Abstract
Chronic pain conditions are difficult to treat and are major health problems. Bone marrow stromal cells (BMSCs) have generated considerable interest as a candidate for cell-based therapy. BMSCs are readily accessible and are easy to isolate and expand ex vivo. Clinical studies show that direct injection of BMSCs does not produce unwanted side effects and is well tolerated and safe. Here, we show that a single systemic (intravenous) or local injection (into the lesion site) of rat primary BMSCs reversed pain hypersensitivity in rats after injury and that the effect lasted until the conclusion of the study at 22 weeks. The pain hypersensitivity was rekindled by naloxone hydrochloride, an opioid receptor antagonist that acts peripherally and centrally, when tested at 1–5 weeks after BMSC infusion. In contrast, naloxone methiodide, a peripherally acting opioid receptor antagonist, only rekindled hyperalgesia in the first 3 weeks of BMSC treatment. Focal downregulation of brainstem mu opioid receptors by RNA interference (RNAi) reversed the effect of BMSCs, when RNAi was introduced at 5- but not 1-week after BMSC transplantation. Thus, BMSCs produced long-term relief of pain and this effect involved activation of peripheral and central opioid receptors in distinct time domains. The findings prompt studies to elucidate the cellular mechanisms of the BMSC-induced pain relieving effect and translate these observations into clinical settings.
Keywords: Adult stem cells, Bone marrow stromal cells, Cellular therapy, Neuropathy, Rat model, Stem cell transplantation
Introduction
Bone marrow stromal cells (BMSCs) are a major type of multipotent mesenchymal stem (or stromal) cells that can also be derived from a variety of sources [1, 2]. Characteristic of mesenchymal stem cells is that BMSCs are capable of differentiating into lineages of the mesenchyme such as osteoblasts, chondrocytes, and adipocytes in vitro [3]. BMSCs have generated considerable interest as a candidate for cell-based therapy because they are easy to isolate and expand in vitro, possess immunoregulatory properties [1, 4, 5], and secrete a variety of trophic mediators [6, 7]. In addition to promising treatment of graft-versus-host disease [4], preclinical studies have shown beneficial effects of BMSCs on neurological disorders. BMSCs facilitate nerve regeneration [8], improve diabetic neuropathy [9], and multiple sclerosis [10] and help functional recovery after stroke [11–16]. Interestingly, BMSCs appear to have potential to treat chronic pain conditions [17–21].
Disorders of the temporomandibular joint (TMJ) and muscles of mastication often lead to chronic pain conditions that are difficult to treat and are a major health problem. In light of the recent development on the therapeutic potential of mesenchymal stromal cells, this study was undertaken to systematically evaluate the effect of BMSCs on persistent pain behavior in rat models. The models involve ligation injury of a tendon of the masseter, one of the four muscles involved in mastication [22] or chronic constriction injury (CCI) of the infraorbital nerve (ION) [23]. After tendon or nerve injury, rats exhibit long-lasting and constant orofacial mechanical hypersensitivity in the orofacial origin, resembling some features of myofacial temporomandibular pain or neuropathic pain. Our results showed that intravenous (i.v.) infusion of BMSCs derived from the rat produced long-term attenuation of mechanical hyperalgesia and allodynia and that endogenous opioids of peripheral and central origins were involved in this effect in distinct time domains.
Materials and Methods
Animals
Male Sprague-Dawley rats, approximately 8-week old at the time of surgery, were used (225–250 g; Harlan, Indianapolis, IN, www.harlan.com). Animals were anesthetized with pentobarbital sodium (50 mg/kg, i.p.). Ligation of the tendon of the anterior superficial part of the rat masseter muscle (TASM) was achieved via an intraoral approach as described elsewhere [22]. Briefly, on the left intraoral site, a 3-mm-long incision was made posteroanteriorly lateral to the gingivobuccal margin in the buccal mucosa, beginning immediately next to the first molar. The TASM was gently freed and tied with two chromic gut (4.0) ligatures, 2 mm apart. The sham-operated rats received the same procedure except that the tendon was not ligated. CCI-ION was performed according to Wei et al. [23]. The wound was checked for hemostasis and the incision closed with three 4.0 silk sutures. In most experiments, four to seven animals were used in each group as indicated in the text and figures. The high-dose BMSC experiments were repeated three times (n = 16) in the tendon ligation model and two times in the CCI-ION model (n = 9). Mu opioid receptors (MOR)–small hairpin RNA (shRNA) experiments were repeated twice (n = 10). All experiments were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) and approved by the Institutional Animal Care and Use Committee, University of Maryland Dental School. All efforts were made to minimize the number of animals used and their suffering.
Behavioral Testing
Mechanical sensitivity of the orofacial region was assessed under blind conditions [22, 24]. A series of calibrated von Frey filaments were applied to the skin above the TASM and ION. An active withdrawal of the head from the probing filament was defined as a response. Each von Frey filament was applied five times at intervals of 5–10 seconds. The response frequencies ([number of responses/number of stimuli] × 100%) to a range of von Frey filament forces were determined and a stimulus-response frequency (S-R) curve plotted. After a nonlinear regression analysis, an EF50 value, defined as the von Frey filament force (g) that produces a 50% response frequency, was derived from the S-R curve (Prism, GraphPad Software, San Diego, CA, www.graphpad.com) [25]. We used EF50 values as a measure of mechanical sensitivity. A leftward shift of the S-R curve, resulting in a reduction of EF50, occurred after ligation of the TASM. This shift of the curve suggests the development of mechanical hypersensitivity or presence of mechanical hyperalgesia and allodynia, as there was an increase in response to suprathreshold stimuli and a decreased response threshold for nocifensive behavior.
BMSC Procedures
Primary cultures of BMSCs were obtained from donor rats under aseptic conditions as described in other reports [13, 26]. The rats were sacrificed with CO2, the both ends of the tibiae, femurs, and humeri were cut off by scissors. A syringe fitted with 18-gauge needle was inserted into the shaft of the bone, and bone marrow was flushed out with culture medium (α-modified Eagle’s medium; Gibco, Carlsbad, CA, www.invitrogen.com; and 10% fetal bovine serum; Hyclone, Logan, UT, www.thermoscientific.com). The bone marrow was then mechanically dissociated and the suspension passed through a 100-µm cell strainer to remove debris. The cells were incubated at 37°C in 5% CO2 in tissue-culture flasks (100 × 200 mm2) (Sarstedt, Nümbrecht, Germany, www.sarstedt.com), and non-adherent cells were removed by replacing the medium. The culture medium was changed next day and every other day thereafter. Before changing the medium, the culture plate was thoroughly washed twice by phosphate-buffered saline (PBS).
At day 7, when the cultures reached 80% confluence, the cells were washed with PBS and harvested by incubation with 1 ml of 0.25% trypsin/1 mM EDTA for 2 minutes at room temperature. Trypsin was neutralized by adding 5 ml of the complete medium. Cells were centrifuged at 1,000g for 2 minutes, the supernatant was removed, and the pellet was washed with PBS. The cell numbers were calculated by the hemocytometer. For i.v. administration, 1.5 × 103–106 cells in 0.2 ml PBS were slowly injected into one tail vein of the anesthetized rat over a 2-minute period using a 22-gauge needle. For local transplantation, 0.15–0.375 × 106 cells were directly injected into the site of TASM ligation.
The property of expanded cells was assessed by flow cytometry. BMSCs were harvested from the tissue culture plate after 7 days ex vivo and centrifuged at 600g for 5 minutes at room temperature. The cells were washed and counted by the hemocytometer. A single cell suspension of 1 × 106 cells was placed in 0.05 ml of staining buffer (eBioscience, San Diego, CA, www.ebioscience.com). The cells were incubated with saturating concentrations of fluorescein isothiocyanate (FITC)–conjugated monoclonal antibodies against CD45 and CD90 (eBioscience) for 20 minutes on ice in the dark. Isotype-matched FITC-conjugated immunoglobulin G antibodies (eBioscience) were used as controls. Cells were then washed three times with staining buffer, centrifuged at 400g for 5 minutes, and resuspended in 0.5 ml ice cold staining buffer. Flow cytometry analyses were performed at the University of Maryland Greenbaum Cancer Center Shared Flow Cytometry Facility. Cell fluorescence was evaluated by flow cytometry in a FACS-can (Becton Dickinson, San Jose, CA, www.bd.com) analytical flowcytometer and the data were analyzed using FlowJo 8.8.6 (TreeStar, Inc., Ashland, OR, www.treestar.com). The specific staining was measured from the cross point of the isotype control with a specific antibody graph.
Drugs
Naloxone hydrochloride and naloxone methiodide were from Sigma (St. Louis, MO, www.sigmaaldrich.com). Drug doses are expressed as milligram salt per kilogram body weight (mg/kg). The injection volume was 1 ml/kg for systemic administration (i.p.).
