Abstract
The amygdala is critically involved in mediating physiological and behavioral responses to threat. In particular, neuroimaging research indicates that the amygdala is highly responsive to facial signals of threat such as fearful and angry expressions. However, individuals differ substantially in both their relative sensitivity to threat and the magnitude of amygdala reactivity to facial signals of threat. Here, we report the novel finding that individual differences in trait anger are positively correlated with bilateral dorsal amygdala reactivity to angry facial expressions, but only among men with elevated trait anxiety scores. These findings add to the growing body of evidence indicating that variability in personality traits contribute to individual differences in threat-related amygdala reactivity and further suggest that heightened amygdala reactivity to angry faces may be uniquely involved in the expression of reactive aggression in men.
Keywords: amygdala, threat, trait anger, trait anxiety, reactive aggression, brain imaging
INTRODUCTION
The amygdala is a limbic structure that plays a critical role in processing potentially threatening stimuli and mediating various autonomic, neuroendocrine and behavioral responses that enable an organism to adapt to social and environmental challenges (see Davis and Whalen, 2001; LeDoux, 2000 for reviews). Previous studies indicate a robust response of the human amygdala to threatening facial expressions (e.g. fear or anger) with substantial inter-individual variability that is stable over time (Johnstone et al., 2005; Manuck et al., 2007). Recent evidence indicates that individual differences in personality are associated with variability in this trait-like amygdala response (see Hariri, 2009 for review). For instance, individual differences in trait anxiety, a component of the behavioral avoidance system (Carver, 2001), are positively correlated with attentional biases and amygdala reactivity to facial expressions of fear and anger (Bishop et al., 2004; Etkin et al., 2004; Mogg et al., 2007; Ewbank et al., 2009; Fakra et al., 2009).
The above findings are consistent with the idea that enhanced sensitivity to threat-related cues, as indexed by amygdala reactivity, may function to promote vigilance and avoidance-like behaviors (Davis and Whalen, 2001). However, another line of research indicates that trait anger, a component of the behavioral approach system (Carver and Harmon-Jones, 2009), and a reliable predictor of reactive aggression (Bettencourt et al., 2006), is positively correlated with attentional biases toward angry facial expressions (van Honk et al., 2001; Putnam et al., 2004). Also, individual differences in approach motivation, a construct closely linked to trait anger (Harmon-Jones, 2003), are positively correlated with amygdala reactivity to angry facial expressions (Beaver et al., 2008), and individuals characterized by excessive bursts of anger and reactive aggression demonstrate amygdala hyper-reactivity to angry facial expressions (Coccaro et al., 2007). These findings suggest that enhanced amygdala reactivity to facial signals of threat may promote vigilance and approach-like reactive aggression (Beaver et al., 2008). Together, both lines of research converge to indicate that amygdala reactivity to facial expressions of threat may depend critically on the type of facial expression displayed by the sender and the personality characteristics of the receiver and may serve to predict important behavioral outcomes.
Here, we extend this literature in two important ways: (i) we investigated amygdala reactivity to both angry and fearful facial expressions; and (ii) we examined the extent to which individual differences in trait anger and trait anxiety may independently or interactively predict amygdala reactivity to angry and/or fearful facial expressions. We chose to examine amygdala responses to fearful and angry faces because although both expressions signal the presence of threat in the environment, angry facial expressions provide clear information concerning the source of the threat, whereas fearful facial expressions, particularly those with eye gaze directed at the viewer, do not (Whalen et al., 2001; Adams and Kleck, 2003, 2005). Thus, the magnitude and/or direction of the association between personality characteristics and amygdala reactivity may depend critically on the type of facial expression displayed by the sender. Because the amygdala is particularly important for the evaluation of environmental threat (Davis and Whalen, 2001) and a prior research has implicated relative amygdala hyper-activity in reactive but not proactive forms of aggression (see Blair, 2010 for review) we specifically considered that trait anxiety, an index of an individual’s relative sensitivity to and attentional bias toward threat, may moderate the relationship between trait anger and threat-related amygdala reactivity. We predicted that individual differences in trait anger would be associated with increased amygdala reactivity only among individuals reporting high trait anxiety (i.e. an attentional bias toward threat). Given gender differences in neural responses to facial signals of threat (Hamann, 2005; Fusar-Poli et al., 2009) and the expression of aggression (Archer, 2009), we also examined the extent to which participant gender would influence any association between individual differences in personality and amygdala reactivity to fearful and/or angry facial expressions.
MATERIALS AND METHODS
Subjects
A total of 103 participants (46 men and 57 women; mean age = 44.45; s.d. = 6.74) were recruited from a larger parent study, the adult health and behavior (AHAB) project, an archival database encompassing detailed measures of behavioral and biological traits among a community sample of middle-aged volunteers. Written informed consent according to the guidelines of the University of Pittsburgh’s institutional review board was provided by all subjects before their participation in our neuroimaging subcomponent of the AHAB. All subjects in the current study were in good general health and free of the following: (i) medical diagnoses of cancer, stroke, diabetes requiring insulin treatment, chronic kidney or liver disease, or a lifetime history of psychotic symptoms and (ii) use of psychotropic, glucocorticoid or cardiovascular medication.
