Abstract
Context:
Although pituitary tumors are common, pituitary carcinoma is very rare and is only diagnosed when pituitary tumor noncontiguous with the sellar region is demonstrated. Diagnosis is difficult, resulting in delays that may adversely effect outcome that is traditionally poor. Barriers to earlier diagnosis and management strategies for pituitary carcinoma are discussed.
Evidence Acquisition:
PubMed was employed to identify relevant studies, a review of the literature was conducted, and data were summarized and integrated from the author's perspective.
Evidence Synthesis:
The available data highlight the difficulties in diagnosis and management and practical challenges in conducting clinical trials in this rare condition. They suggest that earlier diagnosis with aggressive multimodal therapy may be advantageous in some cases.
Conclusions:
Although pituitary carcinoma remains difficult to diagnose and treat, recent developments have led to improved outcomes in selected cases. With broader use of molecular markers, efforts to modify current histopathological criteria for pituitary carcinoma diagnosis may now be possible. This would assist earlier diagnosis and, in combination with targeted therapies, potentially improve long-term survival.
Pituitary carcinoma is defined by the presence of a pituitary tumor that is either not contiguous with the primary sellar tumor and/or a pituitary tumor that has metastasized to sites distant from the pituitary (1, 2). It is important to emphasize that although pituitary tumors commonly infiltrate and actively invade surrounding sellar structures such as dura, bone, and less commonly, blood vessels and nerve sheaths, these so-called “invasive” pituitary adenomas remain confined to the primary sellar tumor site and are not by current definitions pituitary carcinoma (3). This review will only discuss pituitary carcinoma, which fortunately is very uncommon and accounts for only 0.1% of all pituitary tumors. Allowing for recent studies pointing to increasing incidence of pituitary tumors and using a conservative estimate of prevalence of clinically relevant pituitary tumors of 1:1500, this equates with approximately 207 cases in the United States and approximately 4616 cases globally (4). The literature at the time of this writing describes approximately 165 cases in the English literature, which appears to be somewhat of an underestimate pointing to the difficulties in diagnosis and treatment (5–7). In fact, until recently, 75% of cases were only diagnosed at autopsy (8).
Epidemiology of Pituitary Carcinoma
Pituitary carcinoma can present at any age but typically presents in the third to fifth decade of life in patients with preexisting pituitary adenomas (6). Some studies have highlighted more GH-secreting carcinomas in patients between ages 24 and 56 yr than other tumor subtypes, but numbers are small (9). Many pituitary tumors that will ultimately become carcinomas declare their aggressive behavior early and are unresponsive to standard therapy from the outset and/or recur quickly after surgical debulking and progress rapidly to carcinoma. However, other tumors may initially be responsive to standard therapy for prolonged periods and only progress to carcinomas after many years. Therefore, reported latency periods vary from 4 months to 18 yr, with a mean interval of 6.6 yr (5). There is also a suggestion that certain tumor subtypes such as prolactin (PRL)-secreting tumors progress to carcinoma more quickly than ACTH-secreting tumors (4.7 vs. 9.5 yr), but again, the numbers are too small to draw firm conclusions regarding this (7).
No clear gender predilection for pituitary carcinomas has been reported despite the fact that PRL- and ACTH-secreting carcinomas, which are the most commonly encountered carcinoma subtypes, are a more common pituitary tumor subtype in females (7, 9–11). This predominance of benign pituitary adenomas in females may be offset by the observation that PRL-secreting macroadenomas from which pituitary carcinomas typically originate are more common in males. Whether, as in breast or prostate tumors, this points to some form of sex steroid hormone independence as a consequence of transformation is unclear, and some prior studies have actually demonstrated higher estrogen receptor expression in large PRL-secreting tumors in both sexes and PRL-secreting tumors in male patients (12, 13).
Metastatic Pituitary Carcinoma
Cancers metastasize to the pituitary region infrequently and account for only 1.8% of all metastases and 1% of all pituitary cancers. Breast and lung are the most frequent primary sites, and although the majority of patients are asymptomatic and often diagnosed at autopsy, two of the largest reported series noted that symptoms from the pituitary metastasis were the initial manifestation of metastatic disease in over half of patients (14, 15). Diabetes insipidus was by far the most frequent symptom, occurring in 45% of patients, followed by optic nerve dysfunction (28%); anterior pituitary dysfunction (24%); palsies of cranial nerves III, IV, or VI (22%); and headache (16%) (6). In contrast to cancers metastasizing to the sellar region, primary pituitary carcinomas generally do not impair pituitary endocrine function, and partial hypopituitarism has been reported in a single case of gonadotroph carcinoma (1, 16).
PRL-Secreting Pituitary Carcinoma
One of the biggest challenges with diagnosis of pituitary carcinoma is that from an endocrine standpoint, these tumors often behave identically to benign pituitary tumors. For example, no particular factors will help differentiate PRL-secreting adenomas that will follow a benign course from those that will progress to carcinomas (13). Therefore, patients with PRL-secreting carcinomas and elevated serum PRL will manifest typical PRL-mediated symptoms of amenorrhea and galactorrhea in females and erectile dysfunction in males. Serum PRL levels in carcinomas can vary widely (6 to 21,560 ng/ml in one series) but are typically very similar to values measured in PRL-secreting macroadenomas (7, 9). That said, clues to raise clinical suspicion of aggressive tumor phenotype include unresponsiveness and/or escalating serum PRL level and/or tumor growth despite adequate dopamine agonist treatment in a compliant patient. This dopamine agonist unresponsiveness can be seen either de novo or in a previously responsive patient who later exhibits marked discordance between hormonal response and radiological response. Given that the majority of PRL-secreting adenomas respond within 6–9 months on doses typically less than 3 mg/wk, these patients usually eventually come to notice. Given the rarity of pituitary carcinoma, it is often a diagnosis of exclusion, and the possibility that cosecretion of an additional pituitary hormone (typically GH as in so-called fugitive acromegaly) may have been overlooked or actions of sex steroids to interfere with D2-agonist antitumor effects must first be considered (17, 18). Additionally, if the patient has previously undergone pituitary tumor biopsy, repeated review of tumor histopathology may be instructive, including evaluation of specific proliferative markers (see Role of histopathology and molecular studies in diagnosing pituitary carcinoma), although one must be mindful that histopathology and resultant behavior may change in the course of time as illustrated where multiple tumor biopsies are examined in cases that transform from adenoma to carcinoma (19).
Corticotroph Pituitary Carcinomas
Corticotroph pituitary carcinomas are the second most commonly encountered carcinomas and typically occur in three settings. Most commonly (∼64% of ACTH carcinomas) corticotroph tumors secrete ACTH, and patients exhibit typical features of hypercortisolism with central obesity, rounded facies, abdominal and proximal limb striae, hypertension, altered menses, osteoporosis, and delayed wound healing (7, 10, 20). Some of these corticotroph adenomas exhibit increased collagen deposition, so-called “Crooke's cell” change, which can be a marker of potential aggressive behavior. Corticotroph carcinomas have also been observed after bilateral adrenalectomy to control hypercortisolism in patients with corticotroph pituitary tumors. In this setting, the removal of cortisol-mediated negative feedback is not believed to cause the transformation to pituitary cancer but serves as a growth stimulus to an already transformed/transforming corticotroph tumor. However, in approximately 25% of cases, corticotroph carcinomas develop in the setting of “silent” corticotroph tumors where corticotroph tumors secrete precursors of ACTH that may be measurable in the circulation but cannot easily bind and activate the ACTH receptor. Therefore, clinical and biochemical features of Cushing's syndrome are absent despite appropriate investigation. Both the “Crooke's cell” and “silent” corticotroph subtypes of ACTH-secreting pituitary tumors typically exhibit a more aggressive course than other ACTH-secreting tumors and as such might be expected to more frequently progress to pituitary carcinoma, but the presence of these comparatively recently identified corticotroph subtypes is not always clearly documented in older literature.
GH-Secreting Carcinoma
GH-secreting pituitary carcinomas generally present with symptoms and signs that are indistinguishable from benign GH-secreting adenomas with elevated GH and age- and sex-matched IGF-I levels. Like PRL-secreting carcinomas, GH-secreting carcinomas tend to be either unresponsive or only partially responsive to standard therapies. Clearly, the distinction between GH-secreting carcinoma and adenoma cannot be made on this basis alone because approximately 30–40% of benign GH-secreting pituitary tumors may not fully respond biochemically to current surgical and/or medical therapies, and as for other pituitary carcinomas, the demonstration of intracranial and/or systemic metastases needs to be apparent before diagnosing pituitary carcinoma.