Brainstem Microinjections and Targeted Recombinant Plasmid Transfers by Electroporation
Rats were anesthetized with 2%–3% isoflurane in a gas mixture of 30% O2 balanced with 70% nitrogen and placed in a Kopf stereotaxic instrument (Kopf Instruments, Tujunga, CA, www.kopfinstruments.com). A midline incision was made after infiltration of lidocaine (2%) into the skin. A midline opening was made in the skull with a dental drill for inserting an injection needle into the target site. The coordinates for the rostral ventromedial medulla (RVM) were: 10.5 mm caudal to bregma as well as midline and 9.0 mm ventral to the surface of the cerebellum [27, 28]. To avoid penetration of the transverse sinus, the incisor bar was set at 4.7 mm below the horizontal plane passing through the interaural line. Animals were subsequently maintained at approximately 1% halothane. Microinjections were performed by delivering drug solutions slowly over a 10-minute period using a 500-nl Hamilton syringe with a 32-gauge needle. For gene transfer, Sure-silencing shRNA plasmids for rat MOR were used to design the enclosed shRNA (MOR: TCTCGccatcggtctgcctgtaatgtCTTCCTGTCAacattacaggcagaccgatggCT) or scrambled control (control: TCTCggaatctcattcgatgcatacCTTCCTGTCAgtatgcatcgaatgagattccCT) and contained the green fluorescent protein (GFP) gene (SuperArray, Frederick, MD, www.superarray.com). Each vector (500 ng/500 nl) was injected into the RVM. The injection needle was left in place for at least 15 minutes before being slowly withdrawn. A pair of Teflon coated silver positive and negative electrodes were placed along a line rostral and caudal to the injection site. For transfer of negatively charged plasmid into RVM neurons, seven square wave electric pulses (40 V, 50 milliseconds, 1 Hz; model 2100; A–M Systems, Carlsborg, WA, www.a-msystems.com) were delivered [29]. The wound was closed and animals were returned to their cages after recovering from anesthesia. In some experiments, control or MOR shRNA plasmids was injected to the RVM and then followed by placing electrodes without electroporation.
Immunohistochemistry
Rats were deeply anesthetized with pentobarbital sodium (100 mg/kg, i.p.) and perfused transcardially with 4% paraformaldehyde in 0.1 M phosphate buffer at pH 7.4. The brainstem block including RVM was postfixed and transferred to 25% sucrose (w/v) for cryoprotection. Approximately 30 µm-thick coronal sections were cut with a cryostat at −20°C. To determine effects of MOR shRNA or scrambled shRNA on expression of MOR in neurons transferred with GFP, free-floating sections were incubated with primary antibodies (mouse polyclonal anti-GFP, 1:500; Clontech, Palo Alto, CA, www.clontech.com; and rabbit anti-MOR, 1:8,000; ImmunoStar, Hudson, WI, www.immunostar.com) overnight with 1–3% relevant normal sera. For fluorescence staining, the sections were incubated with secondary antibodies coupled to Cy2 or Cy3 (Jackson Immunoresearch Laboratories, www.jacksonimmuno.com). After washes in PBS, all sections were mounted on gelatin-coated slides and cover slipped with Vectashield (Vector Laboratories, Burlingame, CA, www.vectorlabs.com). Images were collected sequentially using a Nikon epifluorescence microscope and a charge-coupled device camera controlled by SPOT software (SPOT, Sterling Heights, MI, www.diaginc.com/software). Immunostaining control studies were performed by omission of the primary or secondary antibodies and by adsorption with an excess (10 µg/ml) of the respective antigens.
Western Blot
The rats were anesthetized with isoflurane (3%) and quickly decapitated. The same block of brainstem tissues as that for immunohistochemistry was collected. The tissue block was turned coronally and the RVM was harvested by taking punches with a 15-gauge needle. The tissues were homogenized in solubilization buffer (50 mM Tris.HCl, pH8.0; 150 mM NaCl, 1 mM EDTA, 1% Nonidet P40, 0.5% deoxycholic acid, 0.1% sodium dodecyl sulfate [SDS], 1 mM Na3VO4, 1 U/ml aprotinin, 20 µg/ml leupetin, and 20 µg/ml pepstatin A). The homogenate was centrifuged at 20,200g for 10 minutes at 4°C. The supernatant was removed. The protein concentration was determined using a detergent-compatible protein assay with a bovine serum albumin standard. Each sample contains proteins from one animal. The proteins (50 µg) were separated on a 7.5% SDS–polyacrylamide gel electrophoresis gel and blotted to a nitrocellulose membrane (Amersham Biosciences, Arlington Heights, IL, www.gelifesciences.com). The blots were blocked with 5% milk in tris-buffered saline (TBS) buffer and then incubated with the anti-MOR antibodies (1:1,000). The membrane was washed with TBS and incubated with anti-rabbit IgG (1:3,000; Cell Signaling Technology, Danvers, MA, www.millipore.com). The immunoreactivity was detected using enhanced chemiluminescence (ECL, Amersham). The loading and blotting of the amount of protein was verified by reprobing the membrane with anti-β-actin antiserum (Sigma, St. Louis, MO) and with Coomassie Blue staining.
Data Analysis
Data are presented as mean (95% confidence interval) for EF50s and mean ± SEM for other data. For western blot, the ECL-exposed films or gel images were digitized and densitometric quantification of immunoreactive cDNA bands was carried out using UN-SCAN-IT gel (ver. 5.3, Silk Scientific Inc., Orem, UT, www.silkscientific.com). The relative protein or mRNA levels were obtained by comparing the respective specific band to the β-actin control from the same membrane or gels. The deduced ratios were further normalized to that of the naïve rats on the same membrane and illustrated as percentage of the naïve controls. Statistical comparisons were made by the use of one-way and two-way analysis of variance (ANOVA). For unbalanced sample size, multiple regressions were performed and Type III sum-of-squares was used to calculate p values (GraphPad Prism). Tukey or Dunnett’s tests were performed for post hoc comparisons. For animals that were subject to repeated testing, ANOVA with repeated measures was used. p < .05 was considered significant for all cases.
Results
Isolation and Characterization of Rat BMSCs
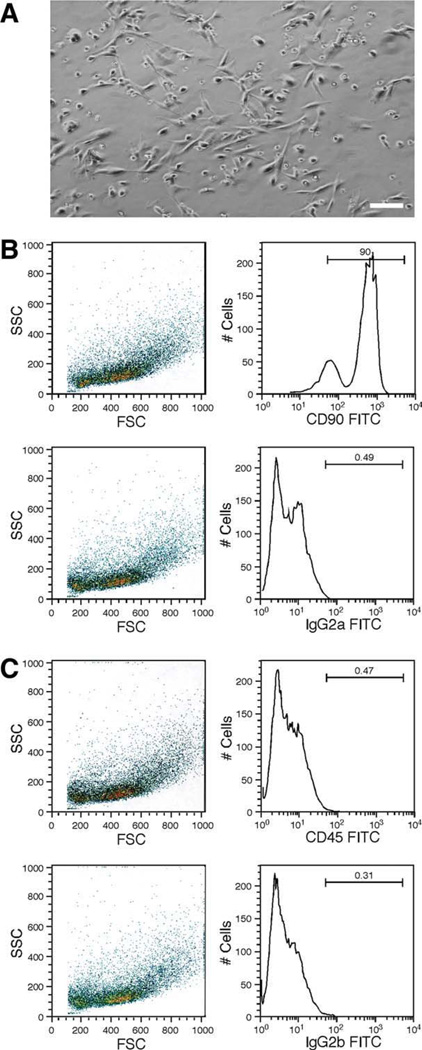
Bone marrow was flushed out from the shaft of the femurs, tibiae, and humeri with culture medium and dissociated. The suspension was passed through a cell strainer to remove debris and plated. Nonadherent cells were removed by washing with PBS and replacing the medium. The cultured cells are mostly spindle-shaped and exhibit a large round nucleus and a few thin cell processes (Fig. 1A), consistent with morphology of bone marrow-derived stromal cells [3, 30]. Flow cytometry confirmed that the cell populations were consistent between samples and were purified and homogeneous (Fig. 1B, 1C). More than 90% of the cultured BMSCs were positive for CD90 (90.0% and 97.5%, n = 2) and less than 1% of the cells were positive for a hematopoietic marker CD45 (0.04% and 0.47%, n = 2), which are consistent with the surface marker phenotype of BMSCs [16, 31] (Fig. 1B, 1C).
Figure 1.
