Abstract
The present study examined the neural correlates of long-term intense romantic love using functional magnetic resonance imaging (fMRI). Ten women and 7 men married an average of 21.4 years underwent fMRI while viewing facial images of their partner. Control images included a highly familiar acquaintance; a close, long-term friend; and a low-familiar person. Effects specific to the intensely loved, long-term partner were found in: (i) areas of the dopamine-rich reward and basal ganglia system, such as the ventral tegmental area (VTA) and dorsal striatum, consistent with results from early-stage romantic love studies; and (ii) several regions implicated in maternal attachment, such as the globus pallidus (GP), substantia nigra, Raphe nucleus, thalamus, insular cortex, anterior cingulate and posterior cingulate. Correlations of neural activity in regions of interest with widely used questionnaires showed: (i) VTA and caudate responses correlated with romantic love scores and inclusion of other in the self; (ii) GP responses correlated with friendship-based love scores; (iii) hypothalamus and posterior hippocampus responses correlated with sexual frequency; and (iv) caudate, septum/fornix, posterior cingulate and posterior hippocampus responses correlated with obsession. Overall, results suggest that for some individuals the reward-value associated with a long-term partner may be sustained, similar to new love, but also involves brain systems implicated in attachment and pair-bonding.
INTRODUCTION
For centuries, humans have speculated about the mysteries of romantic love. One question that has puzzled theorists, therapists and laypeople is whether intense romantic love can last. Some theories suggest that love inevitably declines over time in marriage or after the child-rearing years (Sternberg, 1986; Buss, 1989). Other theories suggest that over time, passionate/romantic love, defined as ‘a state of intense longing for union with another’ generally evolves into companionate love—with deep friendship, easy companionship and sharing of common interests, but not necessarily involving intensity, sexual desire, or attraction (Berscheid and Hatfield, 1969; Grote and Frieze, 1994). Some psychologists even speculated that the presence of intense passion in long-term marriages may sometimes exist, but is an indication of over-idealization or pathology (Freud, 1921; Fromm, 1956). However, other theories suggest that there might be mechanisms through which romantic love may be sustained over time in relationships. Our first hypothesis was that long-term romantic love is similar to early-stage romantic love. We predicted that a group of happily married individuals reporting intense romantic love for their long-term partners (≥10 years) would show neural activity in dopamine-rich regions associated with reward and motivation, particularly the VTA, as in previous studies of early-stage romantic love (Bartels and Zeki, 2000; Aron et al., 2005; Ortigue et al., 2007; Xu et al., 2010). We used functional magnetic resonance imaging (fMRI) approaches used by previous investigations of romantic love (Aron et al., 2005).
Our second hypothesis was that long-term pair-bonds share neural circuitry with parent–infant bonds. Bowlby (1969) developed his theory of human attachment by observing child-caregiver relationships and proposed that the ‘attachment system’ coordinates proximity-seeking to the attachment figure. Since then, studies have applied attachment theory to adult romantic relationships (Hazan and Shaver, 1987; Mikulincer and Shaver, 2007) with some researchers suggesting that pair-bonds and parent–infant bonds share common biological substrates (Fisher, 1992; Carter, 1998). Thus, adult attachment work has been built on the notion that pair-bonds are the adult instantiation of attachment in childhood (Ainsworth, 1991).
We investigated the neural correlates of long-term romantic love and attachment by applying fMRI to a group of long-term happily married, sexually monogamous individuals reporting intense romantic love for their partner. We duplicated procedures used in Aron et al.’s (2005) fMRI study of early-stage intense romantic love, in which participants viewed facial images of their partner and a highly familiar acquaintance permitting a direct, controlled comparison of results between the studies. We predicted that long-term romantic love would involve dopamine-rich subcortical brain regions associated with reward, particularly the ventral tegmental area (VTA), reported in several studies of early-stage romantic love (Bartels and Zeki, 2000; Aron et al., 2005; Ortigue et al., 2007; Xu et al., 2010).
We added a control for social affiliation by including a close, long-term friend as a comparison target. A social affiliation control allowed us to examine attachment-related neural activity in response to the partner. This was important for examining commonalities found for pair-bonds from the present research with previous studies of parent–infant bonds (Bartels and Zeki, 2004; Strathearn et al., 2008). We anticipated activations in response to the partner in brain regions associated with attachment defined as a selective social/emotional bond (Bowlby, 1969). Our regions of interest, notably the globus pallidus (GP), were based on human imaging studies of maternal attachment (Bartels and Zeki, 2004; Strathearn et al., 2008) and animal studies of pair-bonding (Young et al., 2001). Finally, we conducted correlations of neural activity with widely used questionnaire measures of romantic love, obsession, inclusion of other in the self (IOS), friendship-based love, relationship length and sexual frequency.
Relationship researchers have addressed other aspects of love that are useful when considering the possibility of long-term romantic love. Hendrick and Hendrick (1992) speculated that people go through a development sequence of love styles, with Mania (or obsession) being most characteristic of adolescents, evolving into Eros (romantic love) around early adulthood, Storge (akin to companionate love) and Pragma (pragmatic love) in the middle years and eventually into Agape (all-giving love) in the later stages of life.
Other research suggests that there might be mechanisms through which romantic love may be sustained or increased at all stages of relationships. For example, the self-expansion model (Aron and Aron, 1986) proposes that romantic love is the experience of rapid self-expansion by including a particular person in the self (Aron et al., 1996). In the early-stages of relationships, partners experience rapid self-expansion as they learn and integrate new aspects of the beloved. Opportunities for rapid expansion—which tend to decrease as the couple comes to know each other well—may be maintained if partners continue to expand, seeing each other as new and experiencing expansion by way of the relationship. One implication of the model is that couples’ shared participation in novel and challenging activities, if not overly stressful, may promote increases in romantic love as the reward-value associated with the experience becomes associated with the relationship (Aron et al., 2000). Thus, we used the IOS scale to measure its association with reward-related neural activity, particularly in the VTA. Similarly, the intimacy model suggests that rapid increases in intimacy promote increases in passion (Baumeister and Bratslavsky, 1999).
Acevedo and Aron (2009) suggest that intense romantic love (with intensity, engagement and sexual desire) exists in some long-term relationships, but generally without the obsession component common in the early-stages of relationships. Similarly, Tennov (1979) in her book on love and limerance describes how some older people in happy marriages replied affirmatively to being ‘in love’, but unlike those in ‘limerant’ relationships, they did not report continuous and unwanted intrusive thinking. Finally, in-depth interviews carried out by a member of our research team (BPA) suggest that some individuals in long-term love report symptoms common to newly in love individuals: craving for union, focused attention, increased energy when with the partner, motivation to do things that make the partner happy, sexual attraction and thinking about the partner when apart. Thus, we embarked on this study to examine how brain system activity in those who report being intensely in love after 10 years might be similar to and different from early-stage romantic love.
METHOD
Participants
Participants were 17 (10 women) healthy, right-handed individuals, ages 39–67 years (M = 52.85, s.d. = 8.91); married 10–29 years (M = 21.4, s.d. = 5.89) to an opposite sex spouse, and with 0–4 children (M = 1.9) living in the home at the time of the study (three had no children and 10 had children). Seven participants were in a first marriage (for both partners), and 10 were in marriages where one or both partners had been previously divorced. On average, participants had completed 16 years (s.d. = 1.09) of education and had an annual household income ranging from $100 000–$200 000. Ethnic composition of the sample was as follows: 2 (12%) Asian-American, 2 (12%) Latino/a and 13 (76%) Caucasian.
Participants were recruited by word-of-mouth, flyers and newspaper ads in the New York Metropolitan area asking, ‘Are you still madly in love with your long-term partner?’ Individuals were screened by phone for eligibility criteria including relationship length (>10 years), nonuse of antidepressants, fMRI contraindications, sexual monogamy and feelings of intense romantic love. Approximately 40% of potential participants were excluded for not meeting criteria. All participants provided informed consent and received payment for their participation. The study was approved by the human subjects committees at Stony Brook University and New York University.
Questionnaires
Participants completed a battery of questionnaires including the Passionate Love Scale (Hatfield and Sprecher, 1986) and the Eros subscale of the Love Attitudes Scale (Hendrick and Hendrick, 1986) to measure passionate/romantic love; the IOS Scale (Aron et al., 1992) to measure closeness; and the friendship-based love scale (FBLS; Grote and Frieze, 1994) to assess friendship (or companionate love). Participants also reported sexual frequency with their spouse and other relationship demographics.
Stimuli
Facial color photographs of four stimuli for each participant were digitized and presented using E-Prime 2.0 software (Psychological Software Tools, Inc., Pittsburgh, PA, USA). All controls were the same sex and approximately the same age as the partner. Means and s.d.’s of variables describing target stimuli are provided in Supplementary Table S1.
Postscan Ratings
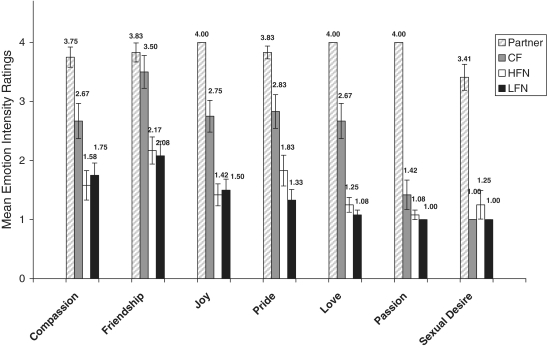
Immediately after the set of image presentations, while still in the scanner, participants rated the emotional intensity elicited by each stimulus. The instructions read ‘for this part of the study you will see a series of emotion words on the screen. Please rate how intensely you felt each emotion while viewing images of {target person}. Please use the following response scale: 1 = not at all, 2 = slightly, 3 = somewhat, 4 = a great deal’. Instructions were identical for each stimulus, except {target person}, was replaced by ‘your partner’, ‘your familiar acquaintance’, ‘your minimally familiar acquaintance’ or ‘your close familiar acquaintance’. The feelings rated were compassion, friendship, joy, pride, love, passion and sexual desire. Results are displayed in Figure 1.