Gonadotroph and Thyrotroph Carcinomas
Few reports of gonadotroph carcinomas exist, and these tumors usually present with sexual impotence in males and oligomenorrhea in females with variable LH, FSH, and α-subunit levels (21–24). Partial hypopituitarism was present in one case of gonadotroph carcinoma, but this appears very unusual (23, 24). TSH-secreting pituitary carcinomas are the most uncommon subtype and may exhibit immunopositivity for other hormones such as PRL or α-subunit (25). Serum TSH levels are reportedly more than 4-fold elevated, and α-subunit levels are usually more than 25-fold elevated. No cases have been reported since 2007, making this the rarest subtype of pituitary carcinoma (11, 25).
Role of Histopathology and Molecular Studies in Diagnosing Pituitary Carcinoma
Although significant advances in our understanding of the molecular mechanisms involved in pituitary tumorigenesis have been made and some evidence indicates that progression from “benign” adenoma to pituitary carcinoma is accompanied by cumulative changes in molecular pathway abnormalities, the details remain unclear (9, 23, 26). For example, although loss of the tumor suppressor gene MENIN commonly results in pituitary adenomas in affected patients, and these tumors can be locally invasive and recurrent, and undetectable menin expression has been reported in pituitary carcinoma biopsies, multiple endocrine neoplasia type 1-associated pituitary adenomas have not been known to progress to pituitary carcinoma (27). Likewise, other genes that are commonly mutated in adenomas from other tissues such as hRas in colorectal and thyroid tumors are rarely mutated in pituitary cancers (28). These features of pituitary tumors are further reflected in efforts to apply standardized morphological and histological criteria in evaluation of pituitary tumors. For example, the presence of increased mitoses, nuclear pleomorphism, and tumor tissue necrosis is often a useful indicator of more aggressive behavior in many tumor tissues, but in pituitary tumors these features are variously demonstrated in “benign,” atypical pituitary tumors and overlap considerably with pituitary carcinomas (29–31).
Efforts to find additional molecular markers of an invasive pituitary tumor phenotype with metastatic potential have included quantitation of proliferation markers in pituitary tumor tissues (6, 32). For example, Ki-67, a well-validated marker of proliferation is immunohistochemically demonstrable during G1, G2–M and S-phase of the cell cycle. Whereas most pituitary adenomas have Ki-67 labeling indices (LI) of 1–2%, levels of 3% and above are unusual and comprise one of several factors (along with pleomorphism and number of mitoses) used to denote an atypical pituitary tumor by World Health Organization (WHO) criteria. Mean Ki-67 LI in pituitary carcinomas in one study was 11.9 ± 3.4%, compared with 1.4 ± 0.15% in noninvasive adenomas, and some experts have proposed that pituitary tumors exhibiting Ki-67 LI of greater than 10% should routinely be classified as atypical, independent of other criteria (3, 33). However, other studies have not observed such a clear distinction between Ki-67 LI in pituitary carcinomas and the other categories of benign/typical, invasive, atypical adenomas (34). Care must also be taken by the interpreting pathologist to rule out Ki-67-labeled inflammatory cells, which can infiltrate the pituitary tumor and confound accurate measurement of Ki-67 LI. Nonetheless, the use of Ki-67 LI along with other measures of proliferation may help the clinician “flag” early in their history the pituitary tumors that manifest the potential to recur and potentially become carcinomas. However, no prospective studies supporting this concept presently exist, and in our own experience, the alteration in Ki-67 and mitoses may coincide with transformation rather than precede it (19). The proliferating cell nuclear antigen (PCNA) is an accessory protein to DNA polymerase required for cell division, and higher PCNA labeling has been noted in metastatic pituitary tumors with a median of 72% (range, 8–98%) compared with adenomas with median of 53% (range, 0–93%). However, PCNA is technically more difficult to quantitate and is considered less reliable than Ki-67 LI (35). The tumor suppressor p53 protein encodes a nuclear phosphoprotein essential for cell proliferation. Commonly mutated in many human cancers, p53 is not mutated in pituitary carcinomas, but nuclear p53 immunoreactivity does correlate with pituitary tumor invasiveness. In one study, all seven of seven (100%) pituitary carcinomas exhibited p53 immunopositivity compared with 5 of 70 pituitary adenomas (7.1%) (36, 37). Considerable intra- and intertumoral variability is a limitation of p53 expression, but like Ki-67, it is included in the 2004 WHO criteria evaluating tumor invasiveness (37).
In summary, present molecular markers have some limitations in predicting which pituitary tumors may become carcinomas, but the presence of a molecular profile characteristic of an aggressive tumor can still be helpful in clinical planning of adjuvant radiation therapy, for example, in the case of residual pituitary tumor (38).
Evaluation and Management
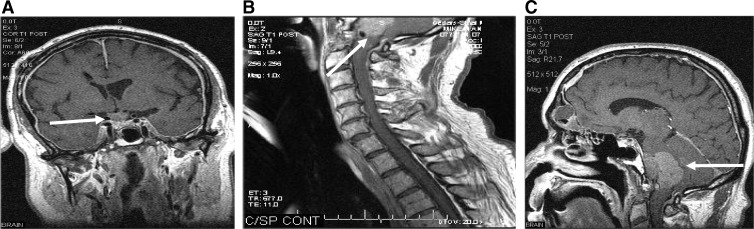
As noted, the diagnosis of pituitary carcinoma is often made surreptitiously when either the patient's clinician observes discordance between tumor and/or biochemical response to therapy prompting imaging or more commonly when routine imaging studies denote metastatic deposits within the central nervous system (39). Examples of persistent elevation of biochemical markers in the absence of residual sellar tumor in a patient who later represents with cervical adenopathy proven to be GH-immunopositive neuroendocrine tumor (NET) on biopsy serves to illustrate how pituitary carcinoma can go unnoticed for many years (40). Furthermore, it is not uncommon that systemic metastases are found incidentally during computed tomography/magnetic resonance scans for seemingly unrelated symptoms and subsequent biopsy confirms NET of pituitary origin (Fig. 1).
Fig. 1.
Magnetic resonance imaging of a pituitary carcinoma depicting (arrows) a large sellar pituitary tumor (A), large cerebellopontine angle (B), and cervical metastatic deposits (C).
No features reliably predict the occurrence of pituitary carcinoma, but the majority develop in the setting of a known macroadenoma that exhibits significant suprasellar extension and cavernous sinus invasion (41). Pituitary carcinomas tend to disseminate systemically via lymphatic and hematogenous spread rather than via craniospinal spread, with a reported frequency of 47% systemic metastases, 40% craniospinal metastases, and 13% exhibiting both (41–43). The extent of involvement can vary from a single lesion to widespread dissemination, and sites of metastasis include the cerebral cortex, cerebellum, spinal cord, leptomeninges, eyes, heart, lung, cervical lymph nodes, pancreas, liver, kidney, pelvic lymph nodes, ovary, myometrium, and bone (10, 44–46). Metastatic pituitary carcinoma cells have also been isolated from cerebrospinal and pleural effusion fluid (25, 32). Unless the index of suspicion is high, cervical or more distant spine imaging to identify so-called “drop metastases” is not routinely performed and is best guided by clinical symptoms (i.e. neck pain) or if there is marked discordance between the volume of the sellar tumor being monitored and the biochemical markers. PRL-secreting carcinomas spread systemically more commonly (71% in one series) than ACTH-secreting carcinomas (57% in the same series), with predilection for the liver (7). In contrast, GH-secreting carcinomas present more commonly with cerebrospinal metastasis, although it must be acknowledged that the numbers of patients included in these series is low (9).
Therapy
Pituitary carcinomas are generally associated with a poor prognosis despite administration of maximal multimodal therapies (47). Patients with systemic metastases have a median survival of 12 months, whereas those with metastases confined to the central nervous system live longer with an average of 2.6 yr (7, 9, 11, 38).
Surgical Therapy
Few studies exist where detailed comments regarding surgical findings are made but studies that have included these typically report that pituitary carcinomas are locally invasive into the sellar floor and/or clivus and/or either cavernous sinus, and therefore surgical resection is rarely curative. On occasion, intracranial metastatic deposits within the third and/or fourth ventricles or other high-risk regions are encountered, and resection of these lesions is associated with significant morbidity and mortality. Nevertheless, it is often possible to surgically debulk and in many instances obtain gross total or subtotal resection of pituitary carcinoma tissue, and this can provide immediate relief of compressive symptoms and, of course, aid definitive diagnosis. Furthermore, repeated surgeries can be performed to remove secondary deposits as they emerge and can complement other therapeutic efforts with systemic and/or radiological therapies to achieve tumor control (48). Recent advances in surgical approaches to the skull base using endoscopic approaches may offer potential advantages over craniotomy in certain circumstances, although transcranial approaches may still be needed for some sites.
