Abstract
Objectives
We sought to measure the strength of association between two indices of obesity, waist hip ratio (WHR) and body mass index (BMI), with left ventricular (LV) dysfunction and mortality in a community cohort.
Background
The distribution of body fat is thought to affect cardiovascular disease risk. The association of BMI (an index of overall obesity) and WHR (an index of central obesity) with LV systolic and diastolic dysfunction in a population-based cohort is unknown.
Methods Results
Anthropomorphic measurements and echocardiographic LV function were measured in a cross-sectional population-based sample of 2042 men and women >45 years old in the Olmsted County Heart Function Study. Five year prospective mortality was measured.
Results
Increased WHR had a stronger association than BMI with: 1) lower LV EF (r= -0.24, p<0.0001 versus r= -0.04, p=0.09), and 2) LV diastolic dysfunction (r = 0.18, p<0.0001 versus r = 0.05, p = 0.02). After adjustment for standard cardiovascular risk factors WHR continued to be significantly associated with diastolic dysfunction, but not with systolic dysfunction. WHR, but not BMI, was strongly predictive of all cause mortality independent of age and sex (HR 23.6, CI 4.0, 139.8; p = 0.0005). This relationship was attenuated on adjustment for diastolic dysfunction.
Conclusions
WHR is a stronger correlate of LV dysfunction and mortality than BMI. These cross-sectional data suggest that the increased risk of mortality from central obesity is mediated at least in part by LV dysfunction, especially diastolic dysfunction.
Keywords: Heart Failure, Epidemiology, Risk Factors, Obesity
INTRODUCTION
Concern has been raised about the growing prevalence of obesity in the US and its potential future impact on longevity.1 Overall obesity, as measured by body mass index (BMI), is an independent risk factor for cardiovascular disease and increased mortality.2-6 Recently, particular attention has been given to patterns of body fat distribution and their comparative impact on cardiovascular disease risk. Central obesity, commonly measured by waist hip ratio (WHR), is increasingly recognized as a more powerful predictor of obesity-related cardiovascular risk factors and death than is overall obesity.3-18, 42
Important unresolved issues in the relationship between obesity, cardiovascular disease and death include the form of obesity (overall or central) most associated with left ventricular (LV) dysfunction, and the form of LV dysfunction (systolic or diastolic) most associated with obesity. Although WHR and LV dysfunction have separately been shown to be predictive of mortality, studies evaluating the relationship between WHR, LV systolic and diastolic function and mortality are lacking. A better understanding of the link between obesity and ventricular dysfunction may help elucidate mechanisms through which obesity contributes to the risk of cardiovascular events and mortality.19
In this report from the Olmsted County Heart Function Study we examined cross-sectional population-based community cohort data to determine the association between obesity indices and echocardiographically determined LV systolic and diastolic function. In addition, we analyzed the relationship between WHR, LV dysfunction and five year all-cause mortality.
METHODS
The Mayo Foundation and Olmsted Medical Center Institutional Review Boards approved this study and all subjects gave written informed consent.
Study setting
. In 1990, 96% of the 106,400 residents of Olmsted County were white. Other characteristics of this population and its unique resources for population based epidemiology research have been previously described.20,21
Population sampling and data collection
Residents of Olmsted County were enumerated using methods previously validated in the Rochester Epidemiology Project.21 A random sample of residents, ≥ 45 years of age on January 1, 1997 was identified. A sampling fraction of 7% was applied within each gender and age (five years) specific stratum. Of the 4203 subjects invited, 2,042 (49%) participated. Analysis of medical records from 500 randomly selected nonparticipants demonstrated that participation rates were not different according to past history of coronary disease, heart failure or other cardiovascular disease, indicating little effect of participation bias.22
Enrollment took place from January 1, 1997 to September 30, 2000. Each subject completed a self-administered questionnaire. Medical records were abstracted by trained nurse abstractors: hypertension, diabetes mellitus, coronary artery disease, cerebrovascular disease, congestive heart failure, myocardial infarction, peripheral vascular disease were diagnosed if recognized criteria were met and if documented by a physician.23-27
Anthropometric measurements
Measurements were carried out by a trained research nurse with the subjects in the standing position. Body mass index (BMI) was measured as weight/height2. Body circumferences were measured in centimeters: for waist at the top of umbilicus, for hip at the greatest diameter and for neck at the lower half of the neck.
Echocardiographic analysis
All subjects underwent echocardiography, performed by one of three registered diagnostic cardiac sonographers, using the same echocardiography machine (HP-2500), and following a standardized protocol.23,24 A single echocardiographer (M.M.R.) interpreted all echocardiograms without knowledge of the subjects’ clinical information.
