Abstract
Background: Primary management of advanced head/neck cancers involves concurrent chemoradiotherapy . Subsequently, regional and local failures are managed with resection but there have been few reports that describe the morbidity and disease control outcomes of surgical salvage in this setting. Methods: Retrospective analysis describes complications, survival, and patterns of failure after salvage resection of isolated local and/or regional failures of head/neck cancer following definitive concurrent chemoradiotherapy. Results: Sixteen patients were identified for inclusion: laryngectomy in 11 patients, oral cavity/oropharynx resection in 2 patients, and neck dissection alone in 4 patients. Ten patients required graft tissue reconstruction (6 pedicle and 4 free flap). Median post-operative hospitalization was 7 days (range 3-19), and 4 patients required hospital re-admission. At a median survivor follow-up of 15.8 months (range 4.3-34.9), 10 patients were alive (6 without evidence of disease). Seven patients experienced disease recurrence at a median 6.7 months (range 0-12.6) following salvage resection (2 with isolated distant failures). Estimated 2-year local/regional control, freedom from failure, and overall survival were 58%, 39%, and 58%, respectively. Conclusions: Surgical salvage after primary definitive concurrent chemoradiotherapy is feasible with toxicity and outcomes similar to prior radiotherapy alone or sequential chemotherapy and radiation. Local andregional recurrence remains the predominant pattern of failure.
KEYWORDS: Head and neck neoplasms, Combined modality therapy, Salvage therapy, Organ preservation therapy
INTRODUCTION
Locoregionally advanced head and neck cancers are optimally treated with definitive concurrent chemoradiotherapy or surgical resection followed by radiotherapy with or without chemotherapy (1). Despite aggressive local treatment, approximately 20-36% of patients will experience locoregional recurrence within 3-5 years, representing 50-67% of all recurrences (1-3). In patients who experience disease recurrence within a previously irradiated field, aggressive salvage surgical resection is the preferred intervention (4). Previously reported series of surgical salvage have generally included patients treated with suboptimal primary
therapy (radiotherapy alone or sequential chemotherapy and radiotherapy), while subsequent randomized trials (5-8) and a meta-analysis (9) have demonstrated superior locoregional control and survival for concurrent chemoradiotherapy over radiotherapy alone or sequential chemoradiotherapy (5), albeit associated with increased risk of adverse effects (5). Few studies have evaluated the complications and outcomes of surgical salvage of locoregional failures following modern platinum-based concurrent chemoradiotherapy (10-13).
The present study is a retrospective analysis of complications, disease control, patterns of failure, and survival in a cohort of patients treated with salvage surgical resection at the Medical University of South Carolina (MUSC).
METHODS
Ethical Considerations
Following Institutional Review Board approval at MUSC, a research spreadsheet was created with study-specific patient, treatment, and outcome data fields. Non-protected health information remained within the primary sources (departmental chart, quality assurance database(s), and/or electronic medical record system).
Selection Criteria
Eligible cases were identified by review of the radiation oncology departmental quality assurance database and office management software. The database contained all patients at MUSC that were initiated on definitive chemoradiotherapy; eligible cases were identified within the database by searching for head and neck cancer patients. Surgical salvage was defined as curative-intent resection for residual disease or recurrent primary tumor, nodal disease, and/or second primary tumor within an irradiated field following platinum-based concurrent chemoradiotherapy. Primary definitive chemoradiotherapy and salvage resection and reconstruction (when necessary) were performed at MUSC in all cases. Exclusion criteria for this study included primary chemoradiotherapy at an outside institution, evidence of distant metastatic disease at the time of surgical salvage and/or post-salvage follow-up of less than 3 months (unless evidence of disease progression/recurrence and/or death). All patients included in descriptive outcomes analysis were required to have had pathologic proof of squamous cell carcinoma recurrence.
Patient Work-Up and Management
All patients were initially evaluated at the MUSC Hollings Cancer Center multidisciplinary head and neck oncology clinic, with evaluations by surgical, medical, and radiation oncology physicians, as well as speech therapy and dental oncology/ maxillofacial-prosthedontic specialists as indicated. Following complete metastatic workup, which consisted of chest computed tomography (CT) and/or positron emission tomography (PET or PET/CT), patients were offered surgical salvage based on feasibility surgical resection and/or patients' medical operability. When microvascular "free flap” reconstruction was required, peripheral vascular studies were performed in order to identify viable graft tissue harvest sites.