Radiation Therapy
Radiation therapy is administered to prevent regrowth in subtotally resected pituitary tumors including carcinomas and to slow growth of expanding sellar tumor and/or metastatic deposits and can be delivered in two primary schedules. Stereotactic radiosurgery (SRS) involves delivery of high-dose radiation typically in a single visit, which offers good efficacy and enhanced patient convenience. If tumor targets approximate radiation-sensitive normal tissues that cannot be spared from the radiation, then radiation treatment given in small daily dose fractions (fractionated radiation therapy) over 5–6 wk is preferred. The various forms of radiation therapy including γ-knife, linear accelerator, cyberknife, and proton beam therapy can all be adapted to deliver either SRS or fractionated therapy, although greater experience to date with SRS has been with γ-knife (49). Because of the physical properties of photons, proton beams allow for improved sparing of surrounding normal tissues from ionizing radiation, although complexity and capital cost of this approach has slowed its expansion. Success rates of any of these treatments in pituitary tumors in general is hard to quantify due to variation in technique, doses administered, and definitions of biochemical response employed (50). However, a few observations appear consistent. First, biochemical responses in irradiated secretory adenomas are improved when hormonal therapy is discontinued approximately 1 month before radiation begins and is held until radiation treatment is completed (51). Additionally, smaller tumors are associated with higher responses to radiation and lower risk of hypopituitarism (51, 52). It is hard to directly extrapolate these findings to pituitary carcinomas where reported comparisons of the forms of radiation therapy (proton beam vs. γ-knife) do not exist and no clinical trial-based data demonstrate that radiotherapy improves survival in patients with pituitary carcinoma. However, anecdotal reports indicate that delay in tumor progression may be obtained in some circumstances, and the observations in adenomas emphasize the important role of debulking surgery when possible (7, 9, 48). Clearly, concerns regarding long-term hypopituitarism in the situation of pituitary carcinoma with shortened life expectancy may be a secondary consideration (9, 39). Furthermore, it must be remembered that unlike repeated surgical debulking which can be considered in suitable patients, radiation treatment is limited by risk of necrosis in the temporal and other brain areas that may be seen as a complication of maximal external radiation therapy (XRT).
Although somatotroph tumors and occasional nonfunctioning tumors express somatostatin receptors and peptide radio-targeted therapy directed at the somatostatin subtype receptor 2 ± 5 subtypes using 111Ind, 90Y and most recently 177Leu has been developed for carcinoid and other NET, this modality has not been employed to date in pituitary carcinoma (53).
Medical Therapy
In pituitary carcinomas, medical therapy can be divided into therapy primarily aimed at controlling biochemical secretion, which may have an indirect action on tumor growth, and therapy that is used primarily for its antiproliferative and/or proapoptotic role, which in turn may have a secondary action to reduce hormone levels. These two aspects clearly overlap, but it must be remembered that in some instances, as in corticotroph carcinomas, the patient may die from complications of hormonal excess (opportunistic infection from immunosuppression, hyperglycemia, and poor wound healing) before the effects of expanding tumor volume. In short, the medical treatments of biochemical hypersecretion in pituitary carcinomas are generally not very different from those employed in benign pituitary adenomas, although higher doses of agents and combinations of agents are often needed. For example, dopamine agonist treatment should be employed at maximally tolerated doses in PRL-secreting carcinomas but may be limited by side effects including orthostatic symptoms and/or nausea. Given the expression of estrogen receptor on PRL-secreting and occasional nonfunctioning tumors, antiestrogens have been tried in occasional cases, but results have been disappointing (54).
Broader use of dopamine agonists in GH-secreting and ACTH-secreting pituitary carcinomas derives from their occasional utility in benign GH- and ACTH-secreting adenomas and certainly justifies consideration of a therapeutic trial, but little objective support for their efficacy exists. GH-secreting and occasional gonadotroph adenomas express somatostatin receptors, particularly subtype 2, whereas ACTH-secreting tumors predominantly express subtype 5. This observation underpins the rationale to use somatostatin-based therapy to lower excess GH secretion in GH-secreting pituitary adenomas, and more recent studies have demonstrated that these ligands can also induce significant shrinkage in approximately 30% of GH-secreting tumors (55). Next generation SMS analogs such as pasireotide exhibit increased affinity for SMS subtype 5, and in a recently completed phase III clinical study in Cushing's disease, 25% of patients exhibited suppression of plasma ACTH and cortisol secretion at 6 months (56). However, the use of octreotide in GH-secreting carcinomas is not well studied, and only one case where combination pasireotide and temozolomide (TMZ) inhibited ACTH secretion and induced shrinkage of cranial, spinal, and hepatic metastases in an ACTH-secreting pituitary carcinoma has been reported (57).
Chemotherapy in Pituitary Carcinomas
Given the rarity of pituitary carcinoma, no randomized studies of systemic chemotherapy have been conducted, and protocols, inclusion or exclusion criteria have varied widely. Although pituitary carcinomas have a high proliferation index, they seem to retain certain aspects of well-differentiated tumors or in some way are importantly different from other cancers because they respond poorly to the standard chemotherapy regimens that offer responses in adenocarcinomas or sarcomas (58). Many single agent and combination chemotherapy regimens have been tried, including cisplatin, carboplatin, etoposide, adriamycin, dacarbazine, cyclophosphamide, procarbazine, vincristine, mitotane, and methotrexate. Reported response rates to chemotherapy have been conflicting and, with exceptions, can be measured in months rather than years and may in part reflect the variability of the tumor proliferative phenotype (59). Until recently, the most commonly reported cytotoxic drugs used in pituitary carcinomas were cyclo-hexyl-chloroethyl-nitrosourea (CCNU) in combination with 5-fluorouracil (5FU) although TMZ, given its recent success in various subtypes of pituitary carcinoma (discussed below), has quickly become first-line therapy.
CCNU + 5FU
5FU is metabolized to 5-dUMP, which inhibits thymidylate synthase to produce thymidine deficiency and thereby decreases DNA synthesis. CCNU (also known as lomustine or CeeNU) is an alkylating agent that inhibits DNA and RNA synthesis by blocking the methylation of deoxyuridylic acid. CCNU has been used for many years to treat breast, colorectal, and pancreatic cancer. CCNU in combination with 5FU has been used in four cases of pituitary carcinoma—three of which had previously undergone surgery followed by external beam radiotherapy, and one case treated previously with radiotherapy alone (47). The regimen was generally well tolerated, and patients received a median of two cycles (range, one to six). Three of the patients developed disease progression during treatment, although survival ranged from 3–65 months, indicating that the therapy may have slowed disease progression in some patients given that the natural history is typically 12 months. However, all four patients eventually died.
Temozolomide
TMZ is a lipophilic imidazotetrazine derivative that is converted to a methylating alkylator agent, MTIC [(methyl-triazene-1-yl)-imidazole-4-carboxamide], that induces DNA damage at any point in the cell cycle through base pair mismatch of O6-methylguanine with thymidine in the sister chromatid instead of cytosine (60). The methylated guanine is misread by the mismatch repair enzymes as an adenosine and thereby replaces thymidine in the sister chromatid. TMZ was originally approved for use in refractory glioblastoma multiforme, where it has transformed the outcome for these patients, although long-term survival is poor (60). TMZ was first used to treat a patient with a PRL-secreting carcinoma that had progressed despite multiple surgeries, radiation therapy, and high-dose dopamine agonist therapy (19).
TMZ depletes the DNA repair enzyme O6-methylguanine-DNA methyltransferase (MGMT), and studies in gliomas have demonstrated that lower methylated MGMT expression correlated with improved TMZ response. This association has also been examined in pituitary carcinomas, and initial studies suggested a correlation between low MGMT expression and TMZ response (61–64). However, a recent study describing eight patients treated with TMZ reported disease progression in a patient whose tumor exhibited low MGMT expression and therefore would have been predicted to respond to TMZ (65). Methodologies including methylation status of the MGMT promoter vs. immunohistochemical MGMT expression may also be important, but at present the relationship between MGMT and TMZ response in pituitary carcinomas is somewhat unclear (64). MGMT status as determined by both MGMT immunohistochemical expression and MGMT promoter methylation-sensitive PCR was also found to predict tumor response to TMZ in only 57% of treated patients (65).