Individual differences
The Spielberger State-Trait Anxiety Inventory (STAI) is a self-report scale indexing the frequency with which individuals generally perceive encountered situations to be threatening and respond to such situations with subjective feelings of apprehension and tension (Spielberger, 1991). The Spielberger State-Trait Anger Expression Inventory (STAXI) is a self-report scale indexing the intensity of anger as an emotional state at a particular time (state) and how often angry feelings are experienced over time (trait). In this study, our primary analyses were centered on the STAI-Trait Anxiety and STAXI-Trait Anger scales, as trait scores better reflect dispositional anxiety and anger (Spielberger, 1991). We also conducted additional analyses using the angry temperament and angry reaction dimensions of the trait anger scale. The angry temperament dimension refers to the propensity to experience anger on minimal provocation, whereas the angry reaction dimension refers to the propensity to experience more or less anger in contexts of reasonable provocation (e.g. when demeaned, criticized or treated unfairly). As in previous studies (van Honk et al., 2001), trait anxiety was positively correlated with trait anger in the current sample (r = 0.44, P < 0.05).
Amygdala reactivity paradigm
Our archival fMRI challenge paradigm has been used extensively to elicit a robust and replicable amygdala response across an array of experimental protocols and sample populations (e.g. Hariri et al., 2002; Hariri et al., 2005; Fisher et al., 2006; Manuck et al., 2007; Zhou et al., 2008; Fisher et al., 2009). The paradigm consists of four blocks of a perceptual face processing task interleaved with five blocks of a sensorimotor control task. During face processing blocks, participants view a trio of faces (expressing either anger or fear) and select one of two faces (bottom) that is identical to a target face (top). Angry and fearful facial expressions can represent honest indicators of an ecologically valid threat and in this context we interpret the amygdala activation elicited by our task as being threat-related. Each face-processing block consists of six images, balanced for gender and target affect (angry or fearful), all of which were derived from a standard set of pictures of facial affect (Ekman and Friesen, 1976). During the sensorimotor control blocks, participants view a trio of simple geometric shapes (circles and vertical and horizontal ellipses) and select one of two shapes (bottom) that are identical to a target shape (top). Each sensorimotor control block consists of six different shape trios. All blocks are preceded by a brief instruction (‘match faces’ or ‘match shapes’) that lasts 2 s. In the face-processing blocks, each of the six face trios is presented for 4 s with a variable inter-stimulus interval (ISI) of 2–6 s (mean = 4 s), for a total block length of 48 s. In the sensorimotor control block, each of the six shape trios is presented for 4 s with a fixed inter-stimulus interval of 2 s, for a total block length of 36 s. Total task time was 390 s. As we were not interested in neural networks associated with face-specific processing per se, but rather in eliciting a maximal amygdala response across all participants that we could then investigate for genotype effects, we chose not to use neutral faces as control stimuli because neutral faces can be subjectively experienced as affectively laden or ambiguous and thus engage the amygdala (Schwartz et al., 2003; Blasi et al., 2009). However, our use of a variable ISI during face processing blocks allows for the estimation of expression-specific neural activation.
Image processing and analysis
Each participant underwent scanning with a Siemens 3T MAGNETOM Allegra (Siemens AG, Erlangen, Germany), which was developed specifically for advanced brain imaging applications and is characterized by increased T2* sensitivity and fast gradients (slow rate, 400 T/m/s), which minimize echo-spacing, thereby reducing echoplanar imaging geometric distortions and improving image quality. Blood oxygenation level-dependent (BOLD) functional images were acquired with a gradient-echo echoplanar imaging sequence (repetition time/echo time = 2000/25 ms, field of view = 20 cm, matrix = 64 × 64), which covered 34 interleaved axial slices (3-mm-thickness slice) aligned with the AC-PC plane and encompassing the entire cerebrum and most of the cerebellum. All scanning parameters were selected to optimize the quality of the BOLD signal while maintaining a sufficient number of slices to acquire whole brain data. Before collecting fMRI data for each participant, we acquired a reference echoplanar imaging scan, which we visually inspected for artifacts (e.g. ghosting) and good signal across the entire volume of acquisition. Additionally, an autoshimming procedure was conducted before the acquisition of BOLD data in each participant to minimize field inhomogeneities. The fMRI data from all 103 participants included in this study were free of such problems.
Whole-brain image analysis was completed using the general linear model of SPM2 (Wellcome Department of Imaging Neuroscience, London, England). Images for each participant were realigned to the first volume in the time series to correct for head motion, spatially normalized into a standard stereotactic space (Montreal Neurological Institute template) using a 12-parameter affine model, and smoothed to minimize noise and residual difference in gyral anatomy with a Gaussian filter set at 6-mm full-width at half-maximum. Voxel-wise signal intensities were ratio-normalized to the whole-brain global mean. These preprocessed data sets were analyzed using second-level random-effects models that accounted for both scan-to-scan and participant-to-participant variability to determine task-specific regional responses. After preprocessing, linear contrasts using canonical hemodynamic response functions were used to estimate condition-specific (i.e. all faces > shapes, angry faces > shapes, fearful faces > shapes) BOLD activation for each individual. These individual contrast images (i.e. weighted sum of the beta images) were then used in second-level random-effects models to determine mean condition-specific amygdala reactivity using one-sample t-tests with a voxel-level statistical threshold of P < 0.05, FWE corrected for multiple comparisons across the entire search volume.