Fig. 1.
Postscan emotion intensity ratings. The y-axis indicates the mean of the intensity ratings given by participants to their long term, intensely loved partner (Partner), close friend (CF), a highly-familiar neutral (HFN) acquaintance and a low-familiar neutral (LFN) acquaintance. A score of 1 indicates not at all and 4 indicates a great deal. Bars indicates ±s.d.
Partner
Partners were known a mean of 24.18 years (s.d. = 6.42). Mean questionnaire scores related to the partner were: passionate love scale (PLS) = 5.51 (s.d. = 0.36), Eros = 5.76 (s.d. = 0.26), IOS = 5.82 (s.d. = 1.59), FBLS = 6.48 (s.d. = 0.77), all on a 7-point scale. Mean weekly sexual frequency was 2.20 (s.d. = 1.85). Postscan emotion intensity ratings were significantly greater (P < 0.01) when participants viewed images of their Partner vs all other target stimuli for all emotions words, except ‘friendship’ was not different from the close friend.
Close friend
The close friend (CF) was someone with whom the participant had a close, positive, interactive (but not romantic) relationship and was known about as long as the Partner. Three were siblings, one was a cousin, two were in-laws, nine were friends and two were co-workers. Participants reported a great degree of closeness and friendship to the CF. As noted, the postscan friendship rating was not significantly different from the CF and Partner, t(11) = 0.94, P > 0.05, supporting the use of the CF as an appropriate control for friendship.
Highly familiar neutral
To help control for familiarity, the highly familiar neutral (HFN) was a ‘neutral’ acquaintance known about as long as the Partner, but substantially less close than the Partner or CF. The HFN was also rated significantly lower on postscan friendship feelings relative to the Partner [t(11) = 5.86 = P < 0.001] and the CF [t(11) = 4.00, P < 0.01], supporting the use of the HFN as a control for familiarity, but not for closeness or friendship.
Low-familiar neutral
To further control for familiarity and provide a more direct examination of familiarity effects by comparison with the HFN, the LFN was known significantly fewer years and was significantly less close than any of the other targets. The HFN and LFN postscan emotion ratings were all relatively low compared to the Partner and CF.
Count-back task
Following procedures in Aron et al. (2005), to reduce carryover effects, all facial images were followed by a count-back distraction task. Participants were asked to count-back from a high number (e.g. 2081) in increments of seven. Instructions were provided before and during scanning.
Attractiveness and image quality
All photos were rated for facial attractiveness and image quality by six independent raters (three females and three males) of around the same age as our participants. Image quality was rated by all six coders, but attractiveness was rated only by coders of the opposite sex as the stimuli. Attractiveness coder ratings were adequately intercorrelated (α = 0.66 for female raters and 0.91 for male raters). For attractiveness, independent coder ratings did not differ significantly across types of target stimuli, F(3, 64) = 0.94, ns. Similarly, there was no significant difference among coder-rated image quality, F(3, 64) = 0.63, ns. There were no significant associations of Partner minus HFN coder-rated attractiveness difference scores with neural responses for the Partner vs HFN contrast or of the Partner minus CF coder rated attractiveness difference scores with neural responses for the Partner vs CF contrast. Thus, it appears that the Partner vs HFN and Partner vs CF activation differences were not due to objective differences in facial attractiveness.
Scanning procedures
The protocol implemented a block design of two 12-min sessions each consisting of six sets of four 30-s tasks in an alternating fashion, followed by stimulus ratings. Each session included two alternating images (starting image counterbalanced), interspersed with a count-back task. Duplicating procedures of Aron et al. (2005), Session 1 displayed Partner and HFN images. For the additional control comparisons, Session 2 displayed CF and LFN images. Participants were instructed to think about experiences with each stimulus person, nonsexual in nature. To assess whether instructions were followed, at debriefing participants were asked to describe their thoughts and feelings while viewing stimuli.
Data acquisition and analysis
MRI scanning was performed at NYU’s Center for Brain Imaging using a 3T Siemens magnetic resonance imaging system with a NOVA head coil. First, anatomical scans were obtained. Next, functional images were obtained. The first four volumes were discarded to allow for scanner calibration, resulting in 360 functional images, in volumes of 30, 3-mm axial slices (0 mm gap) covering the whole brain. Voxel size of functional images was 3 × 3 × 3 mm. A repetition time (TR) of 2000 ms was used, with a TE of 30 ms, a 90° flip.
Data were analysed using SPM2 (http://www.fil.ion.ucl.ac.uk/spm). For preprocessing, functional EPI volumes were realigned to the first volume (motion corrected), smoothed with a Gaussian kernel of 6 mm and then normalized to the anatomical template. Images were inspected for motion and no participant showed movement >3 mm (whole voxel). After preprocessing, activation contrasts were created (Partner vs HFN, Partner vs CF, CF vs HFN, CF vs LFN and HFN vs LFN). Effects for the stimulus conditions were estimated using box-car regressors convolved with a hemodynamic response function, separately for each participant. Analyses were carried out using a mixed effects general linear model, with participants as the random-effects factor and conditions as the fixed effect.
Region of interest analysis
We placed the region of interest (ROI) coordinates at the center of the activations reported by studies of early-stage romantic love (Bartels and Zeki, 2000; Aron et al., 2005; Ortigue et al., 2007) and maternal attachment (Bartels and Zeki, 2004; Strathearn et al., 2008). In addition, we created ROIs based on the peak voxels searched by Aron et al. (2005) in their Table 1 for reward and emotion. We adopted an FDR for multiple comparisons correction (Genovese et al., 2002) with a threshold of P ≤ 0.05. The ROIs occupied a 3-mm radius. Anatomic regions were confirmed with the ‘Atlas of the Human Brain’ (Mai et al., 2008). Given our a priori hypotheses, and our duplication of methods from Aron et al.’s (2005) study of early-stage romantic love, we were first interested in the Partner vs HFN contrast.
Table 1.
Regions of interest activations and deactivations showing responses to images of the Partner vs images of the Highly Familiar acquaintance
| Brain region | Left |
Right |
||||||
|---|---|---|---|---|---|---|---|---|
| x | y | z | P | x | y | z | P | |
| Activations | ||||||||
| VTAa | 2 | −12 | −8 | 0.04 | ||||
| VTA/substantia nigrab,c | 4 | −14 | −16 | 0.02 | ||||
| Substantia nigra, posteriorb,c | −12 | −24 | −12 | 0.01 | 18 | −20 | −10 | 0.04 |
| NAccd | 10 | 4 | −4 | 0.05e | ||||
| Caudate, anteriorb,f | −20 | −2 | 26 | 0.02g | 20 | 4 | 24 | 0.05g |
| Caudate body, anteriora | −20 | 0 | 18 | 0.01 | ||||
| Caudate body, posteriora | 18 | −14 | 22 | 0.03 | ||||
| Putamenb,c | −22 | 2 | 4 | 0.01 | 24 | −18 | 10 | 0.01 |
| GP, posteriorb | −32 | 6 | −8 | 0.03g | 24 | −8 | −8 | 0.03 |
| Dorsal Raphe nucleic | −2 | −24 | −9 | 0.00 | 2 | −26 | −22 | 0.00g |
| Periaqueductal gray (PAG)b | 2 | −32 | −24 | 0.01e | ||||
| Thalamus, lateralb | −12 | −14 | 6 | 0.02 | 4 | −6 | 4 | 0.04 |
| Hypothalamusb | 2 | −8 | −6 | 0.00e | ||||
| Mid-insulab,f | −44 | 4 | −2 | 0.05 | 38 | 10 | −6 | 0.04g |
| Insular cortexc | −38 | 8 | −14 | 0.02 | ||||
| Posterior hippocampusf | −34 | −32 | −4 | 0.05 | 36 | −34 | 0 | 0.00 |
| Amygdalad | −20 | −2 | −12 | 0.01g | ||||
| Anterior cingulateb | −10 | 46 | −4 | 0.04 | ||||
| Posterior cingulateb,c | −12 | −40 | 8 | 0.00 | 10 | −52 | 16 | 0.03 |
| Medial orbitofrontal cortex (mOFC)d | 2 | 58 | −10 | 0.04e | ||||
| Cerebellumf | −6 | −46 | −26 | 0.03e | ||||
| Deactivations | ||||||||
| Accumbens | 8 | 10 | −10 | 0.01 | ||||
| BA 9/46f | 50 | 32 | 30 | 0.03 | ||||
Note. ROIs were identified as the highest intensity voxel in a cluster, for the Partner vs highly-familiar, neutral (HFN) contrast. MNI coordinates (x, y, z) are at the maximum value for the cluster, which may be elongated in any direction. P-values (P) are for false discovery rate (FDR) correction. Symbols indicate activations overlapping with contrasts to control for closeness and familiarity. Letters indicate origin of regions of interest.
aAron et al. (2005) for early stage romantic love.
bBartels and Zeki (2004) for maternal love.
cStrathearn et al. (2008) for maternal attachment.
dResearched by Aron et al. (2005) for reward and emotion.
eBartels and Zeki (2000) for early stage romantic love.
fActivation common with close friend (CF) vs HFN contrast or CF vs low-familiar neutral (LFN) contrast, indicating close friendship effects.
gActivation common with HFN vs LFN contrast, indicating general familiarity effects.
However, we also carried out ROI analyses within the Partner vs CF contrast, which helps control for close friendship, adopting a FDR with a threshold of P ≤ 0.05. In addition, to further examine regions potentially commonly activated for the partner and close friend, the whole-brain t-map for the Partner vs HFN contrast was applied as an inclusive mask for the CF vs LFN contrast, and for the CF vs HFN contrast. We used a voxel-level threshold of P < 0.005 (Kampe et al., 2003; Ochsner et al., 2004), with a minimum spatial extent of ≥15 contiguous voxels. This approach is conservative in that it requires significant activation for both contrasts.