Surgical intervention was left to the discretion of the treating head/neck surgeon and reconstruction specialist. All patients underwent at least selective neck dissection at the time of salvage resection and more aggressive intervention in cases where there was neck recurrence (particularly with radiographic and/or clinical evidence of extranodal extension). When reconstruction was indicated, rotational flaps were constructed from pectoralis major and free flaps were harvested from anterior thigh, radial forearm, or fibula sites.
Following completion of treatment, patients were assessed at a minimum of every 3 months for 2 years, then every 6 months for 3 years, and annually thereafter. Surveillance fiberoptic endoscopy was performed routinely during follow-up appointments. A neck CT or MRI was generally performed 2-3 months following salvage resection, then again 6 months later and/or as indicated based upon clinical suspicion. During the follow-up period, metastatic surveillance with chest X-ray and/or chest CT was generally performed once within 6 months of salvage, then repeated in situations of clinical suspicion or at time of locoregional recurrence.
Endpoints and Definitions
The principal outcome measure of this study was overall survival which was measured from date of salvage resection to last follow-up or death. Secondary outcome measures included duration of post-salvage hospitalization, post-operative complication rates, pattern of failure, locoregional freedom from failure, and overall freedom from failure. Patient status at last follow-up was recorded as "alive, no evidence of disease," "alive with disease," "died of disease," "died of treatmentassociated toxicity," "died of other cause," or "died of unknown cause." Freedom from failure was measured from date of salvage resection to date of second recurrence (earliest sign of clinical, radiographic, or pathologic disease) or last follow-up if there was no evidence of disease recurrence. A patient was considered to have died of treatmentassociated toxicity if there was clear association between toxicity and death or if the patient died during or within 30 days of hospitalization attributed to treatment toxicity (without other evident cause). Treatment-associated mortality was considered an event for freedom from failure. If a patient died of unclear cause, but was known to have had recurrent disease prior to death, he/she was considered to have died of disease. Patterns of failure were recorded by initial site(s) of disease recurrence.
Literature Review Search Criteria
An Ovid Medline search was performed using the search terms "head and neck neoplasms," "local neoplasm recurrence," "recurrence," and "resection." Articles were searched for postchemoradiotherapy salvage resection-specific experiences. A secondary search wasperformed by reviewing references from articles identified from the search.
Statistical Analysis
The Kaplan-Meier method was used to calculate estimated locoregional control, freedom from failure, and overall survival for the entire cohort. Proportionality was tested for each covariate of interest. Those that failed were either not included or were reparameterized to adhere to the proportionality assumption. Cox regression was used to estimate the hazard ratio (HR) comparing risk of timeto- event outcomes by covariates. Disease control estimation, survival estimation, and univariate analyses were performed using R version 2.6.1 (The R Core Development Team; http://www.r-project.org).
RESULTS
Between September 2001 and October 2007, 136 patients initiated platinum-based concurrent chemoradiotherapy for locoregionally advanced head/neck cancer. Isolated head/ neck recurrence developed as the initial site of failure in 28 patients (20.6%) at a median survivor follow-up of 33.1 months (range 4.6-71.1). 17 patients underwent surgical resection as initial salvage intervention; however, one patient had no evidence of invasive disease at salvage resection (post-treatment biopsy at 5.5 weeks post-treatment completion had demonstrated residual disease). This patient was included in assessment of complications but excluded from the survival and patterns of failure analyses. Patient, tumor, and staging characteristics are shown in Table 1. All patients had been prescribed to a dose of 66- 70 Gy, and all but one completed the prescribed course of therapy. Excluding this patient, the median initial chemoradiotherapy duration was 50 days (range 43-63), with one patient requiring a oneweek treatment break (63 total treatment days). All but one patient had pathologic stage III-IV recurrent disease (Table 2) at salvation resection. The primary tumor adverse pathologic features of lymphovascular invasion and perineural invasion were identified in 3 and 4 cases, respectively, and 4 patients had microscopically involved margins at salvage resection. Extranodal extension within the neck was documented in 5 cases.