Characterization of bone marrow stromal cells (BMSCs) derived from the rat. (A): An example of BMSC culture at 5 days after plating. Note spindle-shaped cell bodies with a round nucleus and thin processes. Scale bar = 20 µm. (B, C): Flow cytometry analysis of cultured BMSCs at 5 days after plating. Left, SSC versus FSC scatter graphs; Right, plots of the number of positive immunoreactive cells versus relative fluorescence intensities. In the examples in (B, C), 90% of cells were positive for CD90 (B) and only 0.47% of cells were positive for CD45 (C). The CD90+ and CD45− surface marker phenotype is typical of BMSCs devoid of hematopoietic contamination. Isotype-matched IgG antibodies (IgG2a in (B) and IgG2b in (C)) were used as controls. Abbreviations: #Cells, number of positive immunoreactive cells; FITC, fluorescein isothiocyanate; FSC, forward scatter; SSC, side scatter.
Infusion of BMSC Reversed Pain Hypersensitivity After Injury
Chronic orofacial pain hypersensitivity in rats was produced by ligation of the TASM and CCI-ION, as indicated by significant and persistent reduction in EF50s (Fig. 2). The decreased EF50 indicates the presence of mechanical hyperalgesia, an increased response to a noxious stimulus, and allodynia, a nocifensive response to a normally non-noxious stimulus.
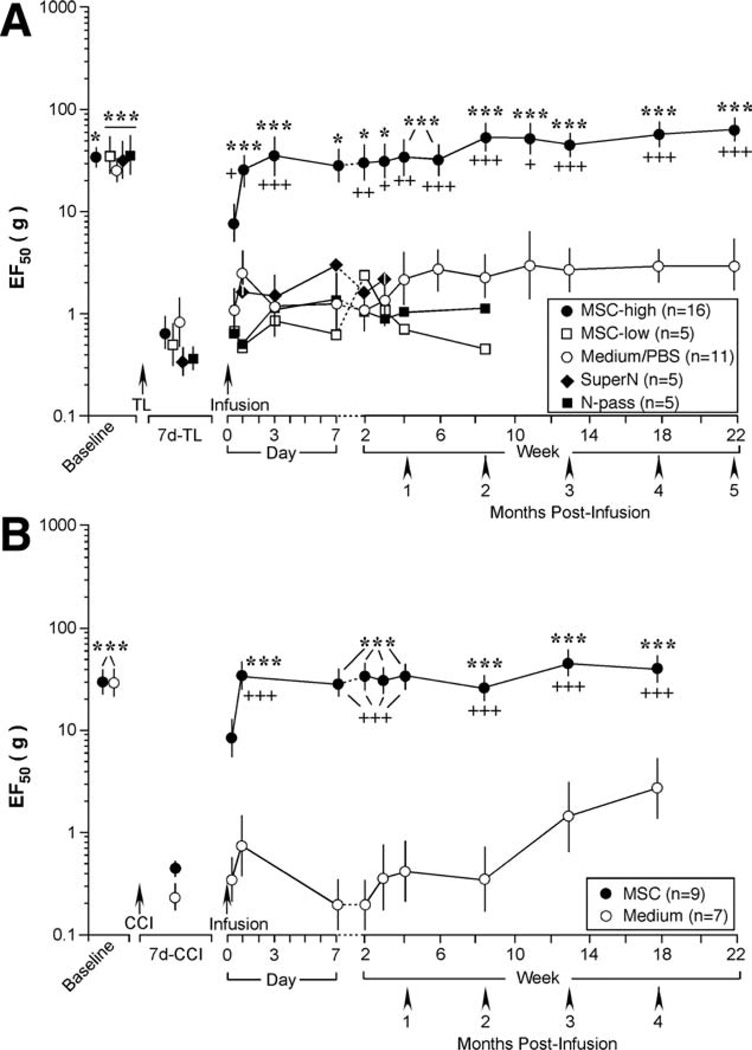
Figure 2.
Bone marrow stromal cell (BMSC) reversed pain hypersensitivity after tendon injury. (A): Cultured cells were infused through a tail vein at 0.2 ml with 1.5 × 103 (low-dose, MSC-low) or 1.5 × 106 (high-dose, MSC-high) BMSCs after 7 day-tendon ligation (TL). Control infusions were culture medium/phosphate-buffered saline, the supernatant of cell suspension (SuperN), and cells (1.5 × 106 BMSCs) after 20 passages (N-pass). When compared with low-dose BMSCs and controls, infusion of 1.5 × 106 BMSCs significantly increased EF50s from 1 day to 5 months after infusion. Asterisks denote significant differences versus 7 day-TL: *, p < .05; ***, p < .001. Cross signs denote significant differences versus medium: +, p < .05; ++, p < .01; +++, p < .001. (B): Infusion of BMSC reversed pain hypersensitivity after chronic constriction injury (CCI) of the infraorbital nerve of the rat. When compared with control (culture medium), infusion of BMSCs significantly increased EF50s from 1 day to 4 months after infusion. Asterisks denote significant differences versus 7 day-CCI: ***, p < .001. Cross signs denote significant differences versus medium: +++, p < .001. Error bars represent 95% confidence intervals of EF50s. Abbreviations: CCI, chronic constriction injury; PBS, phosphate-buffered saline; TL, tendon ligation.
BMSCs derived from the rat grew in the same plate without further passage. The cells were harvested at 7 days after initial plating. At 7 days after tendon ligation, the isolated BMSCs were diluted with PBS and infused through one tail vein of the rat after measuring mechanical sensitivity. The i.v. injection volume was 0.2 ml with 1.5 × 103 (low-dose, n = 5) or 1.5 × 106 (high-dose, n = 16) BMSCs. Control infusions were culture medium (n = 7) or PBS (n = 4). Both the culture medium and PBS did not have an effect on hyperalgesia and their results were combined for analysis (n = 11) (Fig. 2A). Additional controls included the supernatant of cell suspension (n = 5) and cells after 20 passages (1.5 × 106 BMSCs, n = 5) (Fig. 2A). Mechanical sensitivity was assessed for periods of 3 weeks to 5 months after i.v. infusion (Fig. 2A).
In rats receiving a single infusion of 1.5 × 106 BMSCs, the EF50 was significantly increased when compared with the baseline level at 1 day after infusion and maintained throughout the observation period of 5 months (p < .05–.001) in the tendon-injured rats (Fig. 2A) and 4 months in the CCI-ION rats (Fig. 2B). The low-dose of BMSCs (1.5 × 103) did not produce significant changes in mechanical hypersensitivity (Fig. 2A). In rats receiving medium/PBS control, there was fluctuation of EF50s during first 3 weeks of infusion (p > .05), but the mechanical hypersensitivity persisted throughout the 5-month observation period. Infusion of the supernatant and BMSCs of high passages did not affect injury-induced mechanical hypersensitivity (Fig. 2A).
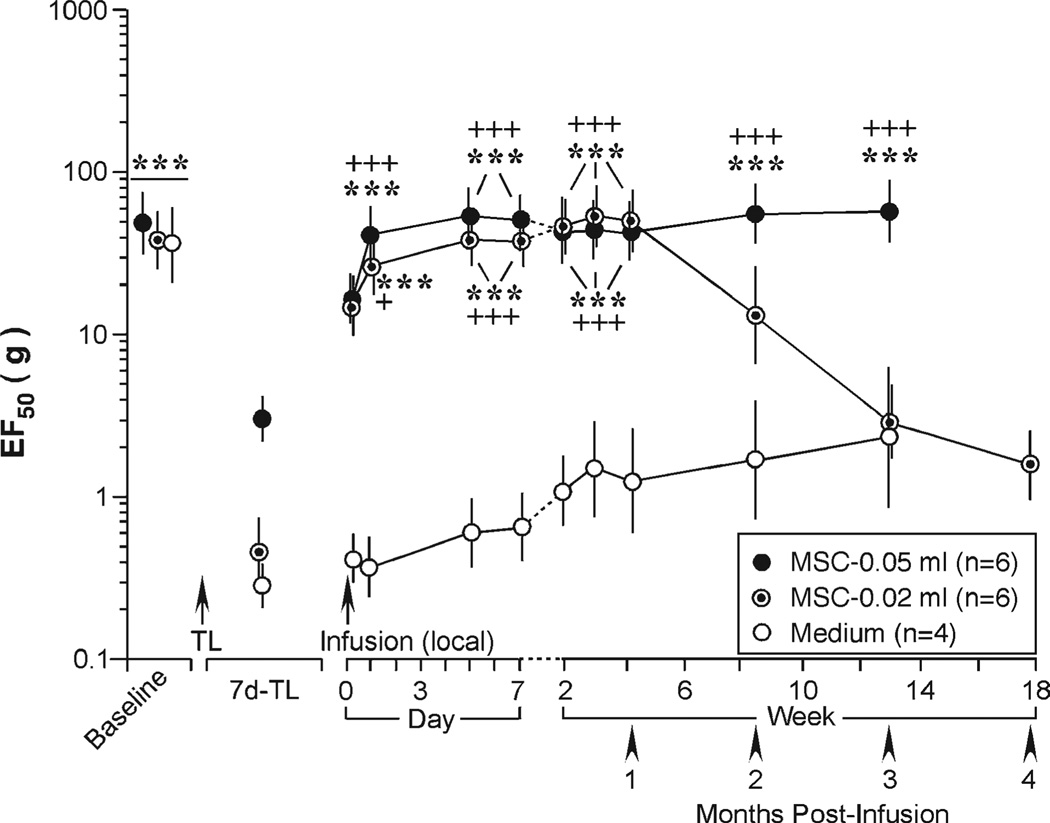
We next tested whether direct injection of BMSCs into the site of injury was effective. As shown in Figure 3, when compared with medium control (n = 4), the local injection of BMSCs (0.05 ml, 0.375 × 106 BMSCs) also reversed mechanical hypersensitivity starting from 1 day after cell injection (p < .001, n = 6). A lower dose of local BMSCs (0.02 ml, 0.15 × 106 cells) similarly reversed increased EF50s but the effect only lasted for about a month (p < .001, n = 6). BMSCs (1.5 × 106 cells) did not affect mechanical sensitivity of the naïve rats (n = 5) (not shown). Rats receiving BMSC treatment groomed normally and did not show abnormal behaviors. The results from Rotarod test suggest that locomotion was not affected by BMSC treatment (n = 6) (not shown).
