Abstract
When you see someone reach into a cookie jar, their goal remains obvious even if you know that the last cookie has already been eaten. Thus, it is possible to infer the goal of an action even if you know that the goal cannot be achieved. Previous research has identified distinct brain networks for processing information about object locations, actions and mental-state inferences. However, the relationship between brain networks for action understanding in social contexts remains unclear. Using functional magnetic resonance imaging, this study assesses the role of these networks in understanding another person searching for hidden objects. Participants watched movie clips depicting a toy animal hiding and an actor, who was ignorant of the hiding place, searching in the filled or empty location. When the toy animal hid in the same location repeatedly, the blood oxygen level-dependent (BOLD) response was suppressed in occipital, posterior temporal and posterior parietal brain regions, consistent with processing object properties and spatial attention. When the actor searched in the same location repeatedly, the BOLD signal was suppressed in the inferior frontal gyrus, consistent with the observation of hand actions. In contrast, searches towards the filled location compared to the empty location were associated with a greater response in the medial prefrontal cortex and right temporal pole, which are both associated with mental state inference. These findings show that when observing another person search for a hidden object, brain networks for processing information about object properties, actions and mental state inferences work together in a complementary fashion. This supports the hypothesis that brain regions within and beyond the putative human mirror neuron system are involved in action comprehension within social contexts.
Keywords: mentalizing, mirror neuron system, action observation, social cognition, fMRI
INTRODUCTION
If a man stands at his front door and searches in his pockets, you can guess that he is looking for his keys even if you know he left them in his car. The man’s ignorance to the location of his keys does not interfere with our ability to make sense of his goal. There is increasing neuroscientific interest in how brain systems for action perception and for mental state inference interact in social tasks, but few studies have directly addressed this question. The current study uses functional magnetic resonance imaging (fMRI) to examine how the brain responds to other people’s searching behaviour when the observer has access to knowledge that an actor does not.
Two distinct brain networks have been associated with action understanding and with mental state inference. A frontoparietal network comprising the posterior portion of inferior frontal gyrus (IFG) plus adjacent ventral premotor cortex (PMv) and inferior parietal lobule (IPL) responds to the execution and observation of actions (Grèzes and Decety, 2001; Gazzola and Keysers, 2009; Caspers et al., 2010). This network is commonly known as the human mirror neuron system (MNS) because it is believed to contain mirror neurons (Kilner et al., 2009; Oosterhof et al., 2010), similar to those observed in the non-human primate brain (Gallese et al., 1996; Fogassi et al., 2005). The MNS responds to action features such as goals and kinematics (Hamilton and Grafton, 2006, 2007, 2008) and is sensitive to at least some aspects of the context surrounding an action (Iacoboni et al., 2005; Newman-Norlund et al., 2007; Liepelt et al., 2009). By contrast, medial prefrontal cortex (mPFC), temporoparietal junction (TPJ) and temporal poles respond when mental states, such as thoughts, beliefs and desires are attributed to other people (Frith and Frith, 1999, 2006). This ‘mentalizing’ network is active when reasoning about the beliefs that a protagonist holds in a story (Fletcher et al., 1995; Gallagher et al., 2000) and when inferring the mental states of another agent in real-time during competitive or cooperative games (McCabe et al., 2001; Gallagher et al., 2002).
The MNS and mentalizing network are believed to be involved in making sense of other people’s non-verbal behaviour. A key theoretical question concerns the relationship between the MNS and the mentalizing network (Keysers and Gazzola, 2007; Uddin et al., 2007; Schilbach, 2010). Some claim that motor simulation, a process of mapping observed actions onto one’s own motor repertoire, is implemented in the MNS and is central to our ability to understand other people’s social behaviour (Rizzolatti et al., 2001; Rizzolatti and Craighero, 2004; Gallese, 2005, 2007; Rizzolatti and Sinigaglia, 2010). Others claim that simulation is not sufficient for social understanding and instead suggest that a more inferential mechanism, possibly implemented in the mentalizing network, is essential to social cognition (Csibra, 2007; Wood and Hauser, 2008). However, most studies that report activation of the mentalizing network have used verbal or abstract tasks (Fletcher et al., 1995; Gallagher et al., 2000; Saxe and Kanwisher, 2003).
Relatively few action observation studies have tested the hypothesis that the mentalizing network has a role in action understanding. In two such studies that have investigated this, Grèzes et al. (2004a, b) showed that mPFC is activated when observing an actor perform whole-body movements with deceptive intent. Furthermore, Brass et al. (2007) showed that TPJ and to a lesser extent mPFC are activated when observing unusual actions in a context where such an action was irrational (e.g. turning on a light with your knee when your hands are free), whereas de Lange et al. (2008) showed that reflecting on the intentions behind unusual actions activated mPFC and TPJ. These studies suggest that in more socially complex contexts, action comprehension can require a form of interpretative processing that is implemented in brain networks beyond the MNS (Csibra, 2007; Wood and Hauser, 2008).
In the present article we take a different approach to examine the links between action perception and mental state inference. We aimed to contrast the roles of the MNS and mentalizing network in a situation where one has access to ‘knowledge’ of the environment that an observed actor does not. For example, when seeing someone reach into a cookie jar, how does your brain respond when you know that the last cookie has already been eaten compared to when you know the cookie jar is full? To address this question we devised a hide and seek paradigm. Participants watched movie clips in which a toy animal moved from the centre of a table and hid in one of two locations, which were positioned to the left or right of the toy animal’s starting position. Subsequently, an actor (who was ignorant of the hiding location) searched for the toy in one of the two locations. Thus, the search could be directed towards the filled or empty location but the outcome of the search was not shown (Figure 1).