Table 1:
Patient, Tumor, and Staging Characteristics
| Salvage Cohort (n=16*) |
|||
|---|---|---|---|
| n | % | ||
|
Age Median (Range) ≥60 yrs |
59 yrs (46-80) |
8 |
50 |
|
Gender Male |
12 |
75 |
|
|
Race White |
10 |
62 |
|
|
Initial Disease Site Oropharynx Larynx Hypopharynx |
5 10 1 |
31 62 6 |
|
|
Interval Since Definitive
ChemoRT# Completion Median (Range) |
7.6 months (1.6-27.6) |
||
|
Prior RT Characteristics Median Dose (Dose Range) |
7000 cGy (4800-7000) |
||
|
Relation to Prior RT Field In-Field Field Margin |
14 2 |
88 12 |
|
|
Recurrent Tumor Site Primary Second Primary Irradiated Neck |
10 1 5 |
62 6 31 |
|
|
Chest Staging at Recurrence None$ Chest Radiograph Chest CT &/or PET |
1 5 10 |
6 31 62 |
|
Excludes one patient without evidence of residual carcinoma at salvage resection.
ChemoRT=Cisplatin-based concurrent chemoradiotherapy.
This patient survived >5 years without recurrence.
Table 2:
Recurrent Tumor Stage Characteristics.
| pT0/Tx | pT1 | pT2 | pT3 | pT4a | pT4b | |
|---|---|---|---|---|---|---|
| pN0 | 4 | 1 | 3 | 1 | ||
| pN1 | 3 | |||||
| pN2a | ||||||
| pN2b | ||||||
| pN2c | 1 | 1 | ||||
| pN3 | 2 |
Salvage surgical intervention involved total laryngectomy in 8 patients, total laryngopharyngectomy in 1 patient, supracricoid laryngectomy in 2 patients, oral cavity/oropharyngeal composite resection in 2 patients, and neck dissection alone in 4 patients. All patients' necks were surgically addressed at salvage, involving unilateral selective dissection in 5 patients, unilateral modified radical neck dissection in 2 patients, unilateral radical neck dissection in 1 patient, bilateral selective neck dissections in 6 patients, and bilateral modified radical neck dissections in 3 patients. 10 patients required flap reconstruction (6 pedicle and 4 free).
The median post-operative hospitalization was 7 days (range 3-19), with significant complications of hematoma (4), wound breakdown (3), and fistula (1). 4 patients required a median duration 13 day (range 4-30) hospital re-admission following initial dischargeReasons for re-hospitalization included pharyngocutaneous fistula, wound breakdown, neck abscess, and bleeding at the gastrostomy site (associated with supratherapeutic anticoagulation). One patient received immediate post-salvage chemotherapy and one concurrent chemoradiotherapy for adverse pathologic features (surgical margin and extranodal neck disease, respectively). 2 out of 10 patients who underwent graft tissue reconstruction at the time of salvage developed complications requiring intervention. One patient developed necrosis along the distal margin of a rotational pectoralis flap, requiring takedown and reconstruction using a second pedicle flap. No further complications were observed. Another patient experienced delayed would dehiscence of a free flap reconstruction, with pharyngocutaneous fistula formation approximately 2 months post-salvage/reconstruction. The flap was removed and the defect was reconstructed with a rotational pectoralis flap, resulting in no further complication.
Two patients died of treatment-associated complications. One patient underwent salvage bilateral modified radical neck dissections for failure within an irradiated neck (4 months after completion of chemoradiotherapy for cT4aN2cM0 hypopharyngeal cancer originating at the pyriform sinus). Recurrent disease in the neck was significant for extension into the soft tissues of the neck as well as perineural and lymphovascular space invasion. The post-operative course was complicated by a neck hematoma requiring evacuation and an orocutaneous fistula, with a salivary leak. The patient was ultimately discharged 10 days after salvage resection but died 3 days later of unspecified cause. Another patient required surgical salvage for residual/ progressive primary tumor 10 weeks following concurrent chemoradiotherapy (initial cT3N0 oropharyngeal cancer). Following salvage palatectomy with inferior maxillectomy and partial glossectomy with anterolateral thigh free flap reconstruction and bilateral selective neck dissections, the patient experienced bleeding from the oronasal and neck incisions. These complications resolved following discontinuation of heparin, after which the patient was discharged (8 days post-salvage). The patient died later that day of an unspecified cause.