We also utilized the masking approach with the HFN vs LFN contrast to examine common activations with the Partner vs HFN contrast.
Exploratory whole-brain analysis
For exploratory purposes, we conducted whole-brain analyses on the Partner vs HFN contrast in which we applied a threshold of P ≤ 0.001 (uncorrected for multiple comparisons) with a minimum spatial extent of ≥15 contiguous voxels. Results are reported in Supplementary Table S2.
Correlations
Finally, we conducted simple regression analyses (correlation) with scores on the PLS, Eros, IOS, FBLS, sexual frequency (controlling for age) and relationship length. We carried out two correlations for the PLS, one with love-related items and one with obsession-related items as suggested by factor analyses of the PLS in long-term relationships (Acevedo and Aron, 2009). Obsessive items were as follows: ‘Sometimes I feel I can’t control my thoughts; they are obsessively on my partner’, ‘An existence without my partner would be dark and dismal’ and ‘I get extremely depressed when things don’t go right in my relationship with my partner’.
Correlations were carried out using each participant’s score on the self-report scale, except for sexual frequency. For sexual frequency, we controlled for age by first carrying out a linear regression (with sexual frequency as the DV and age as the IV) to obtain the residuals. The residuals of sexual frequency (controlling for age) were then correlated with brain activity. For the IOS, we calculated the IOS score difference between the Partner and CF for each participant.
The PLS, Eros and FBLS correlations were carried out on the Partner vs HFN contrast. The IOS and relationship length correlations were carried out within the Partner vs CF contrast to control for closeness. We examined ROIs (adopting a FDR with a threshold of P ≤ 0.05) based on the results of studies of early-stage romantic love (Bartels and Zeki, 2000; Aron et al., 2005; Ortigue et al., 2007) and maternal love (Bartels and Zeki, 2004). We aimed to replicate some previous correlation findings (Aron et al., 2005), and placed the center of ROIs at the same coordinates reported previously to be correlated with the PLS and relationship length.
RESULTS
Partner vs HFN contrast
Table 1 displays coordinates of neural activations based on ROI analyses for the Partner vs HFN contrast. The literature origin of each ROI is indicated by a superscript (a, b, c, d, e) in Table 1.
ROI analyses showed significant activation [P(FDR) < 0.05) in regions of the VTA (Figure 2A), SN (Figure 2A), NAcc, caudate, putamen, posterior GP, mOFC, thalamus, hypothalamus, mid-insula, posterior hippocampus, insular cortex, dorsal Raphe nuclei, PAG, anterior cingulate, posterior cingulate, amygdala and cerebellum.
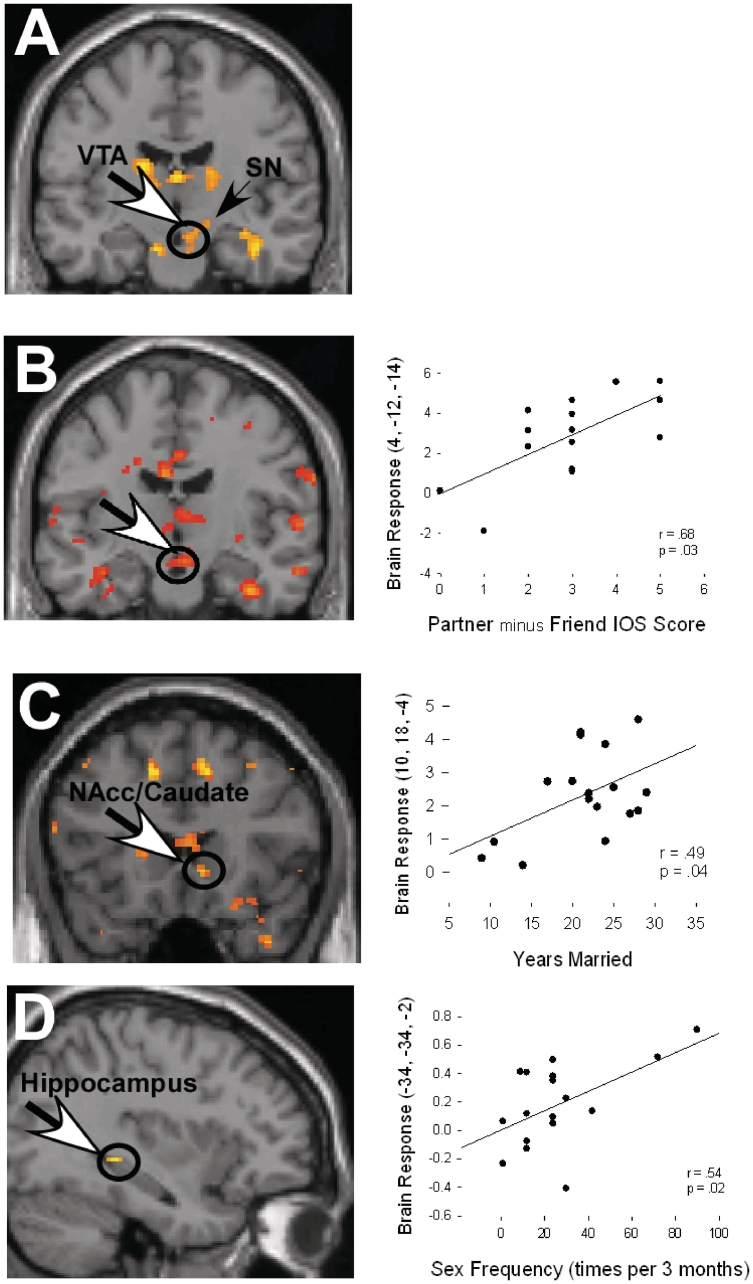
Fig. 2.
(A) Individuals self-reporting intense love for a long-term spouse show significant neural activation in dopamine-rich, reward regions of the VTA/SN in response to images of their partner vs a highly familiar acquaintance (HFN). (B) Image and scatter plot illustrating the association between brain response in the VTA and Partner minus close friend (CF), Inclusion of Other in the Self (IOS) scores. Greater closeness with the Partner was associated with greater response in the VTA for the Partner vs a CF. (C) Image and scatter plot illustrating the association between brain response in the NAcc/Caudate and number of years married to the partner. Greater years married was associated with stronger response in the NAcc/Caudate for the Partner (vs a CF). (D) Image and scatter plot illustrating greater response to the Partner (vs HFN) in the region of the posterior hippocampus is associated with higher sexual frequency.
Thus, as predicted, activations for long-term romantic love were found in mesolimbic, dopamine-rich reward systems. Specifically, we were interested in the VTA, as it has been reported in numerous studies of early-stage romantic love (Bartels and Zeki, 2000; Aron et al., 2005; Ortigue et al., 2007; Fisher et al., 2010; Xu et al., 2010). Inspection of the time course plots showed VTA activity peaked during the first 20 s of each trial in response to the partner.
Regions common to early-stage and long-term romantic love
Regions commonly activated by early-stage romantic love (identified in previous studies) and long-term romantic love included the right VTA and posterior caudate body; bilateral anterior caudate body, mid-insula and posterior hippocampus; and left cerebellum. The right amygdala showed deactivation in early-stage love, while the left amygdala showed activation for the long-term love group.
Regions common to maternal love and long-term romantic love
Regions commonly activated by maternal love (Bartels and Zeki, 2004; Strathearn et al., 2008) and long-term romantic love included the right VTA/SN, PAG and hypothalamus; bilateral SN, anterior caudate, putamen, posterior GP, thalamus, mid-insula, dorsal Raphe and posterior cingulate; the left anterior cingulate and insular cortex.
Regions common to maternal, early-stage and long-term love
As shown in Table 1, regions commonly activated by maternal love (Bartels and Zeki, 2004; Strathearn et al., 2008), early-stage romantic love (Bartels and Zeki, 2000; Aron et al., 2005; Ortigue et al., 2007) and long-term romantic love included the region of the VTA, bilateral anterior caudate body and middle insula.
Deactivations
Activity related to the partner decreased relative to the HFN in the right accumbens and BA 9/46, replicating deactivations found for early-stage romantic love (Bartels and Zeki, 2000).
Partner vs CF Contrast
ROI analyses were carried out for the Partner vs CF contrast based on the studies of early-stage romantic love (Bartels and Zeki, 2004; Aron et al., 2005) and maternal attachment (Strathearn et al., 2008). Significant neural activations were found in regions of the VTA/SN, dorsal Raphe nucleus, caudate, putamen, posterior GP, thalamus, anterior cingulate, posterior cingulate, insular cortex, mid-insula, posterior hippocampus, middle temporal gyrus, amygdala, angular gyrus and cerebellum (Table 2). Regions that were similarly activated to the long-term partner using the two control conditions were the VTA, right anterior caudate, left putamen, posterior GP, dorsal Raphe, insular cortex, posterior hippocampus, amygdala, anterior and posterior cingulate.
Table 2.
