Abstract
The idea of ‘extended self’ refers to the incorporation of personally relevant external stimuli into one’s concept of self. The current study used a transient imagined ownership paradigm to explore brain regions that support the association between self and objects. We hypothesized that incidental associations between self and objects would be manifested by activation in a brain region, medial prefrontal cortex (MPFC), recruited in explicit self-referential processing. We further predicted that a memorial advantage for, and positivity-bias towards, self-relevant objects would be related to activity in MPFC. As anticipated, MPFC showed greater activation when objects were assigned to participants compared to when objects were assigned to another person. Activity in MPFC was also associated with superior subsequent source memory and increased preference for objects assigned to the self. These findings provide neural evidence for the incorporation of self-relevant objects into an extended self, which, in turn, increases their judged value (mere ownership effect).
Keywords: extended self, ownership, self-referential processing, medial prefrontal cortex, fMRI
INTRODUCTION
Given that a central feature of human experience is to develop and maintain a sense of ‘self’ that persists across space and time (James, 1890/1983; Neisser, 1988; Damasio, 1999), it is not surprising that the ‘self’ has long intrigued scholars from diverse academic communities including philosophy, psychology and neuroscience (Conway and Pleydell-Pearce, 2000; Gallagher, 2000; Klein et al., 2002; Heatherton et al., 2004; Boyer et al., 2005; Metcalfe et al., 2010). Furthermore, in philosophy and psychology, different aspects of self have been emphasized, for example, experiential (associated with awareness), instrumental (associated with control) and self-reflective (associated with cognitive complexity—as in taking the self as object of reflection) (Johnson, 1991; Johnson and Reeder, 1997). The pivotal status of self in social-cognitive functioning and its multifaceted role more generally (e.g. as perceiver, cognizer, action-initiator, mind-reader, self-regulator, etc.) are reflected in different concepts of self in neuroscience as well, for example, the ‘proto self’ (Damasio, 1999) in the sensory and motor domain, and ‘autobiographical self’ (Damasio, 1999) or ‘narrative self’ (Gallagher, 2000; Gallagher and Frith, 2003) in the memory domain. In addition, it has been suggested that at a ‘minimal’ level (Gallagher, 2000), a sense of self enables an organism to distinguish itself from its immediate external environment (Neisser, 1988; Boyer et al., 2005), thereby affording a sense of owning a body that processes external stimuli and produces actions.
The concept of self, however, can extend beyond activities of mind and a sense of body. Given the constant interaction between the self and the external environment, a pivotal function of self is to distinguish the external items that require elaborate processing for future reference from those that can be treated in a relatively cursory manner (Tooby and Cosmides, 1995). Belk (1988, 1991) suggested that a diverse range of stimuli (e.g. possessions, acquaintances, places, etc.) are routinely incorporated into people’s representations of self (Wicklund and Gollwitzer, 1982; Beggan, 1992; Burris and Rempel, 2004), and others have suggested that a strong relationship to self gives rise to a conscious experience of ownership (i.e. ‘mineness’: Lambie and Marcel, 2002; Metzinger, 2003).
Once associated with self, objects enjoy a special psychological status. Objects that come in contact with the self elicit feelings of ownership (even when the contact is quite incidental) and increase one’s perceptions of desirability (i.e. mere ownership effect: Belk, 1988, 1991; Beggan, 1992) and worth (i.e. endowment effect: Knetsch and Sinden, 1984; Kahneman et al., 1991). The mere ownership effect has been interpreted as motivational in nature: to satisfy the desire to see oneself in a positive light which extends to overvaluing objects associated with the self (e.g. self-enhancing bias: Beggan, 1991, 1992). Strikingly, this phenomenon extends to the appraisal of artificial/inconsequential stimuli such as abstract symbols (Feys, 1991). Furthermore, a recent study demonstrated that the typical memory advantage of stimuli that have been evaluated with respect to self (i.e. self-reference effect: Rogers et al., 1977; Maki and McCaul, 1985; Symons and Johnson, 1997) extends to objects that were only incidentally associated with self. That is, objects that were transiently assigned to participants were remembered better compared to those assigned to someone else (Cunningham et al., 2008).
Based on such empirical demonstrations of a linkage between self-relevant objects and the self, the current study explored brain regions supporting the incorporation of external objects into one’s self. In searching for the brain structures likely to underlie self-object associations, recent neuroimaging studies that have identified neural correlates of various aspects of self-referential processing provide candidates. Across various domains and stimuli such as the recognition of one’s own physical appearance (Sugiura et al., 2005), awareness of one’s own actions (Blakemore and Frith, 2003), knowledge of one’s own personality traits and abilities (Craik et al., 1999; Johnson et al., 2002; Kelley et al., 2002; D’Argembeau et al., 2005; Ochsner et al., 2005; Heatherton et al., 2006), and thoughts about one’s hopes and duties (Johnson et al., 2006), cortical midline structures are consistently recruited when subjects are engaged in self-referential processing when compared to non-self-referential processing (Northoff and Bermpohl, 2004; Northoff et al., 2006). In particular, the recruitment of medial prefrontal cortex (MPFC) has been most consistently observed, indicating that these structures are important for representing self.
