Abstract
Both breeding activity and abundance and quality of available food are expected to influence daily movements of animals. Animals are predicted to range over large areas to meet high energy demands associated with reproduction (females) or to increase mating success (males). However, animals should expand their range areas whenever food conditions deteriorate. To examine the extent to which breeding activity versus food availability influence space use, we compared the size and location of range areas (home ranges) of the degu (Octodon degus), a diurnal rodent from semiarid environments of north-central Chile, during the austral winter and summer seasons. Degus produce young during the austral spring (September–October) when high-quality food is readily available. In contrast, degus do not breed during the austral summer (January–March) when food is scarce and of low quality. We predicted that degus would range over smaller areas in winter if the availability of food has a greater influence on space than breeding activity. Individuals were radiotracked in winter and the following summer over a 3-year period. Surveys of herbaceous cover were conducted during winter and summer to determine seasonal changes in the abundance and quality of primary food. In summer degus expanded and moved the location of their range areas to locations with available food. Given that preferred food was less abundant in summer than winter, we suggest that degu range areas are strongly influenced by food conditions.
Keywords: breeding activity, degus, food availability, range size, semiarid environment
Intraspecific variation in the range areas (home ranges) of individuals can be caused by numerous extrinsic (e.g., food abundance and quality) and endogenous (e.g., changes in the breeding activity and sex of individuals) factors (Burt 1943; Cooper and Randall 2007; Schradin and Pillay 2006; Slobodchikoff 1984). Separating the roles played by each of these factors is challenging. Difficulties arise when 2 or more factors covary and thus, emerging patterns are equally consistent with alternative scenarios. For instance, individuals are expected to range over smaller areas whenever food conditions and overall habitat productivity are high (Gompper and Gittleman 1991; Harestad and Bunnell 1979). This prediction generally has been supported by correlative studies (Corp et al. 1997; Harris and Leitner 2004; Lurz et al. 2000) and in food-supplementation studies in small mammals (Hubbs and Boonstra 1998; Ims 1987; Ostfeld 1986; Slobodchikoff 1984). An alternative argument is that an association between small range areas and high abundance of food is an indirect effect of food on range areas via density of the consumer (Desy et al. 1990; Taitt 1981; Taitt and Krebs 1981; Wauters and Dhondt 1998). In particular, consumer density can increase in response to food availability and cause a density-dependent decrease of range areas. In contrast, high consumer density also can be the direct consequence of animals decreasing their range areas in response to favorable food conditions (Jones 1990; Mares et al. 1982). Understanding the link between these factors is inherently difficult. Field studies may provide evidence of causation whenever the observed variation in spatial behavior is inconsistent with some of these hypotheses.
The range areas of individuals are influenced by breeding activity. Theory predicts that males range over larger areas than females during breeding (mating) to maximize mating opportunities (Emlen and Oring 1977; Ims 1987; Ostfeld 1985, 1990). Smaller male range areas are predicted during the nonbreeding season or under conditions of high conspecific density due to competition between males over mating (Ostfeld 1985). Compared with males, the range areas of females are expected to be more sensitive to variation in the abundance and distribution of food resources (Brashares and Arcese 2002; Emlen and Oring 1977). Females should have larger range areas in habitats with relatively low-quantity and poor-quality food than in habitats with relatively high quantity and quality of food (Gompper and Gittleman 1991; Harestad and Bunnell 1979; Ims 1987; Ostfeld 1985).
Theory and empirical evidence derived from behavioral energetics and habitat selection indicates that spatial variation in food conditions is a major influence on the size and location of range areas of individuals (Batzli 1985; Brown and Nicoletto 1991). More recently, Schradin and Pillay (2006) proposed the hypothesis that range areas of herbivores, particularly small mammals, change in response to seasonal variation in food resources. They observed that the ranges of striped mice (Rhabdomys pumilio) are influenced by the location of new growth of annual plants in South Africa (Schradin and Pillay 2006). Seasonal changes in food resources also influence the range areas of wood mice (Apodemus sylvaticus—Wolton 1985; but see Todd et al. 2000) and red squirrels (Sciurus vulgaris—Lurz et al. 2000).
In most habitats the abundance and quality of vegetation increases after periods of rain (Gutiérrez et al. 1993, 2000; Hoffman et al. 1977). This relationship can be particularly important in semiarid environments, which are characterized by considerable seasonal variation in rain and temperature. In semiarid environments many species time their breeding to these high-quality food conditions, providing an opportunity to examine the relative influence of breeding activity and food conditions on space use. Irrespective of breeding status, females living in semiarid environments should range over relatively larger areas during periods of low food availability than during periods with abundant food. In contrast, breeding males should have large range areas during breeding periods and relatively small range areas whenever mating has ended. Similar to females and irrespective of breeding status, males should decrease their range areas whenever experiencing abundant food conditions but expand their ranges when food is limited.
To date, few studies have examined the influence of seasonal variation in food availability on the range areas of small mammals. A few studies suggest that range areas increase with decreasing food availability (Eccard et al. 2004; Harris and Leitner 2004), and others suggest that range areas decrease with increasing food availability (Schradin and Pillay 2006) or are unaffected by the availability of food (Cooper and Randall 2007). Thus, we currently do not have a general understanding of the influence of seasonally variable food conditions on the space use of small mammals. We monitored the space use of degus (Octodon degus), representing the New World hystricognaths, an old and distantly related group of rodents compared with the better-known murid species. Evidence of similarities in the space use of murids and hystricognathans in seasonal environments would suggest convergent behavior to similar environmental pressures. On the basis of ecological and space use theory (Ims 1987; Ostfeld 1986, 1990), we hypothesized that degus expand and shift the location of their range areas in response to seasonal changes in food supply. We tested this hypothesis by comparing range areas and the abundance of primary foods during the breeding (winter) and nonbreeding periods (summer).
Materials and Methods
Study animal
Degus are diurnally active (Ebensperger et al. 2004; Kenagy et al. 2002) and semifossorial rodents inhabiting the semiarid Mediterranean environments of central Chile (Ebensperger 1998; Meserve et al. 1984; Yáñez 1976). In these environments the austral winter (June–September) is characterized by low ambient temperatures and abundant rain. In contrast, summers (January–March) are characterized by high ambient temperature and low rain. As a result, the aboveground green parts of annual grasses and forbs, the preferred food of degus (Meserve et al. 1983, 1984), reach a maximum during late winter–early spring and then decrease to a minimum during early summer–autumn (Ebensperger and Hurtado 2005). Degus typically breed once per year during late autumn (May–June—Ebensperger and Hurtado 2005; Rojas et al. 1977). After a 3-month pregnancy females give birth during mid-September. Thus, lactation takes place when food resources are generally abundant and of high quality. In contrast, degus of central Chile do not breed from summer through autumn (December–May), a time when food resources are limited and of poor quality.