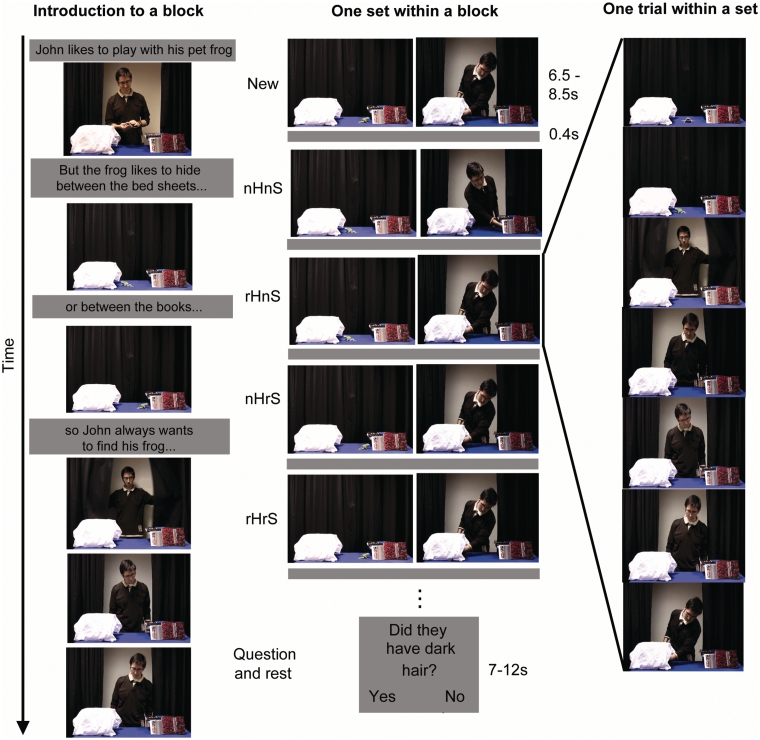
Fig. 1.
Stimuli and experimental set up. The left-side depicts scenes from one introductory video. Before each scanning block two different introductory videos were shown (30 s per video). The centre depicts scenes from a typical movie sequence viewed by participants during fMRI scanning. Each sequence began with a new movie followed by eight experimental clips. For each movie clip the toy animal could hide and the actor could search in the same (repeated) or different (novel) location with respect to the previous movie. As such, each clip fell evenly into a 2 × 2 factorial design for hide and search, novel and repeated (abbreviations are: n = novel, r = repeated, H = hide, S = search). Following a sequence, participants answered a yes-no question regarding the previous movie, then rested. The right side shows six scenes from one trial. On each a trial, a toy animal (e.g. a frog) would hide in one of two locations (e.g. between bed sheets or books) and an actor would open the curtains and search in one of the two locations.
A repetition suppression (RS) approach was used to test for brain regions encoding the location where the toy hid and the location where the actor searched, independently. RS is based on the finding that repeating a stimulus feature attenuates the blood oxygen level-dependent (BOLD) response in brain areas sensitive to that feature (Grill-Spector and Malach, 2001; Grill-Spector et al., 2006). On each trial, the toy animal could hide and the actor could search in the same location as the previous trial or a novel location. Previous action perception studies using RS have shown that anterior intraparietal sulcus (aIPS) is sensitive to action goals (Hamilton and Grafton, 2006, 2007; Ramsey and Hamilton, 2010), whereas IFG is sensitive to action kinematics (Hamilton and Grafton, 2007; Kilner et al., 2009). In the present study, we use the same logic to distinguish the perception of the movement of a toy animal hiding (RS-hide) from the perception of the actor’s searching action (RS-search).
We predicted that RS-hide would engage brain regions involved in processing properties of the hiding location itself, such as form and colour, as well as its spatial location (left vs right). Brain regions associated with coding object properties include fusiform gyrus, middle occipital, occipitotemporal and posterior parietal brain regions (Chao et al., 1999; Grill-Spector et al., 1999; Ishai et al., 1999, 2000; Haxby et al., 2001; Martin, 2007; Simmons et al., 2007). Brain regions associated with reorienting of spatial attention include a frontoparietal network (Corbetta et al., 2008), as well as ventral temporal, middle occipital and occipitotemporal regions (Coull and Nobre, 1998; Martinez et al., 1999). In contrast, we predicted that RS-search would engage brain regions involved in action perception. Consequently, we predicted RS-search in the MNS, which represents observed action features (Hamilton and Grafton, 2006, 2007; Kilner et al., 2009), superior temporal sulcus (STS) and occipitotemporal cortex (OT), which respond to biological motion and body parts, respectively (Downing et al., 2001; Blake and Shiffrar, 2007). We did not expect the RS-search contrast to reveal brain regions encoding high-order features of action such as goals and intentions because participants knew that the goal of the actor on every trial was to find the toy.
Finally, our paradigm enabled a third contrast to be distinguished, which directly compared searches towards filled locations with searches towards empty locations. Critically, the introduction to the videos explicitly stated that the actor did not know which location was filled. In contrast, the participant in the scanner always knew the location of the object. If the MNS distinguishes between actions based on the observer’s knowledge of the goal location, this would be consistent with recent findings that suggest action comprehension abilities in the MNS are more sophisticated than initially outlined (Iacoboni et al., 2005; Newman-Norlund et al., 2007; Liepelt et al., 2009). Such a result would support the claim that the MNS itself is the primary brain network for understanding the meaning of actions (Rizzolatti et al., 2001; Rizzolatti and Craighero, 2004; Gallese, 2005, 2007; Rizzolatti and Sinigaglia, 2010). However, other brain systems might also distinguish reaches towards filled and empty locations. In particular, the mentalizing network responds when individual’s observe actions that occur in ‘irrational’ contexts even without explicit instruction to consider other people’s mental states (Brass et al., 2007). Engagement of the mentalizing network in the current action scenario would suggest that understanding actions involves brain systems beyond the MNS, which are associated with inferential models of action understanding (Gergely and Csibra, 2003; Csibra, 2007; Wood and Hauser, 2008).
METHOD
Twenty-five participants (5 male, mean age 21.8 years, one left-handed) gave their informed consent to complete the experiment in accord with the local medical ethics board. Before scanning, participants were told that they would see movies that were intended for children, which depicted an adult playing with a toy animal. Before each scanning block, an introductory video explained that each actor liked to play with a toy animal, but the animal enjoyed hiding in one of two locations so the actor always wanted to find the animal (Figure 1, left side). These instructions established the actor’s desire to find the animal and his/her ignorance of the animal’s location.
During scanning, participants watched movie clips that were separated by a blank screen for 0.4 s. Movie clip durations ranged from 6.5 to 8.5 s according to the natural length of the event, but were constant within each sequence. Each movie clip comprised two aspects: hide and search. In the hide phase, a toy animal was moved using invisible wire to hide in one of the two locations whilst an actor was standing behind closed curtains, ignorant of the animal’s location. The two hiding locations (one on the left and the other on the right) were clearly distinguished by the different objects available for the animal to hide among, for example, a stack of books vs bed linen (Figure 1, centre). In the search phase, the actor would open the curtains, step forward, look at each location in turn and then reach into one of the locations (Figure 1, right side). Thus, the reach could be performed to the location containing the toy animal (filled) or not containing the toy animal (empty). Following a sequence of nine movies, participants answered an yes–no question about the content of the last movie they had just observed (e.g. Did they have dark hair?), then rested. The content of the question could not be predicted so participants were required to attend to the whole scene in order to answer. The duration of the combined question and rest period before the next sequence of movies began was chosen randomly without replacement from the possible durations (7, 8, 9, 10, 11 or 12 s; Figure 1, centre), and was independent of the stimulus condition. All stimuli were presented with Cogent running under Matlab 6.5 permitting synchronization with the scanner and accurate timing of stimuli presentation.