At a median survivor follow-up of 15.8 months (range 4.3-34.9) post-salvage, 10 patients were alive (6 without evidence of disease) and 6 patients had died (3 of disease, 2 of intervention, and 1 of unknown cause ). Seven patients experienced disease recurrence at a median 6.7 months agpost- salvage (range 0-12.6), involving 1 completely resected primary site, 1 incompletely resected primary site, 2 dissected necks, 1 resected primary and dissected neck, and 2 isolated distant failures. Secondary salvage interventions included chemotherapy in 2 patients, concurrent chemotherapy and re-irradiation in 1 patient, re-resection in 1 patient, and supportive care for 3 patients. The estimated median overall freedom from failure for the study group was 10.8 months, while the median overall survival had not yet been reached (and was thus inestimable). The estimated 1- and 2-year locoregional control were both 58.0% (95% confidence interval: 35.4%-95.2%), as demonstrated in Figure 1. The estimated 1- and 2-year overall freedom from failure were 46.9% (27.0%-81.4%) and 39.1% (20.2%-75.4%), respectively (Figure 2). The estimated 1- and 2-year overall survivals were 78.7% (59.7%-100%) and 58.3% (35.1%-97.0%), respectively (Figure 3).
Figure 1:
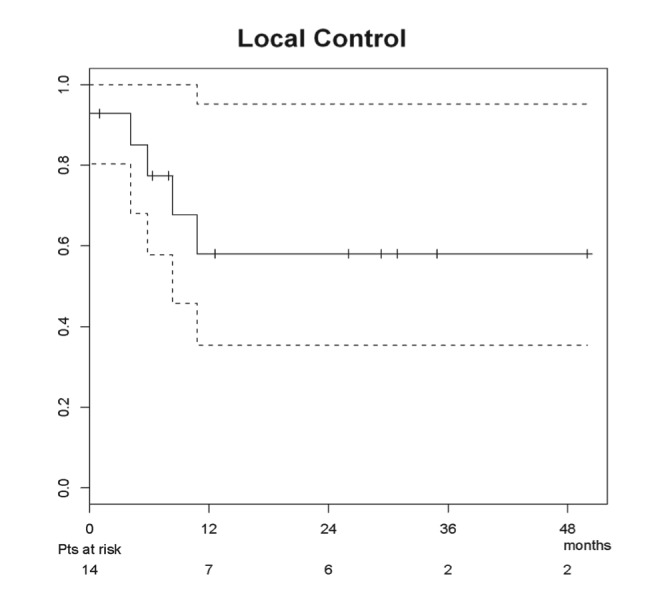
Locoregional control for salvage resection population. Fourteen patients at risk due to subtraction of two patients who died of treatment-associated toxicity within two weeks of salvage resection.
Figure 2:
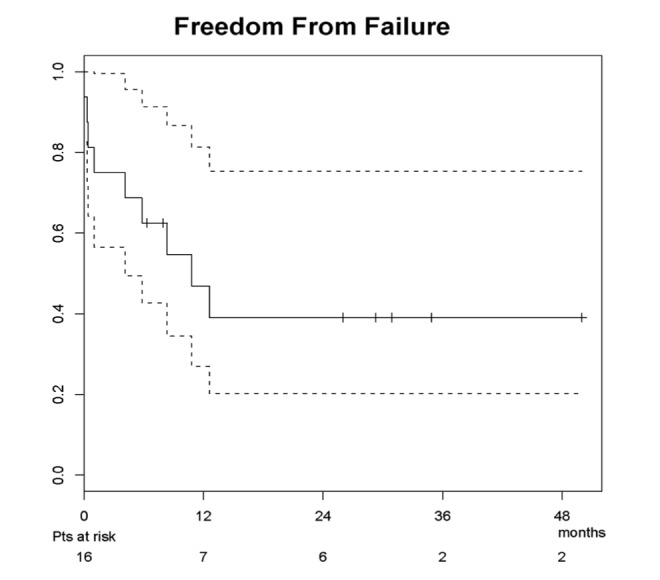
Freedom from disease failure for salvage resection population.