ROI activations showing responses to images of the Partner vs images of the Close Friend
| Brain region | Left |
Right |
||||||
|---|---|---|---|---|---|---|---|---|
| x | y | z | P | x | y | z | P | |
| Activations | ||||||||
| VTA/SNa,b | 4 | −20 | −16 | 0.03 | ||||
| SNa,b | 18 | −18 | −8 | 0.03 | ||||
| Caudate, anteriora,c | 18 | 4 | 22 | 0.02 | ||||
| Putamenc | −22 | 2 | 12 | 0.02 | ||||
| Putamena | 18 | −18 | −8 | 0.03 | ||||
| Putamenb | 24 | −12 | 12 | 0.02 | ||||
| GP, posteriora | −34 | 2 | −6 | 0.02 | 20 | −6 | −8 | 0.03 |
| Dorsal Raphe nucleusb | −2 | −24 | −16 | 0.01 | 2 | −24 | −18 | 0.01 |
| Thalamusb | −8 | −18 | 4 | 0.02 | 8 | −20 | 0 | 0.01 |
| Mid-insulaa,b | −44 | 6 | −2 | 0.04 | 40 | 12 | −2 | 0.00 |
| Mid-insulad | 42 | −4 | 2 | 0.00 | ||||
| Insular cortexb | −38 | 8 | −14 | 0.02 | ||||
| Insula, posteriorb | 36 | −18 | −4 | 0.02 | ||||
| Posterior hippocampusc | −34 | −32 | −4 | 0.01 | 34 | −36 | 0 | 0.04 |
| Amygdalae | −18 | −2 | −14 | 0.01 | ||||
| Anterior cingulateb | −10 | 42 | −10 | 0.02 | 12 | 38 | −10 | 0.01 |
| Posterior cingulateb | −10 | −64 | 10 | 0.03 | ||||
| Middle temporal gyrusb | 46 | 2 | −10 | 0.04 | ||||
| Angular Gyrusf | −64 | −48 | 26 | 0.03 | ||||
| Cerebellumb | 42 | −50 | −26 | 0.05 | ||||
Note. ROIs were identified as the highest intensity voxel in a cluster, for the Partner vs close friend (CF) contrast. MNI coordinates (x, y, z) are at the maximum value for the cluster, which may be elongated in any direction. P-values (P) are for FDR correction. Letters indicate origin of regions of interest.
aBartels and Zeki (2004) for maternal love.
bStrathearn et al. (2008) for maternal attachment.
cBartels and Zeki (2000) for early stage romantic love.
dAron et al. (2005) for early stage romantic love.
eTable 1 by Aron et al. (2005) for reward and emotion
Further controls for closeness
To further examine whether effects found for the Partner vs HFN might be due to close friendship, we applied an inclusive mask of the Partner vs HFN contrast on the CF vs LFN and CF vs HFN contrasts independently. Results are shown in Table 3.
Table 3.
Common Partner and Close Friend activations: general social bonding effects
| Brain region | Left |
Right |
||||||
|---|---|---|---|---|---|---|---|---|
| x | y | z | P | x | y | z | P | |
| CF vs LFN contrast | ||||||||
| mOFC | 2 | 58 | −12 | 0.00 | ||||
| Hypothalamus | 2 | −4 | −6 | 0.00 | ||||
| Periaqueductal gray | 2 | −30 | −20 | 0.00 | ||||
| Tectum | 2 | −30 | −8 | 0.00 | ||||
| Fusiform gyrus | 40 | −32 | −14 | 0.00 | ||||
| Angular gyrus | −34 | −76 | 36 | 0.00 | ||||
| Inferior temporal gyrus | −36 | 4 | −34 | 0.00 | ||||
| Cerebellum | −6 | −48 | −28 | 0.00 | ||||
| CF vs HFN contrast | ||||||||
| NAcc | 10 | 4 | −4 | 0.06 | ||||
Note. Regions for the CF vs LFN contrast were identified as the highest intensity voxel in a cluster for the CF vs LFN contrast masked by the Partner vs HFN contrast. Results are for voxel-level threshold at P (uncorrected multiple comparisons) <0.005, with a spatial extent of ≥15 contiguous voxels. For the CF vs HFN contrast, ROIs were identified as the highest intensity voxel in the cluster. All coordintates (x, y, z) are in MNI space.
Regions commonly activated for the partner (Partner vs HFN) and the CF (CF vs LFN), using voxel-level threshold of P < 0.005, with a minimum spatial extent of 15 or more contiguous voxels, included the right mOFC, hypothalamus, PAG, tectum, fusiform gyrus, the left angular gyrus, inferior temporal gyrus and cerebellum.
The masking approach showed no significant regional activations commonly recruited by the Partner vs HFN and CF vs HFN contrasts. However, applying a ROI analysis on the CF vs HFN contrast revealed a marginally significant activation in a region of the right NAcc (MNI coordinates: 10, 4, −4, P = 0.055).
In sum, effects for the Partner vs HFN contrast commonly activated by close friends were found in the right NAcc, mOFC, hypothalamus, PAG and also in the left hemisphere of the cerebellum. These activations are indicated with the superscript ‘e’ in Table 1 to highlight common activation for Partner and CF effects.
Controls for familiarity
To further investigate familiarity effects, we examined neural activations for the HFN vs LFN contrast. First, we applied an inclusive mask of the Partner vs HFN contrast on the HFN vs LFN contrast. Regions commonly activated for both contrasts (using voxel-level threshold of P < 0.005, with a minimum spatial extent of 15 or more contiguous voxels) included the dorsal Raphe and dorsal caudate head, both on the right side. Since this is a very conservative approach we proceeded with ROI analyses of the HFN vs LFN contrast based on published studies of early-stage and maternal love.
ROI analyses for the HFN vs LFN contrast showed activation in the left GP, amygdala, bilaterally in the dorsal head of the caudate nucleus, on the right side of the middle insula and in the dorsal Raphe nucleus. Results are shown in Supplementary Table S3. In addition, these regions are indicated with the superscript ‘g’ in Table 1 to highlight common activation for partner and familiarity effects, indicating which areas might be playing a role in familiarity of the long-term partner.
Correlations with behavioral self-report measures
ROI analyses for brain-behavior correlations were based on results from studies of early-stage romantic love (Bartels and Zeki, 2000; Aron et al., 2005; Ortigue et al., 2007) and maternal love (Bartels and Zeki, 2004). We adopted a FDR with a threshold of P ≤ 0.05. Results are shown in Table 4.
Table 4.
Significant regional correlations with participants’ scores on passionate love, romantic love, IOS, friendship-based love and sexual frequency
| Brain region | Left |
Right |
||||||
|---|---|---|---|---|---|---|---|---|
| x | y | z | P | x | y | z | P | |
| PLS correlation (love items) | ||||||||
| VTAa | −2 | −12 | −8 | 0.00 | 0 | −14 | −8 | 0.01 |
| Caudate body, mediala | 12 | 16 | 8 | 0.01 | ||||
| Caudate body, antero dorsala | −20 | 4 | 18 | 0.00 | ||||
| Caudate body, postero dorsala | 18 | −18 | 20 | 0.01 | ||||
| Putamenb | −20 | 2 | 6 | 0.00 | ||||
| Posterior hippocampusb | 36 | −38 | 0 | 0.01 | ||||
| PLS correlation (obsession) | ||||||||
| Caudate body, mediala | 12 | 16 | 8 | 0.05 | ||||
| Septum/fornixa | −2 | 0 | 16 | 0.04 | ||||
| Posterior cingulatea | 6 | −52 | 12 | 0.05 | ||||
| Posterior hippocampusb | 36 | −38 | −2 | 0.01 | ||||
| Eros scale correlation | ||||||||
| VTAa | 0 | −12 | −6 | 0.05 | ||||
| Caudate body, mediala | 16 | −20 | −8 | 0.01 | ||||
| Caudate body, postero dorsala | 16 | −12 | 18 | 0.01 | ||||
| Posterior cingulatea | 8 | −52 | 16 | 0.00 | ||||
| Posterior hippocampusb | 36 | −34 | 2 | 0.05 | ||||
| IOS correlation (Partner minus CF scores) | ||||||||
| VTA/SNa | 2 | −12 | −14 | 0.03 | ||||
| Mid-insulab,c | 40 | 12 | −6 | 0.02 | ||||
| Anterior cingulateb,c | −8 | 22 | 40 | 0.04 | ||||
| FBLS correlation | ||||||||
| GPc | 18 | −4 | 2 | 0.02 | ||||
| Insular cortexb,c | −42 | 4 | 4 | 0.03 | ||||
| Parahippocampal gyrusb | 26 | −44 | −8 | 0.00 | ||||
| Sexual frequency correlation | ||||||||
| Posterior hippocampusb | −34 | −34 | −2 | 0.01 | ||||
| Hypothalamus, posterior | 11 | −1 | −9 | 0.03 | ||||
Note. All results are for the Partner vs HFN contrast, except for the correlation with the IOS, which used the Partner vs CF contrast. ROIs were identified as the highest intensity voxel in a cluster. MNI coordinates (x, y, z) are at the maximum value for the cluster, which may be elongated in any direction. P-values (P) are for FDR correction. All results are for regions showing greater brain activation associated with higher scores on the: PLS love items, PLS obsession items, Eros Scale, IOS Scale, FBLS, and sexual frequency (controlling for age). Letters indicate origin of regions of interest.
aROI based on results by Aron et al. (2005) for early stage romantic love.
bROI based on results by Bartels and Zeki (2000) for early stage romantic love.
cROI based on results by Bartels and Zeki (2004) for maternal love.
Passionate love scale
Correlations were conducted separately for passionate love items and obsessive items of the PLS based on findings with factor analyses of the PLS in long-term relationships (Acevedo and Aron, 2009). ROI analyses showed PLS scores (for nonobsession items) were associated with greater neural activity (Partner vs HFN contrast) in regions of the VTA, caudate body, putamen and posterior hippocampus. The positive association between PLS scores with activity in the VTA replicated PLS results reported for early-stage romantic love (Ortigue et al., 2007); activity in the medial caudate body was similar to PLS results reported for early-stage romantic love (Aron et al., 2005) and rejection in love (e.g. those still intensely in love with a person who has rejected them; Fisher et al., 2010).
For obsession-related PLS items, ROI analyses showed greater neural activity in the medial caudate body, posterior cingulate, posterior hippocampus and septum/fornix. An obsession-item PLS correlation in the medial caudate and septum/fornix replicated the PLS correlations found for early-stage romantic love (Aron et al., 2005) and rejection in love (Fisher et al., 2010).
Eros scale
ROI analyses showed Eros scores were positively associated with greater neural activity (Partner vs HFN contrast) in regions of the right VTA, caudate body, posterior cingulate and posterior hippocampus regions found activated for the basic contrasts of early-stage romantic love in other studies.
IOS
To examine the role of closeness using a correlation with the IOS, we tested the association of Partner minus CF IOS scores in the Partner vs CF contrast. ROI analyses showed regional activations in the right VTA/SN (Figure 2B), right mid-insula and the left anterior cingulate.
