Abstract
The aim of vaccination is to induce appropriate immunity against pathogens. Antibody-mediated immunity is critical for protection against many virus diseases, although it is becoming more evident that coordinated, multifunctional immune responses lead to the most effective defense. Specific antibody (Ab) isotypes are more efficient at protecting against pathogen invasion in different locations in the body. For example, compared to other Ab isotypes, immunoglobulin (Ig) A provides more protection at mucosal areas. In this study, we developed a cationic lipopolymer (liposome-polyethylene glycol-polyethyleneimine complex [LPPC]) adjuvant that strongly adsorbs antigens or immunomodulators onto its surface to enhance or switch immune responses. The results demonstrate that LPPC enhances uptake ability, surface marker expression, proinflammatory cytokine release, and antigen presentation in mouse phagocytes. In contrast to Freund’s adjuvant, LPPC preferentially activates Th1- immunity against antigens in vivo. With lipopolysaccharides or CpG oligodeoxynucleotides, LPPC dramatically enhances the IgA or IgG2A proportion of total Ig, even in hosts that have developed Th2 immunities and high IgG1 serum titers. Taken together, the results demonstrate that the LPPC adjuvant not only increases the immunogenicity of antigens but also modulates host immunity to produce an appropriate Ab isotype by combining with immunomodulators.
Keywords: liposome-PEG-PEI complex, adjuvant, class switch, immunomodulator, vaccine
Introduction
The goal of vaccination is to generate a strong immune response that will provide long-term protection against infection. For vaccines that are composed of low immunogenic antigens (such as synthetic peptides or subunit antigens), the addition of an appropriate adjuvant is necessary to improve the immune response. An ideal adjuvant should enhance the immunogenicity of an antigen, reduce the amount of antigens or the number of immunizations needed for protective immunity, increase the biological or immunological half-life of the antigen, improve antigen delivery and/or processing in the antigen-presenting cells (APCs), and induce the production of immunomodulatory cytokines.1,2 The adjuvant categories include mineral salts, tensoactive compounds, microorganism-derived adjuvants, emulsions, cytokines, polysaccharides, nucleic acid-based adjuvants, and particulate antigen delivery systems.1 To date, research has focused on particulate antigen delivery systems in which the particles not only enhance the immunogenicity of encapsulated antigens by themselves but also incorporate other immunomodulatory molecules such as CpG oligodeoxynucleotides (ODN),2 double stranded RNA,3 monophosphoryl lipid A,4 or interleukin (IL)-12,5 which results in a dramatic increase in their efficacy and even in the switching of immune pathways.
Different immune responses have various effects on the pathogenesis of different diseases. For example, a Th1 response efficiently attacks virus-infected cells or tumor cells but inhibits the Th2 response to produce a humoral immune response (antibody production) and vice versa.6 Certain intracellular pathogens, such as Mycobacterium tuberculosis7 and human papilloma virus,8 polarize host immunity toward a Th2 response, which is less effective against that pathogen than the Th1 response. In contrast, three major autoimmune diseases (rheumatoid arthritis, multiple sclerosis, and Type 1 diabetes) are characterized by an excessively dominant Th1 response that results in harm to the host.9 Therefore, if an adjuvant regulates an appropriate immune response, it will protect the host from pathogen infection, and it will reduce the autoimmune response that includes allergic reactions that can damage host tissue. In addition, Th1 or Th2 relative cytokines are involved in antibody class switching10 and antibody class is considered to be important in the pathogenesis of diseases. For example, immunoglobulin (Ig) E antibodies attach to basophils and mast cells by way of their surface IgE receptors. Subsequent exposure to an allergen leads to the activation of the IgE-sensitized cells and results in an allergic reaction.11 In contrast, following treatment with an allergen, an increase in serum IgG levels (particularly IgG4) will lead to the allergen being neutralized and the prevention of IgE cross-linking by the allergen and the degranulation of mast cells.12 In addition, depending on their transporting properties, IgA or IgG1 antibodies provide increased protection at mucosal regions and in infants.13 Thus, if an adjuvant can induce an appropriate antibody isotype, it will be more efficient at protecting the host from disease.
Many adjuvants are surprisingly potent stimulators of humoral and cellular immune responses against a variety of antigens.14 In addition, certain molecules can affect the signal for isotype switching in B cells. It has been demonstrated that CpG ODN enhance the production of IgG2a,15 Escherichia coli lipopolysaccharides (LPS) enhance the production of IgA,16 and cathelin-related antimicrobial peptide increases the production of IgG1.17 Furthermore, Fu-Ling extract enhances IgG and IgA secretions18 and ginseng extract promotes the production of IgG1, IgG2a, and IgG2b.19 Therefore, the combination of particulate antigen delivery systems with these immunomodulators may provide therapeutic benefits for the treatment of pathological symptoms that are induced by improper immunity in humans.
In this study, we evaluated the immunoregulatory potential of the liposome-polyethylene glycol-polyethyleneimine-complex (LPPC) adjuvant. LPPC strongly and quickly adsorbs antigens onto its surface to form complexes.20 Unlike other adjuvants, LPPC binds but does not encapsulate antigen. Therefore, antigens are simply mixed with LPPC before use and do not need to be emulsified. In addition, the positively charged surface of LPPC may bind substances such as LPS or CpG ODN, which specifically enhance the production of a specific antibody isotype. Furthermore, the liposomal core of LPPC may be an immunomodulator carrier. In conclusion, the results demonstrate that the combination of antigen and immunomodulator with the LPPC adjuvant strengthens the efficacy of a vaccine and can switch an improper immune response to an appropriate immune response, thus providing a benefit in the treatment of human disease.
Materials and methods
Reagents
The reagents 1,2-dioleoyl-sn-glycero-3-phosphocholine and 1,2-dilauroyl-sn-glycero-3-phosphocholine were purchased from Avanti Polar Lipids (Alabaster, AL). Polyethylene glycol (PEG; 8000), polyethyleneimine (PEI; branched, MW 25,000), LPS, complete Freund’s adjuvant (CFA), incomplete Freund’s adjuvant (IFA), and fluorescein isothiocyanate (FITC) were purchased from Sigma-Aldrich (St Louis, MO). Bovine serum albumin (BSA) and trypan blue were purchased from Invitrogen (Gaithersburg, MD). CpG ODNs were purchased from InvivoGen (San Diego, CA). FITC-conjugated antimouse major histocompatibility complex (MHC) I mAb, FITC-conjugated antimouse MHC II mAb, and FITC-conjugated antimouse CD86 mAb were purchased from Biolegend (San Diego, CA). PBST is phosphate-buffered saline (PBS; pH 7.4) with 0.5% Tween-20.
Cell lines
P338D1 (mouse macrophage-like cell line; ATCC number: CCL-46) and Balb/3T3 (mouse fibroblast cell line; ATCC number: CCL-163) cell lines were purchased from BCRC (Hsinchu, Taiwan, People’s Republic of China). P338D1 cells were maintained in RPMI-1640 medium (Invitrogen), which was supplemented with heat-inactivated 10% fetal bovine serum (Gibco BRL, Gaithersburg, MD), and 1% penicillin/ streptomycin/ amphotericin (PSA; Biological industries, Beithaemek, Israel) with 5% CO2 at 37°C. Balb/3T3 cells were maintained in Dulbecco’s modified Eagle medium (Invitrogen), which was supplemented with heat-inactivated 10% fetal bovine serum and 1% PSA with 5% CO2 at 37°C.
Protein uptake ability and surface-marker expression of P338D1
LPPCs were prepared in accordance with procedures outlined in a previous study;20 the characteristics of LPPC with or without materials were analyzed. BSA-FITC (50 μg) was used as a fluorescent reporter and was adsorbed with different amounts of LPPC. BSA-FITC alone or LPPC-adsorbed BSA-FITC were cocultured with 5 × 105 P338D1 cells for 4 hours. Before analysis, the fluorescence of the treated cells was monitored by fluorescence-activated cell sorting (FACS; Becton Dickinson, San Jose, CA) with or without the addition of 100 μl trypan blue.
For the surface marker assay, 50 μg BSA was adsorbed on 100 μg LPPC, and the LPPC-BSA or BSA alone was cocultured with 5 × 105 P338D1 cells for 24 hours. The treated cells were probed with FITC-conjugated antimouse CD86, antimouse MHC I, or antimouse MHC II mAbs, and the surface fluorescence of the cells was measured using FACS. The data were expressed as the fold of fluorescent mean – the mean fluorescence intensity of the sample divided by the mean fluorescence intensity of the untreated group.
Enhancement of presentation efficiency by LPPC
Female Balb/c mice (6 to 8 weeks old) were purchased from the National Laboratory Center (Taipei, Taiwan, People’s Republic of China) and were housed in a temperature- and light-controlled room at the Animal Maintenance Facility of National Chiao Tung University. The mice were immunized and boosted on day 1 and day 14, respectively, by the subcutaneous (sc) injection of 100 μg BSA that was emulsified in 100 μL CFA and in 100 μL IFA. The splenocytes of all mice were harvested on day 21. BSA (10 μg), LPPC (4 μg), or 4 μg BSA/LPPC complex (10 μg BSA adsorbed on 4 μg LPPC) were incubated with 5 × 106 splenocytes for 72 hours, and the cytokine concentrations of the culture supernatants were analyzed using an ELISA reader (Tecan Group Ltd, Männedorf, Switzerland).