Every sequence of movies commenced with a randomly chosen movie clip, labelled ‘new’. Subsequently, eight movie clips were presented in a pseudorandom order in a one-back RS design. Each movie was defined in relation to the previous movie as either novel hide location–novel search location (nHnS), repeated hide location–novel search location (rHnS), novel hide location–repeated search location (nHrS) or repeated hide location–repeated search location (rHrS). Further, each movie was defined in terms of whether the reach was directed towards the filled (F) or empty (E) hiding location. Each participant completed three functional runs with six sequences of movies in each run giving 144 RS trials, which evenly filled a 2 × 2 factorial design for hide and search, novel and repeated. Filled–empty trials were classified post hoc with an average of 27 filled trials per run. Six different actors were used, each with a different toy animal and a unique pair of hiding locations. Participants completed three runs, with two distinct actor-toy sets shown in each run, in alternate blocks.
The experiment was performed in a 3T Phillips Achieva scanner using an eight channel-phased array head coil with 38 slices per TR (3-mm thickness); TR: 2500 ms; flip angle: 80°; field of view: 19.2 cm; matrix: 64 × 64. To improve signal detection, double-echo imaging was performed (Gowland and Bowtell, 2007). This method of scanning is designed to optimize signal detection from brain regions that often suffer from dropout (e.g. temporal poles and orbitofrontal cortex) without degradation of signal quality in parietal and occipital regions. Two images were collected in each TR, at echo times of 20 and 40 ms. Two hundred TRs were collected in each of the three runs. Data for each echo time were realigned separately and then combined using a weighted summation based on the signal strength in each brain region (Marciani et al., 2006). From this point onwards only the combined images were analysed further, and were treated like data from typical single-echo fMRI. Data were normalized to the MNI template with a resolution of 2 × 2 × 2 mm using SPM2 software. A design matrix was fitted for each subject with one regressor for each movie type in searches towards the filled (FnHnS, FrHnS, FnHrS, FrHrS) and empty locations (EnHnS, ErHnS, EnHrS, ErHrS) and combined across the three functional runs. Each movie was modelled as a boxcar with the duration of that movie convolved with the standard hemodynamic response function. New and Question trials were modelled in the same way but not analysed further. In order to reduce the impact of movement artefacts each design matrix weighted every raw image according to its overall variability (Diedrichsen and Shadmehr, 2005). After estimation, 9-mm smoothing was applied to the beta images.
In order to localize brain regions showing RS-Hide, a contrast for the main effect of hide (novel > repeated) was calculated across all movies. To localize brain regions showing RS-search, a contrast for the main effect of search (novel > repeated) was calculated across all movies, irrespective of object (toy animal) location. To identify brain regions that are sensitive to searches towards filled versus empty locations, main effects for searches towards filled (filled > empty) and empty locations (filled < empty) were performed across all movies. Contrast images for all participants were taken to the second level for a random effects analysis. Correction for multiple comparisons was performed at the cluster level (Friston et al., 1994), using a voxel-level threshold of P < 0.005 and 50 voxels and a cluster-level correction of P < 0.05. Brain regions that survive a threshold of P < 0.005 uncorrected and 50 voxels over the whole brain are reported in Table 1. To reduce false positives, we focus our discussion on results within our predicted brain networks, the MNS, mentalizing, object-processing and spatial attention networks.
Table 1.
Brain regions showing RS-Hide, RS-Search and the contrasts between searches towards filled and empty locations
| Region | Number of voxels | T | Montreal Neurological Institute co-ordinates |
||
|---|---|---|---|---|---|
| x | y | z | |||
| RS-hide | |||||
| Medial cerebellum | 213 | 6.79 | 0 | −72 | −30 |
| −12 | −80 | −26 | |||
| Left occipitotemporal gyrus | 471 | 5.01 | −36 | −68 | 0 |
| −44 | −72 | −16 | |||
| Right and left superior parietal lobules | 3048 | 4.97 | 16 | −60 | 54 |
| −18 | −48 | 48 | |||
| −14 | −58 | 48 | |||
| Right precentral gyrus | 539 | 4.07 | 32 | −12 | 58 |
| 34 | −20 | 60 | |||
| 26 | −10 | 40 | |||
| Right insula/striatum | 108 | 4.04 | 24 | 18 | −2 |
| Right fusiform, occipitotemporal and middle occipital gyri | 1065 | 3.77 | 34 | −56 | −6 |
| 58 | −66 | −6 | |||
| 32 | −80 | 16 | |||
| Left medial amygdala | 88 | 3.76 | 20 | −6 | −20 |
| Left posterior hippocampus | 117 | 3.74 | −32 | −30 | −4 |
| −24 | −30 | −2 | |||
| Right inferior parietal lobule | 131 | 3.72 | 62 | −42 | 46 |
| 52 | −52 | 52 | |||
| Left insula | 50 | 3.50 | −30 | −6 | 8 |
| Right posterior hippocampus | 83 | 3.50 | 16 | −26 | −4 |
| Right IPL/intraparietal sulcus | 54 | 3.47 | 34 | −30 | 38 |
| 26 | −34 | 34 | |||
| Right insula/putamen | 50 | 3.43 | 36 | −6 | −6 |
| Left inferior temporal gyrus | 77 | 3.42 | −44 | −36 | −20 |
| −40 | −44 | −26 | |||
| Left putamen | 111 | 3.32 | −20 | 2 | −4 |
| −16 | −8 | −6 | |||
| −24 | 8 | −10 | |||
| Right cerebellum | 84 | 3.26 | 20 | −60 | −24 |
| 28 | −62 | −24 | |||
| RS-search | |||||
| Right lateral prefrontal cortex | 353 | 4.46 | 46 | 54 | −4 |
| 50 | 48 | 6 | |||
| 30 | 50 | 16 | |||
| Left lateral prefrontal cortex | 142 | 3.70 | −36 | 54 | −6 |
| Left medial wall of caudate body | 239 | 3.64 | −18 | −8 | 22 |
| −28 | −14 | 24 | |||
| −34 | −16 | 30 | |||
| Right parahippocampul gyrus | 50 | 3.54 | 32 | −12 | −40 |
| Left middle intraparietal sulcus | 120 | 3.44 | −22 | −60 | 46 |
| Right premotor cortex extending into inferior frontal gyrus (pars opercularis) | 186 | 3.26 | 42 | 4 | 60 |
| 42 | 8 | 36 | |||
| 38 | 4 | 48 | |||
| Left inferior frontal gyrus (pars opercularis) | |||||
| 62 | 3.26 | −52 | 12 | 30 | |
| Filled > empty | |||||
| Right temporal pole | 298 | 4.92 | 60 | 4 | −24 |
| 52 | −20 | −22 | |||
| Right anterior inferior frontal gyrus (pars orbitalis) extending into orbitofrontal cortex | 197 | 4.31 | 56 | 34 | −2 |
| 52 | 34 | −10 | |||
| 54 | 26 | −10 | |||
| Medial prefrontal cortex | 171 | 3.95 | −2 | 56 | 38 |
| Right central sulcus | 97 | 3.80 | 48 | −12 | 48 |
| Left insula extending into caudate | 168 | 3.67 | −32 | −18 | 4 |
| −30 | −20 | 12 | |||
| Right middle occipital gyrus | 78 | 3.09 | 8 | −104 | 16 |
| Empty > filled | |||||
| No brain regions | |||||
Only regions surviving a voxel-level threshold of P < 0.005 and 50 voxels are reported. Subpeaks more than 8 mm from the main peak in each cluster are listed. Bold indicates regions that survive the whole-brain cluster-corrected threshold at P < 0.05.