Figure 3.
Local injection of bone marrow stromal cells (BMSCs) at the site of injury reversed tendon injury-induced mechanical sensitivity. When compared with medium control, the local injection of BMSC reversed pain hypersensitivity starting from 1 day after cell injection. Note that the lower dose of BMSCs (0.02 ml, 1.5 × 105 cells) produced shorter lasting increase in EF50s when compared with the higher dose (0.05 ml, 3.75 × 105 cells). Asterisks denote significant differences versus 7 day-tendon ligation: ***, p < .001. Cross signs denote significant differences versus medium: +, p < .05; +++, p < .001. Error bars represent 95% confidence intervals of EF50s. Abbreviations: TL, tendon ligation.
Effect of BMSCs on Early and Late Phases of Mechanical Hypersensitivity After Tendon Injury
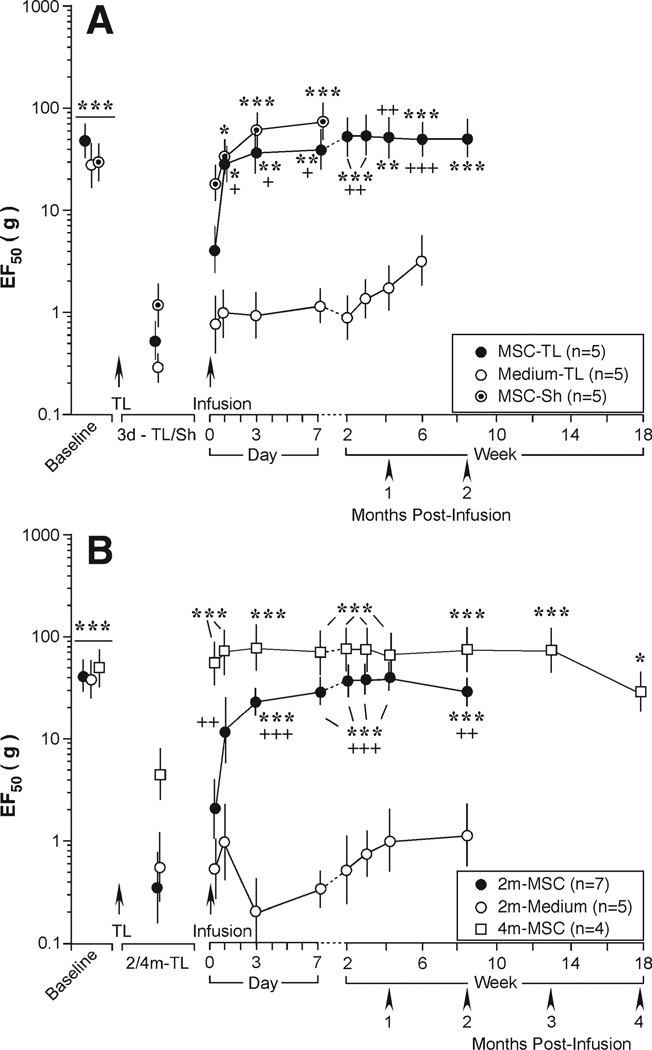
We further evaluated the effect of BMSCs in the tendon injury model. Although ligation of the TASM produces constant long-lasting mechanical hypersensitivity, there are differences between the early acute and late maintenance phases [22]. We asked whether BMSCs could reverse mechanical hypersensitivity when infused at early (3 days) and late (2 and 4 months) time points after tendon injury. At 3 days after injury, tissue injury produced by the ligation procedure per se contributes to mechanical hypersensitivity and the effect lasts for approximately 1 week [22]. This was clearly seen after a sham procedure (Fig. 4A) [22]. Infusion of BMSCs (1.5 × 106 cells) at 3 days after the tendon ligation or sham procedure significantly elevated EF50s (Fig. 4A). When BMSCs were infused at 2 (n = 7) and 4 (n = 4) months after ligation, mechanical hypersensitivity was also reversed, indicated by significant increases in EF50s (p < .05–.001) (Fig. 4B).
Figure 4.
Effect of intravenous bone marrow stromal cells (BMSCs) on early and late phases of mechanical hypersensitivity after tendon injury. BMSCs were infused at 3 days (A) or 2 and 4 months (B) after tendon ligation (TL; MSC-TL). Rats receiving sham operation (MSC-Sh) also was infused with BMSCs at 3 days after the surgery. There were significant increases in EF50s after BMSC infusion. Asterisks denote significant differences versus 3 day-TL/Sh or 2/4 months TL: *, p < .05; **, p < .01; ***, p < .001. Cross signs denote significant differences versus medium: +, p < .05; ++, p < .01; +++, p < .001. Error bars represent 95% confidence intervals of EF50s. Abbreviations: Sh, sham; TL, tendon ligation.
Effects of the Opioid Receptor Antagonists
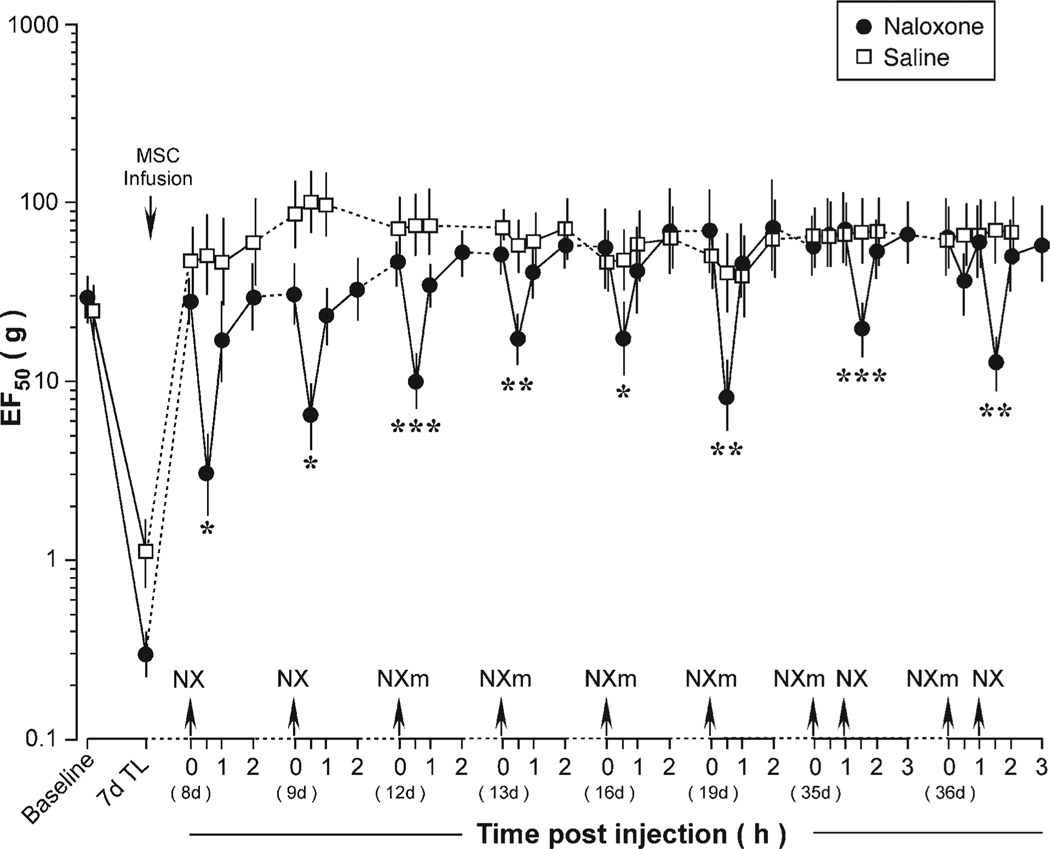
The mechanical hypersensitivity in the TASM injury model is sensitive to morphine [22]. We examined whether the reversal of mechanical hyperalgesia/allodynia after the BMSC treatment involved contribution from opioid receptors. We administered opioid receptor antagonists at different time points of the BMSC treatment in the tendon-injured rats and the results are collectively plotted in Figure 5. After infusion of BMSCs, EF50s of the injured rats returned to the baseline level. At 8 and 9 days after BMSC treatment, naloxone hydrochloride (2 mg/kg, i.p.), an opioid receptor antagonist that acts both peripherally and centrally after systemic administration, was injected. At 30 minutes after naloxone hydrochloride, EF50s were significantly reduced (p < .05, n = 5) when compared with saline-treated rats, indicating rekindling of hyperalgesia. The EF50s were partially recovered at 1 hour and fully returned to the prenaloxone hydrochloride levels at 2-hour-time point (Fig. 5).
