Abstract
A considerable body of evidence derived from terror management theory indicates that the awareness of mortality represents a potent psychological threat engendering various forms of psychological defense. However, extant research has yet to examine the neurological correlates of cognitions about one’s inevitable death. The present study thus investigated in 17 male participants patterns of neural activation elicited by mortality threat. To induce mortality threat, participants answered questions arranged in trial blocks that referred to fear of death and dying. In the control condition participants answered questions about fear of dental pain. Neural responses to mortality threat were greater than to pain threat in right amygdala, left rostral anterior cingulate cortex, and right caudate nucleus. We discuss implications of these findings for stimulating further research into the neurological correlates of managing existential fear.
Keywords: brain, defense mechanism, fear of death, fMRI, mortality salience, terror management theory
INTRODUCTION
Everybody is confronted with the transience of existence and often people are exposed to reminders of mortality in everyday life. As reflected in the oldest known self-referential text, the Sumerian Epic of Gilgamesh, philosophers have long claimed this existential awareness pushes its way through diverse facets of a human’s life, engendering strong cognitive–emotional reactions and psychological efforts to overcome the reality of one’s finitude. But how does confrontation with awareness of death impact patterns of neural activation? Despite considerable study of the psychological consequences of mortality awareness, there is a paucity of research that informs a question that has the potential to bridge some of the oldest philosophical inquires with the methods available to 21st century psychological science.
Extensive research derived from Terror Management Theory (Greenberg et al., 1986) has demonstrated that reminders of mortality, or mortality salience, trigger psychological responses that bolster the individuals’ sense of culturally based meaning and personal value within that enduring belief system. Specifically, mortality salience has led to varied forms of self-esteem striving and securing faith in one’s own cultural worldviews, thus offering ways to achieve at least symbolic immortality (see e.g. Greenberg et al., 2008, for review). For example, in response to experimentally induced mortality cues individuals showed harsh punishment and even aggression towards those criticizing their worldview or holding incompatible worldviews and values, and increased efforts or intentions to live up to the standards of one’s worldview, even if those standards prescribe risky health behavior, or threat to (physical) life. Such effects have been replicated in approximately 20 different countries with varied means of inducing thoughts of death such as fear of death questionnaires, proximity to a funeral home or cemetery, subliminal death primes and a range of different naturally occurring stimuli.
A distinctive feature of these relatively subtle death reminders is that they tend to produce such effects when accessible, but outside of focal awareness, and do not induce conscious experience of negative affective reactions (e.g. Pyszczynski et al., 1999; Greenberg et al., 2003). This follows from terror management theory’s dual model of defense and is also compatible with the psychoanalytic notion that defense mechanisms operate on a non-conscious level of processing preventing potential affective reactions from being experienced (also cf. the existentialist concept of latent angst: Heidegger, 1977).
Despite the breadth of research into the psychological repercussions of mortality awareness, studies have yet to examine the neurological correlates of this activation. Initial research suggests that neural activation does indeed follow reminders of death when participants confront relevant stimuli. Using EEG, Henry et al. (2010) found increased event-related potentials after MS when participants viewed pictures of in-group, rather than out-group, faces. Notably, however, this study did not assess reactions to reminders of death (but rather, reactions to faces), nor did it utilize imaging techniques. The present study thus aims to investigate neural correlates of mortality threat as compared to non-mortality threat. Because the present study pioneers into neural correlates of mortality threat, we have only tentative hypotheses about specific activation patterns. As we elaborate more fully later, given the centrality of the rostral anterior cingulate cortex (ACC) and the amygdala to social, emotional, and anxiety-related responses (e.g. Krendl et al., 2008), we generally expected to see increased activation of these areas with exposure to death-related ideation. Since these areas have been linked to implicit or background feelings rather than to conscious emotion (cf. Lane, 2008), this would extend previous research showing that such mortality threat typically does not lead to conscious emotional processes. In addition, given effects of mortality salience on stereotypical judgment (e.g. Schimel et al., 1999) and relational attachment (e.g. Mikulincer et al., 2003) as terror management strategies, we were also interested in the possibility of activation in neural structures implicating such processes (e.g. ventral tegmental area and caudate nucleus (CN) in attachment processes, Fisher et al., 2005 and caudate nucleus in automatic thought, Packard and McGaugh, 1992).