Study area and observation period
The study was conducted during the austral winter (August–September) and summer (January–February) months of 2005–2006 and 2007–2008 at the Estación Experimental Rinconada de Maipú (33°23′S, 70°31′W; altitude 495 m), a field station of Universidad de Chile. The study site has a characteristic Mediterranean climate and consists of open matorral with scattered shrubs (Proustia pungens, Acacia caven, and Baccharis spp.) and annual grasses and forbs (Ebensperger and Hurtado 2005). In June 2005 we established a 4–5-ha study area in an area in which degus were visually abundant (Hayes et al. 2007).
Trapping and marking of animals
We trapped degus using a combination of Tomahawk (model 201, 14 × 14 × 40 cm; Tomahawk Live Trap Co., Tomahawk, Wisconsin), and locally produced metal live traps (9.5 × 10 × 30 cm; similar to Sherman traps) all baited with rolled oats (Burger et al. 2009). We set traps at previously identified burrow systems (Hayes et al. 2007) for 3–5 consecutive days before emergence of adults (early morning). After 1.5 h traps were checked, and captured degus were radiocollared if they weighed >160 g and were captured previously. Animal identity, sex, body weight, and reproductive condition (females only) were recorded for each individual.
We assigned individuals a unique identification at the time of 1st capture by removing the 1st or 2nd phalanges of 1–4 toes (2005–2006: n = 97 individuals, 29.89% two digits, 70.1% three digits; 2007–2008: n = 278, 2.52% one digit, 29.5% two digits, 18.5% three digits, 49.6% four digits) on no more than 1 toe per foot (Hayes et al. 2009). We used toe clipping because of the need to permanently mark a large number of individuals required to quantify spatial patterns (Ebensperger et al. 2009; Hayes et al. 2007) and reproduction (Hayes et al. 2009; Quan et al. 2009). We minimized pain by making rapid cuts with sharp sterilized clippers. In the event that an individual was bleeding (qualitative estimate is <20%), we applied light pressure to stop bleeding before an individual was released. We applied a topical antibiotic to reduce the risk of subsequent infections. Infections were extremely rare (1 infection for every 100 degus clipped). Tissue samples were kept for genetic analyses (Quan et al. 2009). This study followed the American Society of Mammalogists guidelines (Gannon et al. 2007), was approved by the University of Louisiana at Monroe Institutional Animal Use and Care Committee, and adhered to United States and Chilean laws (permit number 1–58.2005 [2711] by the Servicio Agrícola y Ganadero).
Radiotracking and range areas
In August 2005 we radiotracked 20 adult females. Eight of these females survived until February 2006 and were radiotracked again. We reported the range areas of 7 of these females due to the failure of 1 radiocollar. We radiotracked 28 adult females and 8 adult males during early September 2007. Fifteen of these females and 4 males survived until late January 2008 and were radiotracked again. Radiotracking was performed using LA 12-Q receivers (for radiocollars tuned to 150.000–151.999 MHz frequency) and a 7-element null peak antenna system (AVM Instrument Co., Colfax, California). Individuals were tagged with radiocollars equipped with BR transmitters (AVM Instrument Co.) or with RI-2D transmitters (Holohil Systems, Ltd., Carp, Ontario, Canada), weighing 8 g and representing <5% of body weight.
Daytime ranges were determined by locating the position of animals through triangulation (Kenward 2001) at hourly intervals during the known active period of degus (Kenagy et al. 2002). Two teams of researchers simultaneously recorded bearings of each individual (± 0.5°). Pairs of bearings were then converted to X–Y (north–east) coordinates with Locate II software (Pacer Software, Truro, Nova Scotia, Canada) for subsequent analyses. Two estimates of range areas, 95% minimum convex polygons (MCP) and 95% fixed kernels (FK), were calculated using Ranges VI software (Kenward et al. 2003). We determined MCP range areas on the basis of kernel cores and FK areas with a 40 × 40 matrix. The fixed multiplier was set to “1” in the analysis of FK ranges.
To examine whether individuals modify the size and location of their range areas we used the estimated range cores (generated by Ranges VI when using the kernel algorithm) during winter and summer seasons and combined this information with range overlap between seasons. An individual exhibiting low overlap between its winter and summer ranges coupled to a relatively large distance between the cores calculated during both seasons would indicate that the animal has shifted the overall location of its range area. Overlap of range areas was estimated using 95% MCPs.
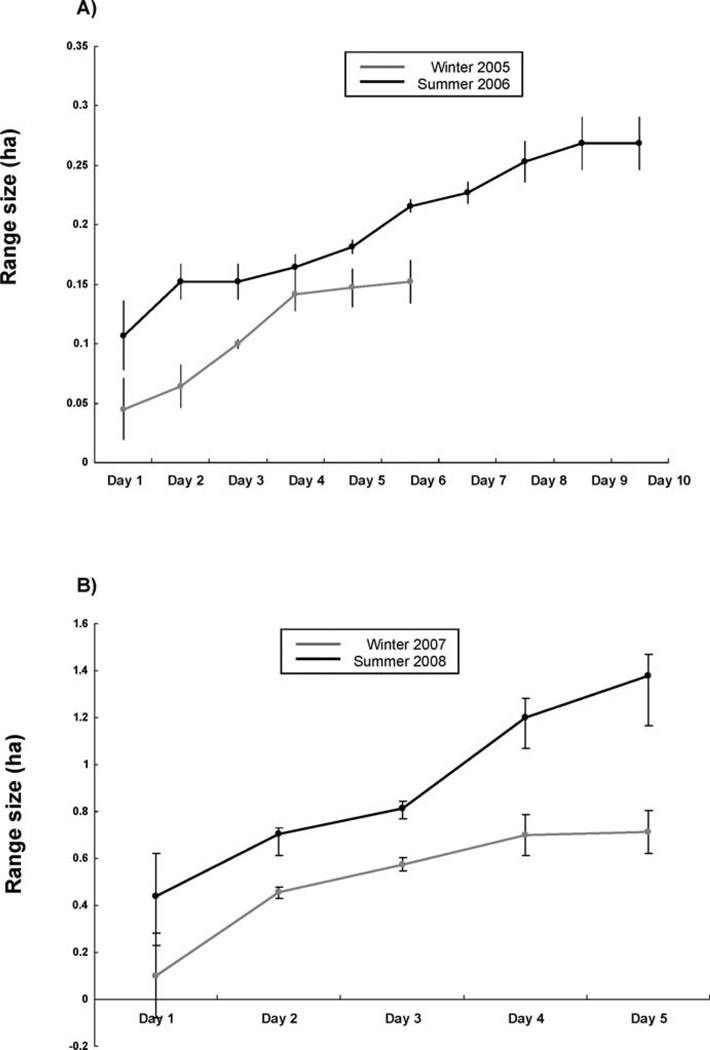
Mean number of locations per animal was 37.3 (SD = 1.9) in winter 2005, 32.4 (SD = 6.6) in summer 2006, 29.2 (SD = 5) in winter 2007, and 26.5 (SD = 3.6) in summer 2008. The sampling saturation was 5–6 days in winter 2005 and 9–10 days in summer 2006 (Fig. 1A). Sampling saturation was achieved after 4–5 days in winter 2007 but not after 5 days in summer 2008 (Fig. 1B). These findings suggested that range areas for summer 2007 were underestimates of the true range areas.