Figure 5.
Effects of opioid receptor antagonists on bone marrow stromal cell-produced attenuation of mechanical hypersensitivity. A significant reduction of EF50 after naloxone (NX and NXm) indicates rekindling of mechanical hypersensitivity. Asterisks denote significant differences between naloxone and saline-treated rats: *, p < .05; **, p < .01; ***, p < .001. Error bars represent 95% confidence intervals of EF50s. Abbreviations: MSC, bone marrow stromal cell; NX, nalox-one hydrochloride; NXm, naloxone methiodide; TL, tendon ligation.
We further evaluated the contribution of peripheral opioid receptors. At 12, 13, 16, and 19 days after BMSC treatment, naloxone methiodide (2 mg/kg, i.p.), a peripherally acting opioid receptor antagonist, was administered. Naloxone methiodide similarly rekindled the hyperalgesia, or produced mechanical hypersensitivity in BMSC-treated rats, in all four cases (p < .05–.001) (Fig. 5). However, at 35 and 36 days after BMSC infusion, naloxone methiodide was not able to reduce EF50s (Fig. 5). Interestingly, naloxone hydrochloride that can cross the blood-brain barrier still produced a significant reduction of EF50s at 35 and 36 days after BMSC infusion (Fig. 5). Naloxone hydrochloride (2 mg/kg, i.p.) did not produce an effect on mechanical sensitivity in naïve rats (n = 4) (not shown).
Effect of Knockdown of MOR in the RVM
The results of the opioid receptor antagonist experiment suggest that endogenous opioid receptors contribute to attenuation of behavioral hyperalgesia/allodynia in BMSC-treated rats in a time-dependent fashion. Peripheral opioid receptors play a major role in the first 3 weeks of the BMSC treatment and central opioid receptors seem to be increasingly engaged from approximately 4–5 weeks after the BMSC treatment (Fig. 5). Opioid receptors in the RVM, a pivotal structure in descending pain modulation, play a key role in descending pain control [32]. We next used RNA interference (RNAi) to produce focal knockdown of MOR in the RVM to examine the role of MOR in the descending pathway in the BMSC-induced effect.
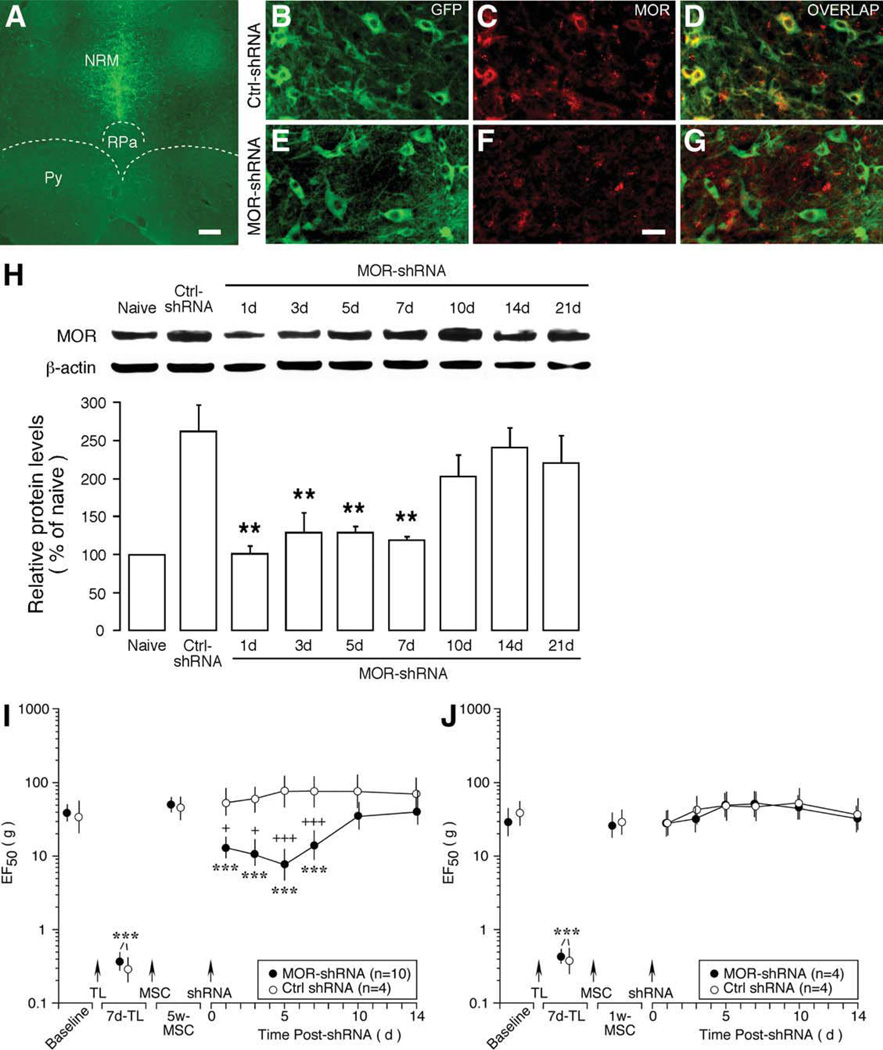
To produce MOR knockdown, shRNA plasmids for rat against MOR (500 ng/500 nl) were injected into the RVM and electroporation was performed to transfer plasmid into RVM neurons. Scrambled shRNA was injected as a control. As indicated by immunostaining of GFPs that was incorporated into the plasmid, shRNA was successfully delivered to the targeted RVM site (Fig. 6A). Double immunofluorescence staining in tissues transferred with scrambled shRNA showed that GFP- labeled cells expressed MOR at 3 days after gene transfer, confirming normal expression of MOR in RVM neurons and transfer of shRNA into MOR-containing neurons (Fig. 6B–6D, control shRNA). In rats receiving MOR-shRNA (Fig. 6E–6G), immunostaining intensity of MOR was clearly reduced at 3 days following the transfer (Fig. 6F) when compared with that of receiving control shRNA (Fig. 6C). The time-dependent downregulation of MOR was further quantified with western blot after transferring MOR-shRNA at 5 weeks after BMSC infusion. RVM tissues were dissected by taking punches and total proteins were isolated. There was a time-dependent reduction of MOR proteins at 1–7 days after MOR-shRNA when compared with that of control shRNA-treated rats (p < .01) (Fig. 6H). The MOR level returned to the control level at 10–21 days after MOR-shRNA transfer (p > .05 vs. Ctrl-shRNA) (Fig. 6H). The expression of serotonin in RVM was not affected by MOR-shRNA (data not shown), suggesting that the downregulation of MOR in RVM neurons was selective.
Figure 6.
Downregulation of mu opioid receptors (MOR) in rostral ventromedial medulla (RVM) on bone marrow stromal cell (BMSCs) produced attenuation of mechanical hypersensitivity. (A): GFP staining illustrates transfer of shRNA into the RVM site. Green fluorescence was distributed in the region of nucleus raphe magnus (NRM), the major nucleus of the RVM. Scale bar = 250 µm. (B–G): Double immunofluorescence staining of RVM neurons in rats receiving scrambled control shRNA (B–D) or MOR-shRNA (E–G). RVM neurons exhibit intense GFP immunostaining (B, E; green), indicating transfer of shRNA into these neurons. GFP colocalizes (D; yellow) with MOR staining (red) (C, D), confirming transfer of shRNA into MOR-containing neurons. MOR-shRNA transfer led to reduction of MOR-like immunoreactivity (C, F). Scale bar = 25 µm. (H): Western immunoblot illustrating downregulation of MOR after transferring MOR-shRNA at 5 weeks after BMSC infusion. Beta-actin was used as a loading control. When compared with the control (5 days after Ctrl-shRNA transfer), the MOR levels were significantly reduced at 1–7 days (p < .01, n = 3–5) and returned to the control level at 10–21 days after MOR-shRNA transfer. Error bars represent SEM. (I, J): shRNAs were transferred into RVM at 5 weeks (I) and 1 week (J) after BMSC infusion. Significant reduction of EF50s was observed in rats receiving shRNA at 5 weeks but not 1 week after BMSC treatment. Asterisks denote significant differences versus 1/5 week-MSC: ***, p < .001. Cross signs denote significant differences versus Ctrl-shRNA: +, p < .05; +++, p < .001. Error bars represent 95% confidence intervals of EF50s. Abbreviations: ctrl, control; GFP, green fluorescent protein; MOR, mu opioid receptors; NRM, nucleus raphe magnus; Py, pyramidal tract; RPa, nucleus raphe pallidus; shRNA, small hairpin RNA; TL, tendon ligation.