METHODS
Participants
Twenty healthy male students (German native speakers only) from Osnabrueck University in Germany voluntarily participated after giving informed consent. Three participants were discarded because of heavy head movements during the scanning session (>1 mm and/or >1°). Thus, the final analysis comprised 17 participants with a mean age of 23.7 years (s.d. = 2.97). Participants reported no neurological or psychiatric problems and met all MRI inclusion criteria requested (no cardiac pacemaker or other electrical body devices, no metallic implants, no vascular operations, no tattoo or piercing above the navel). The protocol described below was reviewed and approved by the local ethics committee.
Experimental design
Participants were scanned in one session consisting of a within-subjects manipulation inducing mortality thoughts and thoughts about dental pain. We chose dental pain as a control condition because of its use in previous studies (Greenberg et al., 2008) contrasting thoughts of death with other types of unpleasant events. These conditions were organized in four mortality blocks (consisting of 3, 4, 5 and 9 trials) and four dental pain blocks (consisting of 3, 5, 5 and 8 trials) alternating in a pseudorandomized order. It should be noted that in behavioral studies mortality salience is typically manipulated in a between-subjects design (see Greenberg et al., 2008). However, our experimental design deviates from this to meet common fMRI standards given the typically large individual variability that exists in these types of data. We return to implications of this design decision in the Discussion section.
To induce thoughts of death, participants responded to 21 items taken from a fear of death scale (Boyar, 1964). In the control condition, participants responded to 21 parallel statements pertaining to dental pain. A typical statement of the mortality condition was I am afraid of a painful death, whereas a typical statement of the dental pain condition was I am getting panicked, when I am sitting in the dentist’s waiting room. This method of inducing thoughts of death has been used in a number of terror management studies that also demonstrate predicted behavioral effects (e.g. Greenberg et al., 1995; Goldenberg et al., 2000; Florian et al., 2001; Arndt et al., 2009; Cox et al., 2009). Stimuli were presented using the stimulus presentation software e-prime 1 (http://www.pstnet.com/).
Before scanning, participants completed a filler questionnaire, and were broadly informed about the experimental procedure and MRI physics. Once in the scanner, participants received two response keys per hand and specific instructions were presented on the screen. Participants were informed that they would be presented with statements that refer to self-descriptions and that they should respond to each statement conscientiously. Specifically, half of the participants were told that the left (right) button should be used for agreeing to the statement and that the right (left) button should be used for disagreeing with the statement.
Each trial lasted 9 s. At the beginning of each trial, a statement was presented for 6 s before it was joined by the response alternatives ‘YES’ and ‘NO’, which appeared beneath the statement until a key press initiated a blank screen for the rest of the trial time. The anatomical scan was applied at the end of the session. Once released from the scanner, participants were fully debriefed.
fMRI data acquisition and analysis
A 3 Tesla Siemens Allegra Head Scanner collected interleaved saggital slices using an echo planar imaging (EPI) pulse sequence (echo time = 30 ms, repetition time = 2000 ms, slice thickness = 3 mm, interslice skip = 3 mm, 80° flip angle, 19.2 × 15.6 cm field of view, 64 × 52 acquisition matrix). T1-weighted scans were acquired using a sagittal magnetization prepared rapid acquisition gradient echo (MPRAGE) sequence (echo time = 4.38 ms, repetition time = 2300 ms, inversion time = 900 ms, slice thickness = 1 mm, 8° flip angle, 25.6 × 25.6 cm field of view, 256 × 256 acquisition matrix).