FBLS
To examine the role of companionate/friendship-based love, we carried out a correlation with the FBLS in response to the Partner vs HFN. ROI analyses showed activations in the right GP, left insular cortex and right parahippocampal gyrus.
Sexual frequency
We carried out a correlation with sexual frequency (controlling for age) within the Partner vs HFN contrast. ROI analyses showed greater sexual frequency with the partner was positively associated with activation of the left posterior hippocampus (Figure 2D), an area found to be activated for the Partner vs HFN and CF contrasts. Exploratory analyses showed a prominent activation in the posterior lateral hypothalamus (MNI coordinates: 10, −2, −7).
Relationship length
We tested the association between number of years married and neural activity within the P vs CF contrast. Exploratory analyses showed greater response associated with years married in the right NAcc/caudate (Figure 2C), (MNI coordinates: 10, 18, −4; 14, 18, 0), and right PAG (MNI coordinates: 2, −28, −20).
DISCUSSION
Long-term romantic love, reward and motivation
This is the first functional imaging study to examine the neural correlates of long-term romantic love. Our first hypothesis was that long-term romantic love is similar to early-stage romantic love. We predicted that subjects would show neural activity in dopamine-rich regions associated with reward and motivation, particularly the VTA, in line with previous studies of early-stage romantic love (Bartels and Zeki, 2000; Aron et al., 2005; Ortigue et al., 2007; Xu, 2009). As predicted, individuals reporting intense, long-term romantic love showed neural activity in response to images of their partners (vs various controls) in mesolimbic, dopamine-rich regions important for reward-processing and motivation. Specifically, early-stage and long-term romantic love commonly recruited the right VTA and caudate, even after controlling for close friendship and familiarity.
The present study is the sixth fMRI study to show significant activation of the right VTA in association with an image of an intensely loved romantic partner (Bartels and Zeki, 2004; Aron et al., 2005; Ortigue et al., 2007; Fisher et al., 2010; Xu et al., 2010). In the present study, activation of the VTA was greater in response to images of the long-term spouse when compared with images of a close friend and a highly familiar acquaintance. The same regions of the VTA showed greater activation as a function of romantic love scores, measured by the Eros scale and PLS (nonobsessive) items. A correlation with closeness scores (measured by the IOS) also showed VTA activation, but also extending into the SN. However, associations with friendship-based love scores, obsession-related items on the PLS and sexual frequency did not show significant effects in the VTA. These correlations are novel findings that provide additional evidence for the involvement of the right VTA in romantic love (without obsession) and with IOS, or perceived closeness in the relationship.
Other studies have shown the important role of the VTA and caudate nucleus in motivation, reinforcement learning and decision making (Delgado et al., 2003; O’Doherty et al., 2004; Carter et al., 2009). The VTA is centrally placed in a wider motivational/reward network associated with behaviors necessary for survival (Camara et al., 2009). It is widely accepted that activation of dopamine-rich sites, such as the VTA and caudate, are evoked in response to rewards such as food (Hare et al., 2008), monetary gains (Delgado et al., 2003; D’Ardenne et al., 2008; Carter et al., 2009), cocaine and alcohol (Heinz et al., 2004; Risinger et al., 2005) and overall highly motivational stimuli (Knutson and Greer, 2008; Carter et al., 2009).
According to one model of reinforcement learning, the ventral and dorsal striatum have distinct functions—the ventral striatum is thought to be involved in reward and motivation, and the dorsal striatum in motor and cognitive control (Doherty et al., 2004). Recruitment of the mesolimbic dopamine system, which mediates reward and motivation, is consistent with notions of romantic love as a ‘desire for union with another’. In addition, recruitment of the dorsal striatum, associated with goal-directed behavior necessary to attain rewarding stimuli, is consistent with characteristics of pair-bonds and romantic love. These activations suggest mechanisms by which humans and other mammals may enact behaviors that maintain (e.g. proximity maintenance and doing things to make the partner happy) and protect (rejection of unfamiliar conspecifics) their pair-bonds (Winslow et al., 1993; Carter et al., 1995; Wang et al., 1997; Aragona et al., 2003).
Long-term love and attachment
We predicted that established, long-term pair-bonds would commonly recruit regions implicated in maternal attachment (Bartels and Zeki, 2004; Strathearn et al., 2008). We based this prediction on the hypothesis that there is an underlying ‘attachment system’ which coordinates proximity seeking, and that it shares common biological substrates for pair-bonds and parent–infant bonds (Hazan and Shaver, 1987; Fisher, 1992; Carter, 1998). Results showed common neural activity for long-term romantic love and maternal attachment in regions of the right posterior GP, bilateral SN, putamen, thalamus, posterior cingulate, left side of the mid-insula, insular cortex, dorsal Raphe and anterior cingulate (after controlling for familiarity and close friendship).
Several of the regions noted above (e.g. the SN, GP and thalamus) have a high density of oxytocin (OT) and vasopressin (AVP) receptors (Jenkins et al., 1984; Loup et al., 1991). OT and AVP have been shown to play a critical role in the regulation of social behaviors (Insel et al., 1994) and monogamous pair-bonding in rodent mammals (Young et al., 2001; Lim and Young, 2004; Young and Wang, 2004). Similar neural activations are observed in the present context for long-term, pair-bonded humans. This is the first human imaging study to show neural activation in pair-bonded humans in regions implicated in pair-bonding of monogamous rodent species.
Activation in the dorsal Raphe is also interesting as the nuclei receive inputs from the VTA/SN (Kirouac et al., 2004), and use serotonin as their neurotransmitter and are involved in the body’s response to pain and stress (Bittar et al., 2005). Activation of the Raphe nuclei may reflect regulatory mechanisms associated with attachment bonds. For example, ‘felt security’ is proposed to be the set goal of the attachment system (Sroufe and Waters 1977). Also, pain and stress reduction have been shown to be associated with the representation of an attachment figure (Coan et al., 2006; Master et al., 2009).
The other major pattern that emerged from examining common activations for long-term pair-bonds and maternal attachment was recruitment of brain systems mediating the ‘liking’ or ‘pleasure’ aspect of reward. That is, according to the incentive motivation model, dopamine mediates ‘wanting’ and the opiate system mediates ‘liking’ (Berridge and Robinson, 1998). Human brain imaging studies have shown that pleasant foods activate the ventral pallidum, NAcc, amygdala, orbitofrontal cortex, anterior cingulate cortex and anterior insular cortex (for review see Smith et al., 2010). Many of these regions were activated in response to the partner.
In addition, the GP is a major site for opiate receptors (Olive et al., 1997; Napier and Mitrovic, 1999) and has been identified as a ‘hedonic hotspot’, mediating both ‘liking’ and ‘wanting’ for primary rewards (Berridge et al., 2010; Smith et al., 2010). Lesion studies have shown that disruptions in the GP cause aversion to previously pleasant foods (Cromwell and Berridge, 1993). Traditionally, the GP was thought of as a major site for motor behavior. More recently, research has shown its important role in mediating reward and motivation (Smith et al., 2009). It is also interesting to note that the posterior GP was uniquely correlated with friendship-based love scores (not with romantic love measures or sex frequency).
In sum, responses to images of a long term, loved partner were associated with brain systems which have been identified as important for ‘liking’ of primary rewards. Moreover, results showed activation of the posterior GP and insular cortex as a function of friendship-based love scores. Activation of these regions in the present context suggest ‘liking’ or ‘pleasure’ aspects related to the relationship with the partner.
Correlation with behavioral measures
Romantic love
Correlations with romantic love measures were carried out separately with nonobsessive items on the PLS and with Eros scores. One result for the correlations with both measures was consistent with previous findings—greater romantic love scores were positively associated with activity in the medial caudate body. Especially interesting to the present study is the correlation of the VTA activity in relation to the PLS (nonobsessive items) and Eros scores, which replicated PLS results for early-stage romantic love (Ortigue et al., 2007). Other correlations with love scores were novel to this group: the posterior cingulate and posterior hippocampus.
Obsession
Obsession-related scores in the present sample were generally low. Our questionnaire findings are consistent with research suggesting long-term romantic relationships are generally low on obsession (Acevedo and Aron, 2009). However, greater scores on obsession-related PLS items were positively associated with activity in the medial caudate body, septum/fornix, posterior cingulate and posterior hippocampus. Activation in the septum/fornix is particularly interesting as it replicated PLS results for early-stage romantic love and rejection in love (Aron et al., 2005; Fisher et al., 2010). Lesions of the septum/fornix have been implicated in anxiety reduction in rats (Decker et al., 1995; Degroot and Treit, 2004). Thus, the septum/fornix may be a target site for social anxiety/obsession reduction in humans. The posterior cingulate is also particularly interesting as it was found for early-stage romantic love (Aron et al., 2005) and it has been implicated in obsessive compulsive disorder (Menzies et al., 2008). More generally, the posterior cingulate has been implicated in autobiographical memory retrieval such as when listening to familiar names (such as a spouse, parent or child) vs nonfamiliar person names (Maddock et al., 2001), or when viewing images of a familiar child vs an unfamiliar child (Leibenluft et al., 2004).
IOS
Greater closeness with the partner (measured as IOS) was associated with activity in neural regions implicated in reward (VTA/SN). Also, consistent with the definition of ‘including the other in the self’, IOS scores were positively associated with activation in areas reflecting self-referential processing, such as the middle insula and anterior cingulate (Northoff et al., 2006; Enzi et al., 2009, meta-analysis). The area of the middle insula where we found activity has been associated in numerous studies involving emotion and gestation (Kurth et al., 2010, meta-analysis). More generally, the insula integrates information from various systems and engenders human awareness (Klein et al., 2007; Craig, 2009). Thus, integration of a partner in the self may be associated with neural activity implicated in reward, emotions, awareness and personal relevance.