Echocardiographic methods have been previously described and validated.23,24 Ejection Fraction (EF) was measured by M-mode, Biplane Simpson's 2-D, and 2-D visual estimate. As EFs from these three methods were very highly correlated, and as the visual estimate was available in >99% of participants, visual estimate was used for analysis.24 Systolic dysfunction was defined as EF <50%.23
As previously described and validated, left ventricular diastolic dysfunction (LVDD) was assessed by pulsed-wave Doppler examination of mitral flow (before and during Valsalva maneuver) and pulmonary venous inflow, as well as by Doppler tissue imaging of the mitral annulus.23,28,29 LVDD was graded on a 4-point ordinal scale: normal; mild DD = abnormal relaxation without increased LV end-diastolic filling pressure (decreased E/A ratio <0.75); moderate or “pseudonormal” DD = abnormal relaxation with increased LV end-diastolic filling pressure (E/A 0.75 to 1.5, deceleration time >140 ms, and 2 other Doppler indices of elevated LV end-diastolic filling pressure); or severe DD = advanced reduction in compliance with restrictive filling (E/A ratio of >1.5, deceleration time <140 ms, and Doppler indices of elevated LV end-diastolic filling pressure). For subjects in atrial fibrillation, diastolic function was classified as indeterminate unless restrictive physiology (E/A >1.5, deceleration time <140 ms) was present. M-mode tracings were measured according to American Society of Echocardiography convention, and LV mass calculated as previously described.29
Mortality data
Mortality data in the Rochester Epidemiology Project are ascertained by review of medical records, death certificates, and obituary notices. Participants were followed until death or November 1 2004, at which time they were censored. This provided 11210 person-years of follow-up (median follow-up 5.5 years) with 128 deaths. Active surveillance by recruitment for follow-up examination in the first 974 (41%) participants identified no deaths additional to those identified by the above mechanisms.
Statistical analysis
Clinical characteristics of subjects were compared using the chi square test for categorical variables and t-test or ANOVA for continuous variables. Due to non-normal bivariate distributions, Spearman's correlation coefficient was used. Spearman's correlation coefficient, and parameter estimate (β) from linear regression were employed to evaluate the strength of association between measures of obesity and LV function. DD was collapsed from six ordinal levels to a dichotomous variable of DD (Present/Absent) for ‘any degree of DD’ used in logistic regression analyses.
A Cox proportional hazards model was used to estimate the association between WHR and all-cause mortality. This association was adjusted separately for confounding variables (age and sex, for potentially intervening variables (LV mass, EF and DD) based on the biologically plausible assumption that if obesity is associated with increased mortality, this would be mediated via LV dysfunction. A decrease in HR (HR) of >20% was considered evidence of significant confounding or intervening effect of the newly introduced variable in the model. Analyses were performed on JMP version 5 and SAS version 8 (SAS Institute, Cary, NC).
RESULTS
Clinical and echocardiographic characteristics of study participants
Men and women had a similar mean age, and similar prevalences of diabetes and hypertension. Men were more likely to have coronary artery disease, heart failure, and systolic dysfunction. (Table I)
Table I.
Characteristics of participants
| All | Men | Women | |
|---|---|---|---|
| N | 2034 | 982 | 1052 |
| Mean Age (yrs) (Range 45,96) | 62 | 62 | 63 |
| Diabetes (%) | 7.5 | 9.6 | 5.6 |
| Hypertension (%) | 30 | 29 | 30 |
| Current smokers (%) | 9 | 10 | 7 |
| Past smokers (%) | 41 | 53 | 31 |
| Never smokers (%)* | 50 | 37 | 62 |
| Congestive heart failure (%)† | 2.5 | 3.3 | 1.8 |
| Coronary Artery Disease (%)* | 12 | 18 | 7 |
| Mean EF (%)* | 63 | 61 | 65 |
| Any Grade Diastolic Dysfunction (%) | 28 | 30 | 27 |
p<0.0001, men vs. women
p<0.05, men vs. women
Out of a total of 2042 subjects, seven were excluded due to indeterminate MI and one due to error in BMI.
The distribution of LV DD categories was similar in women and men: approximately 70% normal diastolic function, 20% mild, 7% moderate and 1% severe LVDD.
Anthropometric Characteristics
Men had greater weight, BMI, waist/neck circumference and WHR than women with much higher prevalence of central obesity (WHR criteria). (Table II)
Table II.