Based on currently available data from 15 TMZ-treated predominantly PRL- and ACTH-secreting pituitary carcinomas (Table 1), TMZ appears effective in all pituitary carcinoma subtypes and exhibited a tumoral and/or hormonal response in nine of 15 (∼60%) of treated cases (7, 13, 19, 21–23, 25, 32, 47, 58, 62, 65–76). The drug is generally well tolerated, fatigue is common, and hematological toxicity with reduced white blood cell or platelet count may require dose reduction and occasionally withdrawal of the agent. Most studies have reported short-term treatment periods of between 6 and 9 months, although a few long-term responses of up to 24 months have been described.
Table 1.
Reported cases of pituitary carcinomas treated with chemotherapy
| No. | Tumor | Disease burden | Treatment | Chemotherapy | Response/outcome | First author, year (Ref.) |
|---|---|---|---|---|---|---|
| 1 | ACTH | Liver, lung, mediastinum, ilea, hip, sacral-lumbar spine | Surgery, RDT, BC, cyproheptadine | Cyclophosphamide, adriamycin, 5FU × four cycles | Alive at 39 months | Kaiser, 1983 (66) |
| 2 | GH | Occipital lobe, pons, cerebellopontine, ankles, spinal cord | Surgery × 3, RDT, BC | Cisplatin, vinblastine, bleomycin × one cycle | Died <1 month after chemo | Hashimoto, 1986 (32) |
| 3 | GH | Frontal lobe | Surgery × 4, RDT | Methotrexate, 5FU | Alive with no recurrence at 24 months | Asai, 1988 (67) |
| 4 | ACTH | Liver, lung, olfactory bulb | Surgery × 3, RDT | Mitotane, carmofur | Died within 4 months | Nawata, 1990 (68) |
| 5 | PRL | Frontal lobe, right ventricle, cerebellopontine angle, vertebral artery | Surgery × 4, RDT, BC | CCNU, procarbazine, etoposide × nine cycles | Initial response but progression, ↑ PRL 500,000, died 12 months after chemo | Petterson, 1992 (69) |
| 6 | PRL | Liver, lungs, hilar nodes | Surgery × 9, RDT, BC, quinagolide, I125 implantation, octreotide | Etoposide, cisplatin, tamoxifen, CCNU, 5FU, folinic acid × two cycles | Progression and death within 1 month | Walker, 1993 (13) |
| 7 | PRL | Thoracic-lumbar vertebrae, femur | Surgery, RDT, BC, pergolide | CCNU, 5FU, folinic acid × one cycle | Progression with death within a few weeks | Walker, 1993 (13) |
| 8 | TSH | Lung, liver, bone, base of brain | Surgery × 3, RDT, BC, sandostatin LAR, octreotide | 5FU, adriamycin, cyclophosphamide × two cycles | Initial response but progression and death within 2 months of chemo | Mixson, 1993 (25) |
| 9 | FSH, LH | Thoracic, lumbar spine, frontal lobe | Surgery × 2, RDT | Cisplatin, etoposide × six cycles | Initially stable, then progression and death 15 months after chemo | Beauchesne, 1995 (23) |
| 10 | PRL | Ovaries, myometrium, pelvic LN, oral submucosa | Surgery × 2, BC, RDT | Cisplatin, etoposide × two cycles, CCNU, procarbazine, vincristine × two cycles, tamoxifen | Initial progression, but then ↓ PRL levels; alive 10 months after chemo | Gollard, 1995 (70) |
| 11 | PRL | Oral, ovaries, myometrium, LN | Surgery, RDT | Cisplatinum, procarbazine, CCNU, vincristine | Alive at 1.25 yr | Pernicone, 1997 (7) |
| 12 | PRL | Spinal subarachnoid | Surgery, RDT, DA | Cisplatinum, procarbazine, CCNU, vincristine | Died within 1.8 yr | Pernicone, 1997 (7) |
| 13 | Null cell | Femur, liver | Surgery | Cisplatinum, procarbazine, CCNU, vincristine | Died within 4 yr | Pernicone, 1997 (7) |
| 14 | PRL | Ethmoid sinus, LN, orbit, jaw, skull, thoracic spine | BC, surgery, cabergoline, octreotide | Carboplatin and etoposide × three cycles | Died 3 months after chemo | Hurel, 1997 (71) |
| 15 | PRL | Liver, lungs | Surgery, RDT | CCNU + 5FU × two cycles | ↓ PRL 550,000 to 90,000 but progression and death 3 months after chemo | Kaltsas, 1998 (47) |
| 16 | PRL | Thoracic, lumbar spine | RDT | CCNU + 5FU × one cycle | Died 6 months after chemo | Kaltsas, 1998 (47) |
| 17 | PRL | Frontal lobe, parietal lobe, orbit | Surgery, RDT | CCNU + 5FU × six cycles, carboplatin × eight cycles, 5FU, α-INF × eight cycles, carboplatin, α-INF × eight cycles | Died 11 yr after chemo | Kaltsas, 1998 (47) |
| 18 | ACTH | Thoracic spine, liver | Surgery, RDT | CCNU + 5FU × six cycles, carboplatin × six cycles, DTIC × two cycles | Died 3 yr after chemo | Kaltsas, 1998 (47) |
| 19 | FSH, LH | Sella, cavernous sinus, temporo-parietal lobe | Surgery × 2, RDT, octreotide | Cyclophosphamide, adriamycin, DTIC × seven cycles | Died 4 months after chemo | McCutcheon, 2000 (21) |
| 20 | FSH, LH | Ribs, spine | BC, RDT, surgery | Cyclophosphamide, vincristine, dacarbazine × seven cycles | ?Slight ↓ tumor but new metastasis in ribs, alive at publication | Roncaroli, 2003 (22) |
| 21 | PRL | Cerebellopontine angle, cervical spine | Cabergoline, RDT, surgery × 2 | TMZ | ↓PRL, size of tumor, alive 24 months after chemo | Lim, 2006 (19) |
| 22 | LH | Cervical, thoracic spine, ribs | Surgery × 3, RDT, SRT | TMZ × 12 cycles, pegylated INF × 1 month | ↓tumor size and pain, asymptomatic 16 months after chemo | Fadul, 2006 (58) |
| 23 | PRL | Cervical, thoracic spine | BC, surgery, proton beam radiation, spine radiation, octreotide, I131 MIBG | Carboplatin, paclitaxel, etoposide × two cycles, TMZ × 10 cycles | ↓ PRL 694 to 50, asymptomatic 15 months after chemo | Fadul, 2006 (58) |
| 24 | PRL | LN | Cabergoline, surgery, sandostatin, RDT | TMZ × 23 cycles | ↓ PRL 738 to 78, ↓ tumor 62%, mets disappeared, alive 34 months after chemo | Hagen, 2009 (62) |
| 25 | ACTH/ Nelson's | Skull, occiput, ear, cervical spine | Surgery × 2, RDT, SRT | TMZ × six cycles | ↓ACTH 2472 to 389, ↓ tumor, alive 6 months after chemo | Moyes, 2009 (72) |
| 26 | PRL | LN | Surgery × 4, RDT, DA | TMZ | Died | Raverot, 2010 (65) |
| 27 | ACTH | Frontal, occipital lobe and spinal metastases | Surgery × 3, BLA, SRT × 2, XRT × 1 | TMZ × 12 cycles and SOM230 900 μg twice daily, then SOM230 900 μg three times daily | Reduced cranial, spinal, and hepatic mets; increased ACTH with TMZ, stable with TMZ and SOM230 | Bode, 2010 (57) |
| 28 | PRL | NA | Surgery × 1, SRT | TMZ × 24 | 60% reduction in tumor size and PRL level; remained stable 10 months of TMZ treatment | Raverot, 2010 (73) |
| 29 | PRL | NA | Surgery × 4, SRT × 2, GK | TMZ × 5 | No response | Raverot, 2010 (73) |
| 30 | PRL | NA | Surgery × 4, SRT × 2 | TMZ × 3 | No response | Raverot, 2010 (73) |
| 31 | ACTH | NA | Surgery × 3 | TMZ alone × six cycles, TMZ and Carm × six cycles | No tumor or hormone response | Raverot, 2010 (73) |
| 32 | ACTH | NA | Surgery × 4, SRT | TMZ × 6 | Reduced sellar tumor and vertebral metastasis, 52% reduction in ACTH | Raverot, 2010 (73) |
| 33 | Null cell | Spinal metastasis | Surgery × 2, RDT | TMZ × 7 cycles | Progressive disease, died | Bush, 2010 (75) |
| 34 | PRL | Middle and posterior fossa, 3rd and 4th ventricles | Surgery × 5, SRT, RDT, DA | TMZ alone × 12 cycles; TMZ, PCZ, and IFN × two cycles; CARB and VP16 × one cycle | Initial response but relapse with progressive disease, died 11 months after chemo | Murakami, 2011 (76) |
NA, Not available; LN, lymph node; RDT, radiation therapy; SRT, stereotactic radiosurgery; DA, dopamine agonist; BC, bromocriptine; DTIC, dacarbazine; Carm, carmustine; PCZ, procarbazine; CARB, carboplatin; chemo, chemotherapy; mets, metastasis; MIBG, 131I-metaiodobenzylguanidine; BLA, bilateral adrenalectomy; GK, gamma knife; IFN, interferon; SOM230, pasireotide; V16, etoposide; up arrow, increased; down arrow, decreased.