BOLD contrast estimates were extracted from functional clusters exhibiting a main effect of task using the above threshold within anatomically defined amygdala regions of interest (ROIs). Because of the structural and functional heterogeneity of the amygdala (Davis and Whalen, 2001; Whalen et al., 2001), we examined the ventral and dorsal amygdala independently to determine whether individual differences in personality map on to the amygdala’s principal input and output regions, respectively. This approach is justified based on previous imaging research indicating that individual difference factors map on to specific regions of the amygdala (Etkin et al., 2004; Manuck et al., 2010). We constructed hemisphere-specific ROIs using Marsbar (v 0.41) for the ventral amygdala, which encompass the basolateral complex, and for the dorsal amygdala, which encompass the central nucleus as well as the sublenticular extended amygdala and nucleus baysalis of Meynert. The ventral amygdala ROIs were anchored by MNI coordinates x = ±21, y = −3, z = −23, with widths of 14, 6 and 6 mm along the x-, y- and z-axes, respectively. The total volume of the ventral amygdala ROI was 1024 mm3 in each hemisphere. The dorsal amygdala ROI was anchored by the MNI coordinates x = ±21, y = −4, z = −13, with widths of 14, 8 and 10 mm along the x-, y- and z-axes, respectively. The total volume of the dorsal amygdala ROI was 1920 mm3 in each hemisphere. The reported widths reflect the total for the ROI along each axis and are centered on the MNI coordinate anchoring each axis (i.e. with x = 21 and width = 14 mm, the range of coordinates included along that axis of the ROI are from x = 14 to x = 28). The posterior extent of both the dorsal and ventral amygdala was carefully defined to exclude the hippocampus.
Statistical analyses
The primary criterion variables were the standardized BOLD contrast estimates extracted bilaterally from clusters of maximal activation in the ventral and dorsal amygdala ROIs. Hierarchical multiple regression analyses were used to investigate the extent to which trait anger, trait anxiety or their interactions influenced amygdala reactivity to angry and/or fearful faces. To minimize multicollinearity, all predictor variables were mean-centered prior to analyses and their product terms were computed using these standardized variables. For these regression analyses, main effects of gender, trait anger, and trait anxiety were entered on Step 1; two-way interactions (i.e. gender-x-trait anger, gender-x-trait anxiety and trait anxiety-x-trait anger) were entered on Step 2; and the three-way interaction (i.e. gender-x-trait anger-x-trait anxiety) was entered on Step 3. Statistical analyses were performed in SPSS Version 15.0, and tests of significance were conducted at conventional alpha (P < 0.05, two-tailed).
RESULTS
Task-related amygdala activation
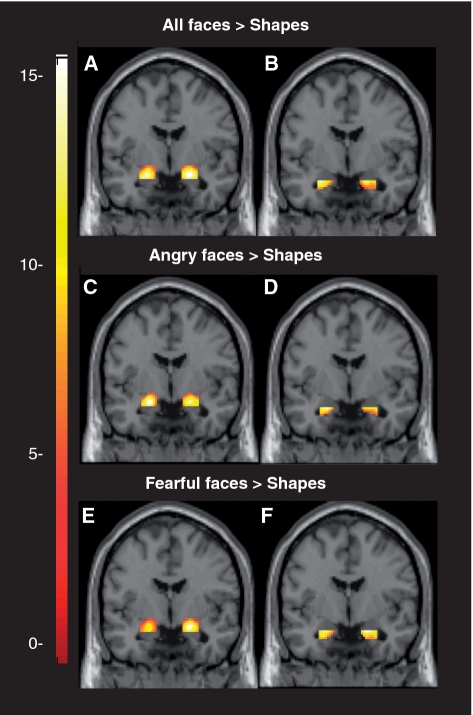
There was robust bilateral condition specific activation within our anatomically defined ventral and dorsal amygdala ROIs for (i) all faces > shapes, (ii) angry faces > shapes and (iii) fearful faces > shapes (Figure 1).
Fig. 1.
Coronal overlays on canonical structural images illustrating mean bilateral amygdala reactivity to threatening faces. (A) Bilateral dorsal amygdala reactivity to all faces > shapes: right hemisphere maximal voxel MNI coordinates x = 20, y = −4, z = −14; 230 voxels; t102 = 14.98, P < 0.05, FWE corrected; left hemisphere maximal voxel MNI coordinates x = −20, y = −6, z = −16; 213 voxels; t102 = 14.77, P < 0.05, FWE corrected. (B) Bilateral ventral amygdala reactivity to all faces > shapes: right hemisphere maximal voxel MNI coordinates x = 20, y = −4, z = −20; 110 voxels; t102 = 11.63, P < 0.05, FWE corrected; left hemisphere maximal voxel MNI coordinates x = −26, y = −2, z = −20; 78 voxels; t102 = 13.60, P < 0.05, FWE corrected. (C) Bilateral dorsal amygdala reactivity to angry faces > shapes: right hemisphere maximal voxel MNI coordinates x = 20, y = −4, z = −16; 205 voxels; t102 = 9.56, P < 0.05, FWE corrected; left hemisphere maximal voxel MNI coordinates x = −20, y = −4, z = −16; 203 voxels; t102 = 10.95, P < 0.05, FWE corrected. (D) Bilateral ventral amygdala reactivity to angry faces > shapes: right hemisphere maximal voxel MNI coordinates x = 28, y = −4, z = −20; 81 voxels; t102 = 6.21, P < 0.05, FWE corrected; left hemisphere maximal voxel MNI coordinates x = −24, y = 0, z = −20; 83 voxels; t102 = 8.78, P < 0.05, FWE corrected. (E) Bilateral dorsal amygdala reactivity to fearful faces > shapes: right hemisphere maximal voxel MNI coordinates x = 20, y = −4, z = −14; 223 voxels; t102 = 14.97, P < 0.05, FWE corrected; left hemisphere maximal voxel MNI coordinates x = −20, y = −6, z = −16; 207 voxels; t102 = 12.54, P < 0.05, FWE corrected. (F) Bilateral ventral amygdala reactivity to fearful faces > shapes: right hemisphere maximal voxel MNI coordinates x = 18, y = −6, z = −20; 111 voxels; t102 = 9.38, P < 0.05, FWE corrected; left hemisphere maximal voxel MNI coordinates x = −22, y = −6, z = −20; 90 voxels; t102 = 8.64, P < 0.05, FWE corrected. Color bar represents t-scores. Each overlay is displayed at y = −4.