RESULTS
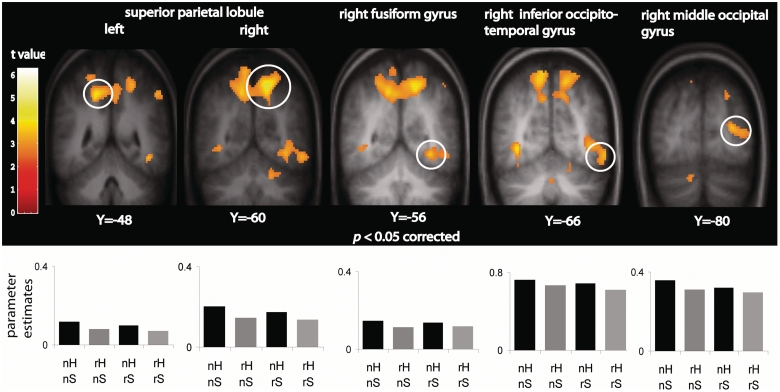
The repetition suppression contrasts yielded results consistent with our predictions. Four brain regions showed RS-hide: bilateral superior parietal lobule (SPL), right middle occipital, occipitotemporal and fusiform gyri. In Figure 2 the pattern of response in these brain regions is depicted with parameter estimate plots showing that irrespective of searching location, the response to a novel hiding location was suppressed when the toy animal hid in the identical location for a second time.
Fig. 2.
Brain regions showing RS-hide. Significant suppression was seen for repeated hide (grey bars) compared to novel hide (black bars) in bilateral superior parietal lobule and right fusiform, occipitotemporal and middle occipital gyri. Parameter estimates (SPM betas) are plotted for each region. n = novel, r = repeated, H = hide, S = search.
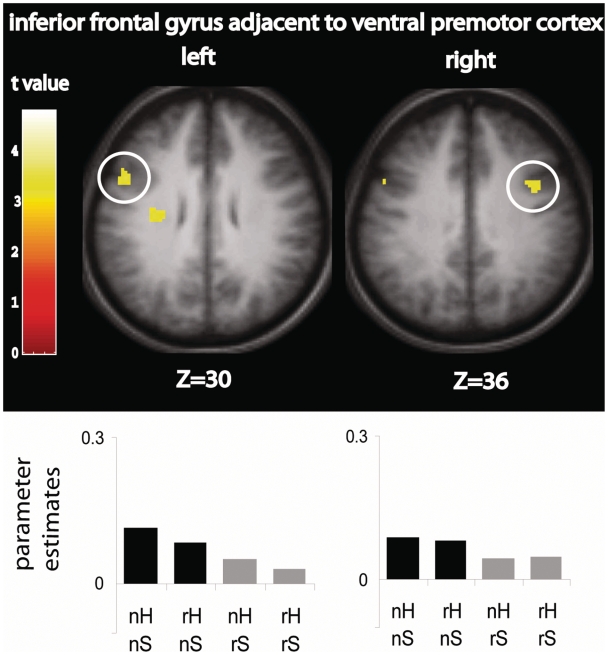
One brain region showed RS-search bilaterally: posterior IFG extending into adjacent PMv. In Figure 3, the pattern of response in these regions is depicted with parameter estimate plots showing that irrespective of the hiding location, the response to seeing an actor search in a novel location was suppressed when the same search was performed for a second time.
Fig. 3.
Brain regions showing RS-search. Significant suppression was seen for repeated search (grey bars) compared to novel search (black bars) in bilateral inferior frontal gyrus and adjacent ventral premotor cortex. Parameter estimates (SPM betas) are plotted for each region. n = novel, r = repeated, H = hide, S = search.
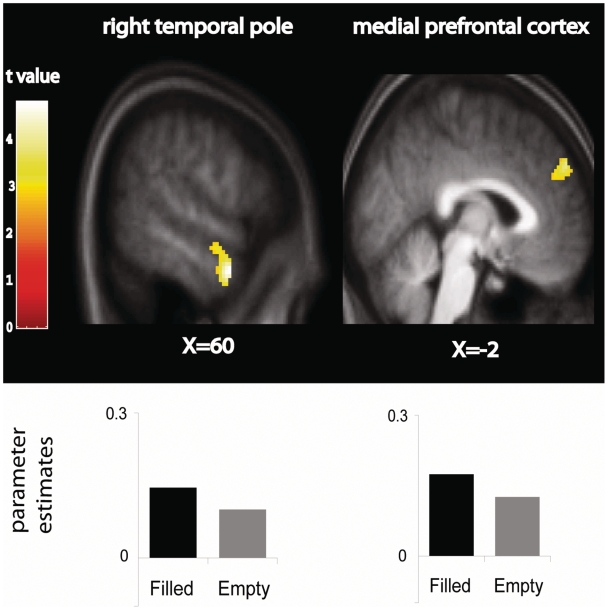
The filled vs empty contrast revealed two brain regions within our predicted networks, which showed a stronger response for searches towards filled than empty locations: mPFC and right temporal pole (Figure 4). Right anterior IFG extending into orbitofrontal cortex also showed the same pattern of response. However, this anterior IFG region is not part of the MNS, which is in posterior IFG adjacent to PMv, and is therefore not part of our predicted networks and thus we do not discuss it further. No brain regions showed a stronger response for false compared to true searches. There were no significant interactions between any of the three contrasts described here.