Figure 3:
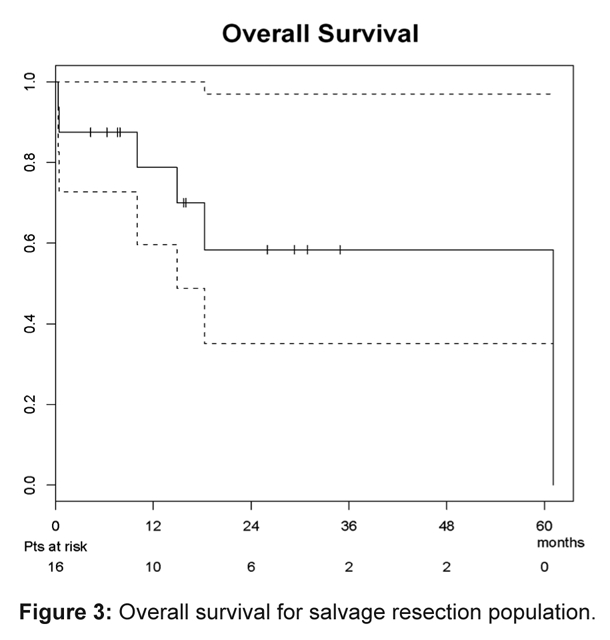
Overall survival for salvage resection population.
Univariate analyses of patient-, tumor-, and treatment-related factor associations with locoregional control, freedom from failure, and overall survival are demonstrated in Table 3. Despite the limitation of small sample size, the pathologic nodal stage and presence of extracapsular carcinoma within the soft tissue of the neck were associated with significantly decreased rates of locoregional control and freedom from disease failure. The association of these factors with overall survival was not demonstrated at a statistically significant level; however, there was a trend toward worse survival for patients with metastatic carcinoma within neck soft tissue.
Table 3:
Univariate Analysis of Factors Associated with Study Endpoints.
| Factor | Locoregional Control |
Freedom From Failure |
Overall Survival | |||
|---|---|---|---|---|---|---|
| HR | P | HR | P | HR | P | |
| Gender | 1.420 | 0.75 | 1.350 | 0.87 | 1.520 | 0.71 |
| Race | 0.865 | 0.87 | 0.396 | 0.25 | 0.280 | 0.26 |
| Age | 0.910 | 0.13 | 0.954 | 0.23 | 1.010 | 0.88 |
| Active Tobacco Use | 1.790 | 0.56 | 0.601 | 0.53 | 0.360 | 0.36 |
| Time to Recurrence | 1.090 | 0.098 | 1.030 | 0.38 | 0.894 | 0.22 |
| rcT-Stage | 1.000 | 0.99 | 0.883 | 0.45 | 0.852 | 0.47 |
| rcN-Stage | 1.480 | 0.056 | 1.360 | 0.058 | 1.120 | 0.62 |
| rcAJCC Stage | 1.660 | 0.27 | 1.570 | 0.2 | 1.080 | 0.87 |
| rpT-Stage | 0.953 | 0.81 | 1.050 | 0.71 | 0.847 | 0.45 |
| rpN-Stage | 1.940 | 0.024 | 1.700 | 0.012 | 1.370 | 0.19 |
| rpAJCC Stage | 2.830 | 0.086 | 2.200 | 0.083 | 1.420 | 0.57 |
| Margin at Salvage | 4.670 | 0.094 | 3.260 | 0.097 | 1.750 | 0.54 |
| Lymphovascular | 1.860 | 0.54 | 1.850 | 0.46 | 0.972 | 0.98 |
| Perineural Invasion | 2.280 | 0.51 | 2.460 | 0.31 | 1.380 | 0.73 |
| Metastatic Carcinoma in Neck Soft Tissue |
12.000 | 0.034 | 8.300 | 0.013 | 4.960 | 0.088 |
Toxicities, local control, and survival outcomes from the present study are pooled with other published series of surgical salvage following concurrent chemoradiotherapy in Table 4.