We next examined the effect of MOR downregulation in the RVM on BMSC-induced reversal of hyperalgesia. At 5 weeks after BMSC infusion, shRNAs were transfected into RVM neurons, and mechanical sensitivity was assessed at 1–14 days after the gene transfer. When compared with control shRNA (n = 4), rats receiving MOR-shRNA showed significant reduction of EF50s at 1–7 days after shRNA treatment, suggesting reappearance of mechanical hypersensitivity (p < .001, n = 10) (Fig. 6I). The time course of the reappearance of mechanical hypersensitivity correlated with that of downregulation of MOR in RVM (Fig. 6H). In contrast, when MOR-shRNA was transferred into RVM at 1 week after the BMSC treatment, the EF50s remained at the elevated levels (Fig. 6J). Together with the selective naloxone experiments, these results suggest that peripheral and central opioid receptors are involved in BMSC-induced effect in distinct time domains. While peripheral opioids contribute earlier, central opioids are engaged 4–5 weeks later after BMSC treatment.
Discussion
The major finding of this study is that i.v. infusion or local injection of BMSCs produced long-term attenuation of mechanical hypersensitivity, or hyperalgesia and allodynia, in rats after tissue and nerve injury. Mesenchymal stromal cells are readily accessible from donor bone marrow, and easy to isolate and expand ex vivo. Clinical studies show that direct injection of mesenchymal stromal cells does not produce unwanted side effects is well tolerated and safe [33, 34]. We did not observe any adverse effect of BMSCs in rats. Mesenchymal stromal cells can persist in tissues for months after systemic administration [35]. The beneficial effect of BMSCs on brain tissue repair has been shown to last for at least 4 months after stroke [12]. Our results indicate that BMSC-induced attenuation of mechanical hypersensitivity lasted up to 5 months of the observation period. Thus, BMSCs possess distinct advantages as a promising alternative and efficacious treatment of chronic pain.
In our pilot studies, we used passage 3 BMSCs and they were effective in alleviating pain (data not shown); however, BMSCs at high passages (>20) were not effective. We decided to use passage 0 BMSC to evaluate their effect on persistent pain. This would minimize any possible genotypic changes during in vitro culturing. The reason that high passage BMSCs lost their pain suppressing function is unclear. BMSCs at passage 0 contain a heterogeneous population of stem and progenitor stromal cells, and it has been well observed that prolonged culturing of BMSCs loses their multiple lineage differentiation potential [36]. Our finding suggests that the pain-relieving therapeutic property of BMSCs may be related to their stemness. It would be interesting to determine the molecular mechanism of this correlation.
The potential of BMSCs for pain treatment has attracted attention only recently. Different from neural stem cells that produce pain hypersensitivity when transplanted for spinal cord injury [37, 38], mesenchymal stromal cells prevent or reverse neuropathic pain [17, 18], attenuate pain hypersensitivity associated with spinal cord injury [19], and reduce nerve injury-induced changes in galanin, neurpeptide Y, and neurpeptide Y Y1-receptor expression [20]. Transplantation of human mesenchymal stem cells into the lateral cerebral ventricle of the mouse also attenuates allodynia [21]. This study extends previous findings by systematically demonstrating a long-lasting attenuation of pain hypersensitivity after BMSC infusion and the involvement of endogenous opioids in this effect.
The endogenous opioid system exhibits plastic changes after peripheral injury [32, 39]. There is a downregulation of central opioid receptors after ligation of the sciatic nerve [39]. Although it is unclear whether there are changes in opioid receptor expression in the TASM and CCI-ION ligation models, mechanical hypersensitivity in these two models was attenuated by systemic morphine or overproduction of enkephalin in trigeminal neurons [22, 40, 41]. Our pharmacological and RNAi analyses suggest that the attenuation of pain by BMSCs involves both peripheral and central actions related to opioids. Studies have shown that transplantation of BMSCs into either the dorsal root ganglion or the brain attenuates neuropathic pain behavior after sciatic nerve injury [18, 21]. However, an interesting finding of this study is that peripheral opioid receptors are activated to play a major role in reducing pain hypersensitivity, but only at the first few weeks (≤4 weeks) after the transplantation. From approximately 4–5 weeks after infusion of BMSCs, the pain attenuation largely depends on activation of central opioid receptors. This differential involvement of peripheral and central opioid receptors seems to correlate with the pathological mechanisms of the development of pain hypersensitivity in the tendon ligation model. It is noted that after TASM ligation, primary afferent input related to initial injury and inflammation diminishes over a few weeks after injury and that decreased growth rate does not occur until 4 weeks after injury [22]. Nevertheless, central neurochemical changes last for at least 8 weeks and pain hypersensitivity is constant for months in the TASM ligation model [22] (this study). Thus, pain hypersensitivity after tendon injury is dependent on peripheral input initially but may be maintained during attenuated primary afferent input, presumably by a central facilitatory drive. Correspondingly, transplantation of BMSCs induces peripheral opioid release initially when peripheral nociceptor sensitization plays a major role and engages central opioid receptors to override descending facilitation when the maintenance of pain hypersensitivity has transitioned into the central site.
Although mesenchymal stromal cells have shown therapeutic promise in a number of clinical situations [4, 34, 42–44], the cellular mechanisms of their action are still elusive. Some animal studies suggest that systemically injected BMSCs are able to migrate to the injured brain site after traumatic or ischemic injury [26, 45]. Other studies indicate that the majority of the intravenously infused BMSCs are localized to the lungs [46–48], less than 5% of the cells reach the circulation, and a very small number of cells appear at the injured brain site in rats [48]. Clinical studies suggest that the infused cells do not need to be localized to the supposed site of activity for their therapeutic effect [reviewed in 49], although homing of stem cells to the site of injury is important for therapies that are intended for tissue repair. Our results showed that both systemic and direct local delivery of BMSCs produced similar attenuation of pain hypersensitivity after tendon injury, suggesting that BMSCs act at the site of injury. It is also possible that BMSCs migrate to the trigeminal ganglion related to the injured orofacial tissue or nerve. BMSCs have been shown to be able to migrate to the dorsal root ganglion associated with an injured sciatic nerve [50].
Mesenchymal stromal cells express a variety of cytokines including chemokines, adhesion molecules, trophic factors, and other soluble molecules [49, 51, 52]. Most likely, the infused BMSCs produce their therapeutic effects through secretion of chemical mediators that interact with the immune system of body [5–7, 45, 49]. Our results suggest that the infused BMSCs stimulate release of endogenous opioid peptides from peripheral and central sites, leading to prolonged attenuation of pain hypersensitivity. This result is consistent with our previous report that tendon ligation-induced hyperalgesia was sensitive to morphine [22]. Opioid peptides β-endorphin, enkephalin, and endomorphins are present in immune cells such as granulocytes, T-cells, and moncytes/macrophages [53–55]. Guided by chemokines and adhesion molecules, immune cells migrate to the site of injury and release analgesic opioid pepides [54]. Conceivably, BMSCs may contribute to this process through release of chemokines and adhesion molecules that interact with their receptors on immune cells [52]. In addition, BMSCs may secrete catecholamines that trigger opioid peptide release [54, 56]. Interestingly, circulating catecholamines are higher in patients reporting chronic pain [57]. Consistently, decreased catecholamine catabolism is related to increased pain sensitivity [58]. In patients with pain associated with dysfunction of the TMJ, the levels of β-endorphin in the synovial fluid of the TMJ are significantly increased [59]. Thus, BMSCs may strengthen endogenous analgesic pathway under chronic pain conditions. While this scenario explains opioid peptide release from peripheral immune cells, it is unclear whether BMSC-triggered opioid action in the central nervous system involves similar mechanisms.