Fig. 4.
Brain regions for filled > empty searches. Significantly greater activity was seen for searches towards filled locations (black bars) compared to empty locations (grey bars) in right temporal pole and medial prefrontal cortex. Parameter estimates (SPM betas) are plotted for each region.
DISCUSSION
Our results demonstrate the involvement of both MNS and mentalizing brain regions in understanding another person’s searching behaviour. When an actor was observed searching in the same location repeatedly, the BOLD response was suppressed in the inferior frontal node of the MNS. In contrast, the mentalizing network distinguished between searches towards filled and empty locations, which suggests that this network has a role in understanding actions in cases of differential knowledge between self and other. These findings suggest functional divisions in the roles played by the MNS and mentalizing network during action perception, which have implications for theories of action understanding in social contexts.
Hiding and searching
Our study is the first human neuroimaging investigation of the brain systems that respond to the observation of another person searching for a hidden object. We found that when the toy repeatedly hid in the same location, the BOLD response was suppressed in superior parietal, middle occipital, occipitotemporal and fusiform brain regions. These brain regions are associated with encoding object properties (Chao et al., 1999; Grill-Spector et al., 1999; Ishai et al., 1999, 2000; Haxby et al., 2001; Martin, 2007; Simmons et al., 2007) as well as with reorienting spatial attention (Coull and Nobre, 1998; Martinez et al., 1999; Corbetta et al., 2008). Both these features are relevant to processing the toy object hiding and we do not attempt to distinguish them. RS-hide was also found in a number of brain regions beyond our predicted network, including the posterior hippocampus, which may reflect spatial memory demands of tracking object locations (Burgess et al., 2002; Bird and Burgess, 2008). Keeping track of objects is typically considered a ‘non-social’ process; it does not entail inferences about other people’s minds (Adolphs, 2009). In the current study, participants needed to track locations in order to interpret the outcome of the actor’s searching action. Thus, our results suggest that non-social and social brain regions can be engaged in concert as the situation demands. This supports recent suggestions that more ecologically-valid models of social information processing can be derived from examining social cognition in contexts that reflect real life interactions to a greater extent (Kingstone et al., 2008; Zaki and Ochsner, 2009; Schippers et al., 2010).
We also examined brain regions sensitive to the actor repeatedly searching in the same location. Bilateral IFG and adjacent PMv showed RS for search, independent of the toy’s actual location. Because high-level features of actions, such as goals or intentions, were kept constant, this pattern of activity reflects sensitivity to action features that changed with search location, such as hand kinematics and body posture. Previous action perception research has shown that IFG responded to kinematic features of hand actions (Hamilton and Grafton, 2007; Kilner et al., 2009) as well as the effector used to perform actions (Jastorff et al., 2010). Our findings suggest that when observing another person search for an object with their hand, IFG and adjacent PMv are sensitive to the direction and configuration of such actions. These data are consistent with emerging hierarchical models of action comprehension (Hamilton and Grafton, 2007; Grafton, 2009; Jastorff et al., 2010), which suggest IFG provides a kinematic or somatotopic description of action in preparation to produce a suitable motor response.
Filled and empty locations
In mPFC and right temporal pole, a stronger BOLD response was observed for searches towards filled compared to empty locations; no brain regions showed stronger responses for searches towards empty locations. Before interpreting these results, we should emphasize the differential knowledge between actor and participant in the current task. The participant knew exactly where the toy was hidden, but the introduction emphasized that the actor was ignorant to the toy animal’s location and consequently had no belief about the location of the toy. When faced with an ignorant actor who wants to find an item that is hidden in one of two locations, adults predict that actors will show no preference to either location (Friedman and Petrashek, 2009). Thus, the engagement of these regions does not reflect fulfilment of the participant’s predictions about the actor’s actions, nor does it reflect encoding of the actor’s belief about the location of the toy animal. Rather, we will consider several possible interpretations of these results.
Only one previous study by Brass and colleagues (2007) reports engagement of the mentalizing network during action observation when participants were not explicitly instructed to consider the intentions (de Lange et al., 2008) or deceptive intent (Grèzes et al., 2004a, b) of the actor. In that study, mPFC and TPJ showed stronger activation when participants observed irrational actions compared to similar actions which, because of a change in context, were rational (Brass et al., 2007). Our results support the findings of Brass and colleagues in the sense that we show that mPFC can be engaged when observing simple actions without the explicit instruction to mentalize. However, our paradigm was quite different. Our data show that brain regions associated with mentalizing respond more when observing actors reach into a location filled with an object compared to an empty location. Numerous cognitive interpretations of this result are plausible, which we will now outline.
One possible interpretation of our data is that when the actor searches in a filled location, there is a clear change in his/her mental state from one of ignorance to one of knowledge. By contrast, on empty trials, the actor does not have direct knowledge of the toy’s location. Therefore, the greater BOLD response for searches to filled compared to empty locations may reflect heightened sensitivity to situations where an actor gains direct and relevant knowledge of the toy location. A second possibility is that participants may predict the consequence of the action in the filled-location searches, for example, that the actor will be happy or will perform further actions, which would not be possible on empty-location searches. Third, prior work has shown that your own action (e.g. lifting a box) can modulate the MNS and occipitotemporal brain regions during the perception of a similar action (Hamilton et al., 2006). The current result may reflect ways that your own knowledge of the environment can modulate brain regions associated with mentalizing during the perception of action. Finally, our results could be considered in terms of teleological reasoning, a possible precursor to mentalizing. Infants are able to interpret actions in relation to contextual and environmental demands without deliberate mental state reasoning (Gergely et al., 1995; Csibra and Gergely, 1998, 2007; Gergely and Csibra, 2003). It is suggested that they may track the relationship between current reality and a future reality or goal state. Participants in our study might have engaged in a similar process when observing actions that will achieve their goal compared to those that will not, and it is possible that this teleological reasoning is sufficient to engage mentalizing brain regions. Future work could distinguish these possibilities.
In sum, a broad network of brain regions respond when deliberately attributing or reasoning about other people’s mental states (Frith and Frith, 1999, 2006), and also when observing actions performed in ‘irrational’ contexts, even when no instruction is given to consider other people’s mental states (Brass et al., 2007). It is therefore possible that different components of the mentalizing network—mPFC and temporal poles—show subtle sensitivity to observed actions based on one’s own knowledge of the environment in relation to an actor’s knowledge. Future work that fractionates possible functional roles of component parts of the mentalizing network would be worthwhile. In addition, it would also be valuable to test the range of cognitive processes that occur in brain regions associated with mentalizing, which do not reflect deliberate mental state reasoning.