Determination of antibody titers in sera
Balb/c mice were divided into seven groups and each group was immunized using sc injection with 100 μg HpHsp60 antigen that was mixed with different adjuvants (100 μL CFA, 100 μL PBS, 100 μg LPPC, or 150 μg LPPC) or with adjuvant alone (100 μL PBS, 100 μg LPPC, or 150 μg LPPC). The mice were boosted 2 weeks after immunization with an sc injection of 100 μg HpHsp60 in different adjuvants (100 μL IFA, 100 μL PBS, 100 μg LPPC, or 150 μg LPPC) or adjuvant alone (100 μL PBS, 100 μg LPPC, or 150 μg LPPC). Starting 1 week after the administration of the booster, blood was collected by eye bleeding every 3 days and HpHsp60-specific antibodies in the sera were measured. The wells of 96-well plates were coated with HpHsp60 proteins (100 ng/well) overnight at 4°C. The wells were blocked with 300 μL PBST plus 2% skim milk (Mediatech, Herndon, VA) for 1 hour and were washed once with PBST plus 0.5% skim milk. One hundred microliters of serially diluted serum samples (1:100 to 1:12,800 in PBS containing 0.5% skim milk) was loaded into the wells and incubated for 2 hours. After washing three times, 100 μL horseradish peroxidase-conjugated antimouse Ig antibody (1:10000 dilution; Sigma Aldrich) was added to each well and incubated for 1 hour. The plates were washed three times and 100 μL of tetramethylbenzidine substrate (Pierce, Rockford, IL) was added to each well. After incubation for 20 minutes, the reaction was stopped using 100 μL 1 N HCl, and the optical density was measured at 450 nm using the ELISA reader.
To determine the effect of immunomodulators on anti-HpHsp60 titers, CpG ODN and LPS were adsorbed by LPPC. LPPC (150 μg) was incubated with 1 μg CpG ODN, 1 μg LPS or 2 μg LPS for 30 minutes and was centrifuged at 5900 × g for 5 minutes; the pellet was then resuspended in 100 μL PBS. Immuno-LPPC was used for immunizations by sc injection as described above. Blood was collected by eye bleeding every 3 days. HpHsp60-specific antibodies in the sera were measured as described.
Determination of anti-HpHsp60 isotype in sera
The mice were divided into seven groups and were immunized and boosted as described above. Blood was collected by eye bleeding, and the isotypes of HpHsp60-specific antibodies in the sera were determined at 21 days. The sera (1:800 dilution) were evaluated using horseradish peroxidase-conjugated antimouse Ig antibodies (total Ig, IgG1, IgG2a, IgG2b, IgA, and IgM; Acris, Herford, Germany). Similarly, the antibody isotypes in the sera of the mice immunized with LPPC-CpG ODN or -LPS were measured.
To determine if LPPC adsorbed with CpG ODN or LPS could change the original immune responses in vivo, mice were preimmunized with Freund’s adjuvant containing HpHsp60. At day 21 (the third week), the mice were immunized with LPPC alone or LPPC containing CpG ODN or LPS. Blood was collected once a week for 7 weeks (49 days) and the isotypes of the anti-HpHsp60 antibodies in the sera were evaluated.
ELISPOT
Mice were immunized and boosted with HpHsp60 alone or HpHsp60 combined with adjuvant (Freund’s adjuvant, 100 μg LPPC, or 150 μg LPPC) as described above. Splenocytes were collected from the treated mice 21 days after immunization, and the number of cells expressing IL-4 or IFN-γ was monitored using a U-CyTech BV mouse ELISpot kit (IFN-γ or IL-4; U-CyTech, Yalelaan, The Netherlands) in accordance with the manufacturer’s standard protocol. The spots in the wells were visualized and counted using an inverted microscope (Olympus, Tokyo, Japan).
Cytokine ELISA
Peripheral blood mononuclear cells (PBMCs) (105 cells per well) or splenocytes (2.5 × 105 cells per well) were dispensed into 96-well culture plates and were treated with 1 μg LPPC. The supernatants were collected at 72 hours and were frozen at −80°C. Supernatant concentrations of TNF-α, IL-1β, IL-4, IL-6, IL-8, IL-10, IL-2, and IFN-γ cytokines were measured by ELISA (ELISA kit; R&D Systems, Minneapolis, MN).
The mice were divided into seven groups and were immunized and boosted as described above. Splenocytes (2.5 × 105 cells per well in a 96-well plate) from treated mice were pulsed with HpHsp60 antigen (1 μg/mL) for 72 hours. The supernatants were analyzed for cytokines (TNF-α, IL-1β, IL-4, IL-10, IL-2, and IFN-γ using ELISA (R&D Systems). To examine the changes in the cytokine profiles of mice that were immunized with LPPC-CpG ODN or LPPC-LPS, splenocytes (2.5 × 105 cells per well in a 96-well plate) from the mice treated with HpHsp60 combined with Freund’s adjuvant, LPPC, LPPC-LPS, or LPPC-CpG were pulsed with HpHsp60 antigen (1 μg/mL) for 72 hours. The supernatants were collected and were analyzed for cytokines (IL-4, IL-5, TGF-β, and IFN-γ) using ELISA (R&D Systems).
The effect of LPPC adjuvant on cytotoxicity
Balb/3T3 cells were transfected with pCJ-HpHsp60 or pCJ-Helicobacter pylori urease B (HpUreaseB) (provided by Dr Wu, National Taiwan Ocean University) and expressed heat shock protein 60 or HpUreaseB, respectively. The transfected Balb/3T3 cells were plated (1 × 104 cells/well in a 96-well plate) and were incubated overnight. Splenocytes that were obtained from the immunized mice were added to the wells as effector cells at an effector:target cell ratio of 100:1, 50:1, 25:1, and 12.5:1. After incubation for 72 hours, the supernatants were removed, the wells were washed six times with PBS, and the cell viabilities were determined by 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay (Sigma-Aldrich). The lysis percentage was calculated using the following equation: the survival rate of the nontreated group (100%) minus the survival rate of the treated individual group.
Statistical analysis
All experiments were carried out independently and in triplicate. Standard deviations (SDs) of the mean were calculate, and data were analyzed using the SAS statistical software package (SAS Institute, Inc, Cary, NC). The results were expressed as the mean ± SD. Differences in mean values were evaluated by a one-way analysis of variance. The level of significance considered was P < 0.05.
Results
The effects of LPPC on antigen presentation
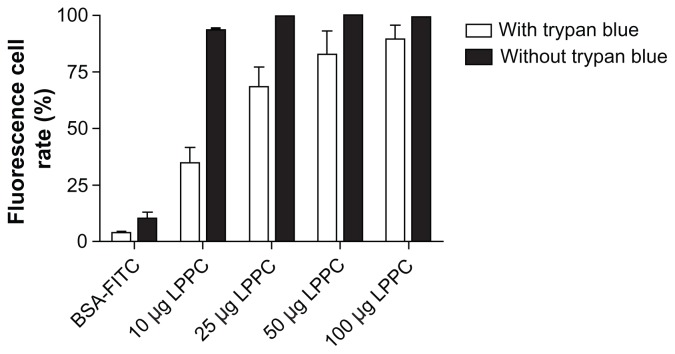
To evaluate the effect of the adjuvant LPPC on the uptake ability, surface marker expression and presentation efficiency of antigen presenting cells were analyzed. First, the ability to facilitate antigen uptake in macrophages by phagocytosis was determined. BSA-FITC (green fluorescence) was used as a reporter protein. Figure 1 indicates that BSA-FITC that is adsorbed on LPPC increased the fluorescence of P338D1 cells compared with BSA-FITC alone. However, it could not be distinguished whether the fluorescence was intracellular or present on the surface of the cells. Therefore, trypan blue, an agent that quenches the green fluorescence of FITC, was used to verify the location of the fluorescence. Because trypan blue cannot cross the membrane of living cells, it can only perform its quenching activity extracellularly. The results show that treatment with trypan blue did not completely block the increases in fluorescence observed after treatment with LPPC/ BSA-FITC complexes, indicating that LPPC facilitates the uptake of BSA-FITC by P338D1 cells (Figure 1).
Figure 1.
The effects of LPPC on the antigen uptake ability of P338D1 cells. The fluorescent protein (BSA-FITC) was absorbed on LPPC as a reporter antigen and was incubated with P338D1 cells. With (open bar) or without (closed bar) the addition of trypan blue, the fluorescence was analyzed using FACS. The cells with a fluorescence higher than that of the untreated cells were counted as positive cells. The fluorescent rate is presented as follows: (the number of positive cells divided by the number of untreated cells) multiplied by 100%.
Abbreviations: BSA, bovine serum albumin; FITC, fluorescein Isothiocyanate; LPPC, liposome-polyethylene glycol (PEG)-polyethyleneimine (PEI) complex.