We used a computerized version of an incidental ownership paradigm (Cunningham et al., 2008) to investigate whether assigning objects to participants and asking them to imagine owning the assigned items recruits brain regions that are involved in explicit self-reference and/or self-knowledge. We hypothesized that compared to objects with little or no relevance to self, those items with potential relevance to one’s own self through transient, imagined ownership would recruit MPFC. We further anticipated that the social-cognitive status of self-relevant objects (i.e. superior memory and more positive evaluation) would be predicted by activity in MPFC.
METHODS
Participants
Twelve young, self-reportedly healthy right-handed adults with normal or corrected-to-normal vision participated in the study (eight females, mean age = 21.5 ± 2.39 years). Participants were screened for MRI compatibility, gave written informed consent, and were compensated. The procedure was approved by the Yale University School of Medicine Human Investigation Committee.
Experimental task
The experiment consisted of four tasks: pre-scan preference rating, object assignment task during scanning, post-scan source memory test and post-scan preference rating.
Pre-scan preference rating
On each trial, following a 500-ms fixation, a picture of an object was presented in the center of a computer screen. The participant’s task was to indicate, upon presentation of each object picture, how much they like it on a 1 (‘Lowest’) to 9 (‘Highest’) scale. Next, they were asked to estimate the preference of other people in general (prompted by the cue ‘People in general’ below the picture) for the same object on the same 1–9 scale. Participants had to make a timed response for each rating (4 and 3 s for their own and others’ preference ratings, respectively), adding up to overall stimulus presentation time of 7 s. A total of 126 objects (i.e. critical items) were rated.
Object assignment task
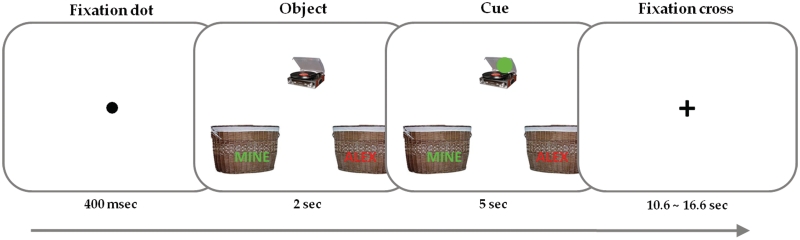
A schematic view of the task procedure is shown in Figure 1.
Fig. 1.
Schematic illustration of the object assignment task performed inside the scanner.
On each trial, participants were presented with a picture of an object at the top center of the screen, along with two baskets presented side by side at the bottom of the screen. The baskets were labeled ‘MINE’ and ‘ALEX’ and the location of the labels was counterbalanced across participants. The participant’s task was to place each item into the appropriate basket according to the color of a dot appearing in a random location on or near the object picture 2 s after the onset of the object picture. The color of the dot matched the color of the label (MINE, ALEX) on one of the baskets and participants simply pressed the appropriate button using left and right index fingers to assign an object to a basket. As soon as participants pressed the button, the object began moving down into the basket to which they assigned it. Participants were asked to imagine that they are going to own the items that were assigned to their basket (the basket labeled ‘MINE’) and that they are not going to own those assigned to the other person’s basket (the basket labeled ‘ALEX’). The whole trial lasted for 7 s. A 400-ms fixation dot signaling the onset of each trial was followed by the presentation of the object picture for each trial. Trials were separated by variable length of intertrial intervals (10.6–16.6 s) to allow jitter in the onset or offset of the hemodynamic response function (HRF). There were nine Mine and nine Alex (Other) trials for each of seven functional runs.
Post-scan source memory test
Each trial consisted of a 250-ms fixation dot, followed by a picture of an object. There were 126 old items (i.e. critical items: 63 Mine and 63 Other) and 63 novel pictures from the same stimulus set. For each object, participants were asked to indicate whether it was assigned to them (Mine) or to another person (Alex) during the object assignment task, or whether it was not seen in the object assignment task at all (New). Objects remained on the screen until a response was made.
Post-scan preference rating
The procedure was exactly the same as the pre-scan preference rating. This phase was included to assess the mere ownership effect (i.e. change in preference rating after the ownership manipulation).
After the post-scan preference rating, participants were asked to rate on a scale ranging from 1 (‘Not at all’) to 7 (‘Very much’) how much they would like to trade their baskets for Alex’s basket if they were given a chance to trade.
Localizer tasks
MPFC localizer
To locate regions of interest (ROIs) independently from our main task, we used a modified version of a typical trait adjective rating task (Kelley et al., 2002). Within a blocked design, participants rated how well trait adjectives describe themselves (i.e. self-referent condition) or former president Bush (i.e. other-referent condition) on a 4-point scale. There were 20 task blocks (10 blocks for each referent condition) with an 8-s fixation period separating the blocks. Each block was prompted by ‘SELF’ or ‘BUSH’ and this cue appeared above the trait adjectives and stayed on the screen throughout the entire block. A total of 100 personality trait adjectives were divided into two lists matched for number of syllables, word length and desirability (Anderson, 1968). Assignment of word lists to the referent condition was counterbalanced and each block consisted of five sequential presentations of adjectives (2.7-s stimulus presentation, 500-ms interstimulus interval).