Fig. 1.
Relationship between MCP home-range size and number of days of radiotracking in A) winter 2005 (7 points per day, n = 7 individuals) and summer 2006 (4 points per day, n = 7 individuals) and B) winter 2007 (8 points per day, n = 19 individuals) and summer 2008 (8 points per day, n = 19 individuals).
Feeding habits and plant surveys
The objective of this study required testing if degus of the studied population were feeding mostly on annual grasses and forbs and that the abundance and quality of these preferred foods were greater in winter than in summer. To examine the 1st aspect we collected 2–10 fecal droppings from burrow systems and livetrapped degus during August 2005 and 2006. Fecal pellets were dried at 60°C for 72 h (Hansson 1970; Korschgen 1969). Fecal material was pooled into common samples according to the burrow systems used by individuals in this study (Hayes et al. 2007, 2009). The mean number of fecal pellets per burrow system in subsequent analyses was 16.3 (SD = 4.7; n = 12) in 2005 and 12.8 (SD = 1.1; n = 5) in 2006. From each burrow system we prepared 10 microscope slides according to standard procedures (Dizeo de Strittmatter 1984). Then 5 subsamples were taken and mounted on microscope slides, covered with a 22 × 22 mm slide cover, and sealed with Entellan Merck. A total of 20 fields from each slide was examined under a Nikon Eclipse E200 microscope equipped with a 10 × 10 quadrant graduated lens. Fields with <50% of area covered with vegetation remains were discarded (Meserve 1981). We compared plant fragments in slides with samples of a voucher collection. The voucher collection was prepared previously from vegetation samples taken during the sampling of food abundance and quality (see below). We quantified the importance of each consumed item as a percentage calculated from the relative surface covered by each plant item in the fields examined (Meserve 1981).
We quantified seasonal variation in the abundance of primary foods (Meserve et al. 1984) by sampling annual herbs during September 2005 and 2007 and January 2008. We collected samples of green herbs at 3 and 9 m from the center of 10 randomly selected burrow systems in the north, east, south, and west cardinal directions (Hayes et al. 2007). At each sampling point we removed the aboveground parts of all green herbs within a 250 × 250 mm2 quadrant (Ebensperger and Hurtado 2005). Samples were dried in an oven (60°C) for 72 h and subsequently weighed (0.01 g) to determine dry mass (g). To compare quality of primary foods we collected 8 samples of green herbaceous vegetation (within 250 × 250 mm2 quadrants) at random points throughout the study area where degus regularly were seen foraging. Two indicators of food quality, insoluble and soluble fiber content, were determined by standard chemical analysis at the Instituto de Nutrición y Tecnología de los Alimentos (Universidad de Chile, Santiago, Chile). Dietary fiber represents a barrier to the extraction of soluble nutrients from cells and is difficult for nonruminants to digest (Van Soest 1982). Lab studies show that degus minimize fiber intake when given a choice of high-fiber and low-fiber foods, supporting the hypothesis that dietary fiber is not a preferred dietary component (Bozinovic 1995; Veloso and Bozinovic 1993). We recorded the locations of perennial shrubs and trees (Acacia floribunda, A. caven, Lithraea caustica, and Quillaja saponaria) in the area using a global positioning system (Garmin, model Summit; Garmin International, Inc., Olathe, Kansas). Our objective was to explore how the location of range areas related to the location of these plants.
Degu density
Given that density of conspecifics can covary with changes in food supply, we also monitored variation of degu density in relation to the abundance of food. The total area trapped during winter 2005 and summer 2006 reached 0.31 ha and during winter 2007 and summer 2008 reached 0.61 ha. We calculated the abundance of degus with a “close capture with heterogeneity model” (Cooch and White 2008; Keesing 1998; Moorhouse and Macdonald 2008; Ribble and Stanley 1998), a method that controls for differences in the trappability of individuals in the population. During our 1st period of observation we used 4 consecutive days of trapping during late winter and summer. The number of consecutive days used during our 2nd observation period in winter and summer was 7 days. These analyses were performed using the MARK software, release 5.1 (White and Burnham 1999). After abundance was estimated with the closed population model, we divided degu abundance by the sampling area to obtain estimates of density.
Statistics
We used a repeated-measures analyses of variance (ANOVA) to examine the effect of season (winter versus summer) on female range areas in the winter and summer seasons in 2005 and 2006. We used a repeated-measures ANOVA with season and sex (males versus females) as factors to analyze male and female range areas in the winter and summer seasons in 2007 and 2008. Before each analysis we used a simple regression model to inspect the potential influence of body mass on range areas.
We used 1-sample Student’s t-tests to test the null hypothesis that percent overlap and core distance between winter and summer ranges were equal to 100% and 0%, respectively (i.e., indicative that the animals did not move their range areas between seasons). Two independent sample Student’s t-tests were used to examine whether potential changes in range location were similar across different observation periods. We used a paired Student’s t-test to examine seasonal changes in the location of range areas in relation to the location of shrubs and trees. To this end we counted the number of shrubs and trees within each range area of a same individual in winter and in summer, controlling for range area (trees/ha).
We used a repeated-measures ANOVA (followed by Tukey post hoc tests) to examine the effect of season-year and distance from main burrow system on food abundance (grams of dry biomass of preferred food). In this analysis season-year was entered as a repeated measure with 3 levels (winter 2005, winter 2007, and summer 2008) and distance from main burrow entrance as a random factor with 2 levels (3 versus 9 m). We compared 2 measures of food quality (soluble fiber, insoluble fiber) between winter 2007 and summer 2008 using multivariate ANOVA (MANOVA).
Assumptions of normality and homogeneity of variance were tested using the Kolmogorov–Smirnov and Levene’s tests, respectively. Data that did not meet model assumptions were either log10(x + 1)-, or in the case of percentage data, arcsine-square root-transformed. Repeated-measures ANOVA and MANOVA were conducted using the general linear module of Statistica 6.0 (StatSoft Inc., Tulsa, Oklahoma); t-tests (Siegel 1956; Sokal and Rohlf 1979) were conducted using the same statistical software. All statistical tests were 2-tailed. We present summary data as mean ± SD. We assumed a significant difference at P < 0.05.
Results
Degu density
Density of degus was 123.4 degus/ha (95% confidence interval [CI] = 119.6–187.0) and 210.4 degus/ha (95% CI = 188.6–290.0) in winter 2005 and summer 2006, respectively. The density of degus increased from 88.9 degus/ha (95% CI = 87.1–104.0) in winter 2007 to 382.9 degus/ha (95% CI = 362.4–424.2) in summer 2008.