Anthropometric measures of obesity
| All | Men | Women | |
|---|---|---|---|
| BMI† | 28.4±5.3 | 28.9±4.6 | 27.9±5.8 |
| Weight (Kg)* | 80.0±18.0 | 89.3±16 | 72.8±16 |
| Waist Circumference (cm)* | 91.7±14.5 | 99±11.6 | 85±13.4 |
| Waist Hip Ratio* | 0.88±0.1 | 0.95±0.1 | 0.81±0.1 |
| Neck Circumference (cm)* | 36.7±4.2 | 39.7±3.1 | 33.9±2.9 |
| Overall Obesity (BMI>30) (%)† | 32 | 33 | 31 |
| Severe Overall Obesity (BMI>40);(%) | 3 | 2.5 | 4 |
| Central Obesity (WHO criteria)(%)*§ | 55 | 83.9 | 27.1 |
p<0.0001 men vs. women
p<0.01 men vs. women‡
‡ Overall obesity was defined as BMI (weight/height2) ≥30.
Central obesity was defined as WHR >0.9 in men and >0.85 in women (WHO criteria).
Continuous variables are presented as mean ± SD.
Among persons with established cardiovascular disease (heart failure, coronary artery disease, myocardial infarction, stroke, peripheral vascular disease, dilated cardiomyopathy), 78% were centrally obese (WHR >0.9 for men and >0.85 for women) whereas only 30% had overall obesity (BMI>30).
Overall obesity and LV function
BMI was not significantly associated with EF in the whole cohort (Table IIIa), nor was it associated in any stratum defined by age, sex, or hypertension. BMI had a weak positive correlation with the severity of DD (Table IIIb). On stratification for age, gender and hypertension, BMI continued to have an association with DD only in women (r=0.1; p = 0.001) and in persons <65 years of age (r=0.14; p = <0.0001).
Table IIIa.
Association of anthropometric measures with left ventricular systolic function*
| EF (r =) | p-value | Parameter estimate (β=) | p-value | |
|---|---|---|---|---|
| Overall Obesity | ||||
| BMI | -0.04 | 0.09 | -0.04 | 0.15 |
| Central Obesity | ||||
| Waist Hip Ratio | -0.24 | <0.0001 | -16.67 | <0.0001 |
| Waist Circumference | -0.18 | <0.0001 | -0.08 | <0.0001 |
| Neck Circumference | -0.25 | <0.0001 | -0.38 | <0.0001 |
In addition to the analysis using visual estimate EF these analyses were also run using EF by Teicholz and M-mode and there was no noticeable difference in the results.
Table IIIb.
Association of anthropometric measures with left ventricular diastolic function
| Grade of LVDD (r =) | p-value | Odds Ratio for abnormal diastolic function for 1 unit increase in BMI, WHR, waist or neck circumference | p-value | Odds Ratio for any DD with 1 SD increase in obesity measure* | |
|---|---|---|---|---|---|
| Overall Obesity | |||||
| BMI | 0.05 | 0.02 | 1.023 (1.00, 1.04) | 0.02 | 1.13 (1.10, 1.22) |
| Central Obesity | |||||
| Waist Hip Ratio | 0.18 | <0.0001 | 81.09 (27.35, 244.98) | <0.0001 | 1.55 (1.39, 1.73) |
| Waist Circumference | 0.13 | <0.0001 | 1.02 (1.01, 1.03) | <0.0001 | 1.36 (1.16, 1.54) |
| Neck Circumference | 0.08 | <0.0001 | 1.03 (1.01, 1.05) | <0.002 | 1.13 (1.04, 1.23) |
1SD equals: for BMI=5 units, for WHR=0.1 unit, for WC=14.5 units, for NC=4.2 units
Central obesity and LV function
WHR was more strongly correlated to EF (Table IIIa). This negative relationship between WHR and EF became insignificant when stratified for gender; men contribute more heavily to the lower end of EF spectrum and women to the higher end. This confounding rendered further analysis of the relationship between WHR and EF meaningless.