Angry facial expressions
Left dorsal amygdala
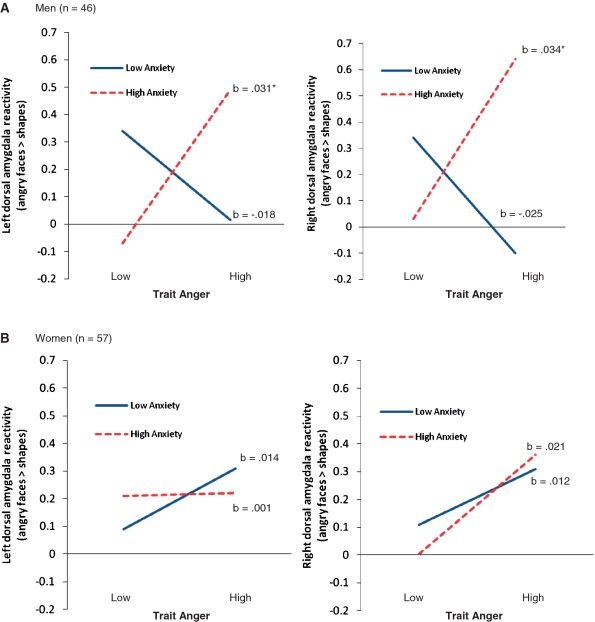
The regression analysis revealed no main effects or two-way interactions (P’s > 0.46), but there was a significant gender-x-trait anger-x-trait anxiety interaction (R2change = 5.1%, F1,95 = 5.37, P = 0.02) (Supplementary Table S1). To interpret this interaction, separate analyses were conducted for men and women. For men, the trait anger-x-trait anxiety interaction was significant (R2change = 11.6%, F1,42 = 5.62, P = 0.02). Simple slopes analyses indicated that there was a positive association between trait anger and left dorsal amygdala activation, but only for men with high trait anxiety scores (b = 0.031, t = 2.27, P = 0.03) (Figure 2). For women, the trait anger-x-trait anxiety interaction was not significant (P = 0.40).
Fig. 2.
Gender-x-trait anxiety-x-trait anger modulation of dorsal amygdala responses to angry faces. (A) For men, trait anger was positively correlated with bilateral dorsal amygdala activation to angry faces, but only among individuals with high trait anxiety scores. (B) For women, trait anger was not correlated with bilateral dorsal amygdala reactivity to angry faces for individuals with high or low trait anxiety scores. Note: High and low values represent +1 and −1 s.d. from the mean for each trait. b indicates simple slopes. Asterisk indicates P < 0.05. Amygdala reactivity is in arbitrary units.
Right dorsal amygdala
The regression analysis indicated that men had greater amygdala reactivity than women (P = 0.01) and that trait anger was positively correlated with amygdala reactivity (r = 0.21, P = 0.04). The second step of the regression model (including all two-way interactions) approached significance (P = 0.065). Here, the trait anxiety-x-trait anger interaction was significant (P = 0.02) (Supplementary Table S1). Simple slopes analyses indicated that trait anger was positively correlated with amygdala reactivity, but only among participants with high trait anxiety scores (b = 0.03, t = 3.13, P = 0.002). Although the three-way interaction was not significant (R2change = 2.2%, F1,95 = 2.63, P = 0.109), we decided to split the analysis by gender based on the pattern observed for the left dorsal amygdala. For men, the trait anger-x-trait anxiety interaction was significant (R2change = 13%, F1,42 = 7.12, P = 0.01), indicating that trait anger was positively correlated with dorsal amygdala reactivity, but only among individuals with high anxiety scores (b = 0.034, t = 2.20, P = 0.03) (Figure 2). For women, the trait anger-x-trait anxiety interaction was not significant (P = 0.59).
Bilateral ventral amygdala responses
For left and right ventral amygdala responses, the main effects (P’s > 0.13), two-way interactions (P’s > 0.15) and three-way interactions (P’s > 0.22) were not significant.
Fearful facial expressions
Left dorsal amygdala
For the left dorsal amygdala, the main effect (P > 0.13), two-way interactions (P’s > 0.60), and the three-way interaction (P = 0.19) were not significant.