Analysis followed established protocols using FSL (Smith et al., 2004); preprocessing included motion correction, slice timing, brain extraction, and spatial smoothing (5 mm full-width half maximum). In a general linear model, we modeled six events: mortality-related thoughts with the participant agreeing to the fear of death question (‘yes’) (My), mortality-related thoughts, participant disagreeing (Mn), dental pain-related thoughts inducing explicit fear (Dy), dental pain-related thoughts not inducing explicit fear (Dn), and responses given by the participant (left vs right key press). Head motion parameters were included as confound experimental variables.
The input waveforms were blurred and delayed. Specifically, experimental variables were set up by separate waveforms describing stimulus presentation (mortality vs control), blank slide, or button-press. The input waveforms were defined by triplets, each of which described a short period of time (onset and duration) and the value of the model during that time. Thus, the corresponding basic waveforms were exactly in time with the stimulation applied. These original waveforms were blurred and delayed in an attempt to match the difference between the stimulus waveforms and the output function (measured fMRI haemodynamic response). For this convolution, a double-gamma HRF (haemodynamic response function) was used, i.e. a mixture of two gamma functions (a standard positive function at normal lag, and a small, delayed, inverted gamma function that attempts to model the late undershoot). Pre-whitening was used to increase the validity and efficiency of the statistics. The main contrast of interest was mortality salience (My + Mn) minus dental pain thoughts (Dy + Dn).
For multi-subject analyses, functional images were linearly registered to the individual participant’s structural image and subsequently non-linearly registered to a T1-weighted image in standard space (MNI 152). Group analyses were conducted using FLAME (Woolrich et al., 2004); the higher level design matrix was applied to each of the lower level contrasts, automatically detecting and deemphasizing outliers in the multi-subject statistics (Woolrich, 2008). The Z-statistic threshold Z > 3.6 (P = 0.00016, uncorrected) was used to define contiguous clusters (Worsley, 2001). The resulting Z statistical images were thresholded to show which clusters were significant at P < 0.05. Activated regions were determined using the MNI Structural Atlas and the Harvard–Oxford Cortical and Subcortical Structural Atlases in combination with an anatomical brain atlas (Mai, 2008).
RESULTS AND DISCUSSION
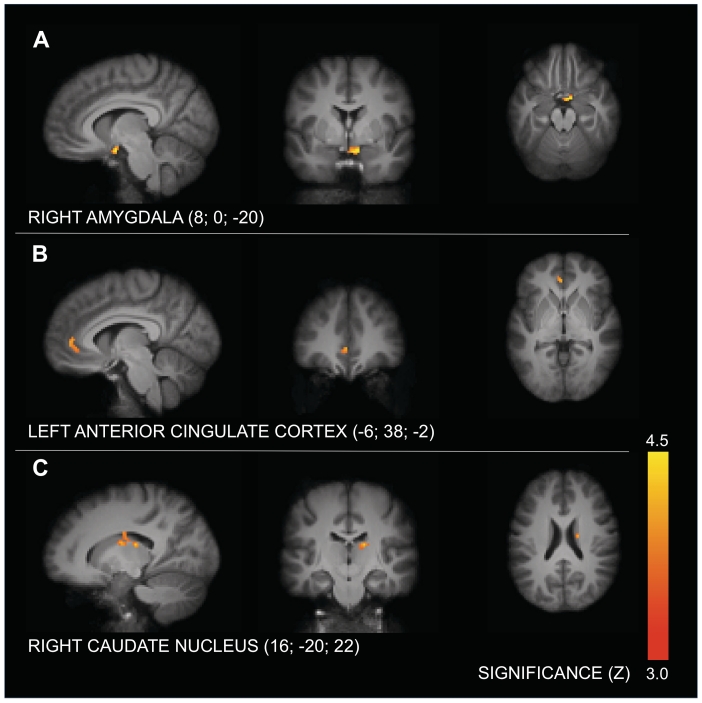
Table 1 and Figure 1 summarize the fMRI findings. Death-related thoughts led to significantly greater activation in comparison to dental pain-related thoughts in the right amygdala (Figure 1a) and in the left rostral ACC (Figure 1b). In addition, we found significantly increased activity in a cluster centering around the tail of the right CN and including the dorsomedial thalamic nucleus (Figure 1c).1 There were no significant activations for the reversed contrast. The ratio between ‘agree’ and ‘disagree’ responses to the fear statements did not significantly differ between the death and pain conditions, t(16) = 0.93, P > 0.36.