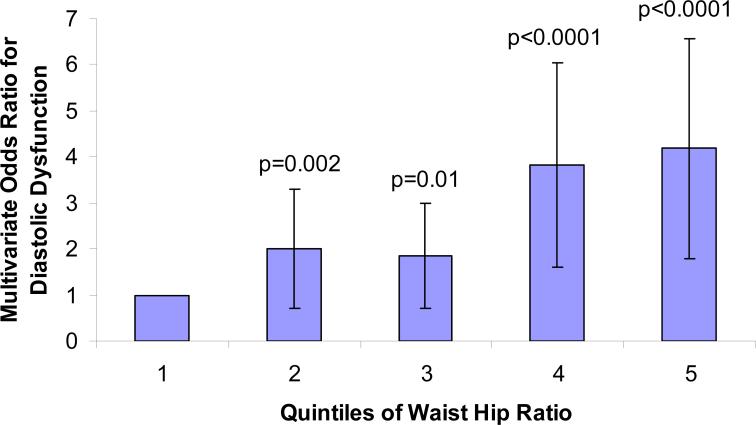
WHR was more highly correlated with the severity of DD than was BMI (Table IIIb). In a multiple logistic regression analysis WHR continued to be strongly associated with DD even after addition of coronary risk factors (age, sex, smoking, diabetes, hypertension), coronary artery disease and systolic dysfunction in the model. Figure 1 depicts a model of multivariable odds ratios (OR) for LVDD plotted against quintiles of WHR, and shows a strong positive relationship (p<0.001 for each OR comparison) between WHR and the odds of having LVDD.
Figure 1.
Multivariable odds of any degree of diastolic function as a function of quintiles of waist hip ratio adjusted for age, sex, diabetes, hypertension and smoking. Second, third, fourth and fifth quintiles of WHR compared against the first quintile as referent (p<0.001 for each comparison).
In addition to the primary observation of the relationship between WHR and DD, it was noted that WHR was consistently more closely associated with other measures of cardiac structure than was BMI: LV end diastolic dimension (r=0.36 for WHR; p<0.0001 vs. r=0.27 for BMI; p<0.0001), LV Mass (r=0.54 for WHR; p<0.0001 vs. r=0.42 for BMI; p<0.0001) and left atrial volume index (r=0.09;p<0.0001 for WHR vs. r=0.04; p=0.05 for BMI).
Since LVDD is highly associated with LV mass we examined LV mass in different forms of obesity. Average LV mass in centrally obese subjects was 120 g/m compared to 112 g/m in overall obese subjects (p<0.0001). Average LV mass increased with worsening systolic and diastolic function (data not shown).
Clinical Heart Failure: Relationship to Forms of Obesity and Ventricular Dysfunction
The odds of having clinical heart failure were more than two fold higher in subjects with central obesity (OR 2.7; CI 1.4,5.4; p=0.003) compared to subjects with overall obesity (OR 1.3; CI 0.7,2.3; p=0.4) in the same logistic regression model, before and after adjustment for sex. After adding DD and systolic dysfunction into this model, WHR was no longer associated with clinical heart failure (OR 1.7; CI 0.6, 5.4). In this multivariable logistic regression model adjusted for WHR, both DD (OR 28.4; CI 5.6, 518.0; p=0.001) and systolic dysfunction (OR 16.6; CI 7.2, 41.3; p<0.0001) were associated with clinical heart failure, suggesting that these are intervening variables between central obesity and clinical heart failure. Adjustment for age and sex did not meaningfully alter these results. All-cause mortality among heart failure patients was much greater than expected, and was similarly increased in those with systolic dysfunction and in those with LVDD. This finding was consistent through the WHR quintiles (data not shown).
Mortality Analysis
After a median follow-up of 5.5 years there were 128 deaths. In the whole cohort BMI was not associated with increased all cause mortality (HR 0.91; CI 0.62-1.31). In contrast, Cox proportional hazard analysis for WHR as a predictor of all cause mortality showed that WHR was associated with a HR of 23.6 (p = 0.0005) for all-cause mortality in the unadjusted model 1. (Table IV) There was no confounding by age or sex (Models 2 and 3). However, adjustment for EF, DD, LV mass, coronary artery disease or congestive heart failure markedly attenuated the effect of WHR on mortality, suggesting that the impact of WHR on mortality may have been substantially mediated through these intervening variables (Models 4-6, 8 and 9). This was further borne out in model 7, which adjusted for both confounding (age, sex) and intervening (EF, LVDD) variables. In model 7, only age, systolic dysfunction and DD continued to be significant independent predictors of mortality.
Table IV.