Friendship-based love
Consistent with research on maternal attachment (Bartels and Zeki, 2004), we found significant correlations in the right GP, insular cortex and parahippocampal gyrus for friendship-based love scores. As noted earlier, the GP is a major opiate receptor site and has been identified as a hedonic hotspot, mediating ‘liking’ of rewards (Smith et al., 2010). Also, the GP has been implicated in pair-bonding research with prairie voles (Young et al., 2001). The insular cortex area where we found activity has been linked with numerous studies of internal body representations and emotional detection and expression (meta-analysis, Kurth et al., 2010). Thus, activation of the insular cortex may reflect sensori-emotive aspects common to attachment bonds, such as warmth and tenderness.
Sexual frequency
Sexual frequency of participants (controlling for age) with their partners was positively correlated with activity in the posterior hypothalamus and left posterior hippocampus. These regions were also found to be specific to the partner after applying close friendship and familiarity controls. Activation of the hypothalamus is consistent with research implicating it in autonomic sexual arousal (Karama et al., 2002).
In the present study, the posterior hippocampus was uniquely activated in response to the partner after controlling for close friendship and familiarity, and correlated with sexual frequency. These findings are consistent with reports suggesting unique activation in this region for romantic love in comparison with maternal love (Bartels and Zeki, 2004). Although little is known about the posterior hippocampal region, some studies have shown increased activation in this area in association with hunger and food craving (LaBar et al., 2001; Pelchat et al., 2004), with particularly greater activity shown in obese individuals (Bragulat et al., 2010). Also, studies show that hippocampal lesions in rodents disrupt the animals’ ability to distinguish hunger and satiation signals (Davidson and Jarad, 1993). Reward system studies suggest that the posterior hippocampus is an important structure in memory, perhaps of stimuli associated with primary rewards (Fernandez and Kroes, 2010). Considering its prominent involvement in the present study as well as others, the posterior hippocampus will be an interesting target for further investigation of relationship research.
Relationship length
Number of years married was positively correlated with neural activity in the right accumbens/caudate. Activity in nearby coordinates of the right accumbens/caudate were found among individuals yearning for a deceased loved one (O’Connor et al., 2008) and for those experiencing cocaine-induced ‘high’ (Risinger et al., 2005). We also found a correlation with activity in the PAG, an area rich in OT, AVP and opioid receptors (Jenkins et al., 1984; Loup et al., 1989; Peckys and Landwehrmeyer, 1999), associated with pain suppression (Bittar et al., 2005). Bartels and Zeki (2004) identified the PAG as specific to maternal love. We found PAG activity for long-term romantic love and close friendship, suggesting the PAG may be implicated more generally in attachment bonds.
Results for relationship length did not overlap with any regions reported by Aron et al. (2005) in their newly in love sample correlations with relationship length (M = 7.40 months). This might be due to time-dependent changes that occur as attachment bonds develop, or differences between long and short-term relationships.
FUTURE DIRECTIONS AND LIMITATIONS
The present study consisted of individuals in heterosexual relationships reporting they were intensely in love with their long-term spouse. It may be useful to recruit individuals in long-term happy (but not intensely in love) marriages in order to distinguish intense romantic love from general relationship happiness. However, this limitation was somewhat controlled for by not finding key activations in the CF vs familiar acquaintance contrast. Nevertheless, the CF is not an all-inclusive control, because happily married (but not intensely in love) individuals may share qualities with in love married individuals which are not typically shared with close friends (e.g. sexual relations, shared investments and offspring).
The present study also showed that for long-term romantic love many more brain regions were affected compared to those found among newly in love subjects (Aron et al., 2005). Some of these differences may reflect time-dependent changes that occur as bonds develop. For example, Aron et al. (2005) recruited a newly in love sample (relationship lengths of 1–17 months, M 7.40 months). Some research suggests that it takes ∼2 years for enduring attachment bonds to become established (Hazan and Zeifman, 1994), thus newly in love individuals may not reflect physiology of full-blown attachment bonds. Future research may seek to address these questions directly by examining neural activations associated with attachment in individuals over time.
This was the first study to examine the neural correlates of intense, long-term romantic love. To reduce noise, we constrained our sample in several ways. For example, we only recruited individuals in heterosexual pair-bonds. Also, our resulting sample had a fairly high house-hold income. Some research suggests that low socioeconomic status is a major stressor for couples (Karney and Bradbury, 2005) that might be an obstacle to experiencing expansion in the relationship and thus sustaining feelings of long-term romantic love. Future research might build on the present findings, and aim to expand the sample demographics to include homosexual couples and more diverse samples overall.
It is important to note although these results are provocative, there are limitations to fMRI research. First, there are several inferences involved in fMRI research that constrain interpretation of the data. In addition, although we find activation in areas rich in receptors for dopamine, OT, AVP, opioids and serotonin, it remains to be determined whether release of these neurochemicals is associated with the experience of romantic love or attachment. Research with prairie voles, a monogamous rodent species, has established that dopamine, OT and AVP are critical in the formation and maintenance of pair-bonds. By extrapolation, we suggest these neurochemicals are implicated in monogamous pair-bonds in humans, but more direct evidence is needed.
IMPLICATIONS
Individuals in long-term romantic love showed patterns of neural activity similar to those in early-stage romantic love. These results support theories proposing that there might be mechanisms by which romantic love is sustained in some long-term relationships. For example, the self-expansion model suggests that continued expansion and novel, rewarding events with the beloved may promote increases in romantic love. Novel, rewarding experiences may use dopamine-rich systems (Schultz, 2001; Guitart-Masip et al., 2010) similar to those activated in this study.
The present results are also consistent with Fisher’s (2006) model of the brain systems involved in mating and reproduction, notably distinguishing between romantic love and attachment. Brain activations associated with romantic love scores (e.g. VTA) were distinct from those brain area activations associated with attachment (e.g. GP, SN). Although there seem to be dissociable neural regions associated with romantic love and attachment both can co-exist in some long-term relationships, as revealed in the present study.
These present results lend support for Berridge and Robinson’s incentive motivation model which distinguishes ‘wanting’ from ‘liking’. According to correlations with self-report measures, passionate/romantic love scores were generally associated with dopamine-rich systems, characteristic of ‘wanting’, while friendship-based love scores were associated with brain regions rich in opiates that mediate ‘liking’ or pleasure aspects of rewarding stimuli. These data are also consistent with models suggesting that romantic love is a motivation or drive, differing from basic emotions to the extent that it is not intrinsically valenced (associated with positive and negative emotions), hard to control, focused on a specific target and associated with the dopamine system (Aron and Aron, 1991; Fisher, 2004; B.P. Acevedo et al., submitted for publication).
Results for long-term romantic love showed recruitment of opioid and serotonin-rich neural regions, not found for those newly in love (Aron et al., 2005). These systems have the capacity to modulate anxiety and pain, and are central brain targets for the treatment of anxiety, obsessive–compulsive disorder and depression. Thus, present findings are in line with behavioral observations suggesting that one key distinction between romantic love in its early and later stages is greater calm associated with the latter (Tennov, 1979; Eastwick and Finkel, 2008; Acevedo and Aron, 2009). These data also serve as preliminary evidence for future research seeking to examine links between relationship quality and brain systems capable of modulating mood and behavior.
The present results also have practical applications, suggesting that educational and therapeutic programs for long-term married couples may be able to set higher standards for what is possible in long-term marriages, as well as a greater potential outcome for those considering whether to commit to a long-term relationship. Indeed, research suggests that romantic love is associated with marital satisfaction (a strong predictor of relationship stability) in long-term marriages (Acevedo and Aron, 2009). Results from the present study add to this body of knowledge suggesting that romantic love—associated with engagement, sexual interest and lower attention to alternative partners (Miller, 1997; Maner et al., 2008)—may promote pair-bond maintenance through sustained reward. Thus, marital therapists and family-focused programs may aim to enhance romantic love feelings among couples as a way to strengthen the relationship and provide cognitive resources associated with pleasure and stress alleviation.
However, the possibility of long-term intense romantic love may create some distress for those in satisfying, but not intensely in love marriages. Indeed, downward comparisons relative to others’ relationships seem to be a cognitive mechanism that may promote relationship commitment (Rusbult et al., 2000). However, these adverse effects may be offset, perhaps by the motivation to enhance one’s relationship. Moreover, even relationships of strongly bonded and in love couples go through ups and downs, and perhaps even longer cycles where the spark of intense romantic love may just be latent. We do not suggest that intensely high feelings, associated with rushes of energy, are sustained constantly. Rather, we propose that dopamine-rich reward systems are involved in long-term intense romantic love, as well as areas important for attachment.
CONCLUSIONS
For the first time, the neural correlates of long-term romantic love were investigated. Results showed activation specific to the partner in dopamine-rich brain regions associated with reward, motivation and ‘wanting’ consistent with results from early-stage romantic love studies. These data suggest that the reward-value associated with a long-term partner may be sustained, similar to new love. The correlation of the IOS score with VTA activity is consistent with the definition of romantic love as a ‘desire for union with another’. Activation of the dorsal striatum, important for goal-directed behavior to attain rewards, suggests regions that are active when partners enact behaviors that maintain and enhance their relationships.
In addition, results showed activations among pair-bonded humans that have been established as critical for pair-bonding in monogamous rodents, namely in the GP. Unlike findings for newly in love individuals, those in long term, in love marriages showed activation in brain regions associated with attachment and ‘liking’ aspect of rewards. In sum, the ‘wanting’, motivation and reward associated with a long-term partner may be sustained, and can co-exist with ‘liking’ and pleasure, aspects of attachment bonding.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Acknowledgement
This research was partially supported by funding from the W. Burghardt Turner Fellowship to Bianca Acevedo and the Psychology Department at Stony Brook University. We thank Keith Sanzenbach for his technical assistance with the fMRI scanner. We thank Suzanna Katz, Zorammawii Ralte, ManChi Ngan, Geraldine Acevedo and Irena Tsapelas for their assistance in data collection and entry.