Methodological implications
Seminal positron emission tomography (PET) and fMRI work that investigated mental-state attribution highlighted the temporal poles as a node in a network of brain regions involved in mentalizing (Fletcher et al., 1995; Gallagher et al., 2000). But, in subsequent neuroimaging studies, these regions have received little attention (but see Olson et al., 2007; Ross and Olson, 2010). One reason may be due to the variability in signal quality across different regions of the brain when using fMRI, and the common problem of signal dropout in temporal poles (Weiskopf et al., 2006). A strength of the current methodology was to use double-echo imaging to improve signal detection in brain areas that are usually impoverished without degradation to any other region (Marciani et al., 2006; Gowland and Bowtell, 2007). In this way, we found activity in temporal poles that may not have been possible using standard fMRI scanning parameters. We suggest that this novel method may be useful to any researcher interested in similar brain regions that suffer from signal dropout.
Theoretical implications and future directions
The current findings advance our understanding of how the MNS and mentalizing network interact during the perception of action. We show the involvement of both the MNS and mentalizing network in action understanding when the observer and actor have different access to knowledge. Components of the MNS were sensitive to the direction of hand motion but were not modulated by the participant’s knowledge. However, components of the mentalizing network distinguished actions that the observer knows are directed to a filled location from those the observer knows are directed to an empty location. Thus, although some studies show the MNS incorporates a wider context surrounding an action (Iacoboni et al., 2005; Newman-Norlund et al., 2007; Liepelt et al., 2009), we show limits to the social competence of the MNS (Csibra, 2007; Wood and Hauser, 2008) and support the hypothesis that the mentalizing network contributes to action comprehension (Grèzes et al., 2004a, b; Brass et al., 2007; de Lange et al., 2008). Together, the findings are compatible with the notion that the mentalzing network and MNS perform complementary roles in understanding other people’s actions (Keysers and Gazzola, 2007; Uddin et al., 2007).
Future work in this area could aim to delineate which specific features of observed actions lead to engagement of the mentalizing network compared to the MNS, and thus define the functional role of each of these networks in action comprehension (Schilbach, 2010). Such an approach would help to distinguish different levels of action perception and revise previous definitions of ‘action understanding’ that may have been too narrow, thus artificially restricting the set of brain regions implicated in this process (Hickok, 2009). On a related note, there is a clear need for more sophisticated neurocognitive models that take into account the time-course, interactions and development of different components of the social brain (Nummenmaa and Calder, 2009). Recent models of action understanding have separated different levels of processing in hierarchical (Hamilton and Grafton, 2007; Grafton, 2009; Jastorff et al., 2010) and dual-route structures (Rumiati and Tessari, 2002), and the extension of these models to more complex situations would be valuable. Finally, the present study suggests that brain regions associated with object processing are engaged when a social stimulus demands information about object locations. Understanding the interaction of social and non-social processes is necessary for a more inclusive and ecologically valid approach to social cognition (Kingstone et al., 2008; Zaki and Ochsner, 2009).
CONCLUSION
Appreciating the meaning of social interactions involves linking an observed individual’s knowledge and actions with your own knowledge, but few studies in social neuroscience have examined this relationship during action perception. We show that brain regions in the MNS respond to visible action features, such as kinematics, whereas mentalizing regions show sensitivity to whether observed actions will achieve their goals. This suggests that different, although complementary, brain networks process visible action features and interpret actions based on one’s own knowledge of the environment. The results point towards a functional dissociation between the MNS and the metalizing network, which supports the hypothesis that action understanding in social contexts requires multiple brain networks both within and beyond the human mirror neuron system.
Acknowledgments
The authors thank Emily Cross for helpful comments on earlier versions of this article. In addition, the authors thank Kay Head and the Sir Peter Mansfield Magnetic Resonance Centre, where fMRI scanning was performed. This research was funded through an Economic and Social Research Council (ESRC RES-061-25-0138) grant awarded to the second author of this article.
REFERENCES
- Adolphs R. The social brain: neural basis of social knowledge. Annual Review of Psychology. 2009;60:693–716. doi: 10.1146/annurev.psych.60.110707.163514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird CM, Burgess N. The hippocampus and memory: insights from spatial processing. Nature Review Neuroscience. 2008;9:182–94. doi: 10.1038/nrn2335. [DOI] [PubMed] [Google Scholar]
- Blake R, Shiffrar M. Perception of human motion. Annual Review of Psychology. 2007;58:47–73. doi: 10.1146/annurev.psych.57.102904.190152. [DOI] [PubMed] [Google Scholar]
- Brass M, Schmitt RM, Spengler S, Gergely G. Investigating action understanding: inferential processes versus action simulation. Current Biology. 2007;17:2117–21. doi: 10.1016/j.cub.2007.11.057. [DOI] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, O’Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35:625–41. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- Caspers S, Zilles K, Laird AR, Eickhoff SB. ALE meta-analysis of action observation and imitation in the human brain. NeuroImage. 2010;50:1148–67. doi: 10.1016/j.neuroimage.2009.12.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao LL, Haxby JV, Martin A. Attribute-based neural substrates in temporal cortex for perceiving and knowing about objects. Nature Neuroscience. 1999;2:913–9. doi: 10.1038/13217. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–24. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JT, Nobre AC. Where and when to pay attention: the neural systems for directing attention to spatial locations and to time intervals as revealed by both PET and fMRI. Journal of Neuroscience. 1998;18:7426–35. doi: 10.1523/JNEUROSCI.18-18-07426.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csibra G. Haggard P, Rossetti Y, Kawato M, editors. Action mirroring and action understanding: an alternative account. Sensorimotor Foundations of Higher Cognition: Attention and Performance. 2007;Vol. XXII Oxford: Oxford University Press, pp. 435–480. [Google Scholar]