Cox proportional hazard analysis of all cause mortality as a function of waist hip ratio and potential confounding variables (age, sex) and potential pathophysiologic intervening variables (LVDD and EF)
| Hazard Ratio | P-value | |
|---|---|---|
| Model 1, unadjusted | ||
| WHR | 23.59 (3.96,139.77) | 0.0005 |
| Models 2-3, adjusting for confounding varaibles | ||
| Model 2, adjusted for age | ||
| WHR | 14.7 (2.08,104.58) | 0.007 |
| Model 3, adjusted for sex | ||
| WHR | 23.82 (2.06,270.43) | 0.001 |
| Models 4-6, adjusting for intervening variables | ||
| Model 4, adjusted for EF | ||
| WHR | 5.01 (0.77,32.92) | 0.09 |
| Model 5, adjusted for LVDD | ||
| WHR | 2.32 (0.25,21.99) | 0.46 |
| Model 6, adjusted for LV mass | ||
| WHR | 0.77 (0.05,11.10) | 0.85 |
| Model 7, adjusted for confounding and intervening variables | ||
| WHR | 0.41 (0.02,11.39) | 0.60 |
| Age | 1.09 (1.06,1.11) | <0.0001 |
| Female sex | 0.78 (0.43,1.41) | 0.40 |
| EF | 0.96 (0.94,,0.97) | <0.0001 |
| LVDD (mild) | 2.87 (1.59,5.13) | 0.0004 |
| Moderate to severe LVDD | 3.36 (1.88,1.72) | 0.0004 |
| Model 8, adjusted for coronary artery disease | ||
| WHR | 5.8 (0.89, 37.74 | 0.06 |
| Model 9, adjusted for congestive heart failure | ||
| WHR | 9.59 (1.56,60.16) | 0.01 |
DISCUSSION
In these cross-sectional data from our population-based community study of adults over age 45 years, central obesity measured by WHR had a stronger correlation with LV systolic and LVDD than did overall obesity measured by BMI. WHR was also more closely associated with the diagnosis of clinical heart failure. Baseline WHR, but not BMI, was predictive of subsequent all cause mortality during follow-up.
Obesity and Cardiovascular Disease
The demonstration of association of obesity with cardiovascular morbidity and mortality started with the definition of obesity based on Metropolotan Weight indices,4 was followed by definitions based on BMI2,5,7,8 and finally has been followed by definitions based on the location of adipose tissue. 15,31-37 This population based study demonstrates much greater prevalence of central obesity than overall obesity (78% vs. 30%) in patients with established cardiovascular disease, highlighting the closer association of central obesity with cardiac disease.
Association of Overall Obesity (BMI) with LV Structure and Function
Prior studies of BMI and LV function include an evaluation of the relationship between overall obesity and ventricular function in a small group of apparently healthy obese young women, which suggested that increasing BMI was associated with higher EF and worse diastolic function.10 In our cohort, the association between BMI and LVDD is confirmed and extended from young obese women(25-30 years)10 to older women (age >45) as well as to middle aged (age 45 to 65 years) community dwelling adults. By contrast, the lack of relationship between BMI and EF may reflect differences in our older population, in whom persons with established cardiovascular disease were included.
Analysis of a large Framingham cohort without cardiovascular disease did not assess diastolic function but established that BMI was an independent predictor of LV mass.38 Our finding of a correlation between BMI and diastolic function, though weak, confirms the relationship between increased LV mass and extends it to include its functional correlate, LVDD.
Association of Central Obesity (WHR) with LV Structure and Function
Prior studies have shown the association of central obesity with coronary heart disease,3 hypertension and diabetes.14,15,19,39 Our study builds on these previous investigations by adding information on cardiac function. It demonstrates that increased central obesity, whether measured by neck or waist circumference or WHR, correlates more powerfully with ventricular dysfunction than do measures of overall obesity such as BMI. The neck correlates with waist (r=0.8; p<0.0001) in our population and may be easier to measure. In addition, WHR has a stronger association with LV function then waist circumference (Table III), indicating that it may be a better measure of obesity. For each standard deviation increase in WHR the odds of LVDD increased 1.55 times. In contrast, for each standard deviation increase in BMI, the odds of LVDD increased 1.13 times. Furthermore, the relationship between WHR and LVDD was not confounded by age, gender or hypertension, indicating a true association independent of these important confounders.
Clinical Heart Failure
Positive associations among BMI, increased left ventricular mass and left ventricular enlargement have been reported by others.38,40,41 In our study BMI and WHR were more strongly associated with LVDD than with LV systolic dysfunction, a finding not reported in other population-based studies. Furthermore, WHR was more strongly associated with LVDD than was BMI, and only WHR was associated with clinical heart failure. A recent study has shown central obesity (WHR) to be a stronger predictor of incident heart failure than overall obesity (BMI). However this study did not include echocardiographic characterization of LV function.42 The strong association between heart failure and LVDD may be related to the higher LV mass in centrally obese persons.38 The effect of WHR was mediated similarly via LVDD and systolic dysfunction, underscoring the importance of LVDD as a factor in heart failure in the community.