Table 4:
Published Series of Toxicities and Outcomes for Salvage Resection of Concurrent Chemoradiotherapy Recurrences in Head/ Neck Cancer.
| Series | N | Methodol ogy | Site(s) | 2y Local Control | 2y Overall | Major Complications |
|---|---|---|---|---|---|---|
| Richey 11 (UNC, 2007) | 38 | Retrospective Review | Multiple | 42% | 27% | Fistula (1/38) Wound Dehiscence (2/38) Respiratory Failure (2/38) Sepsis (1/38) |
| Weber 10 (RTOG 91-11, 2003) | 27 | RCT | Larynx | 74% | 71% | Fistula (8/27) Wound Dehiscence (4/27) |
| Morgan12 (MDACC, 2007) | 38 | Retrospective Review | Multiple | N/R | N/R | Fistula (3/38) Infection (2/38) |
| Yom13 (Multi- Institutional, 2005) | 14* | Retrospective Review | Multiple | 64%* | 57%* | Wound Dehiscence (3/14) |
| Kearney (MUSC) | 17 | Retrospective Review | Multiple | 58%# | 58%# | Fistula (3/17) Wound Dehiscence (3/17) Flap Breakdown (2/10) |
Twelve of fourteen underwent surgical salvage for residual or progressive disease noted at post-chemoradiotherapy assessment, local control and survival reflect crude recurrence rates rather than 2-year estimates.
Survival data based upon 16 patients with pathologic disease at salvage, excludes one patient with squamous cell carcinoma at post-chemoradiotherapy biopsy but no evidence of disease following salvage laryngectomy.
DISCUSSION
The role of surgical resection in locoregionally advanced head/neck cancer has evolved over time. Modern platinum-based concurrent chemoradiotherapy yields equivalent disease control, and is the preferred primary therapy when functional organ preservation is feasible (14). Despite aggressive combined-modality therapy, approximately 20-36% of patients still experience locoregional recurrence within 3-5 years, representing approximately half of all recurrences (1). In patients who experience disease recurrence within a previously irradiated field, aggressive salvage surgical resection is the preferred intervention, yielding 55% local control and 32-39% survival at 5 years (4,15). These have primarily included patients treated with radiotherapy alone or sequential radiotherapy and chemotherapy, which have demonstrated inferior disease control when compared with concurrent chemoradiotherapy (5-10).
Few reports have focused on the complications, disease control, and survival outcomes for concurrent chemoradiotherapy patients. The RTOG 91-11 study provides the best comparative data for salvage resection in the setting of radiotherapy alone versus sequential chemotherapy/radiotherapy versus concurrent chemoradiotherapy (5). Of 517 patients initially randomized, 129 required total laryngectomy (95% for recurrent/residual cancer) (10). While pharyngocutaneous fistula formation was less common in the radiotherapy alone arm (15%) compared to the the concurrent chemoradiotherapy arm (30%), this did not reach statistical significance (p>0.05). Similarly, the overall incidence of major and minor complications was not significantly different between treatment groups (52-59%). These consisted mainly of fistulae formation, infection, wound dehiscence, dysphagia and/or inability to take fluids, which are consistent with complications reported by other published series (10-12). Within the RTOG concurrent chemoradiotherapy group, the rate of fistula was somewhat higher than that described in single-institution series (30% versus 5-8%) (10-12). This may be associated with inherent differences between the studies (disease site, reconstruction techniques) and inconsistent scoring of self-limited or "minor" fistulae in the retrospective analyses relative to the RTOG 91-11 analysis. If only "major" fistulae are considered for the RTOG study, the rate (11%) is more consistent with the retrospective series. Within our own series, 2 of the 3 fistulae were self-limited salivary gland leaks which resolved with conservative measures. Thus, the overall risk of significant fistula formation after surgical salvage for concurrent chemoradiotherapy failures is approximately 5-10%. Graft tissue reconstruction may have a beneficial impact on reducing these major complications. A single-institution analysis of salvage laryngectomy following radiotherapy or chemoradiotherapy demonstrated a reduced risk of fistula for free flap reconstruction compared with primary closure (18% versus 50%, p=0.08) (16). Similarly, the risk of stricture and feeding tube dependence was reduced with free flap reconstruction. It should be noted that this analysis was restricted to patients whose disease was generally limited to the larynx, excluding patients for whom partial pharyngectomy or total laryngopharyngectomy would have been required. Within the present study population, complications were similar regardless of graft tissue reconstruction (4 of 11 reconstructed patients versus 2 of 6 primary closure patients).