Table 1.
Clusters of activation for death greater dental pain reminders
| Anatomical Regiona | Peakb |
Extend | P-levelc | |||
|---|---|---|---|---|---|---|
| Z-score | X | Y | Z | |||
| Right Amygdala | 4.50 | 8 | 0 | −20 | 43 | <0.05 |
| Left ACC | 4.08 | −2 | 38 | −2 | 45 | <0.05 |
| Right CN | 4.06 | 16 | −20 | 16 | 83 | <0.01 |
aBased on MNI Structural Atlas and the Harvard–Oxford Cortical Subcortical Structural Atlases.
bMNI coordinates in millimeter.
cRFT-corrected.
Fig. 1.
Higher activation for mortality versus dental pain reminders. Panel A: right amygdala (P < .05); Panel B: left anterior cingulate cortex (P < .05); Panel C: right cingulate nucleus (P < .01). The results are visualized on the samples' mean structural T1 image.
The present study offers the first investigation of the neural correlates of exposure to thoughts of death. Cognition about mortality as compared to pain produced significantly greater activation in right amygdala, left rostral ACC, as well as right CN. Finding significant activity in limbic system areas is consistent with the notion that mortality threat functions as a potential for anxiety rather than experienced anxiety (Greenberg et al., 2003).
The amygdala is typically involved in detecting and attending to threatening stimuli in general (e.g. Phan et al., 2002) and to socially relevant stimuli such as threatening or foreign faces in particular (e.g. Adolphs et al., 1998), even when presented outside of conscious awareness (e.g. Morris et al., 1998). The rostral ACC plays an important role in anticipating aversive stimuli (e.g. Petrovic et al., 2005; Nitschke et al., 2006) and its activity is associated with levels of anxiety (e.g. Simpson et al., 2001). It has also been implicated in responses to socially oriented threats such as failure and negative evaluation feedback (Pruessner et al., 2008). Consequently, albeit not specifically, amygdala and rostral ACC activity may represent non-conscious, latent markers of threat aroused by mortality salience.
However, a range of studies as well as meta-analytic work indicate that awareness of mortality engenders unique psychological defenses (Burke, Martens, & Faucher, 2010; Greenberg et al., 2008; Hayes et al., 2010). Thus, it is unlikely that activation of theses areas alone mediate effects of mortality threat on such reactions as cultural worldview defense. It is in this light that we observed with particular interest the strong activation in the CN after reminders of death. The CN has been associated with stereotypical, habitual behavior (e.g. Packard and McGaugh, 1992), automatic thought (Schwartz et al., 1996), and more recently, the experience of love (Fisher et al., 2005), rather than threat processing. Although these varied associates of CN render present interpretations speculative, we suggest this may be a generative finding that merits further study. Activation in this region may point to a complimentary clue to understanding the neural processes underlying the ways in which people manage existential fear. Both existential philosophy and terror management theory interpret cultural worldview defense as an unconscious attempt to relieve mortality threat by identifying with attitudes and values of a larger social group (Heidegger, 1977; Pyszczynski et al., 1999) that developmentally come to be associated with existential security. Therefore, finding a region active that is engaged in habitual behaviors may be worth further consideration. Moreover, reminders of death have been found to spontaneously activate romantic attachment cognition (Arndt et al., 2002) and affiliation motivation (Wisman and Koole, 2003; see Mikulincer et al., 2003), which further implicates an interesting role of the CN. Of course, we emphasize that future research is needed to assess these speculations, especially because our within-subjects design thwarted the possibility of validating CN activity with behavioral measures of defensive processes.