In this study, the data show a consistently stronger association of WHR, as compared to BMI, with echocardiographic indices of LV dysfunction (including LV LVDD, left atrial size, LV mass and left ventricular end diastolic dimension), myocardial infarction, stroke, and peripheral vascular disease, adding further credence to the concept that central obesity may be a stronger independent risk factor for diastolic dysfunction and congestive heart failure than overall obesity.9-13, 31, 33
Mortality
Analyses from Framingham have implicated central obesity as a risk factor for mortality.3 Due to the narrow range of WHR measurement units a meaningful effect of WHR on mortality is indicated by a change of 0.1 unit (one standard deviation) in the WHR, instead of 1 unit change. In this study (Table IV, model 1) all cause mortality risk increases 23.59 fold with an increase of 1.0 unit of WHR. An increment of 1.0 unit of WHR is unlikely to occur. A more meaningful expression of this relationship is that mortality risk increases 1.37 fold (p=-0.0005) for each 0.1 unit increase in WHR. Our findings confirm those from Framingham and indicate that WHR is a powerful risk factor for all cause mortality. Furthermore, our analysis demonstrates that after adjustment for EF and diastolic function, WHR was no longer a risk factor for mortality. This suggests that ventricular dysfunction may be an important intervening variable between WHR and mortality. This is further supported by Models 8 and 9 (Table IV), in which coronary artery disease (a cause of systolic and LVDD) and congestive heart failure (the final outcome of ventricular dysfunction) seem to be intervening variables between WHR and mortality.
The impact of LV mass on the relationship between WHR and increased mortality done was as strong as that of systolic and diastolic function combined (Table IV, Models 6 and 7); in either model the mortality HR drops from 23.55 fold to less than 1.0 after adjustment. The current study extends previous findings of LV mass as an independent predictor of mortality; persons with systolic or LVDD have an increased LV mass.
In contrast to the Framingham Study, BMI was not associated with increased mortality risk in this cohort of 2042 persons with 5.5 years follow-up. This difference may be a function of our smaller cohort size, shorter duration of follow-up, as well as stronger correlation of WHR with ventricular dysfunction and mortality than BMI.
Strengths and Limitations
The strengths of this study include a large population-based sample with standardized anthropomorphic and echocardiographic measurements of both systolic and diastolic function. The observational design limits the ability to identify confounders. The cross-sectional nature of the correlation of central obesity with ventricular dysfunction limits the ability to make cause-effect inferences. A potential limitation is the lack of ethnic diversity, and these findings may not apply to nonwhite populations. Anthropomorphic measurements are an imperfect approximation of percent body fat, but are clinically applicable and widely reported in population studies
CONCLUSION
WHR, a measure of central obesity, is more strongly associated with left ventricular systolic and diastolic dysfunction than is BMI, a measure of overall obesity. WHR was more strongly associated with clinical heart failure than was BMI, and this relationship was mediated, in significant part, through ventricular dysfunction. Increased WHR was also a more powerful predictor of all cause mortality than was BMI. The relationship between WHR and increased mortality disappears after adjusting for LVDD, supporting the concept that LVDD could be in the causal pathway between central obesity and increased mortality.