Mesenchymal stromal cells may also affect pain transmission through their immunoregulatory properties. The immune system interacts with the central nervous system to regulate pain sensitivity [60]. Following tissue or nerve injury, there is increased activity of immune cells and glial cells and release of inflammatory cytokines at the spinal and brainstem levels. Ample evidence indicates that injury-induced immune responses contribute to the development of pain hypersensitivity. In the TASM ligation model, there is upregulation of glial cell markers such as glial fibrillary acidic protein (astroglia) and CD11b (microglia), associated with upregulation of neuronal N-methyl-d-aspartate receptors and mechanical hyperalgesia and allodynia [22]. A relevant phenomenon is that BMSCs upregulate anti-inflammatory cytokines such as interleukin-2 (IL-2), IL-4, and IL-10 and downregulate proinflammatory cytokines such as tumor necrosis factor α and IL-17 in animal disease models [5, 10]. This differential regulation of inflammatory cytokines may lead to immunosuppression and pain attenuation. Studies also show that BMSC treatment leads to reduced expression of IL-1β and decreased microglial activity as suggested by reduced glial marker expression in nerve-injured animals [19, 21]. The antiglial activity of BMSCs is in contrast to neural stem cells that differentiate into astrocytes after transplantation into the spinal cord [38], which may explain the appearance of allodynia in animals receiving neural stem cells. Further studying the effect of BMSCs on immune and glial responses to injury will be an important direction toward understanding the underlying mechanisms of the pain-relieving effect of BMSCs.
Conclusion
We have shown in this study that a single infusion of BMSCs produced long-term attenuation of hyperalgesia and allodynia in rats after tissue and nerve injury. The reversal of hyperalgesia/allodynia was naloxone sensitive, suggesting the involvement of endogenous opioids. Further analysis suggests that the early effect of BMSCs on mechanical hypersensitivity mainly involved peripheral opioids and the late effect of BMSCs depended on activation of opioids in brainstem descending pathways. A clinically relevant observation is that treatment at different post-injury time points, as late as 4 months, was effective. These results provide preclinical evidence that supports the use of BMSCs in treatment of long-duration persistent pain associated with TMJ disorders and potentially other chronic pain conditions as well.
Acknowledgments
This work was supported by Grants DE11964, DE018573, NS060735, NS059028, and DE019156 from the NIH. M.W. is supported by Grant-in-Aid for Scientific Research (22592039) from the Japanese Ministry of Education, Science and Culture.
Footnotes
Author contributions: W.G.: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing; H.W., S.Z., and M.W.: collection and assembly of data; M.G.: conception and design, collection and assembly of data, data analysis and interpretation; F.W.: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing, financial support; R.D.: conception and design, data analysis and interpretation, manuscript writing, financial support, final approval of manuscript; G.T.-J. H.: conception and design, data interpretation, manuscript writing, financial support, final approval of manuscript; K.R.: conception and design, assembly of data, data analysis and interpretation, manuscript writing, financial support; final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
REFERENCES
- 1.Bouffi C, Djouad F, Mathieu M, et al. Multipotent mesenchymal stromal cells and rheumatoid arthritis: Risk or benefit? Rheumatology (Oxford) 2009;48:1185–1189. doi: 10.1093/rheumatology/kep162. [DOI] [PubMed] [Google Scholar]
- 2.Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: Their biology and role in regenerative medicine. J Dent Res. 2009;88:792–806. doi: 10.1177/0022034509340867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 4.Le Blanc K, Frassoni F, Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: A phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 5.Mao F, Xu WR, Qian H, et al. Immunosuppressive effects of mesenchymal stem cells in collagen-induced mouse arthritis. Inflamm Res. 2010;59:219–225. doi: 10.1007/s00011-009-0090-y. [DOI] [PubMed] [Google Scholar]
- 6.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 7.Wakabayashi K, Nagai A, Sheikh AM, et al. Transplantation of human mesenchymal stem cells promotes functional improvement and increased expression of neurotrophic factors in a rat focal cerebral ischemia model. J Neurosci Res. 2010;88:1017–1025. doi: 10.1002/jnr.22279. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Ding F, Gu Y, et al. Bone marrow mesenchymal stem cells promote cell proliferation and neurotrophic function of Schwann cells in vitro and in vivo. Brain Res. 2009;1262:7–15. doi: 10.1016/j.brainres.2009.01.056. [DOI] [PubMed] [Google Scholar]
- 9.Shibata T, Naruse K, Kamiya H, et al. Transplantation of bone marrow-derived mesenchymal stem cells improves diabetic polyneuropathy in rats. Diabetes. 2008;57:3099–3107. doi: 10.2337/db08-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bai L, Lennon DP, Eaton V, et al. Human bone marrow-derived mesenchymal stem cells induce Th2-polarized immune response and promote endogenous repair in animal models of multiple sclerosis. Glia. 2009;57:1192–1203. doi: 10.1002/glia.20841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen J, Li Y, Wang L, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Chen J, Zhang CL, et al. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia. 2005;49:407–417. doi: 10.1002/glia.20126. [DOI] [PubMed] [Google Scholar]
- 13.Shen LH, Li Y, Chen J, et al. Intracarotid transplantation of bone marrow stromal cells increases axon-myelin remodeling after stroke. Neuroscience. 2006;137:393–399. doi: 10.1016/j.neuroscience.2005.08.092. [DOI] [PubMed] [Google Scholar]
- 14.Shen LH, Li Y, Chen J, et al. Therapeutic benefit of bone marrow stromal cells administered 1 month after stroke. J Cereb Blood Flow Metab. 2007;27:6–13. doi: 10.1038/sj.jcbfm.9600311. [DOI] [PubMed] [Google Scholar]
- 15.Andrews EM, Tsai SY, Johnson SC, et al. Human adult bone marrow-derived somatic cell therapy results in functional recovery and axonal plasticity following stroke in the rat. Exp Neurol. 2008;211:588–592. doi: 10.1016/j.expneurol.2008.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pavlichenko N, Sokolova I, Vijde S, et al. Mesenchymal stem cells transplantation could be beneficial for treatment of experimental ischemic stroke in rats. Brain Res. 2008;1233:203–213. doi: 10.1016/j.brainres.2008.06.123. [DOI] [PubMed] [Google Scholar]
- 17.Klass M, Gavrikov V, Drury D, et al. Intravenous mononuclear marrow cells reverse neuropathic pain from experimental mononeuropathy. Anesth Analg. 2007;104:944–948. doi: 10.1213/01.ane.0000258021.03211.d0. [DOI] [PubMed] [Google Scholar]
- 18.Musolino PL, Coronel MF, Hökfelt T, et al. Bone marrow stromal cells induce changes in pain behavior after sciatic nerve constriction. Neurosci Lett. 2007;418:97–101. doi: 10.1016/j.neulet.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Abrams MB, Dominguez C, Pernold K, et al. Multipotent mesenchymal stromal cells attenuate chronic inflammation and injury-induced sensitivity to mechanical stimuli in experimental spinal cord injury. Restor Neurol Neurosci. 2009;27:307–321. doi: 10.3233/RNN-2009-0480. [DOI] [PubMed] [Google Scholar]
- 20.Coronel MF, Musolino PL, Brumovsky PR, et al. Bone marrow stromal cells attenuate injury-induced changes in galanin, Npy and NPY Y1-receptor expression after a sciatic nerve constriction. Neuropeptides. 2009;43:125–132. doi: 10.1016/j.npep.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Siniscalco D, Giordano C, Galderisi U, et al. Intra-brain microinjection of human mesenchymal stem cells decreases allodynia in neuropathic mice. Cell Mol Life Sci. 2010;67:655–669. doi: 10.1007/s00018-009-0202-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo W, Wang H, Zou S, et al. Long lasting pain hypersensitivity following ligation of the tendon of the masseter muscle in rats: A model of myogenic orofacial pain. Mol Pain. 2010;6:40. doi: 10.1186/1744-8069-6-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei F, Guo W, Zou S, et al. Supraspinal glial-neuronal interactions contribute to descending pain facilitation. J Neurosci. 2008;28:10482–10495. doi: 10.1523/JNEUROSCI.3593-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ren K. An improved method for assessing mechanical allodynia in the rat. Physiol Behav. 1999;67:711–716. doi: 10.1016/s0031-9384(99)00136-5. [DOI] [PubMed] [Google Scholar]