Lateral occipital complex localizer
People tend to pay more attention to self-relevant information (Markus, 1977) and attention modulates neural responses in higher order visual processing areas (Wojciulik et al., 1998). To examine whether effects of assigning objects to Mine or Other reflect differential attention to self-relevant vs other relevant items, we used a lateral occipital complex (LOC) localizer task consisting of alternating blocks of gray-scale pictures of intact (eight blocks) and scrambled versions (eight blocks) of the same objects, different from the stimulus set used in the main experiment. Each block consisted of 20 sequential presentations of pictures (500-ms stimulus presentation, 200-ms interstimulus interval). An 8-s rest block followed each stimulus block. Participants’ task was to press a button whenever they saw the same picture twice in a row.
Stimuli
The stimulus set comprised 189 photographic images (250 × 250 pixels) of items available for purchase in a large offline/online market (e.g. clothing, stationary, electronic items, etc.). The images were chosen from 260 initial images and were divided into three groups of 63 items each that were matched for preference level (M’s = 5.35, 5.35 and 5.27 on a 1 ‘Lowest’ to 9 ‘Highest’ scale), estimated cost (M’s = $88.94, $90.07 and $90.04), gender-specificity (M’s = 5.14, 5.17 and 5.11 on a scale ranging from 1 ‘Mostly Masculine’ to 9 ‘Mostly Feminine’), and ease of identification (M’s = 2.21, 2.17 and 2.22 on a 1 ‘Easiest’ to 9 ‘Hardest’ scale) through a separate pilot study. Among these stimulus sets, two sets served as critical items for the object assignment task and the remaining set served as ‘new’ items in the post-scan source memory test. The assignment of critical sets to Mine and Other conditions was counterbalanced across participants.
Image acquisition
Imaging data were collected using a 3.0T Siemens Trio scanner with a standard head coil at the Yale University Magnetic Resonance Research Center. A total of 188 functional T2*-weighted echo planar images (EPI) for each of seven functional runs were acquired using a standard echo planar pulse sequence [parameters: repetition time (TR) = 2 s, echo time (TE) = 25 ms, flip angle α = 90°, field of view (FOV) = 240 mm, matrix = 642, slice thickness = 3.5 mm, 34 slices]. At the beginning of each run, four additional volumes were discarded to allow for MR equilibration effects. For localizer runs, a total of 251 and 187 EPI volumes with the same imaging parameters as the main functional runs were acquired for the MPFC and LOC localizer runs, respectively. For registration purpose, two sets of structural images were collected: coplanar images were collected using a T1-flash sequence (TR = 300 ms, TE = 2.47 ms, α = 60°, FOV = 240 mm, matrix = 2562, slice thickness = 3.5 mm, 34 slices). High-resolution images were acquired using a 3D MP-Rage sequence (TR = 2530 ms, TE = 3.34 ms, α = 7°, FOV = 240 mm, matrix = 2562, slice thickness = 1 mm, 160 slices).
Image preprocessing and registration
Functional MRI data were analyzed using FEAT version 5.98, part of FSL software (FMRIB’s Software Library, http://www.fmrib.ox.ac.uk/fsl). Preprocessing included non-brain removal (BET; Smith, 2002), slice-timing correction, motion correction using MCFLIRT (Jenkinson et al., 2002), spatial smoothing (Gaussian, FWHM 5mm), grand-mean intensity normalization and high-pass temporal filtering (cut off = 50 s). Registration was conducted through a 3-step procedure, whereby functional images were first registered to coplanar images, then to MP-Rage structural images, and finally to a standard brain template (Montreal Neurological Institute 152 stereotaxic template) using a rigid body transformations via FLIRT (Jenkinson et al., 2002).
Statistical regression analysis
First-level general linear model (FILM) analysis with time series pre-whitening (Woolrich et al., 2001) was used for each individual EPI sequence using a separate explanatory variable (EV) for each condition of the main analyses. First, the Assignment contrast was modeled using two regressors according to the assignment status (Mine vs Other). The source memory contrast involved two regressors (Remembered Mine vs Remembered Other). The preference contrast was modeled using four regressors which were defined as a function of assignment status and pre- vs post-scan preference change: Mine Higher, Mine Lower, Other Higher and Other Lower. Thus, the trials where there was no difference between the pre- and post-scan preference ratings were excluded from the analysis. For each trial type, the presentation design was convolved with a single-gamma HRF. The second-level analyses combining the seven runs of each participant were performed using a fixed effect model and the group-level analyses were carried out with a mixed effect model, with a random effects component of variances estimated using FSL’s FLAME stage 1 only (Beckmann et al., 2003; Woolrich et al., 2004) to generate Z-static images based on the contrast between conditions. The Z-statistic images were thresholded with clusters determined by Z > 2.0 and a cluster significance threshold of P < 0.05 to correct for multiple comparisons (Worsley, 2001). All coordinates are reported in MNI space.