Size of range areas
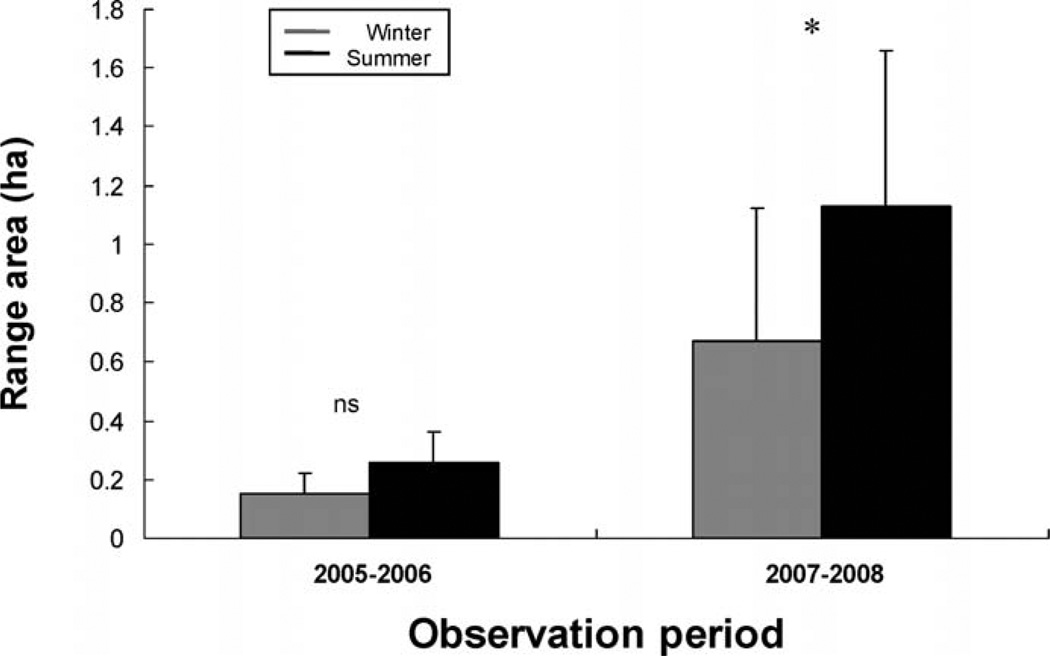
Body mass of radiocollared subjects did not influence the size of range areas during winter 2005 (R2 = 0.13, F1,5 = 0.76, P = 0.42), summer 2006 (R2 = 0.36, F1,5 = 2.87, P = 0.15), winter 2007 (R2 = 0.05, F1,17 = 0.83, P = 0.38), and summer 2008 (R2 = 0.05, F1,17 = 0.98, P = 0.34). During 2005–2006 the daytime range areas of females in winter 2005 were statistically similar to daytime range areas of females in summer 2006 (MCP: F1,6 = 3.58, P = 0.11; FK: F1,6 = 4.49, P = 0.07; Fig. 2). During 2007–2008 MCP (F1,17 = 1.15, P = 0.29) and FK (F1,17 = 1.24, P = 0.28) range areas of males and females did not differ, and no season × sex interaction existed for either the MCP (F1,17 = 0.07, P = 0.79) or FK analyses (F1,17 = 0.40, P = 0.54). Consequently we pooled range areas of males and females for subsequent analyses. In winter 2007 degus had significantly smaller MCP (F1,17 = 5.46, P = 0.03) and FK (F1,17 = 15.54, P = 0.01) ranges than in summer 2008 (Fig. 2).
Fig. 2.
MCP range areas (mean ± SD) of degus during winter and summer in 2005–2006 and 2007–2008. Replicates are 7 degus (all females) during 2005–2006 and 19 degus (15 females and 4 males) during 2007–2008. * = P < 0.05; ns = P > 0.05.
Location shifting of range areas
During 2005–2006 mean overlap of individual range areas in winter and summer (32.0% ± 15.3%, range: 12.5–59.4%) was statistically different from the null expectation of complete overlap (t6 = 17.82, P < 0.001). Likewise, during the 2007–2008 observation period mean overlap of individual range areas in winter and summer (19.7% ± 16.2%, range: 0–58.3%) was statistically different from the null expectation of complete overlap (t18 = 24.52, P < 0.001).
During 2005–2006 the distance between range cores in winter and summer (25.40 ± 12.30 m) was statistically different from the null expectation of no change in the location of core areas between seasons (t6 = 3.85, P = 0.008). During 2007–2008 the distance between range cores in winter and summer (35.7 ± 12.9 m) was statistically different from the null expectation of no change in the location of core areas between seasons (t18 = 9.47, P < 0.001). We observed a seasonal change in the location of range areas in relation to the location of shrubs and trees. Range areas in summer encompassed more shrubs and trees (8.1 ± 3.9 trees/ha) than in winter (3.3 ± 2.8 trees/ha: t18 = 7.35, P < 0.001).
Feeding habits
Our examination of feeding habits confirmed that degus of the study population were consuming mostly annual herbaceous vegetation during the winter season. Overall, 40% of plant material consumed in winter 2005 to 61% in winter 2006 consisted of herbaceous vegetation (Table 1).
Table 1.
Percentages (mean ± SD) for specific items of all plant material found in fecal pellets of Octodon degus in winter 2005 and 2006. Fecal pellets collected from individual degus were pooled per burrow system to constitute 12 replicates (burrow systems) in 2005 and 5 in 2006.