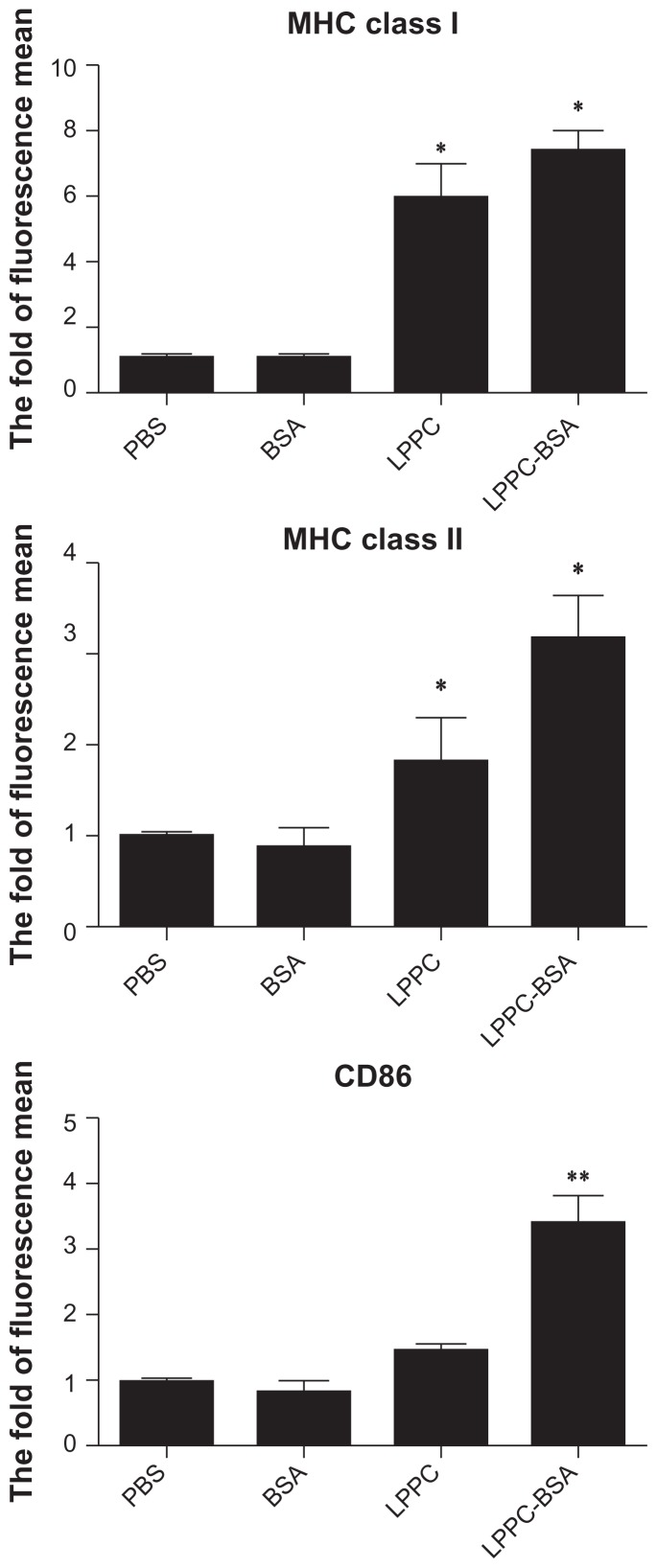
APC maturation is associated with the increased expression of several surface markers that lead to the efficient presentation of antigen. The influence of LPPC on the expression of molecules involved in antigen presentation was determined. For 12 hours following treatment, LPPCs demonstrated their ability to enhance the expression of MHC I, MHC II, and CD86 on the cell surface. Moreover, LPPC complexed with the BSA antigen increased the expression of these molecules compared with LPPC alone (Figure 2).
Figure 2.
The surface maker expression of mouse phagocytes after LPPC treatment. The surface marker expression of P338D1 cells was analyzed by FACS following the incubation of BSA antigen alone or BSA antigen in combination with LPPC. The fold of fluorescence mean was evaluated using the following equation: the mean fluorescence intensity of the sample divided by the mean fluorescence intensity of the untreated group.
Note: A significant difference between BSA antigen in combination with LPPC and the BSA alone group is indicated by *P < 0.05 and **P < 0.01.
Abbreviations: MHC, major histocompatibility complex; PBS, phosphate-buffered saline; BSA, bovine serum albumin, LPPC, liposome-polyethylene glycol (PEG)-polyethyleneimine (PEI) complex.
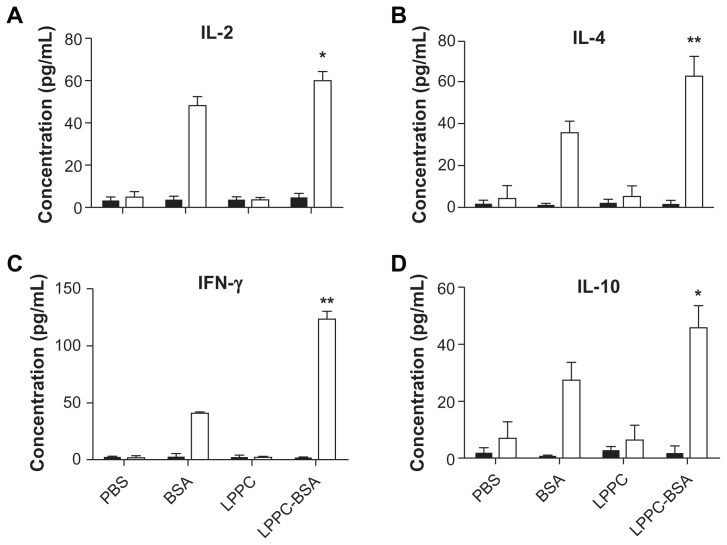
Next, the effect of LPPC on the presentation of specific antigens was verified. Splenocytes from mice immunized or not immunized with BSA were examined for cytokine expression following the addition of different reagents. The results revealed that neither BSA alone nor the BSA/LPPC complex can induce significant cytokine expression in splenocytes derived from mice not immunized with BSA; however, both BSA alone and the BSA/LPPC complex induce significant cytokine expression in splenocytes from mice previously immunized with BSA. In addition, the BSA/LPPC complex induced the release of higher levels of Th1-cytokines (IL-2 and IFN-γ) and Th2-cytokines (IL-4 and IL-10) compared to BSA alone (Figure 3). The release of specific cytokines from T cells requires the presentation of antigen from the antigen-presenting cell. Therefore, LPPC facilitates antigen presentation to allow the more efficient activation of T cells.
Figure 3.
The effects of LPPC on the presentation efficiency of mouse antigen presenting cells. After incubation of splenocytes from naive mice (solid columns) or BSA-immunized mice (open columns) with LPPC complex, the concentration of the cytokines (A) interleukin (IL)-2, (B) IL-4, (C) interferon-gamma (IFN-γ ), and (D) IL-10 in the supernatants was determined by ELISA.
Notes: Data are expressed as the mean ± standard deviation, and experiments were carried out in duplicate (n = 6). A significant difference compared to the BSA group is indicated by *P < 0.05 and **P < 0.01.
Abbreviations: PBS, phosphate-buffered saline; BSA, bovine serum albumin; LPPC, liposome-polyethylene glycol (PEG)-polyethyleneimine (PEI) complex.
Finally, we evaluated whether LPPC alone affects cytokine expression. Table 1 shows that LPPC significantly increased the secretion of the proinflammatory cytokines TNF-α, IL-1β, IL-6, and IL-8. However, LPPC alone did not stimulate the expression of Th1-cytokines (IL-2 and IFN-γ) and Th2-cytokines (IL-4 and IL-10).
Table 1.
The effects of LPPC on the expressions of cytokines in PBMCs or mouse splenocytes
| Cytokine | TNF-α | IL-1β | IL-6 | IL-8 | IL-2 | IFN-γ | IL-4 | IL-10 | |
|---|---|---|---|---|---|---|---|---|---|
| immune cells | |||||||||
| PBMC (±LPPC) | − | 61.6 ± 12 | 8.7 ± 5.3 | 71.1 ± 19.2 | 67.5 ± 26.7 | 30.9 ± 7.3 | 0.5 ± 1.9 | 21.2 ± 7.6 | 38.5 ± 8.0 |
| + | 196.4 ± 32.9* | 29.8 ± 9.0* | 105.7 ± 17.2* | 90.1 ± 14.5* | 29.9 ± 7.4 | 1.0 ± 3.0 | 24.6 ± 9.4 | 30.5 ± 10.4 | |
| Splenocyte (±LPPC) | − | 16.4 ± 5.2 | 18.3 ± 6.9 | 34.9 ± 6.8 | 11.9 ± 3.6 | 13.6 ± 5.6 | 2.9 ± 5.3 | 11.4 ± 4.2 | 17.9 ± 5.8 |
| + | 69.9 ± 9.2* | 32.7 ± 11.5* | 44.5 ± 8.6* | 19.8 ± 4.8* | 13.6 ± 4.3 | 3.7+ ± 4.3 | 14.5 ± 4.5 | 19.5 ± 7.6 | |
Notes: This table summarizes the effects of LPPC on cytokine release from human PBMCs or mouse splenocytes. The secretion of tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1β, IL-6, IL-8, IL-2, IL-4, IL-10, and interferon-gamma (IFN-γ ) was determined.
P < 0.05.
Abbreviations: PBMC, peripheral blood mononuclear cell; LPPC, liposome-polyethylene glycol (PEG)-polyethyleneimine (PEI) complex.
