Brown D. D., Deutch A., Higashinakagawa T., Dawid I. B. Amplified ribosomal DNA from Xenopus laevis has heterogeneous spacer lengths. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2823–2827. doi: 10.1073/pnas.71.7.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]