| Family Species | 2005 | 2006 |
|---|---|---|
| Herbs | ||
| AMARYLLIDACEAE | ||
| Phycella sp. | 1.6 ± 2.2 | 0.6 ± 0.8 |
| BORAGINACEAE | ||
| Pectocarya linearis | 0.2 ± 0.3 | 0.7 ± 1.4 |
| COMPOSITAE | ||
| Madia sativa | 0.4 ± 0.9 | 0.2 ± 0.2 |
| Soliva sessilis | 5.1 ± 3.6 | 33.5 ± 20.8 |
| CRUCIFERAE | ||
| Nasturtium officinale | 0.4 ± 0.7 | 0 |
| GERANIACEAE | ||
| Erodium cicutarium | 2.4 ± 1.5 | 0.3 ± 0.6 |
| Erodium malacoides | 0.2 ± 0.4 | 0.4 ± 0.4 |
| GRAMINEAE | ||
| Nassella sp. | 5.4 ± 5.9 | 2.0 ± 1.8 |
| Graminea sp. | 15.4 ± 4.3 | 21.6 ± 16.2 |
| PAPILIONACEAE | ||
| Medicago polymorpha | 7.0 ± 6.6 | 0.4 ± 0.3 |
| RUBIACEAE | ||
| Galium aparine | 0.4 ± 0.3 | 0.2 ± 0.3 |
| CYPERACEAE | 0.9 ± 1.5 | 1.4 ± 1.5 |
| Subtotal | 39.4 ± 8.8 | 61.1 ± 19.5 |
| Shrub foliage | ||
| MIMOSACEAE | ||
| Acacia caven | 0.2 ± 0.4 | 0 |
| Acacia floribunda | 0.3 ± 0.8 | 0 |
| ANACARDIACEAE | ||
| Lithrea caustica | 0.2 ± 0.3 | 0.1 ± 0.2 |
| Subtotal | 0.7 ± 1.1 | 0.1 ± 0.2 |
| Shrub seeds | ||
| Acacia floribunda (S) | 0.04 ± 0.1 | 0.03 ± 0.1 |
| Lithraea caustica (S) | 2.1 ± 2.6 | 4.0 ± 2.1 |
| Unidentified seeds | 0.9 ± 0.7 | 0.4 ± 0.5 |
| Subtotal | 3.0 ± 2.9 | 4.4 ± 2.5 |
| Unidentified fiber | 43.5 ± 9.3 | 27.2 ± 15.7 |
| Unidentified material | 13.4 ± 5.3 | 7.0 ± 4.8 |
Food abundance and quality
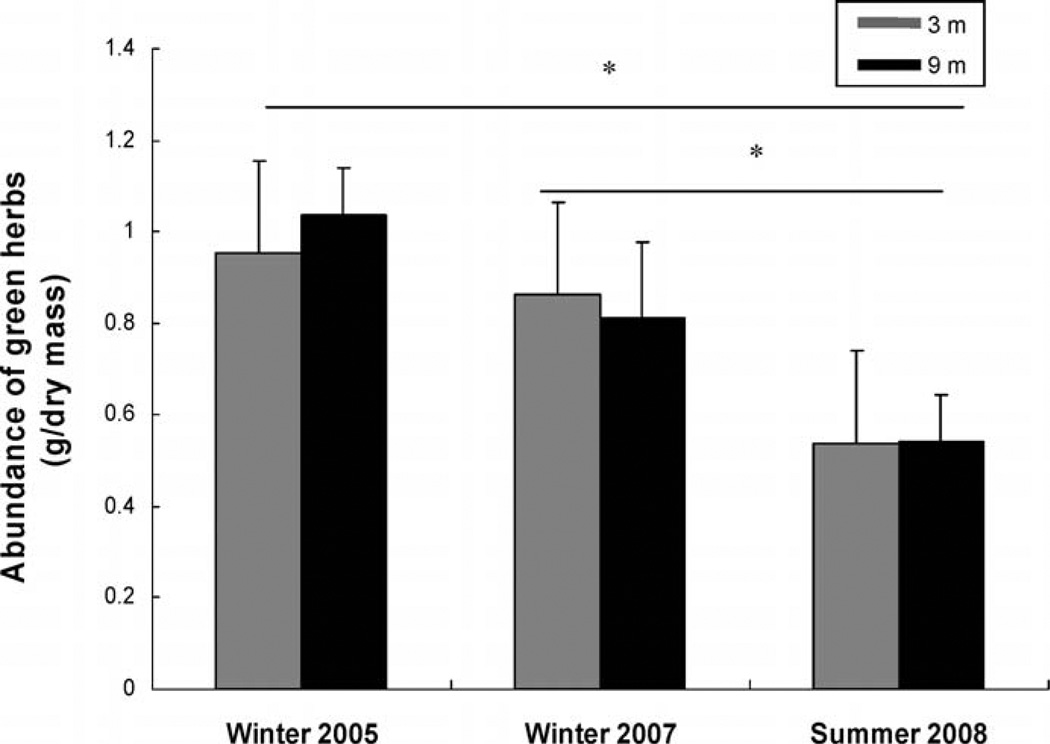
Herbaceous vegetation changed dramatically between winter and summer seasons. Food abundance was lower in January 2008 (summer) compared with September 2005 and September 2007 (winter— F2,44 = 20.68, P < 0.0001; post hoc Tukey test, P < 0.001; Fig. 3). Distance alone (F1,22 = 0.03, P = 0.86), or an interaction between distance and season-year (F2,44 = 0.45, P = 0.64), had no influence on abundance of preferred food.
Fig. 3.
Log-transformed abundance (mean ± SD) of preferred food at 3 and 9 m from main burrows of degus (green herbaceous vegetation) during 2 winter and 1 summer season sampled. * = P < 0.05; ns = P > 0.05.
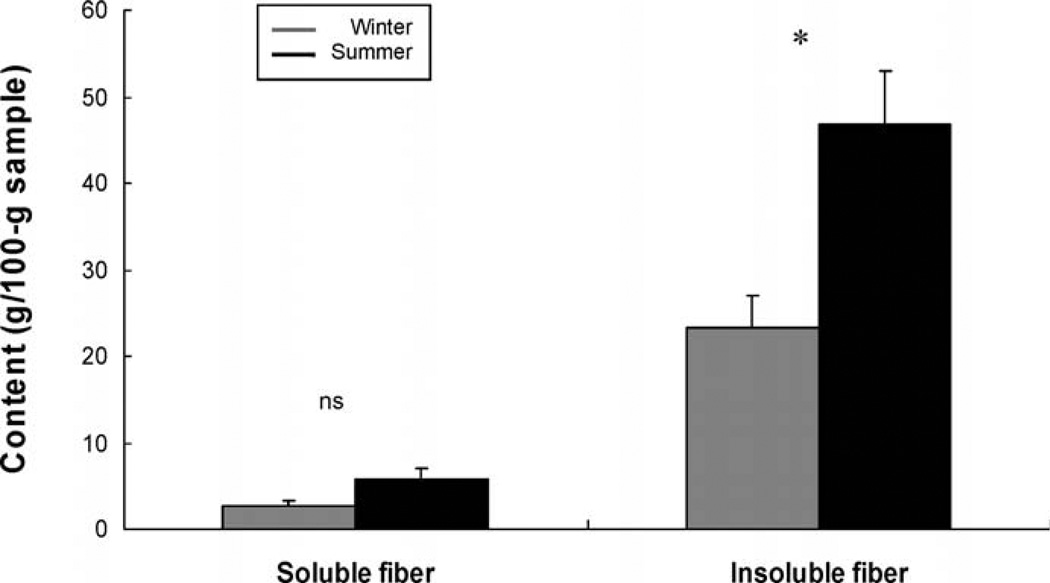
Food quality also changed between the winter and summer months (Wilk’s lambda = 0.23: F2,9 = 10.60, P < 0.004; Fig. 4). Subsequent analyses indicated that although the amount of soluble fiber did not differ significantly (F1,10 = 4.41, P = 0.06), the content of insoluble fiber increased from winter to summer (F1,10 = 10.05, P = 0.009; Tukey test: P = 0.001).
Fig. 4.
Measures (mean ± SD) of food quality available to degus during winter 2007 and summer 2008. * = P < 0.05; ns = P > 0.05.