ROI definition and analysis
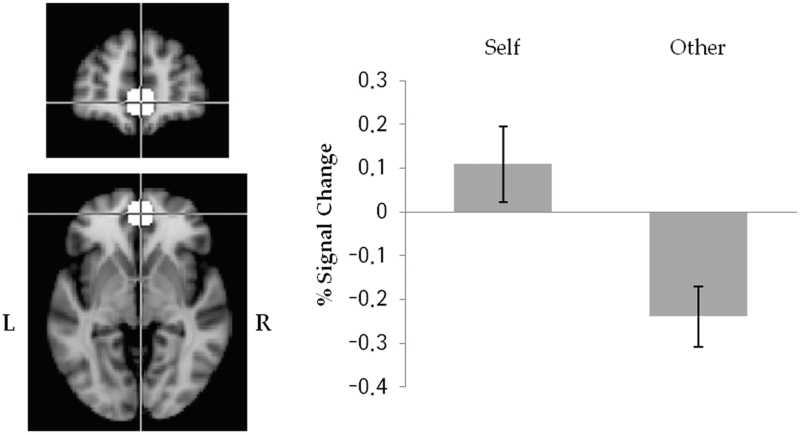
To identify brain regions showing sensitivity to self-referential processing, data from the MPFC localizer scan were analyzed using a FILM and a mixed effect model (FLAME stage 1 only) using two regressors (Self-referent vs Other-referent condition). The group-level contrast map for Self-referent vs Other-referent contrast (Z > 2.5, cluster-corrected, P < 0.05) initially revealed four clusters centered around the MPFC and adjacent anterior cingulate gyrus, lingual gyrus, postcentral gyrus and posterior cingulate gyrus,1 respectively. For the purpose of current analysis, we created a spherical ROI (2, 52, −4; 10-mm radius) centered at a voxel showing the maximal BOLD contrast effect within MPFC (Figure 2).
Fig. 2.
Task-dependent spherical MPFC ROI (2, 52, −4, 10-mm radius) defined from a localizer scan (i.e. trait adjective rating task) and percent signal change for Self- and Other-referent conditions within ROI. Error bars indicate standard error of the mean (SEM).
The LOC ROI was defined as the clusters showing greater activity for intact compared to scrambled object pictures from a group-level activation threshold of Z > 3.7 with a cluster probability of P < 0.05 using two regressors (intact vs scrambled object types).
The time course of the activation within the ROI was assessed by averaging the fMRI signal from each voxel in each participant’s functional data across peri-events created separately for each condition for each of the main analyses, which were then converted to percent signal change relative to an intertrial baseline using the Eventstats program (Gadde et al., 2004) and a custom-made MATLAB script (http://www.mathworks.com). Time courses of signal change were averaged over the voxels contained in our ROI, and then averaged across participants. A two-tailed paired sample t-test was used to compare percent signal change between each condition for each of the main analyses at each point in time (i.e. epochs) from 4 s before the trial onset to 7 s after the trial offset with an emphasis given to epochs from 5 to 9 to account for the delay in the hemodynamic response from the presentation of the assignment cue (i.e. colored dot) in epoch 3.
RESULTS
Behavioral results
Object assignment
No significant difference was found in accuracy (98.81% for Mine and 98.15% for Other condition), t(11) = 1.33, P = 0.21 or in response times (805 and 812 ms for Mine and Other condition, respectively), t(11) = −0.21, P = 0.84.
Source memory
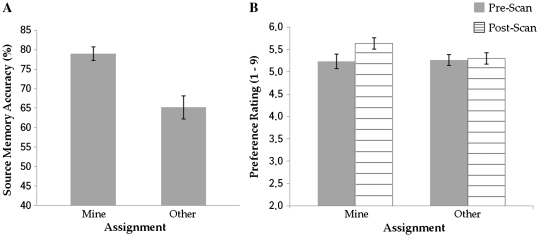
Source memory was calculated by dividing the number of correct source assignments to each assignment type by the total number of items minus the number of items given a ‘New’ response (i.e. misses) for that assignment type. As shown in Figure 3A, participants better remembered an object’s source for Mine compared to Other items, demonstrating a self-reference effect (M = 78.99% vs M = 65.20%), t(11) = 2.35, P = 0.038. Furthermore, participants were faster at correctly identifying objects’ source for items assigned to their own basket (M = 1306 ms) compared to those assigned to the other’s basket (M = 1540 ms), t(11) = −3.84, P = 0.003.
Fig. 3.
(A) Source memory performance and (B) Pre- and post-scan preference as a function of assignment type (Error bars: SEM).
Preference rating
A 2 (assignment; Mine or Other) × 2 (time of rating; pre- or post-scan) repeated-measures analysis of variance (ANOVA) revealed only a significant 2-way interaction, F(1, 11) = 5.27, P = 0.042. As shown in Figure 3B, simple effects revealed that the items assigned to Mine were given significantly higher preference in the post-scan rating task (M = 5.63) than the pre-scan rating task (M = 5.23), F(1, 11) = 6.87, P = 0.024, whereas there was no significant change in preference for those items assigned to Other (M = 5.26 and M = 5.30 for pre- and post-scan ratings, respectively), F(1, 11) = 0.14, P = 0.71, demonstrating the predicted mere ownership effect. Pre-scan ratings for the objects assigned to Mine and Other did not differ, F(1, 11) = 0.05, P = 0.83, confirming that the allocation of objects to conditions based on ratings from pilot participants was successful in equating preferences prior to the assignment manipulation.