Acknowledgments
Grant Support: Supported by grants from the Public Health Service (NIH HL555902 to Dr. Rodeheffer, NIH AR30582 to Dr. Jacobsen, NIH HL63281 to Dr. Redfield), the Miami Heart Research Institute, the Marriott Foundation, the OMC Foundation and the Mayo Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None
REFERENCES
- 1.Preston SH. Deadweight? – The influence of obesity on longevity. N Engl J Med. 2005;352:1135–37. doi: 10.1056/NEJMe058009. [DOI] [PubMed] [Google Scholar]
- 2.Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–13. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 3.Kannel WB, Cupples LA, Ramaswami R, et al. Regional obesity and risk of cardiovascular disease; the Framingham Study. J Clin Epidemiol. 1991;44:183–90. doi: 10.1016/0895-4356(91)90265-b. [DOI] [PubMed] [Google Scholar]
- 4.Hubert HB, Feinleib M, McNamara PM, et al. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67:968–77. doi: 10.1161/01.cir.67.5.968. [DOI] [PubMed] [Google Scholar]
- 5.Rimm EB, Stampfer MJ, Giovannucci E, et al. Body size and fat distribution as predictors of coronary heart disease among middle-aged and older US men. Am J Epidemiol. 1995;141:1117–27. doi: 10.1093/oxfordjournals.aje.a117385. [DOI] [PubMed] [Google Scholar]
- 6.Mora S, Yanek LR, Moy TF, et al. Interaction of body mass index and Framingham risk score in predicting incident coronary disease in families. Circulation. 2005;111:1871–76. doi: 10.1161/01.CIR.0000161956.75255.7B. [DOI] [PubMed] [Google Scholar]
- 7.Wilson PWF, D'Agostino RB, Sullivan L, et al. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med. 2002;162:1867–72. doi: 10.1001/archinte.162.16.1867. [DOI] [PubMed] [Google Scholar]
- 8.Must A, Spadano J, Coakley EH, et al. The disease burden associated with overweight and obesity. JAMA. 1999;282:1523–29. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 9.Berkalp B, Cesur V, Corapcioglu D, et al. Obesity and left ventricular diastolic dysfunction. Int J Cardiol. 1995;52:23–6. doi: 10.1016/0167-5273(95)02431-u. [DOI] [PubMed] [Google Scholar]
- 10.Pascual M, Pascual DA, Soria F, et al. Effects of isolated obesity on systolic and diastolic left ventricular function. Heart. 2003;89:1152–6. doi: 10.1136/heart.89.10.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alpert MA. Obesity cardiomyopathy: pathophysiology and evolution of the clinical syndrome. Am J Med Sci. 2001;321:225–36. doi: 10.1097/00000441-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Alpert MA, Hashimi MW. Obesity and the heart. Am J Med Sci. 1993;306:117–23. doi: 10.1097/00000441-199308000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Fischer M, Baessler A, Hense HW, et al. Prevalence of left ventricular diastolic dysfunction in the community. Results from a Doppler echocardiographic-based survey of a population sample. Eur Heart J. 2003;24:320–8. doi: 10.1016/s0195-668x(02)00428-1. [DOI] [PubMed] [Google Scholar]
- 14.Grundy SM, Hansen B, Smith SC, Jr, et al. Clinical management of metabolic syndrome: Report of the American Heart Association/National Heart, Lung, and Blood Institute/American Diabetes Association Conference on Scientific Issues Related to Management. Circulation. 2004;109:551–6. doi: 10.1161/01.CIR.0000112379.88385.67. [DOI] [PubMed] [Google Scholar]
- 15.Jensen MD. Health consequences of fat distribution. Horm Res. 1997;48(Suppl 5):88–92. doi: 10.1159/000191335. [DOI] [PubMed] [Google Scholar]
- 16.Thomas GN, Ho S, Lam KSL, et al. Impact of obesity and body fat distribution on cardiovascular risk factors in Hong Kong Chinese. Obes Res. 2004;12:1805–13. doi: 10.1038/oby.2004.224. [DOI] [PubMed] [Google Scholar]
- 17.Wellborn TA, Dhaliwal SS, Bennett SA. Waist-hip ratio is the dominant risk factor predicting cardiovascular death in Australia. Med J Aust. 2003;179:580–5. doi: 10.5694/j.1326-5377.2003.tb05704.x. [DOI] [PubMed] [Google Scholar]
- 18.Cassidy AE, Bielak LF, Zhou Y, et al. Progression of subclinical coronary atherosclerosis: Does obesity make a difference? Circulation. 2005;111:1877–82. doi: 10.1161/01.CIR.0000161820.40494.5D. [DOI] [PubMed] [Google Scholar]
- 19.Massie BM. Obesity and heart failure – risk factor or mechanism? N Engl J Med. 2002;347:358–9. doi: 10.1056/NEJMe020065. [DOI] [PubMed] [Google Scholar]
- 20.Kurland LT, Molgaard CA. The patient record in epidemiology. Sci Am. 1981;245:54–63. doi: 10.1038/scientificamerican1081-54. [DOI] [PubMed] [Google Scholar]