Discussion
We suggest that our observations are consistent with the hypothesis that food conditions influence seasonal variation in the size and location of adult range areas in degus. During the winter degus were concentrated in areas with abundant herbs, a low fiber food and preferred dietary component. Degus shifted to areas with shrubs during the summer, when herbs are not available. On the other hand, our observations did not support an indirect influence of consumer density on seasonal variation in the size and location of degu range areas. Range areas were larger during periods of high degu density than during periods of low degu density, a phenomenon most likely driven by the presence of young adults born during the previous late winter (Ebensperger and Hurtado 2005; Rojas et al. 1977). This finding contrasts with previous studies reporting an inverse relationship between density and range areas (Cameron and Spencer 1985; Erlinge et al. 1990; Koford 1982; Moorhouse and MacDonald 2005, 2008; Ostfeld 1986; Rible and Stanley 1998; Wolff and Cicirello 1990), or no relationship (Batzli and Henttonen 1993). It is possible that a greater density of degus during summer increases competition for food resources, thus causing individuals to expand their range areas to meet nutritional needs.
Brown and Nicoletto (1991) hypothesized that herbivorous small mammals are selected to consume forage with low fiber content due to the physiological constraints of digesting and assimilating energy from forage with high fiber content. Degu foraging supports this hypothesis. In the laboratory degus exhibit a preference for food with low fiber content (Bozinovic 1995; Gutiérrez and Bozinovic 1998; Veloso and Bozinovic 1993). During the winter degus consumed mostly green annual herbs, low-fiber food (Table 1). Our observations that degus expanded their ranges to include areas with shrubs (A. floribunda, A. caven) during summer and that they feed on the green foliage of these shrubs (Meserve et al. 1984) suggest that degus attempt to maintain a low-fiber diet. Green annual herbs are not available during summer (Ebensperger and Hurtado 2005). Thus, degus living in semiarid environments adjust their spatial behavior to cope with seasonally varying conditions of food abundance and quality. Subsequent studies are needed to examine whether these changes in spatial and foraging behavior are efficient in reducing the intake of high-fiber food.
Similar changes in diet between seasons have been reported in other rodents (Harris and Leitner 2004; Schradin and Pillay 2006). For example, Harris and Leitner (2004) observed that Mohave ground squirrels (Spermophilus mohavensis) feed on shrub foliage during drought years given that production of forbs is reduced. These authors suggested that an increase in range areas during years of drought (i.e., poor food conditions) is a mechanism for these animals to meet energy demands. Although most previous studies conducted on rodents in arid (or semiarid) habitats conclude that food availability drives the spatial behavior of small mammals (Cooper and Randall 2007; Eccard et al. 2004; Schradin and Pillay 2006), the extent of these responses (in terms of range areas) varies. Only the behavior of the murine nocturnal tree rat, Thallomys nigricauda (Eccard et al. 2004), resembles the seasonal variation in the size of ranges recorded in degus. In contrast, studies on species inhabiting more mesic habitats suggest that range areas are driven more by energetic considerations linked to the cost of thermoregulation and breeding activity (Corp et al. 1997; Hoset et al. 2007; Linders et at. 2004; Lurz et al. 2000; Priotto et al. 2002). Subsequent comparative analyses are needed to confirm this macroecological trend.
Our observations that both males and females have similar range areas regardless of season suggest the possibility that ranges are influenced more heavily by the availability of food than breeding activity. However, several other likely explanations can be proposed for these observations. The most obvious explanation, and important limitation of this study, is that we might not have had an adequate sample size to compare the ranges of males and females. Alternatively, we did not quantify ranges during the autumn (April–May), the mating period for degus at our study site. If degus exhibit a promiscuous or polygynous mating system like other social rodents (Ostfeld 1985, 1990), males should have larger ranges and overlap with multiple females during the mating season in comparison with other periods (Emlen and Oring 1977). Comparisons of degu ranges during the mating season and when females are lactating (winter–spring) are needed to determine the influence of breeding activity on range areas.
In conclusion, like some other rodents that inhabit arid and semiarid environments (Cooper and Randall 2007; Eccard et al. 2004; Schradin and Pillay 2006), degus expand and move their range areas in response to seasonal variation in preferred foods. In contrast, factors such as population density and the reproductive condition of females (nonbreeding versus pregnant/lactating) did not predict seasonal changes in space use. The immediate and long-term fitness implications linked to these changes warrant further study. For example, expanded ranges may come at a cost of greater predation risk (Norrdahl and Korpimäki 1998; Yoder et al. 2004), possibly explaining, in part, the high turnover rates of degu social groups (Ebensperger et al. 2009). Finally, degus are highly social (Ebensperger et al. 2004, 2009; Hayes et al. 2009) and widely distributed within Chile (Ebensperger et al. 2004; Meserve et. al 1984). Given their sensitivity to the availability of preferred foods, degus may be an excellent model for developing theory linking ecological variation, spatial ecology, and social systems (Brashares and Arcese 2002; Emlen and Oring 1977).
Acknowledgments
We thank J. Childers, L. Crespin, A. Farías, S. Januario, P. Lagos, D. Lahr, C. León, M. Pardue, and L. Valenzuela for their assistance. Two anonymous reviewers provided useful comments to improve a previous version of this manuscript. We all are indebted to the Universidad de Chile, particularly to J. D. García, Field Station Administrator, for providing the facilities during field work. VQ was supported by Dirección de Investigación y Postgrado-Pontificia Universidad Católica de Chile (DIPUC), Programa de Mejoramiento de la Calidad y la Equidad de la Educación Superior (MECESUP), and Comisión Nacional Científica y Tecnológica (CONICYT) Ph.D. fellowships. ASC and JRB were funded by the American Society of Mammalogists and Sigma Xi, respectively. LDH was funded by the National Science Foundation EPSCoR (#0553910), the Louisiana Board of Regents (LEQSF 2007-09-RD-A-39), the University of Louisiana at Monroe (ULM) HHMI Program, and the ULM Office of Academic Affairs. LAE was funded by Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECTY) grants (1020861, 1060499, and 1090302) and by the Center for the Advanced Studies in Ecology and Biodiversity (CASEB), Program 1.