The adjuvant effect of LPPC for the production of antibody in vivo
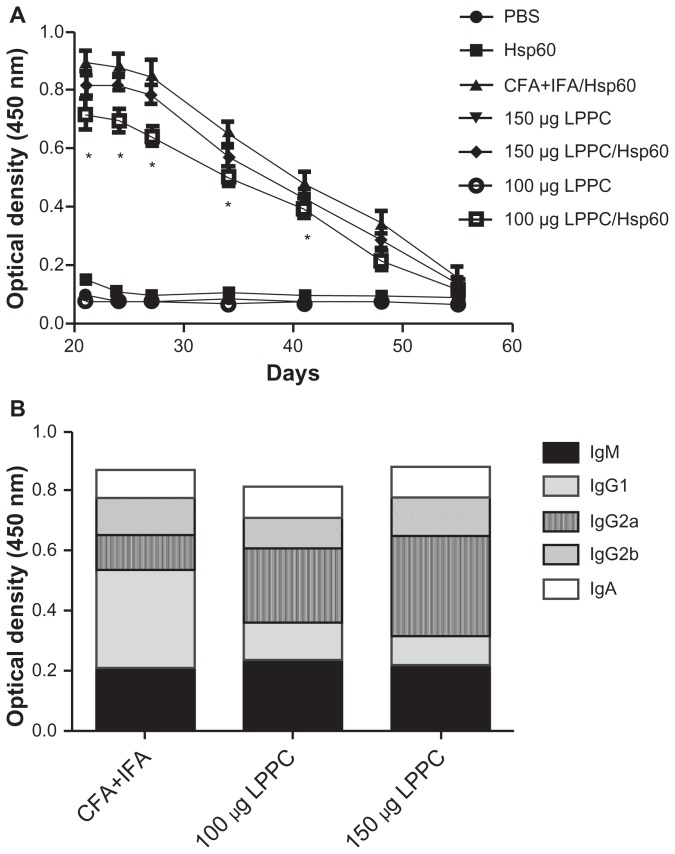
To evaluate the efficiency of LPPC as an effective adjuvant in vivo, we utilized HpHsp60 as an antigen and compared it with the traditional Freund’s adjuvant (CFA and IFA). After completion of the immunization protocol, the titers of anti- HpHsp60 antibodies in the sera were measured. Figure 4A shows that 150 μg LPPC adsorbed with HpHsp60 induced titers of specific anti-HpHsp60 antibodies that were similar to those induced by Freund’s adjuvant. However, 100 μg LPPC adsorbed with HpHsp60 produced lower titers of anti- HpHsp60 antibodies compared with Freund’s adjuvant.
Figure 4.
The adjuvant effects of LPPC on the production of antibody in vivo. (A) The total titers of anti-HpHsp60 antibody were determined every 3 days starting 21 days after immunization and (B) the antibody isotypes (IgM, IgG1, IgG2a, IgG2b, and IgA) were determined 21 days after immunization.
Notes: Data are expressed as the mean ± SD (n = 4). A significant difference compared to the CFA+IFA/Hsp60 group is indicated by *P < 0.05.
Abbreviations: CFA, complete Freund’s adjuvant; IFA, incomplete Freund’s adjuvant; LPPC, liposome-polyethylene glycol (PEG)-polyethyleneimine (PEI) complex; PBS, phosphate-buffered saline; Ig, immunoglobulin.
Although total antibody production in vivo by LPPC adjuvant was similar to Freund’s adjuvant, we were interested to determine whether the two adjuvants induced different antibody isotypes. Therefore, we analyzed the percentages of antibody isotypes that were produced by the two adjuvants. The results demonstrate that the major antibody isotype induced by Freund’s adjuvant was IgG1 (Figure 4B). In contrast, the major antibody isotype induced by both doses of LPPC was IgG2a. With the exception of IgG1 and IgG2a, the other antibody isotypes induced by Freund’s adjuvant or LPPC were similar (Figure 4B). These results indicate that Freund’s adjuvant enhances the production of antibody through the Th2 pathway, whereas LPPC enhances antibody production through the Th1 pathway.
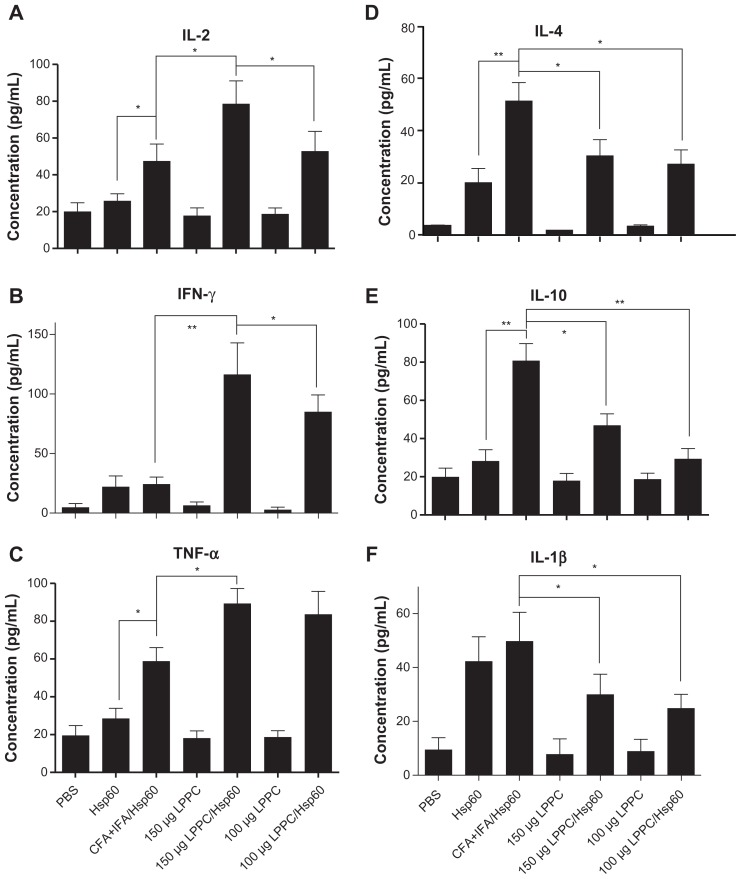
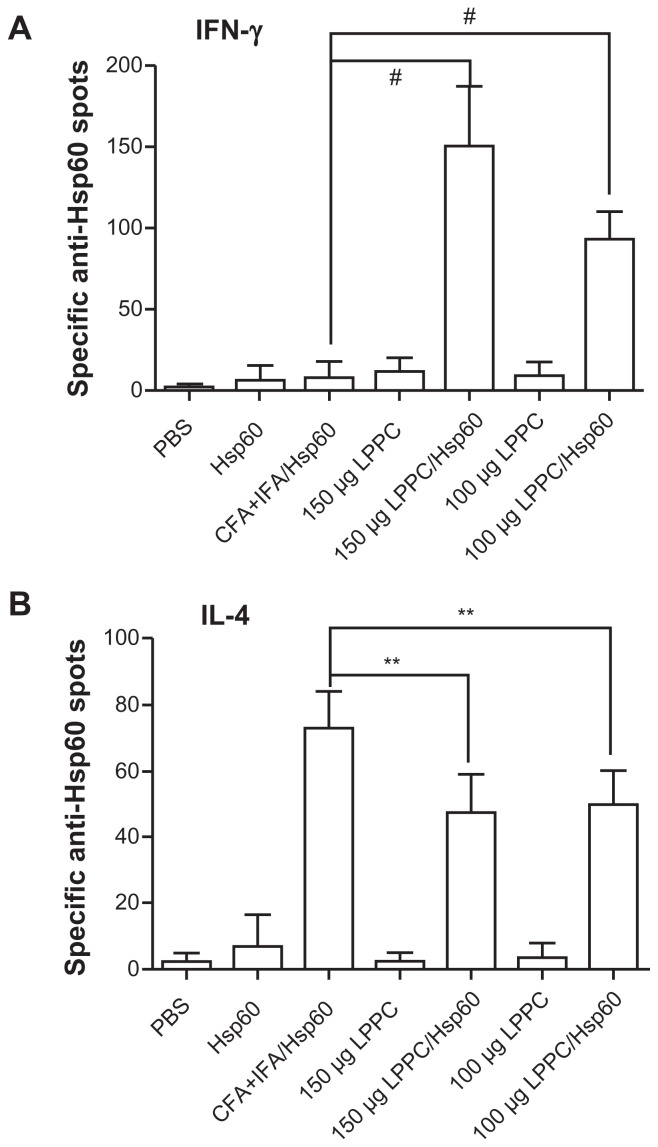
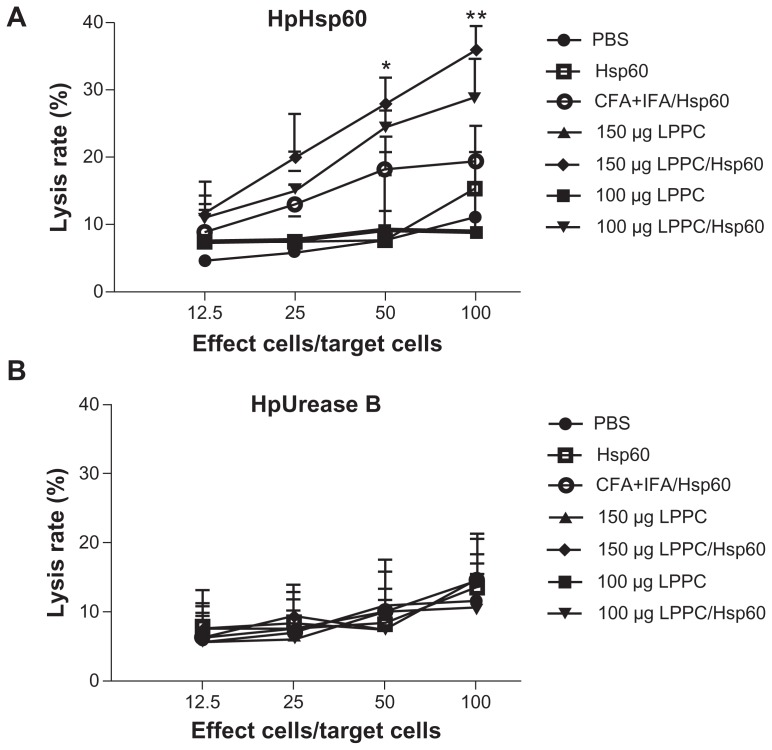
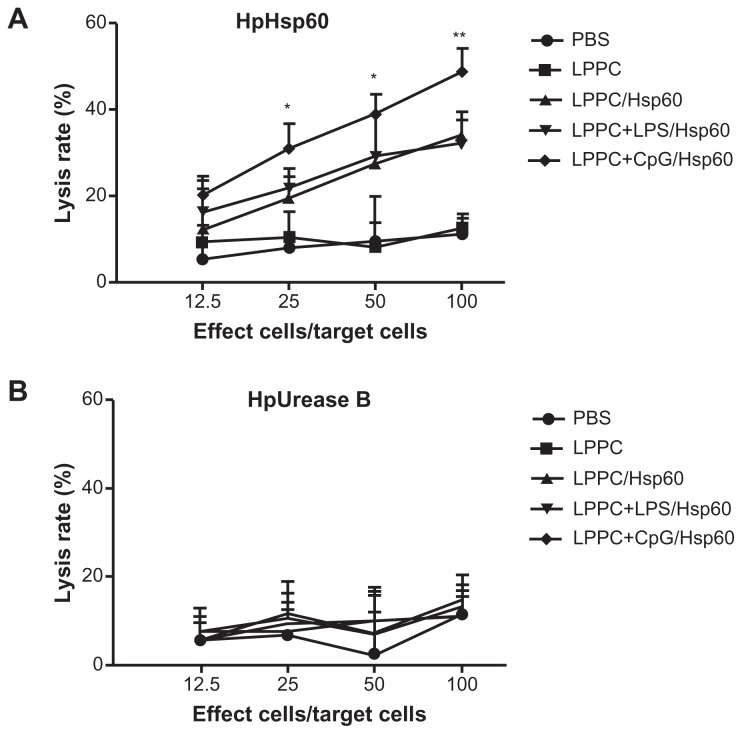
Isotype or antibody switching is dependent on the types of cytokines that are produced following stimulation with antigens. To prove that the two adjuvants induce different immune pathways, we examined the types of cytokines that were induced by antigen stimulation following immunization. The cytokines released from splenocytes that were immunized with Freund’s adjuvant were Th2 cytokines (IL-4 and IL-10) and IL-1β and were different to the Th1 cytokines (IL-2, INF-γ and TNF-α) that were released from splenocytes immunized with LPPC (Figure 5). Figure 6 indicates that antigen immunization with LPPC leads to an increase in the number of INF-γ-expressing T cells (Th1 cells), whereas immunization with Freund’s adjuvant leads to an increase in the number of IL-4-expressing T cells (Th2 cells). The induction of the Th1 pathway is a cytotoxic immune response, and induction of the Th2 pathway is a humoral immune response. Therefore, whether or not LPPC also enhances cytotoxic activity was examined. In accordance with previous results, a cell-mediated immune response (CTL) assay revealed that both doses of LPPC increased the activity of Hsp60-specific CTL against cells transfected with a transgene encoding Hsp60, but not against cells expressing Urease B; this is in contrast to what was observed using Freund’s adjuvant (Figure 7).