Indeed, this is a limitation of the present work that warrants focused study in future research. We exposed each participant to the two conditions of mortality and pain threat, arranging the trials in several blocks. This was done to maximize statistical power in this first study on neural correlates of mortality threat given the well-documented variability that exists across individuals in fMRI data. However, such a design renders it difficult to assess and distinguish different types of psychological defenses (e.g. proximal vs distal defense mechanisms) resulting from mortality salience, as well as the relations between neurological activity and subsequent behavioral manifestations of defense. Moreover, duration of death reminders and distraction from them can have a strong influence on the type of defensive processes expressed (Pyszczynski et al., 1999). Future research may therefore employ alternative designs to enable the investigation of neural correlates of psychological consequences of mortality threat such as cultural worldview defense.
Inferences about the current findings are also limited to the particular control condition used. We drew on thoughts of dental pain to investigate correlates of mortality threat because dental pain refers to physical but not mortality-related threat. A number of prior studies have used this as a control condition and thus it seemed an appropriate starting point for investigating the neural correlates of mortality salience. However, a variety of additional control topics have also been examined in terror management research, and thus future studies including threat conditions other than thoughts of dental pain may prove useful for distinguishing mortality threat from other social threats. Likewise, including a condition unrelated to threat might be useful to determine how neural responses to the threat of death are not just different from other threats but also similar.
Given the nature of the control topic, it is worth considering whether differential affective consequences (e.g. in distress) may be responsible for the effect observed. Although we did not include a separate measure of reported distress, there are two sources of information that inform this possibility. First, participants did not differ in the ratio of agree/disagree responses that they offered to the death and pain items. This offers some indication that overall levels of distress may not have differed between conditions. Second, considerable amounts of prior research have included affective measure after MS manipulations (using various techniques to induce MS including fear of death scales, and including dental pain as a control topic) and generally do not find affective differences. Thus, both the present results and the available literature do not provide support for attributing the neurological differences to general distress. Nonetheless, it will be important to continue to examine this and related issues.
Future research might also explore the extent to which self-referential processes underlie how people respond to mortality awareness, as studies show that threats referring to a high level of self-reference engage anterior cortical midline regions such as the medial PFC or rostral ACC (Northoff et al., 2005; Amodio and Frith, 2006, for reviews). Thus, activity in rostral ACC or even in amygdala may reflect, in part, higher levels of self-reference for mortality than for dental pain threat.
We hope that these issues illustrate the generative capacity of the present research. The present study pioneers the intersection between a well established body of work on terror management and the emerging insights affordable through neuroimaging techniques. Taken together, the present study thus furthers the invitation to explore the underlying neural sensitivity to processes involved with psychological defense against existential fear.
Acknowledgments
This research was facilitated by the University of Osnabrueck (Post Doc Award 2007). The authors would like to thank André Kerber and Melanie Löbe for their efforts concerning recruitment and scanning, as well as Peter Erhardt for discussing the fMRI design and analyses. We also thank Elliot Berkman for helpful advice on data analyses.