Literature Cited
- Batzli GO. Nutrition. In: Tamarin RH, editor. Biology of New World Microtus. Special publication, The American Society of Mammalogists; 1985. pp. 799–806. [Google Scholar]
- Batzli GO, Henttonen H. Home range and social organization of the vole (Microtus mirus) Journal of Mammalogy. 1993;74:868–878. [Google Scholar]
- Bozinovic F. Nutritional energetics and digestive responses of an herbivorous rodent (Octodon degus) to different levels of dietary fiber. Journal of Mammalogy. 1995;76:627–637. [Google Scholar]
- Brashares JS, Arcese P. Role of forage, habitat and predation in the behavioural plasticity of a small African antelope. Journal of Animal Ecology. 2002;71:626–638. [Google Scholar]
- Brown JH, Nicoletto PF. Spacial scaling of species composition: body masses of North American mammals. American Naturalist. 1991;138:1478–1512. [Google Scholar]
- Burger JR, et al. The influence of trap type on evaluating population structure of the semifossorial and social rodent Octodon degus. Acta Theriologica. 2009;54:311–320. [Google Scholar]
- Burt WH. Territoriality and home range concepts as applied to mammals. Journal of Mammalogy. 1943;24:346–352. [Google Scholar]
- Cameron GN, Spencer SR. Assessment of space-use patterns in the hispid cotton rat (Sigmodon hispidus) Oecología. 1985;68:133–139. doi: 10.1007/BF00379485. [DOI] [PubMed] [Google Scholar]
- Cooch E, White G. Program Mark: a gentle introduction. [Accessed 17 June, 2008];2008 http://www.phidot.org/software/mark/index.html. [Google Scholar]
- Cooper LD, Randall JA. Seasonal changes in home ranges of the giant kangaroo rat (Dipodomys ungens): a study of flexible social structure. Journal of Mammalogy. 2007;88:1000–1008. [Google Scholar]
- Corp N, Gorman ML, Speakman JR. Ranging behaviour and time budgets of male wood mice Apodemus sylvaticusin different habitats and seasons. Oecologia. 1997;109:242–250. doi: 10.1007/s004420050079. [DOI] [PubMed] [Google Scholar]
- Desy EA, Batzli GO, Liu J. Effects of food and predation on behaviour of prairie voles: a field experiment. Oikos. 1990;58:159–168. [Google Scholar]
- Dizeo de Strittmatter CG. Métodos de clarificación en materiales vegetales. Parodiana. 1984;3:169–174. [Google Scholar]
- Ebensperger LA. Sociality in rodents: the New World fossorial hystricognaths as study models. Revista Chilena de Historia Natural. 1998;71:65–77. [Google Scholar]
- Ebensperger LA, Hurtado MJ. Seasonal changes in the time budget of degus, Octodon degus. Behaviour. 2005;142:91–112. [Google Scholar]
- Ebensperger LA, Hurtado MJ, Soto-Gamboa M, Lacey EA, Chang AT. Communal nesting and kinship among degus (Octodon degus) Naturwissenschaften. 2004;91:391–395. doi: 10.1007/s00114-004-0545-5. [DOI] [PubMed] [Google Scholar]
- Ebensperger LA, et al. Instability rules social groups in the communal breeder rodent Octodon degus. Ethology. 2009;115:540–554. [Google Scholar]
- Eccard JA, Meyer J, Sundell J. Space use, circadian activity pattern, mating system of the nocturnal tree rat Thallomys nigricauda. Journal of Mammalogy. 2004;85:440–445. [Google Scholar]
- Emlen ST, Oring LW. Ecology, sexual selection, the evolution of mating systems. Science. 1977;197:215–223. doi: 10.1126/science.327542. [DOI] [PubMed] [Google Scholar]
- Erlinge S, Hoogenboom I, Agrell J, Nelson J, Sandell M. Density-related home-range size and overlap in adult field voles (Microtus agrestis) in southern Sweden. Journal of Mammalogy. 1990;71:597–603. [Google Scholar]
- Gannon WL, Sikes RS The Animal Care and Use Committee of the American Society of Mammalogists. Guidelines of the American Society of Mammalogists for the use of wild mammals in research. Journal of Mammalogy. 2007;88:809–823. doi: 10.1093/jmammal/gyw078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gompper ME, Gittleman JL. Home range scaling: intraspecific and comparative trends. Oecologia. 1991;87:343–348. doi: 10.1007/BF00634589. [DOI] [PubMed] [Google Scholar]
- GutiÉrrez JR, Arancio GI, Jaksic FM. Variation in vegetation and seed bank in a Chilean semi-arid community affected by ENSO 1997. Journal of Vegetation Science. 2000;11:641–648. [Google Scholar]
- Gutiérrez JR, Bozinovic F. Diet selection in captivity by a generalist herbivorous rodent (Octodon degus) from the Chilean coastal desert. Journal of Arid Environments. 1998;39:601–607. [Google Scholar]
- Gutiérrez RJ, Meserve PL, Jaksic FM, Contreras LC, Herrera S, Vásquez H. Structure and dynamics of vegetation in a Chilean arid thorn scrub community. Acta Oecologica. 1993;14:271–285. [Google Scholar]
- Hansson L. Methods of morphological diet micro-analysis in rodents. Oikos. 1970;21:255–266. [Google Scholar]
- Harestad AS, Bunnell FL. Home range and body weight—a reevaluation. Ecology. 1979;60:389–402. [Google Scholar]
- Harris JH, Leitner P. Home range size and use of space by adult Mohave ground squirrels, Spermophilus mohavensis. Journal of Mammalogy. 2004;85:517–523. [Google Scholar]
- Hayes LD, Chesh AS, Castro RA, Ortiz Tolhuysen L, Bhattacharjee J, Ebensperger LA. Per capita direct fitness consequences of group-living in the degus (Octodon degus), a plural breeder rodent with communal care. Animal Behaviour. 2009;78:131–139. [Google Scholar]
- Hayes LD, Chesh A, Ebensperger LA. Ecological predictors of range areas and use of burrow systems in the diurnal rodent, Octodon degus. Ethology. 2007;113:155–165. [Google Scholar]
- Hoffmann A, Mooney HA, Kummerow J. Qualitative phenology. In: Thrower NJW, Bradbury DE, editors. Chile–California Mediterranean scrub atlas: a comparative analysis. Stroudsburg, Pennsylvania: Dowden, Hutchinson and Ross, Inc.; 1977. pp. 102–120. [Google Scholar]
- Hoset KS, Le Galliard JF, Gundersen G, Steen H. Behavioral Ecology. Vol. 19. 2007. Home range size and overlap in female root voles: effects of season and density; pp. 139–145. [Google Scholar]
- Hubbs AH, Boonstra R. Effects of food and predators on the home-range of Arctic ground squirrels (Spermophilus parryii) Canadian Journal of Zoology. 1998;76:592–596. [Google Scholar]
- Ims RA. Responses in spatial organization and behaviour to manipulation of food resources in the vole Clethriomys rufocanus. Ecology. 1987;70:607–616. [Google Scholar]
- Jones EN. Effects of forage availability on home range and population density of Microtus pennsylvanicus. Journal of Mammalogy. 1990;71:382–389. [Google Scholar]