Right dorsal amygdala
There was no main effect (P = 0.62), but there was a trait anger-x-trait anxiety interaction (P = 0.03) (Supplementary Table S2). However, simple slopes analyses indicated that trait anger did not predict amygdala reactivity at high or low levels of trait anxiety (b = 0.02, t = 1.69, P = 0.09 and b = −0.01, t = −1.55, P = 0.12, respectively). Although the three-way interaction was not statistically significant (R2change = 2.4%, F1,95 = 2.60, P = 0.11), we decided to split the analysis by gender to examine the gender-specificity of the effect. For men, the trait anger-x-trait anxiety interaction was significant (R2change = 13.1%, F1,42 = 6.79, P = 0.01). Again, however, simple slopes analyses indicated that trait anger did not predict amygdala reactivity at high or low levels of trait anxiety (b = 0.023, t = 1.61, P = 0.11 and b = −0.03, t = −1.82, P = 0.08, respectively). For women, the trait anger-x-trait anxiety interaction was not significant (P = 0.69).
Bilateral ventral amygdala
For left and right ventral amygdala responses, the main effects (P’s > 0.53), two-way interactions (P’s > 0.32), and three-way interactions (P’s > 0.27) were not significant.
Additional analyses
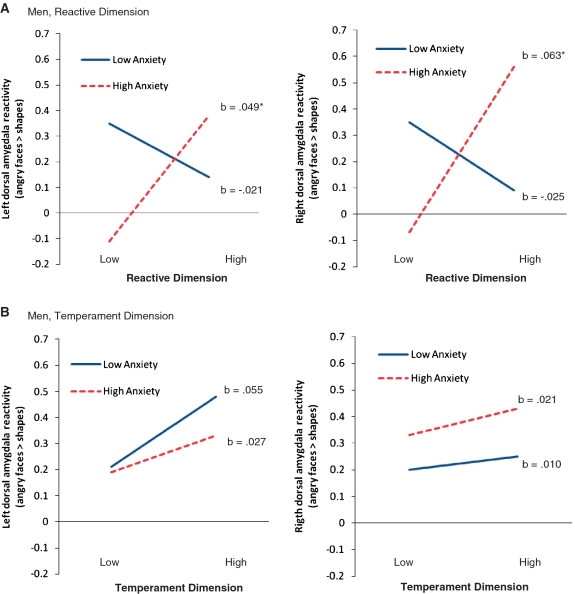
We further probed our data to examine whether the relationship between trait anger and amygdala reactivity to angry facial expressions (among men with high trait anxiety scores) would be specific to the temperament and/or reactive dimensions of the trait anger scale. Regression analyses indicated that individual differences in the reactive dimension of the trait anger scale interacted with trait anxiety to predict left and right dorsal amygdala reactivity to angry facial expressions (R2change = 13.8%, P = 0.01 and R2change = 17.1%, P = 0.003, respectively). Consistent with the results of our primary analyses, the reactive dimension of the trait anger scale was positively correlated with left and right dorsal amygdala reactivity to angry facial expressions for men with high trait anxiety scores (b = 0.049, P = 0.03 and b = 0.063, P = 0.01, respectively), but not men with low trait anxiety scores (P’s = 0.32 and 0.26, respectively). In contrast, the temperament dimension of the trait anger scale did not interact with trait anxiety to predict left or right dorsal amygdala reactivity to angry facial expressions (R2change = 0.3%, P = 0.72 and R2change < 0.01%, P = 0.90, respectively) (Figure 3).
Fig. 3.
Trait anger-x-trait anxiety modulation of dorsal amygdala responses to angry faces for reactive and temperamental dimensions of the trait anger scale. (A) Reactive dimension. For men, the reactive dimension of the trait anger scale was positively correlated with bilateral dorsal amygdala activation to angry faces, but only among individuals with high trait anxiety scores. (B) Temperamental dimension. For men, the temperamental dimension of the trait anger scale did not interact with trait anxiety predict amygdala responses to angry faces. Note: High and low values represent + 1 and −1 s.d. from the mean for each trait. b indicates simple slopes. Asterisk indicates P < 0.05. Amygdala reactivity is in arbitrary units.
DISCUSSION
Consistent with recent evidence (see Hariri, 2009 for review), the current study found that individual differences in stable personality traits map onto variability in threat-related amygdala reactivity. Here, we report the novel finding that individual differences in trait anxiety and trait anger interact to predict dorsal amygdala reactivity in an expression- and gender-specific manner. Specifically, trait anger was positively correlated with bilateral dorsal amygdala reactivity to angry facial expressions, but only among men with high trait anxiety scores.
In general, threat-related amygdala reactivity has been interpreted from a fear perspective, whereby enhanced amygdala reactivity may promote vigilance and avoidance-like behaviors (Davis and Whalen, 2001). However, converging evidence from pre-clinic and clinical populations suggests that the enhanced amygdala reactivity to angry facial expressions observed among the high trait anger/high trait anxiety men in the current study may be a marker for reactive aggression. For instance, psychopathology characterized by elevated anxiety and anger (e.g. depression, post-traumatic stress disorder, intermittent explosive disorder) is associated with heightened levels of reactive aggression and amygdala hyper-reactivity to angry facial expressions (see Siever, 2008; Blair, 2010 for reviews). Similarly, individual differences in approach motivation and trait anger, both constructs linked to reactive aggression, predict enhanced amygdala reactivity and attentional biases toward angry facial expressions (van Honk et al., 2001; Putnam et al., 2004; Beaver et al., 2008). Further, genetic and hormonal factors linked to reactive aggression in men (e.g. ‘low expression’ allele of the MAOA gene and testosterone; McDermott et al., 2009; Carré et al., 2009; 2010) are also associated with heightened amygdala reactivity to facial signals of threat (Meyer-Lindenberg et al., 2006; Hermans et al., 2008; Derntl et al., 2009; Manuck et al., 2010). Importantly, human and animal studies indicate that the amygdala is part of the neural circuitry (including the hypothalamus, orbitofrontal cortex and periaquaductal grey) that modulates reactive aggressive behavior (see Blair, 2004 for review). Indeed, animal models indicate that electrical stimulation of the medial amygdala can potentiate reactive aggression (see Siegel et al., 2007 for review). Interestingly, in a large-scale retrospective study of patients that received bilateral amygdalotomies for untreatable aggression, more than 70% demonstrated moderate to excellent improvement of their reactive outbursts (Ramamurthi, 1988).