- Csibra G, Gergely G. The teleological origins of mentalistic action explanations: a developmental hypothesis. Developmental Science. 1998;1:255–9. [Google Scholar]
- Csibra G, Gergely G. Obsessed with goals': functions and mechanisms of teleological interpretation of actions in humans. Acta Psychologica. 2007;124:60–78. doi: 10.1016/j.actpsy.2006.09.007. [DOI] [PubMed] [Google Scholar]
- de Lange FP, Spronk M, Willems RM, Toni I, Bekkering H. Complementary systems for understanding action intentions. Current Biology. 2008;18:454–7. doi: 10.1016/j.cub.2008.02.057. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Shadmehr R. Detecting and adjusting for artifacts in fMRI time series data. NeuroImage. 2005;27:624–34. doi: 10.1016/j.neuroimage.2005.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing PE, Jiang Y, Shuman M, Kanwisher N. A cortical area selective for visual processing of the human body. Science. 2001;293:2470–73. doi: 10.1126/science.1063414. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Happe F, Frith U, et al. Other minds in the brain: a functional imaging study of ‘theory of mind’ in story comprehension. Cognition. 1995;57:109–28. doi: 10.1016/0010-0277(95)00692-r. [DOI] [PubMed] [Google Scholar]
- Fogassi L, Ferrari PF, Gesierich B, Rozzi S, Chersi F, Rizzolatti G. Parietal lobe: from action organization to intention understanding. Science. 2005;308:662–7. doi: 10.1126/science.1106138. [DOI] [PubMed] [Google Scholar]
- Friedman O, Petrashek AR. Children do not follow the rule ‘ignorance means getting it wrong’. Journal of Experimental Child Psychology. 2009;102:114–21. doi: 10.1016/j.jecp.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Worsley KJ, Frackowiak RSJ, Mazziotta JC, Evans AC. Assessing the significance of focal activations using their spatial extent. Human Brain Mapping. 1994;1:210–20. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. Interacting minds–a biological basis. Science. 1999;286:1692–5. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. The neural basis of mentalizing. Neuron. 2006;50:531–4. doi: 10.1016/j.neuron.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Happe F, Brunswick N, Fletcher PC, Frith U, Frith CD. Reading the mind in cartoons and stories: an fMRI study of 'theory of mind' in verbal and nonverbal tasks. Neuropsychologia. 2000;38:11–21. doi: 10.1016/s0028-3932(99)00053-6. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Jack AI, Roepstorff A, Frith CD. Imaging the intentional stance in a competitive game. NeuroImage. 2002;16:814–21. doi: 10.1006/nimg.2002.1117. [DOI] [PubMed] [Google Scholar]
- Gallese V. Embodied simulation: from neurons to phenomenal experience. Phenomenology and the Cognitive Sciences. 2005;4:23–48. [Google Scholar]
- Gallese V. Before and below a 'theory of mind': embodied simulation and the neural correlates of social cognition. Philosophical Transactions of the Royal Society B: Biological Sciences. 2007;362:659–69. doi: 10.1098/rstb.2006.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallese V, Fadiga L, Fogassi L, Rizzolatti G. Action recognition in the premotor cortex. Brain. 1996;119:593–609. doi: 10.1093/brain/119.2.593. [DOI] [PubMed] [Google Scholar]
- Gazzola V, Keysers C. The observation and execution of actions share motor and somatosensory voxels in all tested subjects: single-subject analyses of unsmoothed fMRI data. Cerebral Cortex. 2009;19:1239–55. doi: 10.1093/cercor/bhn181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gergely G, Csibra G. Teleological reasoning in infancy: the naive theory of rational action. Trends in Cognitive Science. 2003;7:287–92. doi: 10.1016/s1364-6613(03)00128-1. [DOI] [PubMed] [Google Scholar]
- Gergely G, Nadasdy Z, Csibra G, Biro S. Taking the intentional stance at 12 months of age. Cognition. 1995;56:165–93. doi: 10.1016/0010-0277(95)00661-h. [DOI] [PubMed] [Google Scholar]
- Gowland PA, Bowtell R. Theoretical optimization of multi-echo fMRI data acquisition. Physics in Medicine and Biology. 2007;52:1801. doi: 10.1088/0031-9155/52/7/003. [DOI] [PubMed] [Google Scholar]
- Grafton ST. Embodied cognition and the simulation of action to understand others. Annals of the New York Academy of Sciences. 2009;1156:97–117. doi: 10.1111/j.1749-6632.2009.04425.x. [DOI] [PubMed] [Google Scholar]
- Grèzes J, Decety J. Functional anatomy of execution, mental simulation, observation, and verb generation of actions: a meta-analysis. Human Brain Mapping. 2001;12:1–19. doi: 10.1002/1097-0193(200101)12:1<1::AID-HBM10>3.0.CO;2-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grèzes J, Frith C, Passingham RE. Brain mechanisms for inferring deceit in the actions of others. Journal of Neurosciences. 2004a;24:5500–5. doi: 10.1523/JNEUROSCI.0219-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grèzes J, Frith CD, Passingham RE. Inferring false beliefs from the actions of oneself and others: an fMRI study. NeuroImage. 2004b;21:744–50. doi: 10.1016/S1053-8119(03)00665-7. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Henson R, Martin A. Repetition and the brain: neural models of stimulus-specific effects. Trends in Cognitive Science. 2006;10:14–23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Kushnir T, Edelman S, Avidan G, Itzchak Y, Malach R. Differential processing of objects under various viewing conditions in the human lateral occipital complex. Neuron. 1999;24:187–203. doi: 10.1016/s0896-6273(00)80832-6. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Malach R. fMR-adaptation: a tool for studying the functional properties of human cortical neurons. Acta Psychology. 2001;107:293–321. doi: 10.1016/s0001-6918(01)00019-1. [DOI] [PubMed] [Google Scholar]
- Hamilton AF, Grafton ST. Goal representation in human anterior intraparietal sulcus. Journal of Neurosciences. 2006;26:1133–7. doi: 10.1523/JNEUROSCI.4551-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton AF, Grafton ST. The motor hierarchy: from kinematics to goals and intentions. In: Haggard P, Rosetti Y, Kawato M, editors. Sensorimotor Foundations of Higher Cognition: Attention and Performance. Vol. XXII. Oxford, UK: Oxford University Press; 2007. [Google Scholar]
- Hamilton AF, Grafton ST. Action outcomes are represented in human inferior frontoparietal cortex. Cerebral Cortex. 2008;18:1160–8. doi: 10.1093/cercor/bhm150. [DOI] [PubMed] [Google Scholar]
- Hamilton AF, Wolpert DM, Frith U, Grafton ST. Where does your own action influence your perception of another person's action in the brain? NeuroImage. 2006;29:524–35. doi: 10.1016/j.neuroimage.2005.07.037. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Gobbini MI, Furey ML, Ishai A, Schouten JL, Pietrini P. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science. 2001;293:2425–30. doi: 10.1126/science.1063736. [DOI] [PubMed] [Google Scholar]