- 21.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–74. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 22.Jacobsen SJ, Mahoney DW, Redfield MM, et al. Participation bias in a population-based echocardiographic study. Ann Epidemiol. 2004;14:579–84. doi: 10.1016/j.annepidem.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Redfield MM, Jacobsen SJ, Burnett JC, Jr., et al. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 24.Pritchett AM, Jacobsen SJ, Mahoney DW, et al. Left atrial volume as an index of left atrial size: a population-based study. J Am Coll Cardiol. 2003;41:1036–43. doi: 10.1016/s0735-1097(02)02981-9. [DOI] [PubMed] [Google Scholar]
- 25.The sixth report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Arch Intern Med. 1997;157:2413–46. doi: 10.1001/archinte.157.21.2413. [DOI] [PubMed] [Google Scholar]
- 26.Kannel WB, Belanger AJ. Epidemiology of heart failure. Am Heart J. 1991;121:951–7. doi: 10.1016/0002-8703(91)90225-7. [DOI] [PubMed] [Google Scholar]
- 27.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–97. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 28.Ommen SR, Nishimura RA, Appleton CP, et al. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous Doppler-catheterization study. Circulation. 2000;102:1788–94. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- 29.Nishimura RA, Tajik AJ. Evaluation of diastolic filling of left ventricle in health and disease: Doppler echocardiography in the clinician's Rosetta stone. J Am Coll Cardiol. 1997;30:8–18. doi: 10.1016/s0735-1097(97)00144-7. [DOI] [PubMed] [Google Scholar]
- 30.Gardin JM, Siscovick D, Anton-Culver H, et al. Sex, age, and disease affect echocardiographic left ventricular mass and systolic function in the free-living elderly: The Cardiovascular Health Study. Circulation. 1995;91:1739–48. doi: 10.1161/01.cir.91.6.1739. [DOI] [PubMed] [Google Scholar]
- 31.Christoffersen C, Bollano E, Lindegaard ML, et al. Cardiac lipid accumulation associated with diastolic dysfunction in obese mice. Endocrinology. 2003;144:3483–90. doi: 10.1210/en.2003-0242. [DOI] [PubMed] [Google Scholar]
- 32.Cabrol P, Galinier M, Fourcade J, et al. Functional decoupling of left ventricular beta-adrenoceptor in a canine model of obesity-hypertension. Arch Mal Coeur Vaiss. 1998;91:1021–4. [PubMed] [Google Scholar]
- 33.Atzmon G, Yang XM, Muzumdar R, et al. Differential gene expression between visceral and subcutaneous fat depots. Horm Metab Res. 2002;34:622–8. doi: 10.1055/s-2002-38250. [DOI] [PubMed] [Google Scholar]
- 34.Wajchenberg BL, Giannella-Neto D, da Silva ME, et al. Depot-specific hormonal characteristics of subcutaneous and visceral adipose tissue and their relation to the metabolic syndrome. Horm Metab Res. 2002;34:616–21. doi: 10.1055/s-2002-38256. [DOI] [PubMed] [Google Scholar]
- 35.Vague J. The degree of masculine differentiation of obesities: a factor determining predisposition to diabetes, atherosclerosis, gout, and uric acid calculus disease. Am J Clin Nutr. 1956;4:20. doi: 10.1093/ajcn/4.1.20. [DOI] [PubMed] [Google Scholar]
- 36.Goodpaster BH. Measuring body fat distribution and content in humans. Curr Opin Clin Nutr Metab Care. 2002;5:481–7. doi: 10.1097/00075197-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Wang J, Thornton JC, Kolesnik S, et al. Anthropometry in body composition. An overview. Ann N Y Acad Sci. 2000;904:317–26. doi: 10.1111/j.1749-6632.2000.tb06474.x. [DOI] [PubMed] [Google Scholar]
- 38.Lauer MS, Anderson KM, Kannel WB, et al. The impact of obesity on left ventricular mass and geometry. The Framingham Heart Study. JAMA. 1991;266:231–6. [PubMed] [Google Scholar]
- 39.Reilly MP, Rader DJ. The metabolic syndrome: More than the sum if its parts? Circulation. 2003;108:1546–51. doi: 10.1161/01.CIR.0000088846.10655.E0. [DOI] [PubMed] [Google Scholar]
- 40.Tarantini L, Faggiano P, Senni M, et al. Clinical features and prognosis associated with a preserved left ventricular systolic function in a large cohort of congestive heart failure outpatients managed by cardiologists. Data from the Italian Network on Congestive Heart Failure. Ital Heart J. 2002;3:656–64. [PubMed] [Google Scholar]
- 41.Devereux RB, Roman MJ, Liu JE, et al. Congestive heart failure despite normal left ventricular systolic function in a population-based sample: the Strong Heart Study. Am J Cardiol. 2000;86:1090–6. doi: 10.1016/s0002-9149(00)01165-6. [DOI] [PubMed] [Google Scholar]
- 42.Nicklas BJ, Cesari M, Pennix BWJH, et al. Abdominal obesity is an independent risk factor for chronic heart failure in older people. J Am Geriatr Soc. 2006;54:413–420. doi: 10.1111/j.1532-5415.2005.00624.x. [DOI] [PubMed] [Google Scholar]