It can be argued that increased survival of 6 months is modest, but given the natural history of pituitary carcinoma, this increase may still be considered significant. In patients with TMZ-responsive pituitary cancers, the question arises as to how long TMZ treatment should be continued. This is a potential concern because alkylating agents such as TMZ are associated with an increased risk of secondary malignancy, particularly hematological disorders such as leukemia and or lymphoma. Although this risk is small, it may become relevant in pituitary tumor patients who will potentially be treated for many years.
The optimal dosing regimen for TMZ in pituitary carcinoma is poorly defined. Most studies have employed a dosing regimen between 150 and 200 mg/m2, although some have employed daily dosing, whereas others have evaluated a 5 of 28-d regimen. It also remains unclear what the long-term TMZ treatment regimen should be in responsive patients who have exhibited stable disease for a period of time. A recent case where, after a 60% reduction in tumor size and serum PRL level after 24 months, no increase in tumor remnant size or PRL level was noted 10 months after TMZ was stopped (65). In one of our patients with PRL-secreting carcinoma, treated successfully for 2 yr, stopping TMZ in a patient with pituitary carcinoma resulted in tumor recurrence after 18 months, which implies that some therapy may be needed long-term in some patients (19). However, it is unclear at this juncture whether intermittent dosing or low-dose continuous therapy would offer stabilization of disease in some or all of these patients and whether such a schedule would ameliorate the risk of secondary malignancy.
Future Therapeutic Options
As noted, some medical therapies, including dopamine agonists and first- and second-generation somatostatin-based therapies, can inhibit pituitary adenoma growth and/or induce adenoma shrinkage, and therefore consideration of use in pituitary carcinomas is not unreasonable. However, most studies describe minimal effect of these agents on pituitary carcinoma growth (77). Interferon-α gained popularity in the 1980s in carcinoid and NET where it exerted some tumor growth inhibition, but its use in pituitary carcinomas is limited and not encouraging (78). In other areas of oncology, approximately 20 molecular-targeted therapies (MTT) have been approved for medical use in cancer, and clinical experience has established their specificity. Tyrosine kinase inhibitors are most effective when the target is constitutively activated by a mutation or a translocation and is a major driver for transformation and tumor progression. MTT are as yet untested in pituitary tumors including carcinomas, and the absence of a clear mutated target makes identification of optimal agents preclinically challenging. However, as in other tumors, many of these kinase pathways are activated in pituitary tumors, providing some rationale for evaluation, although preclinical activity of MTT has been poorly predictive of antitumor activity and tolerability (79).
Two recent studies examined the actions of a rapamycin analog mTOR (mammalian target of rapamycin) inhibitor (rapalogs), Afinitor, and the multikinase inhibitor sunitinib (Sutent) in patients with low and intermediate grade NET who had demonstrated disease progression despite standard of care therapy and are potentially relevant to pituitary carcinoma (80, 81). NET patients treated with the mTOR inhibitor, Afinitor, exhibited improved progression-free survival of 11 months, compared with 4.6 months with best standard of care, and this agent has been shown to reduce cell viability in in vitro cultures of human pituitary tumors (80, 82). Similarly, Sutent-treated patients exhibited improved progression-free survival of 11.4 vs. 5.5 months (80), and both of these agents are approved for management of metastatic pancreatic NET. Similar to pituitary carcinomas, low- and intermediate-grade NET manifest a broad range of Ki-67 LI of 1–2% for low grade and 3–10% for intermediate grade. Tumors exhibiting Ki-67 LI above 20% define these tumors as highly aggressive and set themselves apart from other carcinomas (as in other NET), and arguably chemotherapy should be the mainstay of therapy (83). In pituitary carcinomas, however, reliance on Ki-67 LI alone to diagnose pituitary carcinoma has not yet gained support, but one could argue that a pituitary tumor regardless of size and presence or lack of local invasion with a Ki-67 LI greater than 20–30% is in fact in situ carcinoma.
However, it must be cautioned that despite frequent loss of potential “rapalog” targets in various cancers including phosphatase and tensin homolog, and/or phosphoinositide-3 kinase (PI3K) activation, and/or overexpression of IGF-I receptor or epidermal growth factor receptor, rapalogs have not demonstrated dramatic efficacy. This has fostered development of next-generation TOR-kinase inhibitors (KI) and dual action PI3K/mTOR catalytic site inhibitors (PI3K/TOR-KI). These agents more potently inhibit mTORC1-dependent protein synthesis, impair mTORC1 inhibition-mediated activation of PI3K pathway, and directly inhibit mTORC2. Early indications are that TOR-KI and PI3K/TOR-KI more profoundly inhibit protein and lipid synthesis, more effectively arrest tumor cell growth, and unlike rapalogs, can induce apoptosis and autophagy (84).
Although pituitary tumors are typically identified as hypoenhancing lesions on contrast-enhanced magnetic resonance imaging, they are highly vascular tumors on resection and express high levels of angiogenic factors including vascular endothelial growth factor (VEGF). Sunitinib, a multikinase inhibitor that targets several growth factor receptors, including VEGFR-2 as noted above, has demonstrated efficacy in pancreatic NET, and other more potent VEGFR inhibitors including Axitinib are also in development. The extensive array of these compounds is beyond the scope of this review and includes targeting of many other pathways altered in tumors such as histone deacetylase, heat shock protein, proteasome and topoisomerase inhibitors (85). One can be optimistic that some of these agents will offer additional therapeutic options in pituitary cancers.
Conclusion
Overall, there remain many unanswered questions regarding the diagnosis and management of pituitary carcinomas. The rarity of pituitary carcinoma precludes the likelihood of large-scale randomized clinical trials, and treatment options at present must be based on available case reports or small series. Most studies have shown that multimodal therapy with surgery, radiation therapy, and chemotherapy, although not capable in most situations of offering full or partial disease regression, can lead to disease stabilization (i.e. nonprogression), which in this setting is a very acceptable outcome. What is not clear is that earlier intervention, before repeated surgeries and/or XRT with a drug such as TMZ would offer similar or improved outcomes in some patients. However, given the increasing use of TMZ in recurrent and/or atypical pituitary adenomas that have not metastasized, are not yet by definition carcinomas, and yet behave aggressively, it will be interesting to see whether the progression to true carcinoma in these cases is averted. It is particularly important that the long-term effects of TMZ therapy are better understood before the drug is more broadly used for aggressive pituitary adenomas. Combination conventional chemotherapy with kinase-based agents has offered enhanced efficacy in other cancers, but the use of these types of combination therapy is as yet unexplored in pituitary carcinomas. These combinatorial approaches may offer additional improved therapeutic options for these rare but rapidly fatal cancers.
Acknowledgments
Disclosure Summary: The author has nothing to disclose.
Footnotes
- CCNU
- Cyclo-hexyl-chloroethyl-nitrosourea
- 5FU
- 5-fluorouracil
- KI
- kinase inhibitor
- LI
- labeling indices
- MGMT
- O6-methylguanine-DNA methyltransferase
- mTOR
- mammalian target of rapamycin
- MTT
- molecular-targeted therapy
- NET
- neuroendocrine tumor
- PCNA
- proliferating cell nuclear antigen
- PI3K
- phosphoinositide-3 kinase
- PRL
- prolactin
- SRS
- stereotactic radiosurgery
- TMZ
- temozolomide
- VEGF
- vascular endothelial growth factor
- XRT
- external radiation therapy.