Figure 5.
The adjuvant effects of LPPC on cytokine release ex vivo. Following different treatments, splenocytes were harvested from mice, were incubated with HpHsp60 and supernatants were analyzed for expression of (A) IL-2, (B) IFN-γ, (C) TNF-α, (D) IL-4, (E) IL-10, and (F) IL-1β cytokines.
Notes: *P < 0.05; **P < 0.01.
Abbreviations: CFA, complete Freund’s adjuvant; IFA, incomplete Freund’s adjuvant; IFN-γ, interferon-gamma; IL, interleukin; LPPC, liposome-polyethylene glycol (PEG)-polyethyleneimine (PEI) complex; PBS, phosphate-buffered saline; TNF-α, tumor necrosis factor-alpha.
Figure 6.
The adjuvant effects of LPPC on the differentiation of T cells in vivo. The numbers of (A) interferon-gamma (IFN)-γ or (B) interleukin (IL)-4-expressing T cells responding to HpHsp60 in treated mice were evaluated by ELISPOT kit. The spots were observed, and the data were calculated and expressed as the mean ± SD.
Notes: n = 6; **P < 0.05; #P < 0.0001.
Abbreviations: CFA, complete Freund’s adjuvant; IFA, incomplete Freund’s adjuvant; LPPC, liposome-polyethylene glycol (PEG)-polyethyleneimine (PEI) complex; PBS, phosphate-buffered saline; SD, standard deviation.
Figure 7.
The adjuvant effects of LPPC on CTL. Following immunization with different treatments, the cytotoxicity of splenocytes from different mice against (A) pCJ-HpHsp60-or (B) pCJ-HpUrease B-transfected Balb/3T3 cells was determined. The lysis rate was calculated using the following equation: the survival rate of the phosphate-buffered treated group (100%) minus the survival rate of individual group. Statistically significant differences were found for the 150 μg LPPC/Hsp60 group compared with the CFA+IFA/ Hsp60 group.
Notes: n = 6; *P < 0.05; **P < 0.01.
Abbreviations: CFA, complete Freund’s adjuvant; IFA, incomplete Freund’s adjuvant; LPPC, liposome-polyethylene glycol (PEG)-polyethyleneimine (PEI) complex; PBS, phosphate-buffered saline.
The ability of LPPC to regulate the production of different antibody isotypes
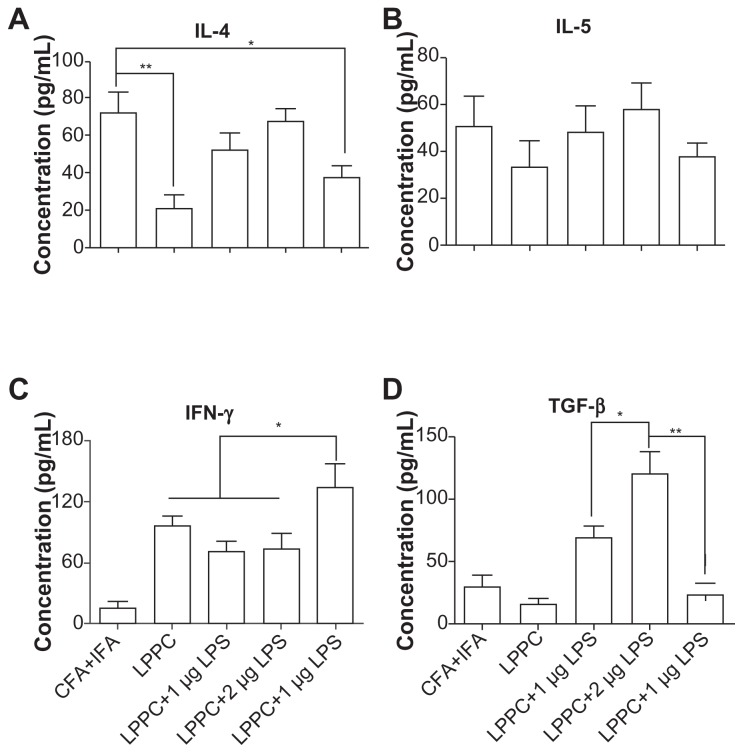
According to the results, certain reagents enhance the production of specific antibody isotypes. First, the ability of E. coli LPS to raise IgA production following adsorption onto LPPC was examined. CpG oligonucleotides were also examined for their ability to increase IgG2a production. LPPC easily adsorbs LPS or CpG oligonucleotides (results not shown). Figure 8A shows that both LPS and CpG increase the total titer of specific antibodies induced by LPPC. Following isotyping, the results indicate that LPS significantly increases the production of IgA and CpG significantly increases the production of IgG2a (Figure 8B). Consistent with a published study,2 CpG facilitates the ability of LPPC to increase the activity of CTL against cells expressing the specific antigen Hsp60 (HpHsp60) but not against cells expressing Urease B (Figure 9), whereas LPS has no effect on the ability of LPPC to increase specific CTL (Figure 9). Antibody isotype switching is dependent on certain cytokines, such as IL-4, IL-5, IFN-γ, and TGF-β. The cytokine assays indicated the following: immunization with Freund’s adjuvant resulted in the highest expression of IL-4; LPS in combination with LPPC increased the expression of IL-4, IL-5, and TGF-β; and CpG ODN in combination with LPPC increased the production of INF-γ (Figure 10).