Footnotes
1Additional analyses correlating activity in these clusters with fear of death versus dental pain scores (i.e. the sum of responses indicating fear of death versus dental pain, respectively) were not significant, all p’s > 0.26. This is consistent with previous research suggesting that people do not necessarily have conscious access to fears of mortality (e.g. Pyszczynksi et al., 1999)
REFERENCES
- Adolphs R, Tranel D, Damasio AR. The human amygdala in social judgment. Nature. 1998;393:470–4. doi: 10.1038/30982. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7(4):268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Arndt J, Greenberg J, Cook A. Mortality salience and the spreading activation of worldview-relevant constructs: exploring the cognitive architecture of terror management. Journal of Experimental Psychology: General. 2002;131:307–24. doi: 10.1037//0096-3445.131.3.307. [DOI] [PubMed] [Google Scholar]
- Arndt J, Vess M, Cox CR, Goldenberg JL, Lagle S. The psychosocial effect of thoughts of personal mortality on cardiac risk assessment by medical students. Medical Decision Making. 2009;29:175–81. doi: 10.1177/0272989X08323300. [DOI] [PubMed] [Google Scholar]
- Boyar JI. The construction and partial validation of a scale for the measurement of the fear of death. Dissertation Abstracts International. 1964;25:20–1. [Google Scholar]
- Burke BL, Martens A, Faucher EH. Two decades of terror management theory: a meta-analysis of mortality salience research. Personality and Social Psychology Review. 2010;14:155–95. doi: 10.1177/1088868309352321. [DOI] [PubMed] [Google Scholar]
- Cox CR, Cooper DP, Vess M, Arndt J, Goldenberg JL, Routledge C. Bronze is beautiful but pale can be pretty: the effects of appearance standards and mortality salience on sun-tanning outcomes. Health Psychology. 2009;28:746–52. doi: 10.1037/a0016388. [DOI] [PubMed] [Google Scholar]
- Fisher H, Aron A, Brown LL. Romantic love: an fMRI study of a neural mechanism for mate choice. Journal of Comparative Neurology. 2005;493:58–62. doi: 10.1002/cne.20772. [DOI] [PubMed] [Google Scholar]
- Florian V, Mikulincer M, Hirschberger G. Validation of personal identity as a terror management mechanism – evidence that sex-role identity moderates mortality salience effects. Personality and Social Psychology Bulletin. 2001;8:1011–22. [Google Scholar]
- Goldenberg JL, McCoy SK, Pyszczynksi T, Greenberg J, Solomon S. The body as a source of self-esteem: the effects of mortality salience on identification with one's body, interest in sex, and appearance monitoring. Journal of Personality and Social Psychology. 2000;79:118–30. doi: 10.1037//0022-3514.79.1.118. [DOI] [PubMed] [Google Scholar]
- Greenberg J, Pyszczynski T, Solomon S. The causes and consequences of a need for self-esteem: a terror management theory. In: Baumeister RF, editor. Public Self and Private Self. New York: Springer; 1986. pp. 189–212. [Google Scholar]
- Greenberg J, Martens A, Jonas E, Eisenstadt D, Pyszczynski T, Solomon S. Psychological defense in anticipation of anxiety: eliminating the potential for anxiety eliminates the effect of mortality salience on worldview defense. Psychological Science. 2003;14(5):516–9. doi: 10.1111/1467-9280.03454. [DOI] [PubMed] [Google Scholar]
- Greenberg J, Simon L, Harmon-Jones E, Solomon S, Pyszczynski T, Chatel D. Testing alternative explanations for mortality effects: terror management, value accessibility, or worrisome thoughts? European Journal of Social Psychology. 1995;12:417–33. [Google Scholar]
- Greenberg J, Solomon S, Arndt J. A basic but uniquely human motivation: terror management. In: Shah JY, Gardner WL, editors. Handbook of Motivation Science. New York: Guilford Press; 2008. pp. 114–34. [Google Scholar]
- Hayes J, Schimel J, Arndt J, Faucher EH. A theoretical and empirical review of the death-thought accessibility concept in terror management research. Psychological Bulletin. 2010;136:699–739. doi: 10.1037/a0020524. [DOI] [PubMed] [Google Scholar]
- Heidegger M. Sein und Zeit. In: von Hermann F-W, editor. Heidegger's Gesamtausgabe 2. Stuttgart: Vittorio Klostermann; 1977. p. 586. [Google Scholar]
- Henry EA, Bartholow BD, Arndt J. Death on the brain: effects of mortality salience on the neural correlates of race bias. Social Cognitive and Affective Neuroscience. 2010;5:77–87. doi: 10.1093/scan/nsp041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krendl AC, Richeson JA, Kelley WM, Heatherton TF. The negative consequences of threat: an fMRI investigation of the neural mechanisms underlying women's underperformance in math. Psychological Science. 2008;19(2):168–75. doi: 10.1111/j.1467-9280.2008.02063.x. [DOI] [PubMed] [Google Scholar]