fMRI results
Mine vs Other during object assignment and imagined ownership
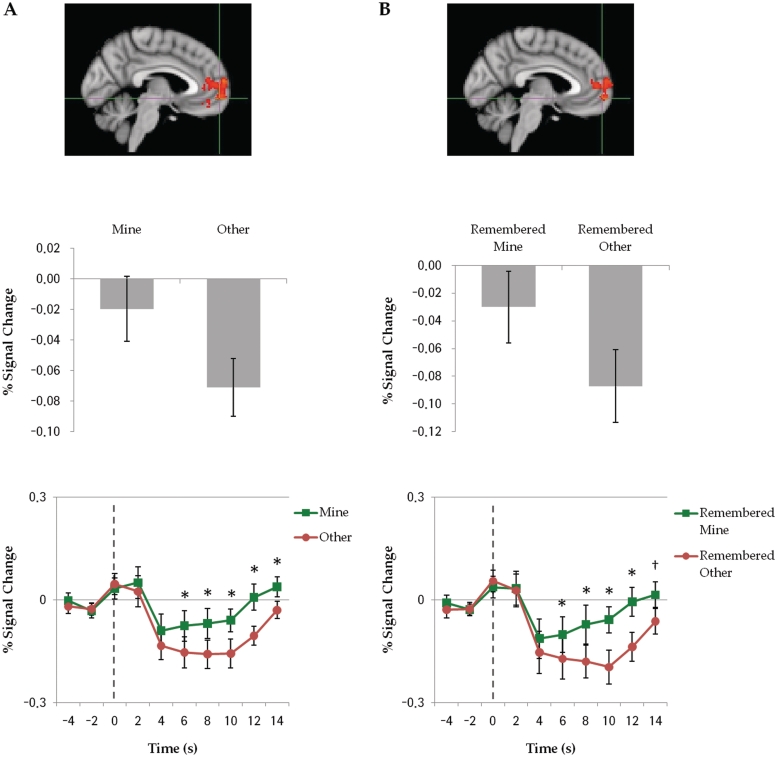
A whole-brain analysis contrasting Mine vs Other conditions showed greater activation in MPFC, paracingulate gyrus and frontal pole for objects assigned to the Mine compared to Other condition (Figure 4A, top panel; peak voxel: −10, 68, 6; Z-max = 3.6). The mean signal change in the MPFC ROI (Figure 2) across all the epochs for each event type is shown in Figure 4A middle panel (Mine > Other, t(11) = 3.70, P = 0.003) and, as can be seen in Figure 4B (bottom panel), the mean signal change for each of the 5 time points of interest was significantly greater for the Mine than Other condition, P’s < 0.05, t’s > 2.70. As in many studies, the overall pattern reflects greater deactivation in MPFC for processing unrelated to the self than processing related to the self. These results are consistent with the hypothesis that even transient, incidental relevance to the self (i.e. moving objects to Mine vs Other baskets and imagined ownership) increases activity in MPFC, supporting the role of this brain region in incorporating external stimuli into an extended self.
Fig. 4.
(A) Assignment contrast (Mine > Other) and (B) Source Memory contrast (Remembered Mine > Remembered Other). Top: activation map from whole-brain regression analysis; middle: mean percentage of signal change across all epochs of each event type within MPFC ROI; bottom: average time course of activity within ROI. Dotted line represents the trial onset. Time points with significant differences between event types are indicated with asterisk (†P < 0.08). Error bars indicate SEM.
Self-reference memory effect
In line with previous findings (Macrae et al., 2004), we found greater activity in MPFC for subsequently Remembered Mine than subsequently Remembered Other items in the whole-brain contrast as shown in Figure 4B (top panel; peak voxel: −12, 68, 6; Z-max = 3.75). The averaged signal for Remembered Mine items across all the epochs in the MPFC ROI was significantly greater compared to that of Remembered Other items, t(11) = 2.62, P = 0.024 (Figure 4B, middle). Whereas activity in MPFC for Remembered Mine items did not significantly differ from baseline, MPFC showed significantly lower activity compared to baseline for subsequently Remembered Other items, t(11) = −3.33, P = 0.007. A significant signal difference between Remembered Mine compared to Remembered Other items was observed in four out of five time points of interest (Figure 4B, bottom), P’s < 0.05, t’s > 2.30.