- Hickok G. Eight problems for the mirror neuron theory of action understanding in monkeys and humans. Journal of Cognitive Neuroscience. 2009;21:1229–43. doi: 10.1162/jocn.2009.21189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M, Molnar-Szakacs I, Gallese V, Buccino G, Mazziotta JC, Rizzolatti G. Grasping the intentions of others with one's own mirror neuron system. PLoS Biology. 2005;3:e79. doi: 10.1371/journal.pbio.0030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishai A, Ungerleider LG, Martin A, Haxby JV. The representation of objects in the human occipital and temporal cortex. Journal of Cognitive Neuroscience. 2000;12:35–51. doi: 10.1162/089892900564055. [DOI] [PubMed] [Google Scholar]
- Ishai A, Ungerleider LG, Martin A, Schouten JL, Haxby JV. Distributed representation of objects in the human ventral visual pathway. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:9379–84. doi: 10.1073/pnas.96.16.9379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastorff J, Begliomini C, Fabbri-Destro M, Rizzolatti G, Orban GA. Coding observed motor acts: different organizational principles in the parietal and premotor cortex of humans. Journal of Neurophysiology. 2010;104:128–40. doi: 10.1152/jn.00254.2010. [DOI] [PubMed] [Google Scholar]
- Keysers C, Gazzola V. Integrating simulation and theory of mind: from self to social cognition. Trends in Cognitive Sciences. 2007;11:194–6. doi: 10.1016/j.tics.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Kilner JM, Neal A, Weiskopf N, Friston KJ, Frith CD. Evidence of mirror neurons in human inferior frontal gyrus. Journal of Neurosciences. 2009;29:10153–9. doi: 10.1523/JNEUROSCI.2668-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingstone A, Smilek D, Eastwood JD. Cognitive ethology: a new approach for studying human cognition. British Journal of Psychology. 2008;99:317–40. doi: 10.1348/000712607X251243. [DOI] [PubMed] [Google Scholar]
- Liepelt R, Ullsperger M, Obst K, Spengler S, von Cramon DY, Brass M. Contextual movement constraints of others modulate motor preparation in the observer. Neuropsychologia. 2009;47:268–75. doi: 10.1016/j.neuropsychologia.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Marciani L, Pfeiffer JC, Hort J, et al. Improved methods for fMRI studies of combined taste and aroma stimuli. Journal of Neuroscience Methods. 2006;158:186–94. doi: 10.1016/j.jneumeth.2006.05.035. [DOI] [PubMed] [Google Scholar]
- Martin A. The representation of object concepts in the brain. Annual Review of Psychology. 2007;58:25–45. doi: 10.1146/annurev.psych.57.102904.190143. [DOI] [PubMed] [Google Scholar]
- Martinez A, Anllo-Vento L, Sereno MI, et al. Involvement of striate and extrastriate visual cortical areas in spatial attention. Nature Neurosciences. 1999;2:364–9. doi: 10.1038/7274. [DOI] [PubMed] [Google Scholar]
- McCabe K, Houser D, Ryan L, Smith V, Trouard T. A functional imaging study of cooperation in two-person reciprocal exchange. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:11832–5. doi: 10.1073/pnas.211415698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman-Norlund RD, van Schie HT, van Zuijlen AM, Bekkering H. The mirror neuron system is more active during complementary compared with imitative action. Nature Neurosciences. 2007;10:817–18. doi: 10.1038/nn1911. [DOI] [PubMed] [Google Scholar]
- Nummenmaa L, Calder AJ. Neural mechanisms of social attention. Trends in Cognitive Sciences. 2009;13:135–43. doi: 10.1016/j.tics.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Olson IR, Plotzker A, Ezzyat Y. The Enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 2007;130:1718–31. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- Oosterhof NN, Wiggett AJ, Diedrichsen J, Tipper SP, Downing PE. Surface-based information mapping reveals crossmodal vision-action representations in human parietal and occipitotemporal cortex. Journal of Neurophysiology. 2010;104:1077–89. doi: 10.1152/jn.00326.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey R, Hamilton AFde C. Triangles have goals too: understanding action representation in left aIPS. Neuropsychologia. 2010;48:2773–6. doi: 10.1016/j.neuropsychologia.2010.04.028. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annuual Review of Neurosciences. 2004;27:169–92. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V. Neurophysiological mechanisms underlying the understanding and imitation of action. Nature Review Neurosciences. 2001;2:661–70. doi: 10.1038/35090060. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Sinigaglia C. The functional role of the parieto-frontal mirror circuit: interpretations and misinterpretations. Nature Review Neuroscience. 2010;11:264–74. doi: 10.1038/nrn2805. [DOI] [PubMed] [Google Scholar]
- Ross LA, Olson IR. Social cognition and the anterior temporal lobes. NeuroImage. 2010;49:3452–62. doi: 10.1016/j.neuroimage.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumiati RI, Tessari A. Imitation of novel and well-known actions: the role of short-term memory. Experimental Brain Research. 2002;142:425–33. doi: 10.1007/s00221-001-0956-x. [DOI] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N. People thinking about thinking people. The role of the temporo-parietal junction in ‘theory of mind’. NeuroImage. 2003;19:1835–42. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Schilbach L. A second-person approach to other minds. Nature Review Neurosciences. 2010;11:449. doi: 10.1038/nrn2805-c1. [DOI] [PubMed] [Google Scholar]
- Schippers MB, Roebroeck A, Renken R, Nanetti L, Keysers C. Mapping the information flow from one brain to another during gestural communication. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:9388–93. doi: 10.1073/pnas.1001791107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons WK, Ramjee V, Beauchamp MS, McRae K, Martin A, Barsalou LW. A common neural substrate for perceiving and knowing about color. Neuropsychologia. 2007;45:2802–10. doi: 10.1016/j.neuropsychologia.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Iacoboni M, Lange C, Keenan JP. The self and social cognition: the role of cortical midline structures and mirror neurons. Trends in Cognitive Sciences. 2007;11:153–7. doi: 10.1016/j.tics.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Weiskopf N, Hutton C, Josephs O, Deichmann R. Optimal EPI parameters for reduction of susceptibility-induced BOLD sensitivity losses: a whole-brain analysis at 3 T and 1.5 T. NeuroImage. 2006;33:493–504. doi: 10.1016/j.neuroimage.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Wood JN, Hauser MD. Action comprehension in non-human primates: motor simulation or inferential reasoning? Trends in Cognitive Sciences. 2008;12:461–5. doi: 10.1016/j.tics.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Zaki J, Ochsner K. The need for a cognitive neuroscience of naturalistic social cognition. Annals of the New York Academy of Sciences. 2009;1167:16–30. doi: 10.1111/j.1749-6632.2009.04601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]