Figure 8.
The effects of LPPC in combination with different immunomodulators on antibody isotype expression. LPPC adsorbed with HpHsp60 and different immunomodulators (LPS or CpG ODN) was used to immunize and boost mice. (A) The total titer of anti-HpHsp60 antibody was determined every 3 days starting on day 21 after immunization and (B) the antibody isotype titers (IgM, IgG1, IgG2a, IgG2b, and IgA) were determined 21 days after immunization.
Notes: Data are expressed as the mean ± SD (n = 4). A significant difference compared to the CFA+IFA/Hsp60 group is indicated by *P < 0.05.
Abbreviations: CFA, complete Freund’s adjuvant; IFA, incomplete Freund’s adjuvant; LPPC, liposome-polyethylene glycol (PEG)-polyethyleneimine (PEI) complex; PBS, phosphate-buffered saline; LPS, lipopolysaccharides; Ig, immunoglobulin.
Figure 9.
The effects of LPPC in combination with different immunomodulators on the cytotoxicity of mouse splenocytes. Following immunization with LPPC adsorbed with different immunomodulators (LPS or CpG ODN), the cytotoxicity of splenocytes from different mice against (A) pCJ-HpHsp60- or (B) pCJ-HpUrease B-transfected Balb/3T3 cells was determined. The lysis rate was calculated using the following equation: the survival rate of the nontreated group (100%) minus the survival rate of the individual group. Significant differences were found for the LPPC+CpG/Hsp60 group compared with the LPPC+LPS/Hsp60 group.
Notes: n = 6; *P < 0.05; **P < 0.01.
Abbreviations: LPPC, liposome-polyethylene glycol (PEG)-polyethyleneimine (PEI) complex; PBS, phosphate-buffered saline; LPS, lipopolysaccharides.
Figure 10.
The effects of LPPC in combination with different immunomodulators on cytokine expression. Following treatment, mouse splenocytes were harvested, incubated with HpHsp60 and the supernatants were analyzed for cytokine concentration. The expression of (A) IL-4, (B) IL-5, (C) IFN-γ, and (D) TGF-β was evaluated.
Notes: *P < 0.05; **P < 0.01.
Abbreviations: CFA, complete Freund’s adjuvant; IFA, incomplete Freund’s adjuvant; IL, interleukin; IFN-γ, interferon-gamma; LPPC, liposome-polyethylene glycol (PEG)-polyethyleneimine (PEI) complex; LPS, lipopolysaccharides; TGF-β, transforming growth factor-beta.
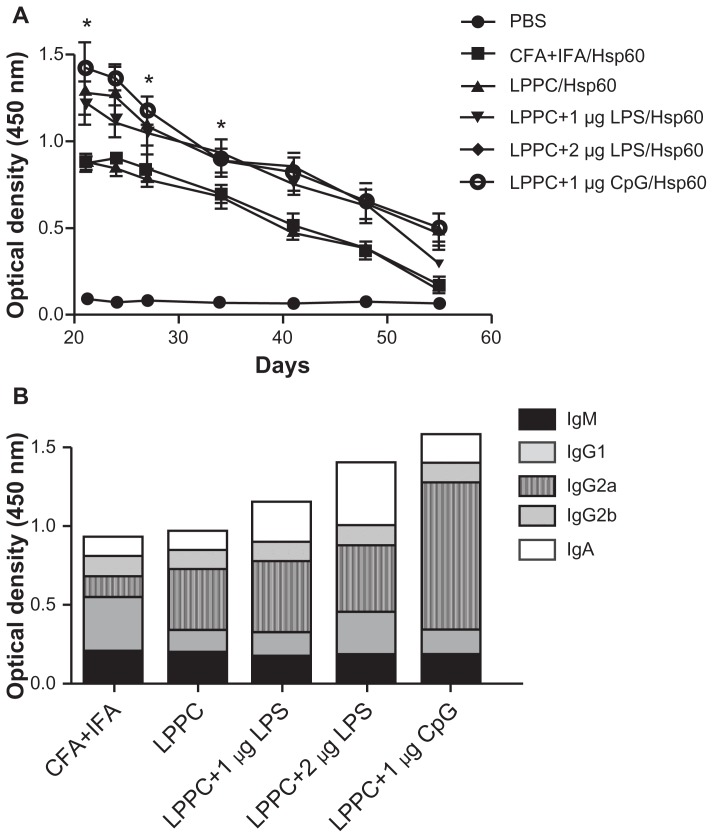
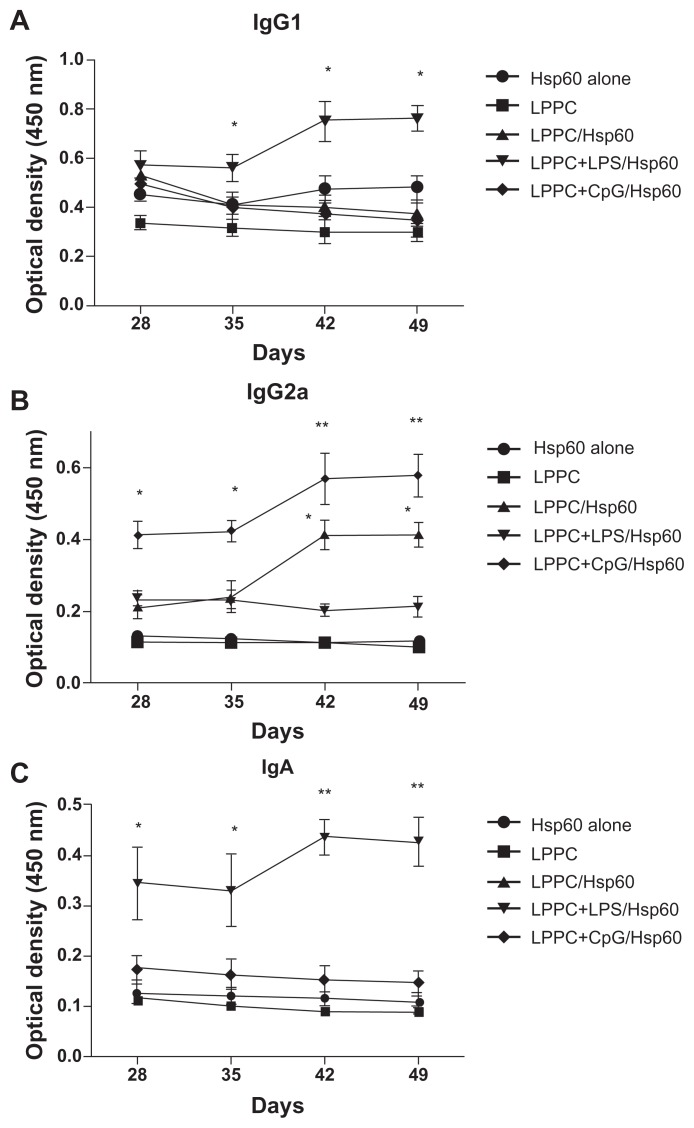
Finally, we examined if the “LPPC-based adjuvant” can switch the antibody isotype in animals that have been immunized. Interestingly, Figure 6 shows that the boost with Hsp60 and LPS antigens increased IgA and IgG1 production after 7 days compared with the boost with Hsp60 alone. CpG ODN enhanced IgG2a production at 7 days, but at 28 days, the IgG1 titer in the sera was decreased (Figure 11). Our results have revealed that LPPC easily adsorbs antigen and immunomodulatory molecules, and is a vaccine that possesses dramatic properties that can control the production of antibody isotypes.
Figure 11.
The effects of LPPC in combination with different immunomodulators on the changes in antibody isotypes of mice immunized with Freund’s adjuvant. Mice were immunized with HpHsp60 that was mixed with Freund’s adjuvant and were further treated with LPPC with or without immunomodulators (LPS or CpG ODN). IgG1, IgG2a, or IgA expression was determined every 7 days and the kinetics are shown (in A, B, and C, respectively).
Note: A significant difference compared to the Hsp60 alone group is indicated by *P < 0.05 and **P < 0.01.
Abbreviations: Ig, immunoglobulin; LPPC, liposome-polyethylene glycol (PEG)-polyethyleneimine (PEI) complex; LPS, lipopolysaccharides.