Mere ownership effect
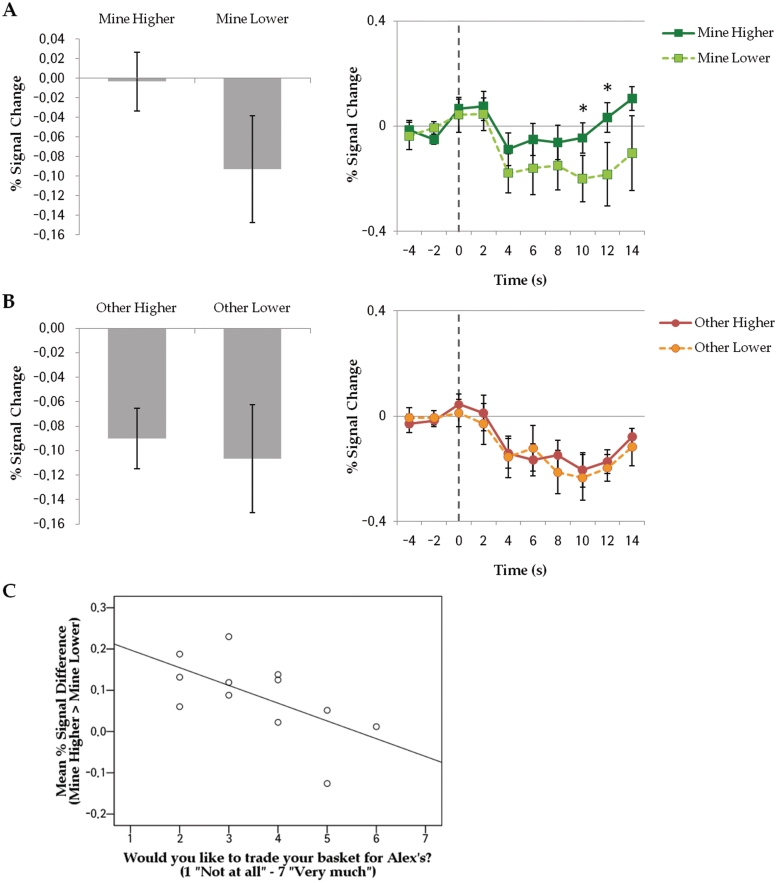
The whole-brain contrast for Mine Higher vs Mine Lower did not identify any significant clusters at a threshold level of Z > 2.0 with a cluster probability of P < 0.05. However, as shown in the left panel of Figure 5A, when the same contrast was done for our independently identified MPFC ROI, there was significantly greater activation for Mine Higher than Mine Lower items in mean signal change across all epochs, t(11) = 2.22, P = 0.048, and at two individual critical time points (Figure 5A, right), P’s < 0.05, t’s > 2.33. Given that MPFC supports self-relevant processing, greater activity in this region presumably reflects stronger engagement of the self for some objects, which in turn later resulted in a stronger self-object association as manifested in an increased post-scan preference. Importantly, this modulation of activity in MPFC was not evident when objects assigned to Other were contrasted according to higher vs lower post-scan preference as shown in Figure 5B, t(11) = 0.34, P = 0.74. The non-significant differences in activity between Mine Lower vs Other Higher items, t(11) = −0.06, P = 0.96 or between Mine Lower vs Other Lower items, t(11) = −0.27, P = 0.79, also suggest that objects generating only a minimal sense of self-relevance (i.e. less self-referential processing), resulted in an activity pattern resembling other-referential processing and no subsequent mere ownership effect.
Fig. 5.
Mean signal change across all epochs and average time courses within ROI for (A) Mine Higher vs Mine Lower and (B) Other Higher vs Other Lower. Time points with significant differences between event types are indicated with asterisk. (C) Mean signal difference between Mine Higher and Mine Lower across all epochs in relation to participants’ rating on post-scan questionnaire measuring ownership effect (Pearson r = −0.61, P = 0.036). Error bars indicate SEM.
The post-scan measure of participant’s willingness to trade their own baskets for Alex’s basket was negatively correlated with the mean percent signal difference in MPFC between Mine Higher and Mine Lower items, Pearson r = −0.61, P = 0.036, as illustrated in Figure 5C, providing further support for the modulation of MPFC activity according the overall strength of self-object association.
Signal change within LOC ROI
None of the critical comparisons reported above showed a significant difference in activity in LOC (P’s > 0.44 and > 0.41 for left- and right-LOC ROI, respectively). Along with the behavioral results provided above, these results indicate that differential attention to objects in the Mine vs Other conditions is unlikely to alone account for the findings.
DISCUSSION
The current findings support the notion of extended self: even when objects were only transiently relevant to the self as participants ‘moved’ objects to the assigned basket and imagined ownership of items assigned to them, a brain region involved in explicit self-referential processing, the MPFC, was more engaged for Mine than Other objects. In addition, the memory advantage of self-relevant over other-relevant objects was also associated with greater activity in MPFC. Furthermore, a behavioral mere ownership effect was reflected in greater activation in MPFC: objects that were rated as more preferred after than before they were assigned to the self generated greater activity in MPFC than those that were not rated as more preferred, providing further support for the role of self-referential brain areas in incorporating personally-relevant aspects of external stimuli into the concept of one’s self.
In everyday life, self-object associations occur in many ways. For instance, one can be given objects (e.g. gifts are a type of ‘assignment’) or one can actively obtain them (e.g. buying objects is a type of choice). Mather et al. (2003) found a choice-supportive memory bias (i.e. mis-attribution of positive and negative features to a chosen option and to an unchosen option, respectively) only when individuals voluntarily chose an option and not when the option was assigned to them. The findings of Mather et al. are consistent with the idea that agency (self-generation and goal-directed intentionality of an action) can modulate the strength of a self-stimulus association (Fink et al., 1999; Jeannerod, 2001). The present findings, together with these previous observations, suggest that compared to a procedure where the objects are experimentally assigned to Mine and Other conditions, there would be even greater activation of MPFC and a larger mere ownership effect in preferences if participants were allowed to choose which objects they placed in their own basket (i.e. engaging self-as-agent). Given that a self also includes the subjective experience of a willful power of making a choice (i.e. one’s choice as a part of self-concept, James, 1890/1983), the individual and interactive effects of ownership and choice in creating linkages between one’s concept of self and external objects deserve further exploration. Other intriguing directions for future research include exploring the differences in neural activity associated with imagined transient vs actual persisting ownership or in ‘central’ vs ‘extended’ aspects of self. In short, there are many yet to be explored issues that would contribute to a fuller understanding of the neural activity related to such a complex concept as ‘self’.