References
- 1. Amar AP, Hinton DR, Krieger MD, Weiss MH. 1999. Invasive pituitary adenomas: significance of proliferation parameters. Pituitary 2:117–122 [DOI] [PubMed] [Google Scholar]
- 2. Thapar K, Kovacs K, Scheithauer BW, Stefaneanu L, Horvath E, Pernicone PJ, Murray D, Laws ER., Jr 1996. Proliferative activity and invasiveness among pituitary adenomas and carcinomas: an analysis using the MIB-1 antibody. Neurosurgery 38:99–106; discussion 106–107 [DOI] [PubMed] [Google Scholar]
- 3. Scheithauer BW, Kovacs KT, Laws ER, Jr, Randall RV. 1986. Pathology of invasive pituitary tumors with special reference to functional classification. J Neurosurg 65:733–744 [DOI] [PubMed] [Google Scholar]
- 4. Daly AF, Tichomirowa MA, Beckers A. 2009. The epidemiology and genetics of pituitary adenomas. Best Pract Res Clin Endocrinol Metab 23:543–554 [DOI] [PubMed] [Google Scholar]
- 5. Roncaroli F, Scheithauer BW, Horvath E, Erickson D, Tam CK, Lloyd RV, Kovacs K. 2010. Silent subtype 3 carcinoma of the pituitary: a case report. Neuropathol Appl Neurobiol 36:90–94 [DOI] [PubMed] [Google Scholar]
- 6. Kontogeorgos G. 2005. Classification and pathology of pituitary tumors. Endocrine 28:27–35 [DOI] [PubMed] [Google Scholar]
- 7. Pernicone PJ, Scheithauer BW, Sebo TJ, Kovacs KT, Horvath E, Young WF, Jr, Lloyd RV, Davis DH, Guthrie BL, Schoene WC. 1997. Pituitary carcinoma: a clinicopathologic study of 15 cases. Cancer 79:804–812 [DOI] [PubMed] [Google Scholar]
- 8. Cusimano MD, Ohori P, Martinez AJ, Jungreis C, Wright DC. 1994. Pituitary carcinoma. Skull Base Surgery 4:46–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kaltsas GA, Grossman AB. 1998. Malignant pituitary tumors. Pituitary 1:69–81 [DOI] [PubMed] [Google Scholar]
- 10. Scheithauer BW, Fereidooni F, Horvath E, Kovacs K, Robbins P, Tews D, Henry K, Pernicone P, Gaffrey TA, Jr, Meyer FB, Young WF, Jr, Fahlbusch R, Buchfelder M, Lloyd RV. 2001. Pituitary carcinoma: an ultrastructural study of eleven cases. Ultrastruct Pathol 25:227–242 [DOI] [PubMed] [Google Scholar]
- 11. Brown RL, Muzzafar T, Wollman R, Weiss RE. 2006. A pituitary carcinoma secreting TSH and prolactin: a non-secreting adenoma gone awry. Eur J Endocrinol 154:639–643 [DOI] [PubMed] [Google Scholar]
- 12. Stefaneanu L, Kovacs K, Horvath E, Lloyd RV, Buchfelder M, Fahlbusch R, Smyth H. 1994. In situ hybridization study of estrogen receptor messenger ribonucleic acid in human adenohypophysial cells and pituitary adenomas. J Clin Endocrinol Metab 78:83–88 [DOI] [PubMed] [Google Scholar]
- 13. Lv H, Li C, Gui S, Zhang Y. 21 March 2011. Expression of estrogen receptor alpha and growth factors in human prolactinoma and its correlation with clinical features and gender. J Endocrinol Invest 10.3275/7607 [DOI] [PubMed] [Google Scholar]
- 14. Branch CL, Jr, Laws ER., Jr 1987. Metastatic tumors of the sella turcica masquerading as primary pituitary tumors. J Clin Endocrinol Metab 65:469–474 [DOI] [PubMed] [Google Scholar]
- 15. Morita A, Meyer FB, Laws ER., Jr 1998. Symptomatic pituitary metastases. J Neurosurg 89:69–73 [DOI] [PubMed] [Google Scholar]
- 16. Mountcastle RB, Roof BS, Mayfield RK, Mordes DB, Sagel J, Biggs PJ, Rawe SE. 1989. Pituitary adenocarcinoma in an acromegalic patient: response to bromocriptine and pituitary testing: a review of the literature on 36 cases of pituitary carcinoma. Am J Med Sci 298:109–118 [DOI] [PubMed] [Google Scholar]
- 17. Gillam MP, Middler S, Freed DJ, Molitch ME. 2002. The novel use of very high doses of cabergoline and a combination of testosterone and an aromatase inhibitor in the treatment of a giant prolactinoma. J Clin Endocrinol Metab 87:4447–4451 [DOI] [PubMed] [Google Scholar]
- 18. Lim JS, Ku CR, Lee MK, Kim TS, Kim SH, Lee EJ. 2010. A case of fugitive acromegaly, initially presenting as invasive prolactinoma. Endocrine 38:1–5 [DOI] [PubMed] [Google Scholar]
- 19. Lim S, Shahinian H, Maya MM, Yong W, Heaney AP. 2006. Temozolomide: a novel treatment for pituitary carcinoma. Lancet Oncol 7:518–520 [DOI] [PubMed] [Google Scholar]
- 20. Sheldon WH, Golden A, Bondy PK. 1954. Cushing's syndrome produced by a pituitary basophil carcinoma with hepatic metastases. Am J Med 17:134–142 [DOI] [PubMed] [Google Scholar]
- 21. McCutcheon IE, Pieper DR, Fuller GN, Benjamin RS, Friend KE, Gagel RF. 2000. Pituitary carcinoma containing gonadotropins: treatment by radical excision and chemotherapy: case report. Neurosurgery 46:1233–1239; discussion 1239–1240; discussion 1239–1240 [DOI] [PubMed] [Google Scholar]
- 22. Roncaroli F, Nosé V, Scheithauer BW, Kovacs K, Horvath E, Young WF, Jr, Lloyd RV, Bishop MC, Hsi B, Fletcher JA. 2003. Gonadotropic pituitary carcinoma: HER-2/neu expression and gene amplification—report of two cases. J Neurosurg 99:402–408 [DOI] [PubMed] [Google Scholar]
- 23. Beauchesne P, Trouillas J, Barral F, Brunon J. 1995. Gonadotropic pituitary carcinoma: case report. Neurosurgery 37:810–816 [DOI] [PubMed] [Google Scholar]
- 24. Spertini F, Deruaz JP, Perentes E, Pelet B, Gomez F. 1986. Luteinizing hormone (LH) and prolactin-secreting pituitary tumor: possible malignant transformation of the LH cell line. J Clin Endocrinol Metab 62:849–854 [DOI] [PubMed] [Google Scholar]
- 25. Mixson AJ, Friedman TC, Katz DA, Feuerstein IM, Taubenberger JK, Colandrea JM, Doppman JL, Oldfield EH, Weintraub BD. 1993. Thyrotropin-secreting pituitary carcinoma. J Clin Endocrinol Metab 76:529–533 [DOI] [PubMed] [Google Scholar]
- 26. Heaney AP, Melmed S. 2004. Pituitary tumors: molecular targets and new medical therapies. Nat Rev Cancer 4:285–295 [DOI] [PubMed] [Google Scholar]
- 27. Theodoropoulou M, Cavallari I, Barzon L, D'Agostino DM, Ferro T, Arzberger T, Grübler Y, Schaaf L, Losa M, Fallo F, Ciminale V, Stalla GK, Pagotto U. 2004. Differential expression of menin in sporadic pituitary adenomas. Endocr Relat Cancer 11:333–344 [DOI] [PubMed] [Google Scholar]
- 28. Pei L, Melmed S, Scheithauer B, Kovacs K, Prager D. 1994. H-ras mutations in human pituitary carcinoma metastasis. J Clin Endocrinol Metab 78:842–846 [DOI] [PubMed] [Google Scholar]
- 29. Thapar K, Kovacs K, Muller PJ. 1995. Clinical-pathologic Correlations of Pituitary Tumors. Baillieres Clin Endocrinol Metab 9:243–270 [DOI] [PubMed] [Google Scholar]
- 30. Challa VR, Marshall RB, Hopkins MB, 3rd, Kelly DL, Jr, Civantos F. 1985. Pathobiologic study of pituitary tumors: report of 62 cases with a review of the recent literature. Hum Pathol 16:873–884 [DOI] [PubMed] [Google Scholar]
- 31. Saeger W, Lüdecke DK, Buchfelder M, Fahlbusch R, Quabbe HJ, Petersenn S. 2007. Pathohistological classification of pituitary tumors: 10 years of experience with the German Pituitary Tumor Registry. Eur J Endocrinol 156:203–216 [DOI] [PubMed] [Google Scholar]