Discussion
In this study, we describe an interesting adjuvant, LPPC, which easily and strongly adsorbs antigen to form a complex. Previously, it was shown that LPPC strongly binds DNA and many kinds of proteins, and that these adsorbed substances cannot be replaced by the addition of other proteins.20 Accordingly, antigens bind tightly to LPPC to form a stable complex. LPPC, similar to Freund’s adjuvant, enhances the production of specific anti-antigen antibodies (Figure 4A). It is obvious from our results that LPPC preferentially induces Th1 responses including increases in the levels of IgG2a (Figure 4B), IFN-γ release (Figure 5), and CTL (Figure 7), and compared to Freund’s adjuvant, LPPC negatively regulates Th2 responses. Interestingly, LPPC can easily combine with immunomodulators to polarize the immune response. When complexed with LPS, LPPC increases the production of IgA in sera without decreasing the production of IgG2a. This indicates that the addition of LPS to LPPC may increase the expression of IgA in mucosal areas and still allow a strong CTL in a host in which such an immune response is considered a good immunity and is induced by a vaccine that provides protection against viral infections via the respiratory or digestive tracts. A study has shown that CpG ODN are good adjuvants that induce a Th1 response,2 including a significant increase in Th1-biased cytokine and chemokine gene transcripts and the regulation of co-stimulatory and surface marker molecule expression.2 Compared with LPPC alone, LPPC in combination with CpG ODN not only significantly facilitates LPPC’s ability to produce higher levels of IgG2a, but also dramatically raises CTL activity, which suggests that it may be a good therapeutic vaccine against cancer. In addition, the increase of LPPC induces higher Ab response to Hsp60, which might be explained by an improved binding and stability of the adsorbed Ab. Furthermore, combining LPPC with immunomodulators can induce antibody isotype switching (Figure 11), suggesting that LPPC can be used as an efficient and flexible vector for the development of vaccines and to facilitate the switch from an improper immunity to an immunity that is appropriate to fight disease.
To date, many biomaterials have been developed as vectors that enhance the immunogenicity of antigens. However, the immunogenicity of these adjuvants is often considered insufficient to activate APCs and efficiently eliminate the pathogen. Therefore, to achieve a higher immunogenicity or to induce proper immunity, these systems have been modified by incorporating immunomodulators. For example, poly(D, L-lactideco-glycolide), a copolymer codelivering antigen, enhances the humoral immune response (Th2-cell dependent) by increasing phagocytic activity,21–23 whereas monophosphory lipid A, a Toll-like receptor (TLR) 4 agonist, significantly increases this system’s ability to induce a cytotoxic T cell response.4 In addition, the adjuvant chitosan has been developed as a vaccine24 due to its ability to increase macrophage uptake when it is combined with mannose for intranasal immunizations25 or when it is combined with IL-12 to improve Th1 cytokine release.5 Cationic liposomal vectors are also considered good adjuvants that enhance Th1 immune responses. However, the capabilities of some of these adjuvants were also increased by incorporating immunomodulators, such as CpG ODN,2,26 LPS,27 and IFN-γ.28 Some immunomodulators, because they are natural TLR ligands, activate immunity by engaging with TLRs on/in APCs.29 Other immunomodulators include cytokines, that are proposed to activate a Th1 response such as IL-2, IFN-γ, and IL-12, and other immunomodulators that are considered to be Th2 activators, such as IL-4, IL-10, and IL-5.9 Certain compounds that are derived from the extracts of herbs, such as saponins30 and Fu- Ling, are considered good immunomodulators.18 Studies have combined particulate antigen delivery systems with immunomodulators to create an efficient adjuvant that strongly induces appropriate immune responses against disease.2–5 In addition to the adsorption of protein antigens and modulators such as CpG ODN or LPS, our studies also reveal that LPPC easily incorporates hydrophobic compounds, such as 3,3-Dioctadecyloxacarbocyanine perchlorate dye20 or curcumin,31 following a short incubation period of blank LPPC with the compound. This indicates LPPC is a useful adjuvant and is a convenient platform for examining combinations of antigens and immunomodulators that can best induce proper immunity.
In contrast, antibody isotype switching has been investigated as a therapy for some diseases. In this phenomenon, specific cytokines are considered critical factors in host switching of antibody isotypes. Chow et al demonstrated that the incorporation of a cytokine gene (IL-12, IFN-γ, and IL-4) in a DNA vaccine formulation influences the differentiation of Th cells and the nature of an immune response and may, therefore, provide a strategy to improve the prophylactic and therapeutic efficacy of the vaccine.32 Furthermore, IL-21 regulates IgE-production in B cells in vivo.33 Moreover, Mueller et al utilized recombinant adeno-associated virus vectors encoding IL-13 and IL-17ɛ soluble receptors to neutralize IL-13 and IL-17 to modulate IgE expression.34
This immunostimulatory activity of LPPC may be due to an increase in the antigen-presenting activity of APC. It has been established that the activation of T cells is closely associated with the presentation of APC, which results in a humoral response of varying strengths. T cells recognize processed antigen that is presented in MHC I or MHC II molecules but not the intact antigen itself. Therefore, additional antigens must be presented on MHC I or MHC II molecules on APCs to induce cytokine expression in activating T cells. Figure 3 indicates that compared with antigen alone, LPPC increases the expression of Th1 (IL-2 and IFN-γ) and Th2 (IL-4 and IL-10) cytokines in T cells, suggesting that LPPC has an increased ability to facilitate BSA antigen presentation compared with BSA alone. This increase in presentation may result from facilitating antigen uptake (Figure 1), increasing the expression of presenting molecules (MHC I, MHC II, and CD86; Figure 2) and inducing the expression of proinflammatory cytokines, including TNF-α, IL-1β, IL-6, and IL-8 (Table 1). We propose that these properties are associated with the PEI component of LPPC. PEI activates Th1 and Th2 gene expression in murine splenocytes.35 Although only a few PEI molecules are incorporated into LPPC (1 μg/100 μg LPPC), they contribute to the positive charge on the surface zeta potential of LPPC.20 As a cationic liposome, the positive charge of PEI aids particle uptake by the electronic interaction between LPPC and APCs.36,37
Compared with Freund’s adjuvant, antigen immunization with LPPC enhances the production of IgG2a but not IgG1 (Figure 4B), triggers increased expression of Th1 cytokines (IL-2, IFN-γ, and TNF-α; Figure 5), and increases CTL activity (Figure 7). Therefore, LPPC activates the Th1 pathway. Figure 6 demonstrates that immunization with LPPC induces an increase in Th1 cells, whereas immunization with Freund’s adjuvant induces an increase in Th2 cells in mice. An endogenous antigen (eg, an intracellular virus antigen), presented in MHC I, preferentially promotes the Th1 immune response.38 Our unpublished results demonstrate that LPPC adsorbs transgenes and delivers them into cells where they are expressed. The transfection activity of LPPC is associated with PEI, which transfects transgenes into cells using the endosome pathway39 and releases the transfected DNA from the endosome into cytosol via the hydrogen sponge effect.40 Furthermore, PEI delivers proteins into cells and facilitates protein release from the endosome toward the MHC I pathway.41–43 Therefore, we propose that LPPC delivers proteins into cells resulting in the exogenous antigen becoming an endogenous antigen that is processed and presented on MHC I molecules.
Conclusion
In this study, we demonstrated that LPPC is an adjuvant that enhances mouse immunity against antigens and specifically increases Th1-related immune responses. In addition, LPPC in combination with immunomodulators promotes antibody isotype switching. Therefore, LPPC may have clinical applications for the induction of appropriate immunity against pathogens and disease.
Footnotes
Disclosure
The authors declare no conflicts of interest in this work.