- 25.Guo W, Wei F, Zou S, et al. Group I metabotropic glutamate receptor NMDA receptor coupling and signaling cascade mediate spinal dorsal horn NMDA receptor 2B tyrosine phosphorylation associated with inflammatory hyperalgesia. J Neurosci. 2004;24:9161–9173. doi: 10.1523/JNEUROSCI.3422-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen J, Li Y, Wang L, et al. Therapeutic benefit of intracerebral transplantation of bone marrow stromal cells after cerebral ischemia in rats. J Neurol Sci. 2001;189:49–57. doi: 10.1016/s0022-510x(01)00557-3. [DOI] [PubMed] [Google Scholar]
- 27.Guo W, Robbins MT, Wei F, et al. 2006 Supraspinal brain-derived neurotrophic factor signaling: A novel mechanism for descending pain facilitation. J Neurosci. 2006;26:126–137. doi: 10.1523/JNEUROSCI.3686-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 5th ed. Amsterdam, Netherlands: Elsevier; 2005. [Google Scholar]
- 29.Wei F, Dubner R, Zou S, et al. Molecular depletion of descending serotonin unmasks its novel facilitatory role in the development of persistent pain. J Neurosci. 2010;30:8624–8636. doi: 10.1523/JNEUROSCI.5389-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crisostomo PR, Wang Y, Markel TA, et al. Human mesenchymal stem cells stimulated by TNF-alpha, LPS, or hypoxia produce growth factors by an Nf kappa B- but not JNK-dependent mechanism. Am J Physiol Cell Physiol. 2008;294:C675–C682. doi: 10.1152/ajpcell.00437.2007. [DOI] [PubMed] [Google Scholar]
- 31.Carr CA, Stuckey DJ, Tatton L, et al. Bone marrow-derived stromal cells home to and remain in the infarcted rat heart but fail to improve function: An in vivo cine-MRI study. Am J Physiol Heart Circ Physiol. 2008;295:H533–H542. doi: 10.1152/ajpheart.00094.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ren K, Dubner R. Descending control mechanisms. In: Basbaum AI, Kaneko A, Shepherd GM, et al., editors. The Senses: A Comprehensive Reference. Vol 5. San Diego, CA: Academic Press; 2008. pp. 723–762. Pain, Ed. [Google Scholar]
- 33.Lazarus HM, Haynesworth SE, Gerson SL, et al. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): Implications for therapeutic use. Bone Marrow Transplant. 1995;16:557–564. [PubMed] [Google Scholar]
- 34.Mazzini L, Mareschi K, Ferrero I, et al. Autologous mesenchymal stem cells: Clinical applications in amyotrophic lateral sclerosis. Neurol Res. 2006;28:523–526. doi: 10.1179/016164106X116791. [DOI] [PubMed] [Google Scholar]
- 35.Liechty KW, MacKenzie TC, Shaaban AF, et al. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med. 2000;6:1282–1286. doi: 10.1038/81395. [DOI] [PubMed] [Google Scholar]
- 36.Digirolamo CM, Stokes D, Colter D, et al. Propagation and senescence of human marrow stromal cells in culture: A simple colony-forming assay identifies samples with the greatest potential to propagate and differentiate. Br J Haematol. 1999;107:275–281. doi: 10.1046/j.1365-2141.1999.01715.x. [DOI] [PubMed] [Google Scholar]
- 37.Hofstetter CP, Holmström NA, Lilja JA, et al. Allodynia limits the usefulness of intraspinal neural stem cell grafts; directed differentiation improves outcome. Nat Neurosci. 2005;8:346–353. doi: 10.1038/nn1405. [DOI] [PubMed] [Google Scholar]
- 38.Macias MY, Syring MB, Pizzi MA, et al. Pain with no gain: Allodynia following neural stem cell transplantation in spinal cord injury. Exp Neurol. 2006;201:335–348. doi: 10.1016/j.expneurol.2006.04.035. [DOI] [PubMed] [Google Scholar]
- 39.Niikura K, Narita M, Butelman ER, et al. Neuropathic and chronic pain stimuli downregulate central mu-opioid and dopaminergic transmission. Trends Pharmacol Sci. 2010;31:299–305. doi: 10.1016/j.tips.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 40.Christensen D, Gautron M, Guilbaud G, et al. Combined systemic administration of the glycine/NMDA receptor antagonist, (+)-HA966 and morphine attenuates pain-related behaviour in a rat model of trigeminal neuropathic pain. Pain. 1999;83:433–440. doi: 10.1016/S0304-3959(99)00126-8. [DOI] [PubMed] [Google Scholar]
- 41.Meunier A, Latrémolière A, Mauborgne A, et al. Attenuation of pain-related behavior in a rat model of trigeminal neuropathic pain by viral-driven enkephalin overproduction in trigeminal ganglion neurons. Mol Ther. 2005;11:608–616. doi: 10.1016/j.ymthe.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 42.Djouad F, Bouffi C, Ghannam S, et al. Mesenchymal stem cells: Innovative therapeutic tools for rheumatic diseases. Nat Rev Rheumatol. 2009;5:392–399. doi: 10.1038/nrrheum.2009.104. [DOI] [PubMed] [Google Scholar]
- 43.Horwitz EM, Gordon PL, Koo WK, et al. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc Natl Acad Sci USA. 2002;99:8932–8937. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holzer U, van Royen-Kerkhof A, van der Torre P, et al. Successful autologous stem cell transplantation in two patients with juvenile dermatomyositis. Scand J Rheumatol. 2010;39:88–92. doi: 10.3109/03009740903096622. [DOI] [PubMed] [Google Scholar]
- 45.Mahmood A, Lu D, Chopp M. Intravenous administration of marrow stromal cells (MSCs) increases the expression of growth factors in rat brain after traumatic brain injury. J Neurotrauma. 2004;21:33–39. doi: 10.1089/089771504772695922. [DOI] [PubMed] [Google Scholar]
- 46.Barbash IM, Chouraqui P, Baron J, et al. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: Feasibility, cell migration, and body distribution. Circulation. 2003;108:863–868. doi: 10.1161/01.CIR.0000084828.50310.6A. [DOI] [PubMed] [Google Scholar]
- 47.Fischer UM, Harting MT, Jimenez F, et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery: The pulmonary first-pass effect. Stem Cells Dev. 2009;18:683–692. doi: 10.1089/scd.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harting MT, Jimenez F, Xue H, et al. Intravenous mesenchymal stem cell therapy for traumatic brain injury. J Neurosurg. 2009;110:1189–1197. doi: 10.3171/2008.9.JNS08158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Horwitz EM, Prather WR. Cytokines as the major mechanism of mesenchymal stem cell clinical activity: Expanding the spectrum of cell therapy. Isr Med Assoc J. 2009;11:209–211. [PubMed] [Google Scholar]
- 50.Coronel MF, Musolino PL, Villar MJ. Selective migration and engraftment of bone marrow mesenchymal stem cells in rat lumbar dorsal root ganglia after sciatic nerve constriction. Neurosci Lett. 2006;405:5–9. doi: 10.1016/j.neulet.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 51.Noël D, Djouad F, Bouffi C, et al. Multipotent mesenchymal stromal cells and immune tolerance. Leuk Lymphoma. 2007;48:1283–1289. doi: 10.1080/10428190701361869. [DOI] [PubMed] [Google Scholar]
- 52.Salem HK, Thiemermann C. Mesenchymal stromal cells: Current understanding and clinical status. Stem Cells. 2010;28:585–596. doi: 10.1002/stem.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Machelska H. Targeting of opioid-producing leukocytes for pain control. Neuropeptides. 2007;41:355–363. doi: 10.1016/j.npep.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 54.Stein C, Lang LJ. Peripheral mechanisms of opioid analgesia. Curr Opin Pharmacol. 2009;9:3–8. doi: 10.1016/j.coph.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 55.Jessop DS, Fassold A, Wolff C, et al. Endomorphins in rheumatoid arthritis, osteoarthritis, and experimental arthritis. Ann N Y Acad Sci. 2010;1193:117–122. doi: 10.1111/j.1749-6632.2009.05294.x. [DOI] [PubMed] [Google Scholar]
- 56.Maestroni GJ, Cosentino M, Marino F, et al. Neural and endogenous catecholamines in the bone marrow. Circadian association of norepinephrine with hematopoiesis? Exp Hematol. 1998;26:1172–1177. [PubMed] [Google Scholar]
- 57.Harden RN, Rudin NJ, Bruehl S, et al. Increased systemic catecholamines in complex regional pain syndrome and relationship to psychological factors: A pilot study. Anesth Analg. 2004;99:1478–1485. doi: 10.1213/01.ANE.0000132549.25154.ED. [DOI] [PubMed] [Google Scholar]
- 58.Diatchenko L, Slade GD, Nackley AG, et al. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum Mol Genet. 2005;14:135–143. doi: 10.1093/hmg/ddi013. [DOI] [PubMed] [Google Scholar]
- 59.Kajii TS, Okamoto T, Yura S, et al. Elevated levels of beta-endorphin in temporomandibular joint synovial lavage fluid of patients with closed lock. J Orofac Pain. 2005;19:41–46. [PubMed] [Google Scholar]
- 60.Ren K, Dubner R. Interactions between the immune and nervous systems in pain. Nat Med. 2010;16:1267–1276. doi: 10.1038/nm.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]