REFERENCES
- Acevedo BP, Aron A. Does a long-term relationship kill romantic love? Review of General Psychology. 2009;13:59–65. [Google Scholar]
- Ainsworth MDS. Attachments and other affectional bonds across the life cycle. In: Parkes CM, Stevenson-Hinde J, Marris P, editors. Attachment across the life cycle. New York: Tavistock/Routledge; 1991. pp. 33–51. [Google Scholar]
- Aragona BJ, Liu Y, Curtis JT, Stephan FK, Wang Z. A critical role for nucleus accumbens dopamine in partner-preference formation in male prairie voles. Journal of Neuroscience. 2003;23(8):3483–90. doi: 10.1523/JNEUROSCI.23-08-03483.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron A, Aron E. Love and the Expansion of Self: Understanding Attraction and Satisfaction. New York: Hemisphere; 1986. [Google Scholar]
- Aron A, Aron EN, Smollan D. Inclusion of other in the Self Scale and the structure of interpersonal closeness. Journal of Personality and Social Psychology. 1992;63:596–612. [Google Scholar]
- Aron A, Fisher H, Mashek D, Strong G, Li H, Brown L. Reward, motivation and emotion systems associated with early-stage intense romantic love. Journal of Neurophysiology. 2005;93:327–37. doi: 10.1152/jn.00838.2004. [DOI] [PubMed] [Google Scholar]
- Aron A, Henkemeyer L. Marital satisfaction and passionate love. Journal of Social and Personal Relationships. 1995;12:141–9. [Google Scholar]
- Aron A, Norman CC, Aron E, McKenna C, Heyman RE. Couples' shared participation in novel and arousing activities and experienced relationship quality. Journal of Personality and Social Psychology. 2000;78(2):273–84. doi: 10.1037//0022-3514.78.2.273. [DOI] [PubMed] [Google Scholar]
- Aron A, Westbay L. Dimensions of the prototype of love. Journal of Personality and Social Psychology. 1996;70:535–51. [Google Scholar]
- Bartels A, Zeki S. The neural basis of romantic love. NeuroReport. 2000;11(17):3829–34. doi: 10.1097/00001756-200011270-00046. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The neural correlates of maternal and romantic love. NeuroImage. 2004;21(3):1155–66. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Bratslavsky E. Passion, intimacy, and time: Passionate love as a function of change in intimacy. Personality and Social Psychology Review. 1999;3(1):49–67. doi: 10.1207/s15327957pspr0301_3. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Research Reviews. 1998;28:309–69. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Ho C, Richard JM, DiFelicieantonio AG. The tempted brain eats: pleasure and desire circuits in obesity and eating disorders. Brain Research. 2010;1350:43–64. doi: 10.1016/j.brainres.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berscheid E, Hatfield [Walster] EH. Interpersonal Attraction. New York: Addison-Wesley; l969. [Google Scholar]
- Bittar RG, Kar-Purkayastha I, Owen SL, et al. Deep brain stimulation for pain relief: a meta-analysis. Journal of Clinical Neuroscience. 2005;5:515–9. doi: 10.1016/j.jocn.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Bowlby J. Attachment and Loss. Vol. 1. Attachment. London: Hogarth; 1969. [Google Scholar]
- Bragulat V, Dzemidzic M, Bruno C, et al. Food-related odor probes of brain reward circuits during hunger: a pilot fMRI study. Obesity. 2010;18:1566–1571. doi: 10.1038/oby.2010.57. [DOI] [PubMed] [Google Scholar]
- Buss DM. Sex differences in human mate preferences: Evolutionary hypotheses tested in 37 cultures. Behavioral & Brain Sciences. 1989;12:1–49. [Google Scholar]
- Camara E, Rodriguez-Fornells A, Ye Z, Munte TF. Reward networks in the brain captured by connectivity measures. Frontiers in Neuroscience. 2009;3:350–62. doi: 10.3389/neuro.01.034.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23:779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- Carter CS, DeVries AC, Getz LL. Physiological substrates of mammalian monogamy: the prairie vole model. Neuroscience and Biobehavioral Reviews. 1995;19:303–14. doi: 10.1016/0149-7634(94)00070-h. [DOI] [PubMed] [Google Scholar]
- Carter RM, MacInnes JJ, Huettel SA, Adcock RA. Activation in the VTA and nucleus accumbens increases in anticipation of both gains and losses. Frontiers in Behavioral Neuroscience. 2009;3(21):1–15. doi: 10.3389/neuro.08.021.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan JA, Schaefer HS, Davidson RJ. Lending a hand: Social regulation of the neural response to threat. Psychological Science. 2006;17(12):1032–9. doi: 10.1111/j.1467-9280.2006.01832.x. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel—now? The anterior insula and human awareness. Nature Review Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Cromwell HC, Berridge KC. Where does damage lead to enhanced food aversion: the ventral pallidum/sunstantia innominata or lateral hypothalamus? Brain Research. 1993;624:1–10. doi: 10.1016/0006-8993(93)90053-p. [DOI] [PubMed] [Google Scholar]
- D’Ardenne K, McClure SM, Nystrom LE, Cohen JD. BOLD responses reflecting dopaminergic signals in the human ventral tegmental area. Science. 2008;319:1264–7. doi: 10.1126/science.1150605. [DOI] [PubMed] [Google Scholar]
- Davidson TL, Jarad LE. A role for hippocampus in the utilization of hunger signals. Behavioral and Neural Biology. 1993;59:161–71. doi: 10.1016/0163-1047(93)90925-8. [DOI] [PubMed] [Google Scholar]
- Decker MW, Curzon P, Brioni JD. Influence of separate and combined septal and amygdala lesions on memory, acousticstartle, anxiety, and locomotor activity in rats. Neurobiology of Learning and Memory. 1995;64:156–68. doi: 10.1006/nlme.1995.1055. [DOI] [PubMed] [Google Scholar]
- Degroot A, Treit D. Anxiety is functionally segregated within the septo-hippocampal system. Brain Research. 2004;1001:60–71. doi: 10.1016/j.brainres.2003.10.065. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Locke HM, Stenger VA, Fiez JA. Dorsal striatum responses to reward and punishment: Effects of valence and magnitude manipulations. Cognitive Affective & Behavioral Neuroscience. 2003;3:27–38. doi: 10.3758/cabn.3.1.27. [DOI] [PubMed] [Google Scholar]
- Eastwick PW, Finkel EJ. The attachment system in fledgling relationships: An activating role for attachment anxiety. Journal of Personality and Social Psychology. 2008;95:628–47. doi: 10.1037/0022-3514.95.3.628. [DOI] [PubMed] [Google Scholar]
- Enzi B, Greck M, Prosch U, Templemann C, Northoff G. Is our self nothing but reward? Neuronal overlap and distinction between reward and personal relevance and its relation to human personality. PLoS ONE. 2009;4:1–12. doi: 10.1371/journal.pone.0008429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez G, Kroes MC. Protecting endangered memories. Nature Neuroscience. 2010;13:408–10. doi: 10.1038/nn0410-408. [DOI] [PubMed] [Google Scholar]
- Fromm Erich. The Art of Loving. New York: Harper Perennial; 1956. [Google Scholar]
- Freud S. 1921. Group Psychology and the Analysis of the Ego. New York: W.W. Norton and Company. SE, XVIII, 90–91. [Google Scholar]
- Fisher HE. Anatomy of Love: The Natural History of Monogamy, Adultery, and Divorce. New York: W.W. Norton; 1992. [Google Scholar]
- Fisher H. Why We Love: The Nature and Chemistry of Romantic Love. New York: Henry Holt; 2004. [Google Scholar]
- Fisher HE. The drive to love. In: Sternberg R, Weis K, editors. The New Psychology of Love. New Haven: Yale University Press; 2006. pp. 87–115. [Google Scholar]
- Fisher HE, Brown LL, Aron A, Strong G, Mashek D. Reward, addiction, and emotion regulation systems associated with rejection in love. Journal of Neurophysiology. 2010;104:51–60. doi: 10.1152/jn.00784.2009. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging data using the false discovery rate. NeuroImage. 2002;15:870–8. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Grote NK, Frieze IH. The measurement of friendship-based love in intimate relationships. Personal Relationship. 1994;1:275–300. [Google Scholar]
- Guitart-Masip M, Bunzeck N, Stephan KE, Dolan RJ, Düzel E. Contextual novelty changes reward representations in the striatum. Journal of Neuroscience. 2010;30(5):1721–6. doi: 10.1523/JNEUROSCI.5331-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, O’Doherty J, Camerer CF, Schultz W, Rangel A. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. Journal of Neuroscience. 2008;28:5623–30. doi: 10.1523/JNEUROSCI.1309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield E, Sprecher S. Measuring passionate love in intimate relations. Journal of Adolescence. 1986;9:383–410. doi: 10.1016/s0140-1971(86)80043-4. [DOI] [PubMed] [Google Scholar]
- Hazan C, Shaver PR. Romantic love conceptualized as an attachment process. Journal of Personality and Social Psychology. 1987;52:511–24. doi: 10.1037//0022-3514.52.3.511. [DOI] [PubMed] [Google Scholar]
- Hazan C, Zeifman D. Sex and the psychological tether. In: Bartholomew K, Perlman D, editors. Advances in personal relationships: Vol 5. Attachment processes in adulthood. London: Jessica Kingsley; 1994. pp. 151–177. [Google Scholar]
- Hazan C, Zeifma D. Pair-bonds as attachments: Evaluating the evidence. In: Cassidy J, Shaver PR, editors. Handbook of Attachment: Theory, Research, and Clinical Applications. New York: Guilford Press; 1999. pp. 336–354. [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, et al. Correlation between dopamine D2 receptors in the ventral striatum and central processing of alcohol cues and craving. The American Journal of Psychiatry. 2004;161:1783–9. doi: 10.1176/appi.ajp.161.10.1783. [DOI] [PubMed] [Google Scholar]
- Hendrick C, Hendrick SS. A theory and method of love. Journal of Personality and Social Psychology. 1986;50:392–402. [Google Scholar]