Acknowledgments
This work was supported by the National Institutes of Health (Grant R37AG009253). The authors wish to thank Gregory McCarthy for helpful comments on the study design and data analysis and Tamar Gendler for her contributions to a pilot version of this study.
Footnotes
1 We did not find any significant assignment-related modulation of activity in posterior cingulate cortex (PCC) either in the whole-brain contrast or in the PCC ROI (−4, −22, 44; 10-mm radius) defined in an analogous way as the MPFC ROI. For a discussion of the dissociation between MPFC and PCC in self-reflection, see Johnson et al. (2006), and for a discussion of the role of PCC in memory see Wagner et al. (2005).
REFERENCES
- Anderson NH. Likableness ratings of 555 personality trait words. Journal of Personality and Social Psychology. 1968;9:272–9. doi: 10.1037/h0025907. [DOI] [PubMed] [Google Scholar]
- Beckmann C, Jenkinson M, Smith SM. General multi-level linear modeling for group analysis in FMRI. NeuroImage. 2003;20:1052–63. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Beggan JK. Using what you own to get what you need: the role of possessions in satisfying control motivation. Journal of Social Behavior and Personality. 1991;6:129–46. [Google Scholar]
- Beggan JK. On the social nature of nonsocial perception: the mere ownership effect. Journal of Personality and Social Psychology. 1992;62:229–37. [Google Scholar]
- Belk RW. Possessions and the extended self. Journal of Consumer Research. 1988;15:139–68. [Google Scholar]
- Belk RW. The ineluctable mysteries of possessions. Journal of Social Behavior and Personality. 1991;6:17–55. [Google Scholar]
- Blakemore SJ, Frith C. Self-awareness and action. Current Opinion in Neurobiology. 2003;13:219–24. doi: 10.1016/s0959-4388(03)00043-6. [DOI] [PubMed] [Google Scholar]
- Boyer P, Robbins P, Jack AI. Varieties of self-systems worth having. Consciousness and Cognition. 2005;14:647–60. doi: 10.1016/j.concog.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Burris CT, Rempel JK. “It's the end of the world as we know it”: threat and the spatial-symbolic self. Journal of Personality and Social Psychology. 2004;86:19–42. doi: 10.1037/0022-3514.86.1.19. [DOI] [PubMed] [Google Scholar]
- Conway MA, Pleydell-Pearce CW. The construction of autobiographical memories in the self-memory system. Psychological Review. 2000;107:261–88. doi: 10.1037/0033-295x.107.2.261. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Moroz TM, Moscovitch M, et al. In search of the self: a positron emission tomography study. Psychological Science. 1999;10:26–34. [Google Scholar]
- Cunningham SJ, Turk DJ, Macdonald LM, Macrae CN. Yours or mine? Ownership and memory. Consciousness and Cognition. 2008;17:312–8. doi: 10.1016/j.concog.2007.04.003. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A, Collette F, Van der Linden M, et al. Self-referential reflective activity and its relationship with rest: a PET study. NeuroImage. 2005;25:616–24. doi: 10.1016/j.neuroimage.2004.11.048. [DOI] [PubMed] [Google Scholar]
- Damasio AR. The Feeling of What Happens: Body and Emotion in the Making of Consciousness. New York: Harcourt Brace; 1999. [Google Scholar]
- Feys J. Briefly induced belongingness to self and preference. European Journal of Social Psychology. 1991;21:547–52. [Google Scholar]
- Fink GR, Marshall JC, Halligan PW, et al. The neural consequences of conflict between intention and the senses. Brain. 1999;122:497–512. doi: 10.1093/brain/122.3.497. [DOI] [PubMed] [Google Scholar]
- Gadde S, Michelich CR, Voyvodic J. An xml-based data access interface for image analysis and visualization software. 2004. Poster session presented at the Annual Meetings of the Organization for Human Brain Mapping, Budapest, Hungary.