The finding that the reactive but not the temperament dimension of the trait anger scale interacted with trait anxiety to predict bilateral dorsal amygdala reactivity to angry faces (Figure 3) lends support to our proposition that heightened amygdala responses to angry facial expressions may represent a specific neurobiological marker for aggressive behavior in response to provocation (i.e. reactive aggression). Nevertheless, it will be important for future studies to examine the extent to which the reactive dimension of the trait anger scale is more closely linked to reactive aggression then the temperament dimension. Interestingly, individuals characterized by callous unemotional traits and/or high levels of unprovoked or ‘proactive aggression’ demonstrated amygdala hypo-reactivity to angry facial expressions (see Blair, 2010 for review). These findings raise the intriguing possibility that heightened amygdala reactivity to angry facial expressions may be a neurobiological marker for reactive (or provoked) forms of aggression, whereas amygdala hypo-reactivity may be a marker for proactive (or unprovoked) forms of aggression.
The basolateral complex of the amygdala plays a more prominent role in the processing and encoding of an environmental stimulus as threatening. The central nucleus of the amygdala and dorsal aspects including the sublenticular extended amygdala and the nucleus basalis of Meynert mediate autonomic, neuroendocrine and behavioral responses to threat through feed forward projections onto hypothalamic and brainstem target areas as well as prefrontal and other cortical circuits (Davis and Whalen, 2001). Within humans, these regions encompass the ventral and dorsal portions of the amygdala, respectively. As such, we sought to model unique contributions of these amygdala nuclei through a dorsal and ventral specific ROI. Our finding that trait anxiety and trait anger interact to predict dorsal amygdala reactivity in men is consistent with angry facial expressions driving response-related arousal circuitry in individuals with a predisposition towards anger, anxiety and possibly reactive aggressive behavior.
Analysis of main effects indicated that trait anger was positively correlated with right dorsal amygdala reactivity to angry facial expressions. This finding is consistent with a previous study in which individual differences in approach motivation, a construct positively correlated with trait anger (Harmon-Jones, 2003), map onto variation in amygdala reactivity to angry facial expressions (Beaver et al., 2008). However, unlike previous studies (Bishop et al., 2004; Etkin et al., 2004; Dickie and Armony, 2008), we did not observe a main effect of anxiety on dorsal or ventral amygdala reactivity to consciously presented angry or fearful facial expressions. Importantly, Kim and Whalen (2009) noted that the relationship between anxiety and amygdala reactivity has typically been found in studies that have used unconsciously presented and/or unattended facial stimuli. Moreover, the relationship between anxiety and amygdala reactivity to facial signals of threat appears to be most robust when examining state anxiety, as opposed to trait anxiety (Ewbank et al., 2010). Also, the importance of contextual factors was recently highlighted in a study in which social support moderated the association between threat-related amygdala reactivity and trait anxiety (Hyde et al., 2011). And finally, it should be noted that we used a relatively conservative statistical approach in our analysis of the relationship between individual differences in personality and threat-related amygdala reactivity. Specifically, as noted in the methods, we extracted our amygdala reactivity values from anatomical regions of interest based on the main effects of our fMRI challenge paradigm. This conservative approach eliminates the possibility of correlations that are artificially inflated due to extraction and correlation techniques that capitalize on the same data twice (Viviani, 2010). Thus, given the above issues, the lack of an association between trait anxiety and threat-related amygdala reactivity is perhaps not surprising.
The findings from the current study suggest that the relationship between individual differences in personality and amygdala reactivity to facial signals of threat are influenced by gender. Most previous fMRI studies examining the link between personality and amygdala reactivity have been based on relatively small samples sizes, and thus, did not have sufficient statistical power to explore the potential influence of gender. Here, using a relatively large sample size, we demonstrate that the modulation of amygdala reactivity to angry faces by the trait anger-x-trait anxiety interaction was specific to men. This effect was statistically significant in the left dorsal amygdala, but the pattern of findings was similar in the right dorsal amygdala. However, because the three-way interaction between gender, trait anxiety and trait anger was not statistically significant (P = 0.109) for the right dorsal amygdala, the gender specific effect of the trait anxiety-x-trait anger should be interpreted with caution. Nevertheless, to the extent that amygdala reactivity to angry faces is a marker for reactive aggression, our gender-specific effects are consistent with behavioral studies indicating that the association between trait anxiety and aggressive behavior is more pronounced in men than women (Marsee et al., 2008).
In summary, the present findings add to the growing body of evidence indicating that individual differences in personality traits contribute to variability in threat-related amygdala reactivity. The extent to which individual differences in amygdala reactivity to angry facial expressions map onto behavioral responses will be an important question for future research. A direct assessment of reactive and proactive forms of aggression using well-validated behavioral measures will be needed to support our proposition that enhanced amygdala reactivity to angry faces may be a neurobiological risk factor for one’s propensity to respond aggressively to actual (or perceived) provocation, whereas decreased amygdala responses to angry faces may be a risk factor for one’s propensity to engage in more instrumental/proactive forms of aggressive behavior.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Acknowledgments
Funding for this work and preparation of the article were provided by National Institutes of Health grants HL040962 and HL065137 (to S.B.M.) and MH072837 (to A.R.H.).