- Keesing F. Ecology and behavior of the pouched mouse, Saccostomus mearnsiin central Kenya. Journal of Mammalogy. 1998;79:919–931. [Google Scholar]
- Kenagy GJ, Vásquez RA, Nespolo RF, Bozinovic F. A time-energy analysis of daytime surface activity in degus, Octodon degus. Revista Chilena de Historia Natural. 2002;75:149–156. [Google Scholar]
- Kenward RE. A manual for wildlife radio tagging. San Diego, California: Academic Press; 2001. [Google Scholar]
- Kenward RE, South AB, Walls SS. Ranges 6, version 1.2: for the analysis of tracking and location data. Wareham, United Kingdom: Anatrack Ltd.; 2003. [Google Scholar]
- Koford RR. Journal of Mammalogy. Vol. 63. 1982. Mating system of a territorial tree squirrel (Tamiasciurus douglasii) in California; pp. 274–283. [Google Scholar]
- Korschgen LJ. Procedures for food-habits analysis. In: Giles RH, editor. Management techniques wildlife. Washington, DC: The Wildlife Society; 1969. pp. 233–250. [Google Scholar]
- Linders MJ, West SD, Haegen WMV. Seasonal variability in the use of space by western gray squirrels in southcentral Washington. Journal of Mammalogy. 2004;85:511–516. [Google Scholar]
- Lurz PWW, Garson PJ, Wauters LA. Effects of temporal and spatial variation in food supply on the space and habitat use of red squirrels (Sciurus vulgarisL.) Journal of Zoology (London) 2000;251:167–178. [Google Scholar]
- Mares MA, Lacher TE, Willing MR, Bitar NA. An experimental analysis of social spacing in Tamias striatus. Ecology. 1982;63:267–273. [Google Scholar]
- Meserve PL. Trophic relationships among small mammals in a Chilean semiarid thorn scrub community. Journal of Mammalogy. 1981;62:304–314. [Google Scholar]
- Meserve PL, Martin RE, Rodríguez J. Feeding ecology of two Chilean caviomorphs in a central Mediterranean savanna. Journal of Mammalogy. 1983;64:322–325. [Google Scholar]
- Meserve PL, Martin RE, Rodríguez J. Comparative ecology of the caviomorph rodent Octodon degusin two Chilean Mediterranean-type communities. Revista Chilena de Historia Natural. 1984;57:79–89. [Google Scholar]
- Moorhouse TP, MacDonald DW. Temporal patterns of range use in water voles: do female territories drift? Journal of Mammalogy. 2005;86:655–661. [Google Scholar]
- Moorhouse TP, Macdonald DW. What limits male range sizes at different population densities? Evidence from three populations of water voles. Journal of Zoology (London) 2008;274:395–402. [Google Scholar]
- Norrdahl K, Korpimäki E. Does mobility or sex of voles affect risk of predation by mammalian predators? Ecology. 1998;79:226–232. [Google Scholar]
- Ostfeld RS. Limiting resources and territoriality in microtine rodents. American Naturalist. 1985;126:1–15. [Google Scholar]
- Ostfeld RS. Territoriality and mating system of California voles. Journal of Animal Ecology. 1986;55:691–706. [Google Scholar]
- Ostfeld RS. The ecology of territoriality in small mammals. Trends in Ecology and Evolution. 1990;5:411–415. doi: 10.1016/0169-5347(90)90026-A. [DOI] [PubMed] [Google Scholar]
- Priotto J, Strinmann A, Polop J. Factors affecting home range size and overlap in Calomys venustus(Muridae: Sigmodontidae) in Argentine agroecosystems. Mammalian Biology. 2002;67:97–104. [Google Scholar]
- Quan YF, MacManes MD, Ebensperger LA, Lacey EA, Hayes LD. Isolation and characterization of polymorphic microsatellite loci from Octodon degus. Molecular Ecology Resources. 2009;9:999–1001. doi: 10.1111/j.1755-0998.2009.02536.x. [DOI] [PubMed] [Google Scholar]
- Ribble DO, Stanley S. Home ranges and social organization of syntopic Peromyscus boylli and P. truei. Journal of Mammalogy. 1998;79:932–941. [Google Scholar]
- Rojas M, Rivera O, Montenegro G, Barros C. Algunas observaciones en la reproducción de la hembra silvestre de Octodon degusMolina y su posible relación con la fenología de la vegetación. Medio Ambiente (Chile) 1977;3:78–82. [Google Scholar]
- Schradin C, Pillay N. Female striped mice (Rhabdomys pumilio) change their home ranges in response to seasonal variation in food availability. Behavioral Ecology. 2006;17:452–458. [Google Scholar]
- Siegel S. Nonparametric statistics for the behavioral sciences. New York: McGraw Hill; 1956. [Google Scholar]
- Slobodchikoff CN. Resources and the evolution of social behavior. In: Price PW, Slobodchikoff CN, Gaud WS, editors. A new ecology: novel approaches to interactive systems. New York: John Wiley & Sons, Inc.; 1984. pp. 227–251. [Google Scholar]
- Sokal R, Rohlf F. Principios y métodos estadísticos en la investigacio’n biológica. Spain: Blume, Barcelona; 1979. [Google Scholar]
- Taitt MJ. The effect of extra food on small rodent populations: I. Deermice (Peromyscus maniculatus) Journal of Animal Ecology. 1981;50:125–137. [Google Scholar]
- Taitt MJ, Krebs CJ. The effect of extra food on small rodent populations: II. Voles (Microtus townsendii) Journal of Animal Ecology. 1981;50:111–129. [Google Scholar]
- Todd IA, Tew TE, MacDonald DW. Arable habitat use by wood mice (Apodemus sylvaticus). 1. Macrohabitat. Journal of Zoology (London) 2000;250:299–303. [Google Scholar]
- Van Soeste IJ. Nutritional ecology of the ruminant. Ithaca, New York: Cornell University Press; 1982. [Google Scholar]
- Veloso C, Bozinovic F. Dietary and digestive constraints on basal energy metabolism in a small herbivorous rodent. Ecology. 1993;74:2003–2010. [Google Scholar]
- Wauters LA, Dhondt AA. Variation in spacing behavior of Eurasian red squirrels, Sciurus vulgarisin winter: effects of density and food abundance. In: Steele MA, Merritt JF, Zegerse DA, editors. Ecology and evolutionary biology of tree squirrels. 1998. pp. 71–77. Virginia Museum of Natural History, Special Publication 6. [Google Scholar]
- White GC, Burnham KP. Program MARK: survival estimation from populations of marked animals. Bird Study. 1999;46 Suppl.:120–138. [Google Scholar]
- Wolff JO, Cicirello DM. Mobility versus territoriality: alternative reproductive strategies in white-footed mice. Animal Behaviour. 1990;39:1222–1224. [Google Scholar]
- Wolton RJ. The ranging and nesting behaviour of wood mice, Apodemus sylvaticus(Rodentia: Muridae), as revealed by radio tracking. Journal of Zoology (London) 1985;206:203–224. [Google Scholar]
- Yáñez JL. B.S. thesis. Universidad de Chile, Santiago, Chile; 1976. Ecoetología de Octodon degus. [Google Scholar]
- Yoder JM, Marschall EA, Swanson DA. The cost of dispersal: predation as a function of movement and site familiarity in ruffed grouse. Behavioral Ecology. 2004;15:469–476. [Google Scholar]