- Gallagher HL, Frith CD. Functional imaging of ‘theory of mind’. Trends in Cognitive Sciences. 2003;7:77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Gallagher II. Philosophical conceptions of the self: implications for cognitive science. Trends in Cognitive Sciences. 2000;4:14–21. doi: 10.1016/s1364-6613(99)01417-5. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Macrae CN, Kelley WM. What the social brain sciences can tell us about the self. Current Directions in Psychological Science. 2004;13:190–3. [Google Scholar]
- Heatherton TF, Wyland CL, Macrae CN, Demos KE, Denny BT, Kelley WM. Medial prefrontal activity differentiates self from close others. Social Cognitive and Affective neuroscience. 2006;1:18–25. doi: 10.1093/scan/nsl001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James W. The Principles of Psychology. Cambridge, MA: Harvard University Press; 1890/1983. [Google Scholar]
- Jeannerod M. Neural simulation of action: a unifying mechanism for motor cognition. NeuroImage. 2001;14:S103–9. doi: 10.1006/nimg.2001.0832. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Johnson MK. Reflection, reality monitoring, and the self. In: Kunzendorf R, editor. Mental Imagery. New York: Plenum; 1991. pp. 3–16. [Google Scholar]
- Johnson MK, Raye CL, Mitchell KJ, Touryan SR, Greene EJ, Nolen-Hoeksema S. Dissociating medial frontal and posterior cingulate activity during self-reflection. Social Cognitive and Affective Neuroscience. 2006;1:56–64. doi: 10.1093/scan/nsl004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MK, Reeder JA. Consciousness as meta-processing. In: Cohen JD, Schooler JW, editors. Scientific Approaches to Consciousness. Mahwah, NJ: Erlbaum; 1997. pp. 261–93. [Google Scholar]
- Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP. Neural correlates of self-reflection. Brain Research. 2002;125:1808–14. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- Kahneman D, Knetsch JL, Thaler RH. The endowment effect, loss aversion, and status quo bias. The Journal of Economic Perspectives. 1991;5:193–206. [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. Journal of Cognitive Neuroscience. 2002;14:785–94. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Klein SB, Rozendal K, Cosmides L. A social-cognitive neuroscience analysis of the self. Social Cognition. 2002;20:105–35. [Google Scholar]
- Knetsch JL, Sinden JA. Willingness to pay and compensation demanded: experimental evidence of an unexpected disparity in measures of value. The Quarterly Journal of Economics. 1984;99:507–21. [Google Scholar]
- Lambie JA, Marcel AJ. Consciousness and the varieties of emotion experience: a theoretical framework. Psychological Review. 2002;109:219–59. doi: 10.1037/0033-295x.109.2.219. [DOI] [PubMed] [Google Scholar]
- Macrae CN, Moran JM, Heatherton TF, Banfield JF, Kelley WM. Medial prefrontal activity predicts memory for self. Cerebral Cortex. 2004;14:647–54. doi: 10.1093/cercor/bhh025. [DOI] [PubMed] [Google Scholar]
- Maki RH, McCaul KD. The effects of self-reference versus other reference on the recall of traits and nouns. Bulletin of the Psychonomic Society. 1985;23:169–72. [Google Scholar]
- Markus HR. Self-schemata and processing of information about the self. Journal of Personality and Social Psychology. 1977;35:63–78. [Google Scholar]
- Mather M, Shafir E, Johnson MK. Remembering chosen and assigned options. Memory & Cognition. 2003;31:422–433. doi: 10.3758/bf03194400. [DOI] [PubMed] [Google Scholar]
- Metcalfe J, Eich TS, Castel A. Metacognition of agency across the lifespan. Cognition. 2010;116:267–82. doi: 10.1016/j.cognition.2010.05.009. [DOI] [PubMed] [Google Scholar]
- Metzinger T. Being No One. Cambridge, MA: MIT Press; 2003. [Google Scholar]
- Neisser U. Five kinds of self-knowledge. Philosophical psychology. 1988;1:35–59. [Google Scholar]
- Northoff G, Bermpohl F. Cortical midline structures and the self. Trends in Cognitive Sciences. 2004;8:102–7. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain–A meta-analysis of imaging studies on the self. NeuroImage. 2006;31:440–57. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Beer JS, Robertson ER, et al. The neural correlates of direct and reflected self-knowledge. NeuroImage. 2005;28:797–814. doi: 10.1016/j.neuroimage.2005.06.069. [DOI] [PubMed] [Google Scholar]
- Rogers TB, Kuiper NA, Kirker WS. Self-reference and the encoding of personal information. Journal of Personality and Social Psychology. 1977;35:677–88. doi: 10.1037//0022-3514.35.9.677. [DOI] [PubMed] [Google Scholar]
- Smith S. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–55. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura M, Watanabe J, Maeda Y, Matsue Y, Fukuda H, Kawashima R. Cortical mechanisms of visual self-recognition. NeuroImage. 2005;24:143–49. doi: 10.1016/j.neuroimage.2004.07.063. [DOI] [PubMed] [Google Scholar]
- Symons CS, Johnson BT. The self-reference effect in memory: a meta-analysis. Psychological Bulletin. 1997;121:371–94. doi: 10.1037/0033-2909.121.3.371. [DOI] [PubMed] [Google Scholar]
- Tooby J, Cosmides L. Mapping the evolved functional organization of mind and brain. In: Gazzaniga MS, editor. The Cognitive Neurosciences. Cambridge, MA: MIT Press; 1995. pp. 1185–97. [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends in Cognitive Sciences. 2005;9:445–53. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Wicklund RA, Gollwitzer PM. Symbolic Self-completion. Hillsdale, NJ: Lawrence Erlbaum; 1982. [Google Scholar]
- Wojciulik E, Kanwisher N, Driver J. Covert visual attention modulates face-specific activity in the human fusiform gyrus: fMRI study. Journal of Neurophysiology. 1998;79:1574–8. doi: 10.1152/jn.1998.79.3.1574. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TEJ, Beckman CF, Jenkinson M, Smith SM. Multi-level linear modeling for fMRI group analysis using Bayesian inference. NeuroImage. 2004;21:1732–47. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady JM, Smith SM. Temporal autocorrelation in univariate linear modeling of fMRI data. NeuroImage. 2001;14:1370–86. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Worsley KJ. Statistical analysis of activation images. In: Jezzard P, Matthews PM, Smith SM, editors. Functional Magnetic Resonance Imaging: An Introduction to Methods. New York: Oxford University Press; 2001. pp. 251–70. [Google Scholar]