REFERENCES
- Adams RB, Kleck RE. Perceived gaze direction and the processing of facial displays of emotion. Psychological Science. 2003;14:644–7. doi: 10.1046/j.0956-7976.2003.psci_1479.x. [DOI] [PubMed] [Google Scholar]
- Adams RB, Kleck RE. The effects of direct and averted gaze on the perception of facially communicated emotion. Emotion. 2005;5:3–11. doi: 10.1037/1528-3542.5.1.3. [DOI] [PubMed] [Google Scholar]
- Archer J. Does sexual selection explain human sex differences in aggression? Behavioral and Brain Sciences. 2009;32:249–66. doi: 10.1017/S0140525X09990951. [DOI] [PubMed] [Google Scholar]
- Beaver JD, Lawrence AD, Passamonti L, Calder AJ. Appetitive motivation predicts the neural response to facial signals of aggression. Journal of Neuroscience. 2008;28:2719–25. doi: 10.1523/JNEUROSCI.0033-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettencourt BA, Talley A, Benjamin AJ, Valentine J. Personality and aggressive behavior under provoking and neutral conditions: a meta-analytic review. Psychological Bulletin. 2006;132:751–77. doi: 10.1037/0033-2909.132.5.751. [DOI] [PubMed] [Google Scholar]
- Bishop SJ, Duncan J, Lawrence AD. State anxiety modulation of the amygdale response to unattended threat-related stimuli. Journal of Neuroscience. 2004;24:10364–8. doi: 10.1523/JNEUROSCI.2550-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJ. The roles of the orbital frontal cortex in the modulation of antisocial behavior. Brain and Cognition. 2004;55:198–208. doi: 10.1016/S0278-2626(03)00276-8. [DOI] [PubMed] [Google Scholar]
- Blair RJ. Neuroimaging of psychopathy and antisocial behavior: a target review. Current Psychiatry Reports. 2010;12:76–82. doi: 10.1007/s11920-009-0086-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi G, Hariri AR, Alce G, et al. Preferential amygdala reactivity to the negative assessment of neutral faces. Biological Psychiatry. 2009;66:847–53. doi: 10.1016/j.biopsych.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carré JM, Gilchrist JD, Morrissey MD, McCormick CM. Motivational and situational factors and the relationship between testosterone dynamics and human aggression during competition. Biological Psychology. 2010;84:346–53. doi: 10.1016/j.biopsycho.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Carré JM, Putnam SK, McCormick CM. Testosterone responses to competition predict future aggressive behaviour at a cost to reward in men. Psychoneuroendocrinology. 2009;34:561–70. doi: 10.1016/j.psyneuen.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Carver C. Affect and the functional bases of behavior: on the dimensional structure of affective experience. Personality and Social Psychology Review. 2001;5:345–56. [Google Scholar]
- Carver CS, Harmon-Jones E. Anger is an approach-related affect: evidence and implications. Psychological Bulletin. 2009;135:183–204. doi: 10.1037/a0013965. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, McCloskey MS, Fitzgerald DA, Phan KL. Amygdala and orbitofrontal reactivity to social threat in individuals with impulsive aggression. Biological Psychiatry. 2007;62:168–78. doi: 10.1016/j.biopsych.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Derntl B, Windischberger C, Robinson S, et al. Amygdala activity to fear and anger in healthy young males is associated with testosterone. Psychoneuroendocrinology. 2009;34:687–693. doi: 10.1016/j.psyneuen.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Dickie EW, Armony JL. Amygdala responses to unattended fearful faces: interaction between sex and trait anxiety. Psychiatry Research. 2008;162:51–7. doi: 10.1016/j.pscychresns.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Pictures of Facial Affect. Palo Alto, CA: Consulting Psychologists Press; 1976. [Google Scholar]
- Etkin A, Klemenhagen KC, Dudman JT, et al. Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron. 2004;44:1043–55. doi: 10.1016/j.neuron.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Ewbank MP, Fox E, Calder AJ. The interaction between gaze and facial expression in the amygdala and extended amygdala is modulated by anxiety. Frontiers in Human Neuroscience. 2010;4:1–11. doi: 10.3389/fnhum.2010.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewbank MP, Lawrence AD, Passamonti L, Keane J, Peers PV, Calder AJ. Anxiety predicts a differential neural response to attended and unattended facial signals of anger and fear. Neuroimage. 2009;44:1144–51. doi: 10.1016/j.neuroimage.2008.09.056. [DOI] [PubMed] [Google Scholar]
- Fakra E, Hyde LW, Gorka A, et al. Effects of HTR1A C(-1019)G on amygdala reactivity and trait anxiety. Archives of General Psychiatry. 2009;66:33–40. doi: 10.1001/archpsyc.66.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher PM, Meltzer CC, Price JC, et al. Medial prefrontal cortex 5-HT2A density is correlated with amygdala reactivity, response habituation, and functional coupling. Cerebral Cortex. 2009;19:2499–507. doi: 10.1093/cercor/bhp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher PM, Meltzer CC, Ziolko SK, et al. Capacity for 5-HT1A-mediated autoregulation predicts amygdala reactivity. Nature Neuroscience. 2006;9:1362–3. doi: 10.1038/nn1780. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, et al. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. Journal of Psychiatry and Neuroscience. 2009;34:418–32. [PMC free article] [PubMed] [Google Scholar]