- 32. Hashimoto N, Handa H, Nishi S. 1986. Intracranial and intraspinal dissemination from a growth hormone-secreting pituitary tumor. J Neurosurg 64:140–144 [DOI] [PubMed] [Google Scholar]
- 33. Kovacs K. 2006. The 2004 WHO classification of pituitary tumors: comments. Acta Neuropathol 111:62–63 [DOI] [PubMed] [Google Scholar]
- 34. Salehi F, Agur A, Scheithauer BW, Kovacs K, Lloyd RV, Cusimano M. 2009. Ki-67 in pituitary neoplasms: a review—Part I. Neurosurgery 65:429–437 [DOI] [PubMed] [Google Scholar]
- 35. Scheithauer BW, Gaffey TA, Lloyd RV, Sebo TJ, Kovacs KT, Horvath E, Yapicier O, Young WF, Jr, Meyer FB, Kuroki T, Riehle DL, Laws ER., Jr 2006. Pathobiology of pituitary adenomas and carcinomas. Neurosurgery 59:341–353 [DOI] [PubMed] [Google Scholar]
- 36. Tanizaki Y, Jin L, Scheithauer BW, Kovacs K, Roncaroli F, Lloyd RV. 2007. p53 Gene mutations in pituitary carcinomas. Endocr Pathol 18:217–222 [DOI] [PubMed] [Google Scholar]
- 37. Thapar K, Scheithauer BW, Kovacs K, Pernicone PJ, Laws ER., Jr 1996. p53 Expression in pituitary adenomas and carcinomas: correlation with invasiveness and tumor growth fractions. Neurosurgery 38:765–770; discussion 770–771 [PubMed] [Google Scholar]
- 38. Scheithauer BW, Kurtkaya-Yapicier O, Kovacs KT, Young WF, Jr, Lloyd RV. 2005. Pituitary carcinoma: a clinicopathological review. Neurosurgery 56:1066–1074 [PubMed] [Google Scholar]
- 39. Manahan MA, Dackiw AP, Ball DW, Zeiger MA. 2007. Unusual case of metastatic neuroendocrine tumor. Endocr Pract 13:72–76 [DOI] [PubMed] [Google Scholar]
- 40. Ragel BT, Couldwell WT. 2004. Pituitary carcinoma: a review of the literature. Neurosurg Focus 16:E7. [DOI] [PubMed] [Google Scholar]
- 41. Lopes MB, Scheithauer BW, Schiff D. 2005. Pituitary carcinoma: diagnosis and treatment. Endocrine 28:115–121 [DOI] [PubMed] [Google Scholar]
- 42. Saeger W, Lubke D. 1996. Pituitary carcinomas. Endocr Pathol 7:21–35 [DOI] [PubMed] [Google Scholar]
- 43. Garrão AF, Sobrinho LG, Pedro-Oliveira, Bugalho MJ, Boavida JM, Raposo JF, Loureiro M, Limbert E, Costa I, Antunes JL. 1997. ACTH-producing carcinoma of the pituitary with haematogenic metastases. Eur J Endocrinol 137:176–180 [DOI] [PubMed] [Google Scholar]
- 44. Frost AR, Tenner S, Tenner M, Rollhauser C, Tabbara SO. 1995. ACTH-producing pituitary carcinoma presenting as the cauda equina syndrome. Arch Pathol Lab Med 119:93–96 [PubMed] [Google Scholar]
- 45. Ayuk J, Natarajan G, Geh JI, Mitchell RD, Gittoes NJ. 2005. Pituitary carcinoma with a single metastasis causing cervical spinal cord compression: case report. J Neurosurg Spine 2:349–353 [DOI] [PubMed] [Google Scholar]
- 46. Cartwright DM, Miller TR, Nasr AJ. 1994. Fine-needle aspiration biopsy of pituitary carcinoma with cervical lymph node metastases: a report of two cases and review of the literature. Diagn Cytopathol 11:68–73 [DOI] [PubMed] [Google Scholar]
- 47. Kaltsas GA, Mukherjee JJ, Plowman PN, Monson JP, Grossman AB, Besser GM. 1998. The role of cytotoxis chemotherapy in the management of aggressive and malignant pituitary tumors. J Clin Endocrinol Metab 83:4233–4238 [DOI] [PubMed] [Google Scholar]
- 48. Swords FM, Allan CA, Plowman PN, Sibtain A, Evanson J, Chew SL, Grossman AB, Besser GM, Monson JP. 2003. Stereotactic radiosurgery XVI: a treatment for previously irradiated pituitary adenomas. J Clin Endocrinol Metab 88:5334–5340 [DOI] [PubMed] [Google Scholar]
- 49. Loeffler JS, Shih HA. 2011. Radiation therapy in the management of pituitary adenomas. J Clin Endocrinol Metab 96:1992–2003 [DOI] [PubMed] [Google Scholar]
- 50. Rowland NC, Aghi MK. 2010. Radiation treatment strategies for acromegaly. Neurosurg Focus 29:E12. [DOI] [PubMed] [Google Scholar]
- 51. Sheehan JP, Pouratian N, Steiner L, Laws ER, Vance ML. 2011. Gamma knife surgery for pituitary adenomas: factors related to radiological and endocrine outcomes. J Neurosurg 114:303–309 [DOI] [PubMed] [Google Scholar]
- 52. Castinetti F, Nagai M, Dufour H, Kuhn JM, Morange I, Jaquet P, Conte-Devolx B, Regis J, Brue T. 2007. Gamma knife radiosurgery is a successful adjunctive treatment in Cushing's disease. Eur J Endocrinol 156:91–98 [DOI] [PubMed] [Google Scholar]
- 53. Kwekkeboom DJ, de Herder WW, Krenning EP. 2011. Somatostatin receptor-targeted radionuclide therapy in patients with gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am 40:173–185, ix [DOI] [PubMed] [Google Scholar]
- 54. Walker JD, Grossman A, Anderson JV, Ur E, Trainer PJ, Benn J, Lowy C, Sönksen PH, Plowman PN, Lowe DG, 1993. Malignant prolactinoma with extracranial metastases: a report of three cases. Clin Endocrinol (Oxf) 38:411–419 [DOI] [PubMed] [Google Scholar]
- 55. Melmed S, Sternberg R, Cook D, Klibanski A, Chanson P, Bonert V, Vance ML, Rhew D, Kleinberg D, Barkan A. 2005. A critical analysis of pituitary tumor shrinkage during primary medical therapy in acromegaly. J Clin Endocrinol Metab 90:4405–4410 [DOI] [PubMed] [Google Scholar]
- 56. Biller BM, Grossman AB, Stewart PM, Melmed S, Bertagna X, Bertherat J, Buchfelder M, Colao A, Hermus AR, Hofland LJ, Klibanski A, Lacroix A, Lindsay JR, Newell-Price J, Nieman LK, Petersenn S, Sonino N, Stalla GK, Swearingen B, Vance ML, Wass JA, Boscaro M. 2011. Pasireotide (SOM230) demonstrates efficacy in patients with Cushing disease: results from a larger randomized-dose, double-blind, phase III study. Endocr Rev 32;OR09-6 (Abstract) [Google Scholar]
- 57. Bode H, Seiz M, Lammert A, Brockmann MA, Back W, Hammes HP, Thomé C. 2010. SOM230 (pasireotide) and temozolomide achieve sustained control of tumour progression and ACTH secretion in pituitary carcinoma with widespread metastases. Exp Clin Endocrinol Diabetes 118:760–763 [DOI] [PubMed] [Google Scholar]
- 58. Fadul CE, Kominsky AL, Meyer LP, Kingman LS, Kinlaw WB, Rhodes CH, Eskey CJ, Simmons NE. 2006. Long-term response to pituitary carcinoma to temozolomide: report of two cases. J Neurosurg 105:621–626 [DOI] [PubMed] [Google Scholar]
- 59. Komninos J, Vlassopoulou V, Protopapa D, Korfias S, Kontogeorgos G, Sakas DE, Thalassinos NC. 2004. Tumors metastatic to the pituitary gland: case report and literature review. J Clin Endocrinol Metab 89:574–580 [DOI] [PubMed] [Google Scholar]
- 60. Esteller M, Garcia-Foncillas J, Andion E, Goodman SN, Hidalgo OF, Vanaclocha V, Baylin SB, Herman JG. 2000. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med 343:1350–1354 [DOI] [PubMed] [Google Scholar]
- 61. McCormack AI, McDonald KL, Gill AJ, Clark SJ, Burt MG, Campbell KA, Braund WJ, Little NS, Cook RJ, Grossman AB, Robinson BG, Clifton-Bligh RJ. 2009. Low O6–methylguanine-DNA methyltransferase (MGMT) expression and response to temozolomide in aggressive pituitary tumors. Clin Endocrinol (Oxf) 71:226–233 [DOI] [PubMed] [Google Scholar]