The present study demonstrated an estimated 2-year overall survival of 58%, which is comparable to other single-institution series of post-chemoradiotherapy salvage resection (11,13) as well as mixed-treatment populations (4). Patients with smaller tumor burden (rT1-2) appear to have improved survival over larger tumors, as do laryngeal recurrences over pharyngeal sites (4,10,15,17,18). Within our own population, all 4 patients with longterm disease-free and overall survivals (>2 years) were locoregional failures of larynx primary sites, despite locally advanced disease at recurrence (rp- T4aN0 in two patients, rpT3N0 in one, and rpTxN1 in one). The interval from primary therapy completion to disease failure does not appear to impact disease control or survival outcomes (4). Though limited by small numbers, no association was identifiable within the present study population.
The role of immediate post-salvage chemotherapy and/or re-irradiation remains to be determined. A recently published French trial randomized 130irradiated patients to adjuvant chemoradiotherapy or observation following salvage resection (19). Despite improved local control and disease-free survival, no overall survival benefit was observed, and severe late toxicities (including subcutaneous fibrosis, osteoradionecrosis, and trismus) were higher in the adjuvant chemoradiotherapy arm (39% vs 11% at 2 years). It is possible that the benefit may be limited to a specific "high-risk" subset of patients requiring salvage resection. One single-institution series of postchemoradiotherapy failures demonstrated a trend toward poorer survival in patients with the specific high-risk features of involved surgical margins and/ or extranodal neck disease (11). Alternatively, these patients may simply have more advanced disease and would have a worse prognosis regardless of post-salvage intervention. Within the present study, two patients underwent immediate postsalvage therapy for high-risk pathologic features. One patient received immediate chemotherapy for involved surgical margins and another underwent concurrent chemotherapy and re-irradiation for extranodal carcinoma within their previously irradiated neck. Neither patient experienced significant postsalvage complications; however, both experienced local disease failure within 6 months.
Despite aggressive surgical salvage, the majority of disease recurrences involve the primary tumor site. Of 7 patients from the present series who experienced disease recurrence, 5 recurrences involved the tumor bed. This is consistent with other published data (11); however, the pattern of failure appears to shift toward more distant metastasis for advanced node-positive recurrences (11,13) and those treated with radiotherapy alone (10,20). The timing of post-salvage disease failure was also consistent with previously published series (median 9 months) (11,18); all but one patient within the present series failed within one year (isolated distant failure at 12.6 months). As suggested by Goodwin's comprehensive review (4), the rapidity of post-salvage recurrence may be attributable to advanced stage at initial recurrence presentation. Specifically, the analysis demonstrated an inverse relationship between recurrent disease stage and post-salvage median disease-free survival (>22.1, >11.5, 14.4, and 5.5 months for stages I, II, III, and IV disease, respectively). Within the present series, all patients who experienced post-salvage disease failure had recurrent pathologic stage III (n=2) or IV (5) disease.
Salvage resection of local and/or regional head/neck cancer failures following platinum-based concurrent chemoradiotherapy is feasible and can provide the opportunity for disease control and survival. Post-operative complication rates, timing and patterns of disease failure, locoregional control, freedom from disease failure, and overall survival rates are similar to those described in series of surgically salvaged patients treated with prior radiotherapy alone or sequential chemotherapy and radiation.