- Hamann S. Sex differences in the responses of the human amygdala. The Neuroscientist. 2005;11:288–93. doi: 10.1177/1073858404271981. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E. Anger and the behavioural approach system. Personality and Individual Differences. 2003;35:995–1005. [Google Scholar]
- Hariri AR. The neurobiology of individual differences in complex behavioral traits. Annual Reviews of Neuroscience. 2009;32:225–47. doi: 10.1146/annurev.neuro.051508.135335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Drabant EM, Munoz KE, et al. A susceptibility gene for affective disorders and the response of the human amygdala. Archives of General Psychiatry. 2005;62:146–52. doi: 10.1001/archpsyc.62.2.146. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–3. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E. Anger and the behavioural approach system. Personality and Individual Differences. 2003;35:995–1005. [Google Scholar]
- Hermans EJ, Ramsey M, van Honk J. Exogenous testosterone enhances responsiveness to social threat in the neural circuitry of social aggression in humans. Biological Psychiatry. 2008;63:263–70. doi: 10.1016/j.biopsych.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Hyde LW, Gorka A, Manuck SB, Hariri AR. Perceived social support moderates the link between threat-related amygdala reactivity and trait anxiety. Neuropsychologia. 2011;49:651–6. doi: 10.1016/j.neuropsychologia.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone T, Somerville LH, Alexander AL, et al. Stability of amygdala BOLD response to fearful faces over multiple scan sessions. Neuroimage. 2005;25:1112–23. doi: 10.1016/j.neuroimage.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Whalen PJ. The structural integrity of an amygdala-prefrontal pathway predicts trait anxiety. Journal of Neuroscience. 2009;29:11614–8. doi: 10.1523/JNEUROSCI.2335-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Reviews of Neuroscience. 2000;23:155–84. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Brown SM, Forbes EE, Hariri AR. Temporal stability of individual differences in amygdala reactivity. American Journal of Psychiatry. 2007;164:1613–4. doi: 10.1176/appi.ajp.2007.07040609. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Marsland AL, Flory JD, Gorka A, Ferrell RE, Hariri AR. Salivary testosterone and a trinucleotide (CAG) length polymorphism in the androgen receptor gene predict amygdala reactivity in men. Psychoneuroendocrinology. 2010;35:94–104. doi: 10.1016/j.psyneuen.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsee MA, Weems CF, Taylor LK. Exploration of the association between aggression and anxiety in youth: a look at aggressive subtypes, gender, and social cognition. Journal of Child and Family Studies. 2008;17:154–68. [Google Scholar]
- McDermott R, Tingley D, Cowden J, Frazzeto G, Johnson DD. Monoamine oxidase A gene (MAOA) predict behavioral aggression following provocation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:2118–23. doi: 10.1073/pnas.0808376106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Buckholtz JW, Kolachana B, et al. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:6269–74. doi: 10.1073/pnas.0511311103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogg K, Garner M, Bradley PB. Anxiety and orienting of gaze to angry and fearful faces. Biological Psychology. 2007;76:163–9. doi: 10.1016/j.biopsycho.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putman P, Hermans E, van Honk J. Emotional stroop performance for masked angry faces: it’s BAS, not BIS. Emotion. 2004;4:305–11. doi: 10.1037/1528-3542.4.3.305. [DOI] [PubMed] [Google Scholar]
- Ramamurthi B. Stereotactic operation in behaviour disorders. Amygdalotomy and hypothalamotomy. Acta Neurochirurgica. 1988;44:152–7. doi: 10.1007/978-3-7091-9005-0_29. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Wright CI, Shin LM, et al. Differential amygdalar response to novel versus newly familiar neutral faces: a functional MRI probe developed for studying inhibited temperament. Biological Psychiatry. 2003;53:854–62. doi: 10.1016/s0006-3223(02)01906-6. [DOI] [PubMed] [Google Scholar]
- Siegel A, Bhatt S, Bhatt R, Zalcman SS. The neurobiological basis for development of pharmacological treatments of aggressive disorders. Current Neuropharmacology. 2007;5:135–47. doi: 10.2174/157015907780866929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siever LJ. Neurobiology of aggression and violence. American Journal of Psychiatry. 2008;165:429–42. doi: 10.1176/appi.ajp.2008.07111774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD. State-trait Anger Expression Inventory. Odessa, FL: Psychological Assessment Resources Inc; 1991. [Google Scholar]
- van Honk J, Tuiten A, de Haan E, van den Hout M, Stam H. Attentional biases for angry faces: relationships to trait anger and anxiety. Cognition and Emotion. 2001;15:279–97. [Google Scholar]
- Viviani R. Unbiased ROI selection in neuroimaging studies of individual differences. Neuroimage. 2010;50:184–9. doi: 10.1016/j.neuroimage.2009.10.085. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Shin LM, McInerney SC, Fischer H, Wright CI, Rauch SL. A functional MRI study of human amygdala responses to facial expressions of fear vs. anger. Emotion. 2001;1:70–83. doi: 10.1037/1528-3542.1.1.70. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Zhu G, Hariri AR, et al. Genetic variation in human NPY expression affects stress response and emotion. Nature. 2008;452:997–1001. doi: 10.1038/nature06858. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.