- 62. Hagen C, Schroeder HD, Hansen S, Hagen C, Andersen M. 2009. Temozolomide treatment if a pituitary carcinoma and two pituitary macroadenomas resistant to conventional therapy. Eur J Endocrinol 161:631–637 [DOI] [PubMed] [Google Scholar]
- 63. Kovacs K, Horvath E, Syro LV, Uribe H, Penagos LC, Ortiz LD, Fadul CE. 2007. Temozolomide therapy in a man with an aggressive prolactin-secreting pituitary neoplasm: morphological findings. Hum Pathol 38:185–189 [DOI] [PubMed] [Google Scholar]
- 64. Kovacs K, Scheithauer BW, Lombardero M, McLendon RE, Syro LV, Uribe H, Ortiz LD, Penagos LC. 2008. MGMT immunoexpression predicts responsiveness of pituitary tumors to temozolomide therapy. Acta Neuropathol 115:261–262 [DOI] [PubMed] [Google Scholar]
- 65. Raverot G, Sturm N, de Fraipont F, Muller M, Salenave S, Caron P, Chabre O, Chanson P, Cortet-Rudelli C, Assaker R, Dufour H, Gaillard S, François P, Jouanneau E, Passagia JG, Bernier M, Cornélius A, Figarella-Branger D, Trouillas J, Borson-Chazot F, Brue T. 2010. Temozolomide treatment of aggressive pituitary tumors and pituitary carcinomas: a French multicenter experience. J Clin Endocrinol Metab 95:4592–4599 [DOI] [PubMed] [Google Scholar]
- 66. Kaiser FE, Orth DN, Mukai K, Oppenheimer JH. 1983. A pituitary parasellar tumor with extracranial metastases and high, partially suppressible levels of adrenocorticotropin and related peptides. J Clin Endocrinol Metab 57:649–653 [DOI] [PubMed] [Google Scholar]
- 67. Asai A, Matsutani M, Funada N, Takakura K. 1988. Malignant growth hormone-secreting pituitary adenoma with haematogenous dural metastasis: case report. Neurosurgery 22:1091–1094 [DOI] [PubMed] [Google Scholar]
- 68. Nawata H, Higuchi K, Ikuyama S, Kato K, Ibayashi H, Mimura K, Sueishi K, Zingami H, Imura H. 1990. Corticotropin-releasing hormone- and adrenocorticotropin-producing pituitary carcinoma with metastases to the liver and lung in a patient with Cushing's disease. J Clin Endocrinol Metab 71:1068–1073 [DOI] [PubMed] [Google Scholar]
- 69. Petterson T, MacFarlane IA, MacKenzie JM, Shaw MDM. 1992. Prolactin secreting pituitary carcinoma. J Neurol Neurosurg Psychiatry 55:1205–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gollard R, Kosty M, Cheney C, Copeland B, Bordin G. 1995. Prolactin-secreting pituitary carcinoma with implants in the cheek pouch and metastases to the ovaries. Cancer 76:1814–1820 [DOI] [PubMed] [Google Scholar]
- 71. Hurel SJ, Harris PE, McNicol AM, Foster S, Kelly WF, Baylis PH. 1997. Metastatic prolactinoma: effect of octreotide, cabergoline, carboplatin and etoposide; immunocytochemical analysis of proto-oncogene expression. J Clin Endocrinol Metab 82:2962–2965 [DOI] [PubMed] [Google Scholar]
- 72. Moyes VJ, Alusi G, Sabin HI, Evanson J, Berney DM, Kovacs K, Monson JP, Plowman PN, Drake WM. 2009. Treatment of Nelson's syndrome with temozolomide. Eur J Endocrinol 160:115–119 [DOI] [PubMed] [Google Scholar]
- 73. Raverot G, Wierinckx A, Dantony E, Auger C, Chapas G, Villeneuve L, Brue T, Figarella-Branger D, Roy P, Jouanneau E, Jan M, Lachuer J, Trouillas J. 2010. Prognostic factors in prolactin pituitary tumors: clinical, histological, and molecular data from a series of 94 patients with a long postoperative follow-up. J Clin Endocrinol Metab 95:1708–1716 [DOI] [PubMed] [Google Scholar]
- 74. Moin T, Yong WH, Heaney AP. 2011. Atypical adenoma, pituitary carcinoma and the role of chemotherapy in the management of refractory pituitary adenoma. In: Schwartz TH, Anand VK. eds. Endoscopic pituitary surgery. New York: Thieme Publishers [Google Scholar]
- 75. Bush ZM, Longtine JA, Cunningham T, Schiff D, Jane JA, Jr, Vance ML, Thorner MO, Laws ER, Jr, Lopes MB. 2010. Temozolomide treatment for aggressive pituitary tumors: correlation of clinical outcomes with DNA methylation and MGMT expression. J Clin Endocrinol Metab 95:E280–E290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Murakami M, Mizutani A, Asano S, Katakami H, Ozawa Y, Yamazaki K, Ishida Y, Takano K, Okinaga H, Matsuno A. 2011. A mechanism of acquiring temozolomide resistance during transformation of atypical prolactinoma into prolactin-producing pituitary carcinoma: case report. Neurosurgery 68:E1761–E1767; discussion E1767 [DOI] [PubMed] [Google Scholar]
- 77. Greenman Y, Woolf P, Coniglio J, O'Mara R, Pei L, Said JW, Melmed S. 1996. Remission of acromegaly caused by pituitary carcinoma after surgical excision of growth hormone-secreting metastasis detected by 111-indium pentetreotide scan. J Clin Endocrinol Metab 81:1628–1633 [DOI] [PubMed] [Google Scholar]
- 78. Ahmed M, Kanaan I, Alarifi A, Ba-Essa E, Saleem M, Tulbah A, McArthur P, Hessler R. 2000. ACTH-producing pituitary cancer: experience at the King Faisal Specialist Hospital, Research Center. Pituitary 3:105–112 [DOI] [PubMed] [Google Scholar]
- 79. Pivot X, Bedairia N, Thiery-Vuillemin A, Espie M, Marty M. 2011. Combining molecular targeted therapies: clinical experience. Anticancer Drugs 22:701–710 [DOI] [PubMed] [Google Scholar]
- 80. Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, Valle J, Metrakos P, Smith D, Vinik A, Chen JS, Hörsch D, Hammel P, Wiedenmann B, Van Cutsem E, Patyna S, Lu DR, Blanckmeister C, Chao R, Ruszniewski P. 2011. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 364:501–513 [DOI] [PubMed] [Google Scholar]
- 81. Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, Hobday TJ, Okusaka T, Capdevila J, de Vries EG, Tomassetti P, Pavel ME, Hoosen S, Haas T, Lincy J, Lebwohl D, Öberg K. 2011. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 364:514–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zatelli MC, Minoia M, Filieri C, Tagliati F, Buratto M, Ambrosio MR, Lapparelli M, Scanarini M, Degli Uberti EC. 2010. Effect of everolimus on cell viability in nonfunctioning pituitary adenomas. J Clin Endocrinol Metab 95:968–976 [DOI] [PubMed] [Google Scholar]
- 83. Kulke MH, Siu LL, Tepper JE, Fisher G, Jaffe D, Haller DG, Ellis LM, Benedetti JK, Bergsland EK, Hobday TJ, Van Cutsem E, Pingpank J, Oberg K, Cohen SJ, Posner MC, Yao JC. 2011. Future directions in the treatment of neuroendocrine tumors: consensus report of the National Cancer Institute neuroendocrine tumor clinical trials planning meeting. J Clin Oncol 29:934–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Wander SA, Hennessy BT, Slingerland JM. 2011. Next-generation mTOR inhibitors in clinical oncology: how pathway complexity informs therapeutic strategy. J Clin Invest 121:1231–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Perez CA, Santos ES, Arango BA, Raez LE, Cohen EE. 4 May 2011. Novel molecular targeted therapies for refractory thyroid cancer. Head Neck 10.1002/hed.21755 [DOI] [PubMed] [Google Scholar]