REFERENCES
- 1.Forastiere A, Koch W, Trotti A, Sidransky D. Head and neck cancer. N Engl J Med. 2001;345:1890–1900. doi: 10.1056/NEJMra001375. [DOI] [PubMed] [Google Scholar]
- 2.Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937–1944. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 3.Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350:1945–1952. doi: 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 4.Goodwin WJ. Salvage surgery for patients with recurrent squamous cell carcinoma of the upper aerodigestive tract: when do the ends justify the means? Laryngoscope. 2000;110(suppl 93):1–18. doi: 10.1097/00005537-200003001-00001. [DOI] [PubMed] [Google Scholar]
- 5.Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091–2098. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 6.Calais G, Alfonsi M, Bardet E, et al. Randomized trial of radiation therapy versus concomitant chemotherapy and radiation therapy for advanced-stage oropharynx carcinoma. J Natl Cancer Inst. 1999;91:2081–2086. doi: 10.1093/jnci/91.24.2081. [DOI] [PubMed] [Google Scholar]
- 7.Adelstein DJ, Li Y, Adams GL, et al. An Intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol. 2003;21:92–98. doi: 10.1200/JCO.2003.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Adelstein DJ, Saxton JP, Lavertu P, et al. A phase III randomized trial comparing concurrent chemotherapy and radiotherapy with radiotherapy alone in resectable stage III and IV squamous cell cancer: preliminary results. Head Neck. 1997;19:567–575. doi: 10.1002/(sici)1097-0347(199710)19:7<567::aid-hed2>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 9.Pignon JP, Maitre A, Bourhis J. Meta-analyses of chemotherapy in head and neck cancer (MACH-NC): an update. Int J Radiat Oncol Biol Phys. 2007;69(2 suppl 1):S112–S114. doi: 10.1016/j.ijrobp.2007.04.088. [DOI] [PubMed] [Google Scholar]
- 10.Weber RS, Berkey BA, Forastiere A, et al. Outcome of salvage total laryngectomy following organ preservation therapy. The Radiation Therapy Oncology Group trial 91- 11. Arch Otolaryngol Head Neck Surg. 2003;129:44–49. doi: 10.1001/archotol.129.1.44. [DOI] [PubMed] [Google Scholar]
- 11.Richey LM, Shores CG, George J, et al. The effectiveness of salvage surgery after the failure of primary concomitant chemoradiation in head and neck cancer. Otolaryngol Head Neck Surg. 2007;136:98–103. doi: 10.1016/j.otohns.2006.06.1267. [DOI] [PubMed] [Google Scholar]
- 12.Morgan JE, Breau RL, Suen JY, Hanna EY. Surgical wound complications after intensive chemoradiotherapy for advanced squamous cell carcinoma of the head and neck. Arch Otoloryngol Head Neck Surg. 2007;133:10–14. doi: 10.1001/archotol.133.1.10. [DOI] [PubMed] [Google Scholar]
- 13.Yom SS, Machtay M, Biel MA, et al. Survival impact of planned restaging and early surgical salvage following definitive chemoradiation for locally advanced squamous cell carcinomas of the oropharynx and hypopharynx. Am J Clin Oncol. 2005;28:385–392. doi: 10.1097/01.coc.0000162422.92095.9e. [DOI] [PubMed] [Google Scholar]
- 14.Cognetti DM, Weber RS, Lai SY. Head and neck cancer: an evolving treatment paradigm. Cancer. 2008;113(7 suppl):1911–1932. doi: 10.1002/cncr.23654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agra IMG, Carvalho AL, Ulbrich FS, et al. Prognostic factors in salvage surgery for recurrent oral and oropharyngeal cancer. Head Neck. 2006;28:107–113. doi: 10.1002/hed.20309. [DOI] [PubMed] [Google Scholar]
- 16.Withrow KP, Rosenthal EL, Gourin CG, et al. Free tissue transfer to manage salvage laryngectomy defects after organ preservation failure. Laryngoscope. 2007;117:781–784. doi: 10.1097/MLG.0b013e3180332e39. [DOI] [PubMed] [Google Scholar]
- 17.Ganly I, Patel SG, Matsuo J, et al. Results of surgical salvage after failure of definitive radiation therapy for earlystage squamous cell carcinoma of the glottic larynx. Arch Otolaryngol Head Neck Surg. 2006;132:59–66. doi: 10.1001/archotol.132.1.59. [DOI] [PubMed] [Google Scholar]
- 18.Kim AJ, Suh JD, Sercarz JA, et al. Salvage surgery with free flap reconstruction: factors affecting outcome after treatment of recurrent head and neck squamous carcinoma. Laryngoscope. 2007;117:1019–1023. doi: 10.1097/MLG.0b013e3180536705. [DOI] [PubMed] [Google Scholar]
- 19.Janot F, Raucourt D, Benhamou E, et al. Randomized trial of postoperative reirradiation combined with chemotherapy after salvage surgery compared with salvage surgery alone in head and neck carcinoma. J Clin Oncol. 2008;26:5518–5523. doi: 10.1200/JCO.2007.15.0102. [DOI] [PubMed] [Google Scholar]
- 20.Forastiere AA, Maor M, Weber RS, et al. Long-term results of Intergroup RTOG 91-11: a phase III trial to preserve the larynx – induction cisplatin/5-FU and radiation therapy versus concurrent cisplatin and radiation therapy versus radiation therapy. J Clin Oncol. 2006;24(18 suppl):A5517–A5517. [Google Scholar]