References
- 1.Aguilar JC, Rodriguez EG. Vaccine adjuvants revisited. Vaccine. 2007;25(19):3752–3762. doi: 10.1016/j.vaccine.2007.01.111. [DOI] [PubMed] [Google Scholar]
- 2.Erikçi E, Gursel M, Gürsel I. Differential immune activation following encapsulation of immunostimulatory CpG oligodeoxynucleotide in nanoliposomes. Biomaterials. 2011;32(6):1715–1723. doi: 10.1016/j.biomaterials.2010.10.054. [DOI] [PubMed] [Google Scholar]
- 3.Hafner AM, Corthésy B, Textor M, Merkle HP. Tuning the immune response of dendritic cells to surface-assembled poly(I:C) on microspheres through synergistic interactions between phagocytic and TLR3 signaling. Biomaterials. 2011;32(10):2651–2661. doi: 10.1016/j.biomaterials.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Z, Tongchusak S, Mizukami Y, et al. Induction of anti-tumor cytotoxic T cell responses through PLGA-nanoparticle mediated antigen delivery. Biomaterials. 2011;32(14):3666–3678. doi: 10.1016/j.biomaterials.2011.01.067. [DOI] [PubMed] [Google Scholar]
- 5.Heffernan MJ, Zaharoff DA, Fallon JK, Schlom J, Greiner JW. In vivo efficacy of a chitosan/IL-12 adjuvant system for protein-based vaccines. Biomaterials. 2011;32(3):926–932. doi: 10.1016/j.biomaterials.2010.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383(6603):787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 7.Hernández-Pando R, Orozcoe H, Sampieri A, et al. Correlation between the kinetics of Th1, Th2 cells and pathology in a murine model of experimental pulmonary tuberculosis. Immunology. 1996;89(1):26–33. [PMC free article] [PubMed] [Google Scholar]
- 8.Bais AG, Beckmann I, Lindemans J, et al. A shift to a peripheral Th2-type cytokine pattern during the carcinogenesis of cervical cancer becomes manifest in CIN III lesions. J Clin Pathol. 2005;58(10):1096–1100. doi: 10.1136/jcp.2004.025072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kidd P. Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern Med Rev. 2003;8(3):223–246. [PubMed] [Google Scholar]
- 10.Stavnezer J. Immunoglobulin class switching. Curr Opin Immunol. 1996;8(2):199–205. doi: 10.1016/s0952-7915(96)80058-6. [DOI] [PubMed] [Google Scholar]
- 11.Singh VK, Mehrotra S, Agarwal SS. The paradigm of Th1 and Th2 cytokines: its relevance to autoimmunity and allergy. Immunol Res. 1999;20(2):147–161. doi: 10.1007/BF02786470. [DOI] [PubMed] [Google Scholar]
- 12.García BE, Sanz ML, Gato JJ, Fernández J, Oehling A. IgG4 blocking effect on the release of antigen-specific histamine. J Investig Allergol Clin Immunol. 1993;3(1):26–33. [PubMed] [Google Scholar]
- 13.Parham P. The Immune System. 3rd ed. New York, NY: Garland Science; 2009. [Google Scholar]
- 14.Nordly P, Madsen HB, Nielsen HM, Foged C. Status and future prospects of lipid-based particulate delivery systems as vaccine adjuvants and their combination with immunostimulators. Expert Opin Drug Deliv. 2009;6(7):657–672. doi: 10.1517/17425240903018863. [DOI] [PubMed] [Google Scholar]
- 15.Hemmi H, Takeuchi O, Kawai T, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408(6813):740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 16.Park SR, Kim HA, Chun SK, Park JB, Kim PH. Mechanisms underlying the effects of LPS and activation-induced cytidine deaminase on IgA isotype expression. Mol Cells. 2005;19(3):445–451. [PubMed] [Google Scholar]
- 17.Kin NW, Chen Y, Stefanov EK, Gallo RL, Kearney JF. Cathelin-related antimicrobial peptide differentially regulates T- and B-cell function. Eur J Immunol. 2011;41(10):3006–3016. doi: 10.1002/eji.201141606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liou CJ, Tseng J. A Chinese herbal medicine, fu-ling, regulates interleukin-10 production by murine spleen cells. Am J Chin Med. 2002;30(4):551–560. doi: 10.1142/S0192415X02000600. [DOI] [PubMed] [Google Scholar]
- 19.Liou CJ, Li ML, Tseng J. Intraperitoneal injection of ginseng extract enhances both immunoglobulin and cytokine production in mice. Am J Chin Med. 2004;32(1):75–88. doi: 10.1142/S0192415X04001771. [DOI] [PubMed] [Google Scholar]
- 20.Liu YK, Lin YL, Chen CH, et al. A unique and potent protein binding nature of liposome containing polyethylenimine and polyethylene glycol: a nondisplaceable property. Biotechnol Bioeng. 2010;108(6):1318–1327. doi: 10.1002/bit.23048. [DOI] [PubMed] [Google Scholar]
- 21.Bennewitz NL, Babensee JE. The effect of the physical form of poly(lactic-co-glycolic acid) carriers on the humoral immune response to co-delivered antigen. Biomaterials. 2005;26(16):2991–2999. doi: 10.1016/j.biomaterials.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 22.Coombes AG, Major D, Wood JM, Hockley DJ, Minor PD, Davis SS. Resorbable lamellar particles of polylactide as adjuvants for influenza virus vaccines. Biomaterials. 1998;19(11–12):1073–1081. doi: 10.1016/s0142-9612(98)00035-0. [DOI] [PubMed] [Google Scholar]
- 23.Matzelle MM, Babensee JE. Humoral immune responses to model antigen co-delivered with biomaterials used in tissue engineering. Biomaterials. 2004;25(2):295–304. doi: 10.1016/s0142-9612(03)00531-3. [DOI] [PubMed] [Google Scholar]
- 24.Ghendon Y, Markushin S, Akopova I, Koptiaeva I, Krivtsov G. Chitosan as an adjuvant for poliovaccine. J Med Virol. 2011;83(5):847–852. doi: 10.1002/jmv.22030. [DOI] [PubMed] [Google Scholar]
- 25.Jiang HL, Kang ML, Quan JS, et al. The potential of mannosylated chitosan microspheres to target macrophage mannose receptors in an adjuvant-delivery system for intranasal immunization. Biomaterials. 2008;29(12):1931–1939. doi: 10.1016/j.biomaterials.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 26.Kwong B, Liu H, Irvine DJ. Induction of potent anti-tumor responses while eliminating systemic side effects via liposome-anchored combinatorial immunotherapy. Biomaterials. 2011;32(22):5134–5147. doi: 10.1016/j.biomaterials.2011.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chhibber S, Wadhwa S, Yadav V. Protective role of liposome incorporated lipopolysaccharide antigen of Klebsiella pneumoniae in a rat model of lobar pneumonia. Jpn J Infect Dis. 2004;57(4):150–155. [PubMed] [Google Scholar]
- 28.van Slooten ML, Storm G, Zoephel A, et al. Liposomes containing interferon-gamma as adjuvant in tumor cell vaccines. Pharm Res. 2000;17(1):42–48. doi: 10.1023/a:1007514424253. [DOI] [PubMed] [Google Scholar]
- 29.McGettrick AF, O’Neill LA. Toll-like receptors: key activators of leucocytes and regulator of haematopoiesis. Br J Haematol. 2007;139(2):185–193. doi: 10.1111/j.1365-2141.2007.06802.x. [DOI] [PubMed] [Google Scholar]
- 30.Rajput ZI, Hu SH, Xiao CW, Arijo AG. Adjuvant effects of saponins on animal immune responses. J Zhejiang Univ Sci B. 2007;8(3):153–161. doi: 10.1631/jzus.2007.B0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin YL, Liu YK, Tsai NM, et al. A Lipo-PEG-PEI complex for encapsulating curcumin that enhances its antitumor effects on curcumin-sensitive and curcumin-resistance cells. Nanomedicine. 2011 doi: 10.1016/j.nano.2011.06.011. Epub June 24, 2011. [DOI] [PubMed] [Google Scholar]
- 32.Chow YH, Chiang BL, Lee YL, et al. Development of Th1 and Th2 populations and the nature of immune responses to hepatitis B virus DNA vaccines can be modulated by codelivery of various cytokine genes. J Immunol. 1998;160(3):1320–1329. [PubMed] [Google Scholar]
- 33.Suto A, Nakajima H, Hirose K, et al. Interleukin 21 prevents antigen-induced IgE production by inhibiting germ line C(epsilon) transcription of IL-4-stimulated B cells. Blood. 2002;100(13):4565–4573. doi: 10.1182/blood-2002-04-1115. [DOI] [PubMed] [Google Scholar]
- 34.Mueller C, Keeler A, Braag S, Menz T, Tang Q, Flotte TR. Modulation of Exaggerated-IgE Allergic Responses by Gene Transfer-mediated Antagonism of IL-13 and IL-17e. Mol Ther. 2010;18(3):511–518. doi: 10.1038/mt.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Regnström K, Ragnarsson EG, Köping-Höggård M, Torstensson E, Nyblom H, Artursson P. PEI – a potent, but not harmless, mucosal immuno-stimulator of mixed T-helper cell response and FasL-mediated cell death in mice. Gene Ther. 2003;10(18):1575–1583. doi: 10.1038/sj.gt.3302054. [DOI] [PubMed] [Google Scholar]
- 36.Korsholm KS, Agger EM, Foged C, et al. The adjuvant mechanism of cationic dimethyldioctadecylammonium liposomes. Immunology. 2007;121(2):216–226. doi: 10.1111/j.1365-2567.2007.02560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Christensen D, Agger EM, Andreasen LV, Kirby D, Andersen P, Perrie Y. Liposome-based cationic adjuvant formulations (CAF): past, present, and future. J Liposome Res. 2009;19(1):2–11. doi: 10.1080/08982100902726820. [DOI] [PubMed] [Google Scholar]
- 38.Grommé M, Neefjes J. Antigen degradation or presentation by MHC class I molecules via classical and non-classical pathways. Mol Immunol. 2002;39(3–4):181–202. doi: 10.1016/s0161-5890(02)00101-3. [DOI] [PubMed] [Google Scholar]
- 39.Godbey WT, Wu KK, Mikos AG. Poly(ethylenimine) and its role in gene delivery. J Control Release. 1999;60(2–3):149–160. doi: 10.1016/s0168-3659(99)00090-5. [DOI] [PubMed] [Google Scholar]
- 40.Akinc A, Thomas M, Klibanov AM, Langer R. Exploring polyethylenimine-mediated DNA transfection and the proton sponge hypothesis. J Gene Med. 2005;7(5):657–663. doi: 10.1002/jgm.696. [DOI] [PubMed] [Google Scholar]
- 41.Chen J, Li Z, Huang H, et al. Improved antigen cross-presentation by polyethyleneimine-based nanoparticles. Int J Nanomedicine. 2011;6:77–84. doi: 10.2147/IJN.S15457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kitazoe M, Murata H, Futami J, et al. Protein transduction assisted by polyethylenimine-cationized carrier proteins. J Biochem. 2005;137(6):693–701. doi: 10.1093/jb/mvi081. [DOI] [PubMed] [Google Scholar]
- 43.Didenko VV, Ngo H, Baskin DS. Polyethyleneimine as a transmembrane carrier of fluorescently labeled proteins and antibodies. Anal Biochem. 2005;344(2):168–173. doi: 10.1016/j.ab.2005.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]