- Hendrick S, Hendrick C. Romantic Love. Newbury Park, CA: Sage Publications, Inc; 1992. [Google Scholar]
- Insel TR, Wang ZX, Ferris CF. Patterns of brain vasopressin receptor distribution associated with social organization in microtine rodents. Journal of Neuroscience. 1994;14:5381–92. doi: 10.1523/JNEUROSCI.14-09-05381.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins JS, Ang VT, Hawthorn J, Rossor MN, Iversen LL. Vasopressin, oxytocin and neurophysins in the human brain and spinal cord. Brain Research. 1984;291(1):111–7. doi: 10.1016/0006-8993(84)90656-5. [DOI] [PubMed] [Google Scholar]
- Kampe K, Frith CD, Frith U. "Hey John": Signals conveying communicative intention towards the self activate brain regions associated with mentalising regardless of modality. Journal of Neuroscience. 2003;23:5258–63. doi: 10.1523/JNEUROSCI.23-12-05258.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karama S, Lecours AR, Leroux J-M, et al. Areas of brain activation in males and females during viewing of erotic film excerpts. Human Brain Mapping. 2002;16:1–13. doi: 10.1002/hbm.10014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karney BR, Bradbury TN. Contextual influences on marriage: Implications for policy and intervention. Current Directions in Psychological Science. 2005;14:171–4. [Google Scholar]
- Kirouac GJ, Li S, Mabrouck G. GABAergic projections from the ventral tegmental area and substantia nigra to the periaqueductal gray region and the dorsal Raphe nucleus. The Journal of Comparative Neurology. 2004;469:170–84. doi: 10.1002/cne.11005. [DOI] [PubMed] [Google Scholar]
- Klein TA, Endrass T, Kathmann N, et al. Neural correlates of error awareness. Neuroimage. 2007;34:1774–81. doi: 10.1016/j.neuroimage.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Knutson B, Greer SM. Anticipatory affect: neural correlates and consequences for choice. Philosophical Transactions of the Royal Society B. 2008;363:3771–86. doi: 10.1098/rstb.2008.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth F, Ziles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: Functional differentiation and integration within the human insula revealed by meta-analysis. Brain Structure and Function. 2010;214:519–34. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Gitelman DR, Mesulam MM, Parrish TB. Impact of signal-to-noise on functional MRI of the human amygdala. Neuroreport. 2001;12:3461–4. doi: 10.1097/00001756-200111160-00017. [DOI] [PubMed] [Google Scholar]
- Lee, John A. A typology of styles of loving. Personality & Social Psychology Bulletin. 1977;3:173–82. [Google Scholar]
- Leibenluft E, Gobbini MI, Harrison T, Haxby JV. Mothers' neural activation in response to pictures of their children and other children. Biological Psychiatry. 2004;56(4):225–32. doi: 10.1016/j.biopsych.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Lim MM, Young LJ. Vasopresson-dependent neural circuits underlying pair-bond formation in the monogamous prairie vole. Neuroscience. 2004;125:35–45. doi: 10.1016/j.neuroscience.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Loup FE, Tribollet E, Dubois-Dauphin M, Dreifuss JJ. Localization of high-affinity binding sites for oxytocin and vasopressin in the human brain. An autoradiographic study. Brain Research. 1991;555:220–32. doi: 10.1016/0006-8993(91)90345-v. [DOI] [PubMed] [Google Scholar]
- Loup FE, Tribollet E, Dubois-Dauphin M, Pizzolato G, Dreifuss JJ. Localization of oxytocin binding sites in the human brainstem and uppoer spinal cord: An auto radiographic study. Brain Research. 1989;500:223–30. doi: 10.1016/0006-8993(89)90317-x. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH. Remembering familiar people: the posterior cingulate cortex and autobiographical memory retrieval. Neuroscience. 2001;104(3):667–76. doi: 10.1016/s0306-4522(01)00108-7. [DOI] [PubMed] [Google Scholar]
- Mai JK, Paxinos G, Voss T. Atlas of the Human Brain. 3rd edn. San Diego: Elsevier Academic Press; 2008. [Google Scholar]
- Maner JK, Rouby DA, Gonzaga GC. Automatic inattention to attractive alternatives: the evolved psychology of relationship maintenance. Evolution and Human Behavior. 2008;29:343–9. [Google Scholar]
- Master SL, Eisenberger NI, Taylor SE, Naliboff BD, Shirinyan D, Lieberman MD. A picture’s worth. Partner photographs reduce experimentally induced pain. Psychological Science. 2009;20:1316–8. doi: 10.1111/j.1467-9280.2009.02444.x. [DOI] [PubMed] [Google Scholar]
- Menzies L, Chamberlain SR, Laird AR, et al. Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: The orbitofrontal-straitel model revisited. Neuroscience and Behavioral Reviews. 2008;32:525–49. doi: 10.1016/j.neubiorev.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikulincer M, Shaver PR. Attachment in Adulthood: Structure, Dynamics, and Change. New York: The Guilford Press; 2007. [Google Scholar]
- Miller RS. Inattentive and contented: Relationship commitment and attention to alternatives. Journal of Personality and Social Psychology. 1997;73:758–66. [Google Scholar]
- Napier TC, Mitrovic I. Opiod modulation of ventral pallidal inputs. Annals of the New York Academy of Sciences. 1999;877:176–201. doi: 10.1111/j.1749-6632.1999.tb09268.x. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, deGreck M, et al. Self-referential processing in our brain—a meta-analysis of imaging studies on the self. Neuroimage. 2006;31:440–57. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- O'Connor M-F, Wellisch DK, Stanton AL, et al. Craving love? Enduring grief activates brain's reward center. NeuroImage. 2008;42:969–72. doi: 10.1016/j.neuroimage.2008.04.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304:452–4. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, et al. Reflecting upon feelings: An fMRI study of neural systems supporting the attribution of emotion to self and other. Journal of Cognitive Neuroscience. 2004;16:1746–72. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Olive MF, Anton B, Micevych P, Evans CJ, Maidment NT. Presynaptic vs. Postsynaptic locoalization of opioid receptors in dorsal and ventral striatopallidal pathways. J Neuroscience. 1997;17:7471–9. doi: 10.1523/JNEUROSCI.17-19-07471.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortigue S, Bianchi-Demicheli F, Hamilton AF, Grafton ST. The neural basis of love as a subliminal prime: An event-related fMRI study. Journal of Cognitive Neuroscience. 2007;19:1218–30. doi: 10.1162/jocn.2007.19.7.1218. [DOI] [PubMed] [Google Scholar]
- Peckys D, Landwehrmeyer GB. Expression of mu, kappa, and delta opioid receptor messenger RNA in the human CNS: a 33P in situ hybridization study. Neuroscience. 1999;88:1093–135. doi: 10.1016/s0306-4522(98)00251-6. [DOI] [PubMed] [Google Scholar]
- Pelchat ML, Johnson A, Chan R, Valdez J, Ragland JD. Images of desire: food-craving activation during fMRI. Neuroimage. 2004;23:1486–93. doi: 10.1016/j.neuroimage.2004.08.023. [DOI] [PubMed] [Google Scholar]
- Risinger RC, Salmeron BJ, Ross TJ, et al. Neural correlates of high and craving during cocaine self-administration using BOLD fMRI. NeuroImage. 2005;26(4):1097–108. doi: 10.1016/j.neuroimage.2005.03.030. [DOI] [PubMed] [Google Scholar]
- Rusbult CE, Van Lange PAM, Wildschut T, Yovetich NA, Verette J. Perceived superiority in close relationships: Why it exists and persists. Journal of Personality and Social Psychology. 2000;79:521–45. [PubMed] [Google Scholar]
- Schultz W. Reward signaling by dopamine neurons. The Neuroscientist. 2001;7:293–302. doi: 10.1177/107385840100700406. [DOI] [PubMed] [Google Scholar]
- Smith KS, Tindell AJ, Aldridge JW, Berridge KC. Ventral pallidum roles in reward and motivation. Behavioral Brain Research. 2009;196(2):155–67. doi: 10.1016/j.bbr.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sroufe LA, Waters E. Attachment as an organizational construct. Child Development. 1977;48:1184–99. [Google Scholar]
- Sternberg RJ. A triangular theory of love. Psychological Review. 1986;93:119–35. [Google Scholar]
- Strathearn L, Li J, Fonagy P, Montague P. What's in a smile? Maternal brain responses to infant facial cues. Pediatrics. 2008;122(1):40–51. doi: 10.1542/peds.2007-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennov D. Love and Limerence: The Experience of Being in Love. New York: Stein & Day; 1979. [Google Scholar]
- Walum H, Westberg L, Henningsson S, et al. Genetic variation in the vasopressin receptor 1a gene (AVPR1A) associates with pair-bonding behavior in humans. Proceedings of the National Academy of Sciences. 2008;105:14153–6. doi: 10.1073/pnas.0803081105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZX, Hulihan TJ, Insel TR. Sexual and social experience is associated with different patterns of behavior and neural activation in male prairie voles. Brain Research. 1997;767(2):321–32. doi: 10.1016/s0006-8993(97)00617-3. [DOI] [PubMed] [Google Scholar]
- Winslow J, Hastings N, Carter CS, Harbaugh C, Insel TR. A role of central vasopressin in pair bondings in monogamous prairie voles. Nature. 1993;365:545–8. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Xu X, Aron A, Brown L, Cao G, Feng T, Weng X. Reward and motivation systems: a brain mapping study of early-stage intense romantic love in Chinese participants. Human Brain Mapping. 2010;32:249–57. doi: 10.1002/hbm.21017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Lim MM, Gingrich B, Insel TR. Cellular mechanisms of social attachment. Hormones and Behavior. 2001;40(2):133–8. doi: 10.1006/hbeh.2001.1691. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z. The neurobiology of pair-bonding. Natural Neurosciences. 2004;7(10):1048–54. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.