- Lane RD. Neural substrates of implicit and explicit emotional processes: a unifying framework for psychosomatic medicine. Psychosomatic Medicine. 2008;70(2):214–31. doi: 10.1097/PSY.0b013e3181647e44. [DOI] [PubMed] [Google Scholar]
- Mai JK, Paxinos G, Voss T. Atlas of the Human Brain. Amsterdam, The Netherlands: Elsevier Academic Press; 2008. [Google Scholar]
- Mikulincer M, Florian V, Hirschberger G. The existential function of close relationships. Introducing death into the science of love. Personality and Social Psychology Review. 2003;7:20–40. doi: 10.1207/S15327957PSPR0701_2. [DOI] [PubMed] [Google Scholar]
- Morris JS, Öhman A, Dolan RJ. Conscious and unconscious emotional learning in the human amygdala. Nature. 1998;393:467–70. doi: 10.1038/30976. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Sarinopoulos I, Mackiewicz KL, Schaefer HS, Davidson RJ. Functional neuroanatomy of aversion and its anticipation. NeuroImage. 2006;29:106–16. doi: 10.1016/j.neuroimage.2005.06.068. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain–a meta-analysis of imaging studies on the self. Neuroimage. 2005;31:440–57. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Packard MG, McGaugh JL. Double dissociation of fornix and caudate nucleus lesions on acquisition of two water maze tasks: further evidence for multiple memory systems. Behavioral Neuroscience. 1992;106:439–46. doi: 10.1037//0735-7044.106.3.439. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Dietrich T, Fransson P, Andersson J, Carlsson K, Ingvar M. Placebo in emotional processing - induced expectations of anxiety relief activate a generalized modulatory Network. Neuron. 2005;46:957–69. doi: 10.1016/j.neuron.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. NeuroImage. 2002;16(2):331–48. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Dedovic K, Khalili-Mahani N, et al. Deactivation of the limbic system during acute psychosocial stress: evidence from positron emission tomography and functional magnetic resonance imaging studies. Biological Psychiatry. 2008;63(2):234–40. doi: 10.1016/j.biopsych.2007.04.041. [DOI] [PubMed] [Google Scholar]
- Pyszczynski T, Greenberg J, Solomon S. A dual-process model of defense against conscious and unconscious death-related thoughts: an extension of terror management theory. Psychological Review. 1999;106(4):835–45. doi: 10.1037/0033-295x.106.4.835. [DOI] [PubMed] [Google Scholar]
- Schimel J, Simon L, Greenberg J, et al. Support for a functional perspective on stereotypes: evidence that mortality salience enhances stereotypic thinking and preferences. Journal of Personality and Social Psychology. 1999;77:905–26. doi: 10.1037//0022-3514.77.5.905. [DOI] [PubMed] [Google Scholar]
- Schwartz JM, Stoessel PW, Baxter LR, Martin KM, Phelps ME. Systematic changes in cerebral glycose metabolic rate after successful behavior modification treatment of obsessive-compulsive disorder. Archives of General Psychiatry. 1996;53:109–13. doi: 10.1001/archpsyc.1996.01830020023004. [DOI] [PubMed] [Google Scholar]
- Simpson JR, Jr, Drevets WC, Snyder AZ, Gusnard DA, Raichle ME. Emotion induced changes in human medial prefrontal cortex: II. During anticipatory anxiety. Proceedings of National Academy of Science. 2001;98:688–93. doi: 10.1073/pnas.98.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(S1):208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Wisman A, Koole SL. Hiding in the crowd: can mortality salience promote affiliation with others who oppose one's worldviews? Journal of Personality and Social Psychology. 2003;84(3):511–26. [PubMed] [Google Scholar]
- Woolrich M. Robust group analysis using outlier inference. Neuroimage. 2008;41(2):286–301. doi: 10.1016/j.neuroimage.2008.02.042. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TEJ, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for fMRI group analysis using bayesian inference. Neuroimage. 2004;21(4):1732–47. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Worsley KJ. Statistical analysis of activation images. In: Jezzard P, Matthews PM, Smith SM, editors. Functional MRI: An Introduction to Methods. 1st edn. Oxford: Oxford University Press; 2001. pp. 251–70. [Google Scholar]