Abstract
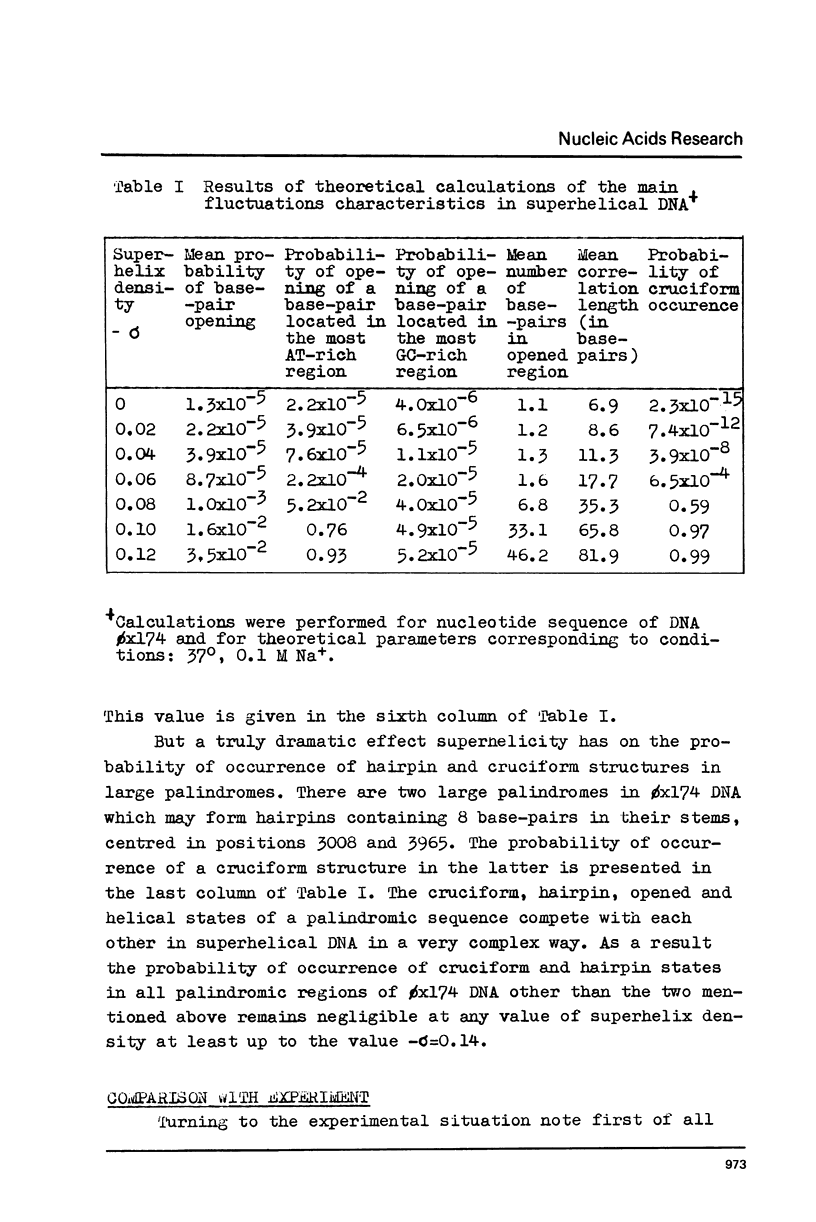
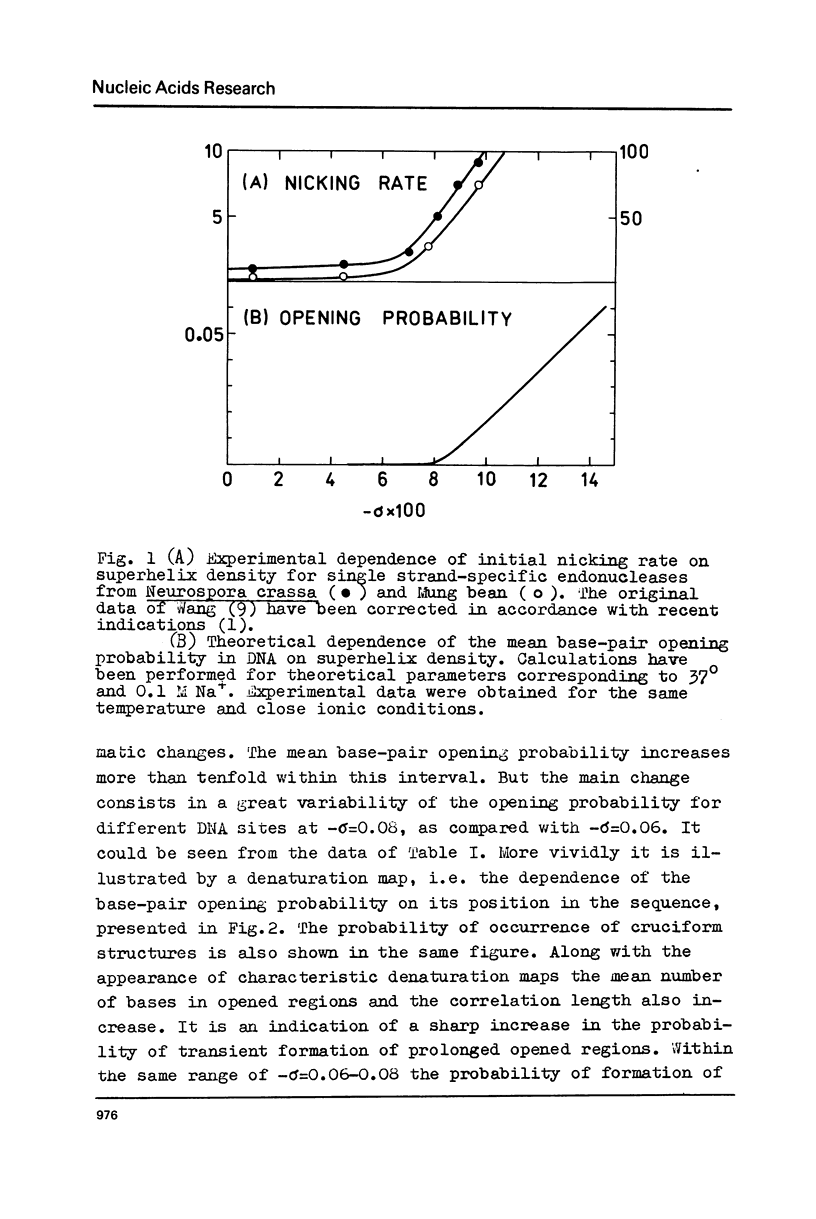
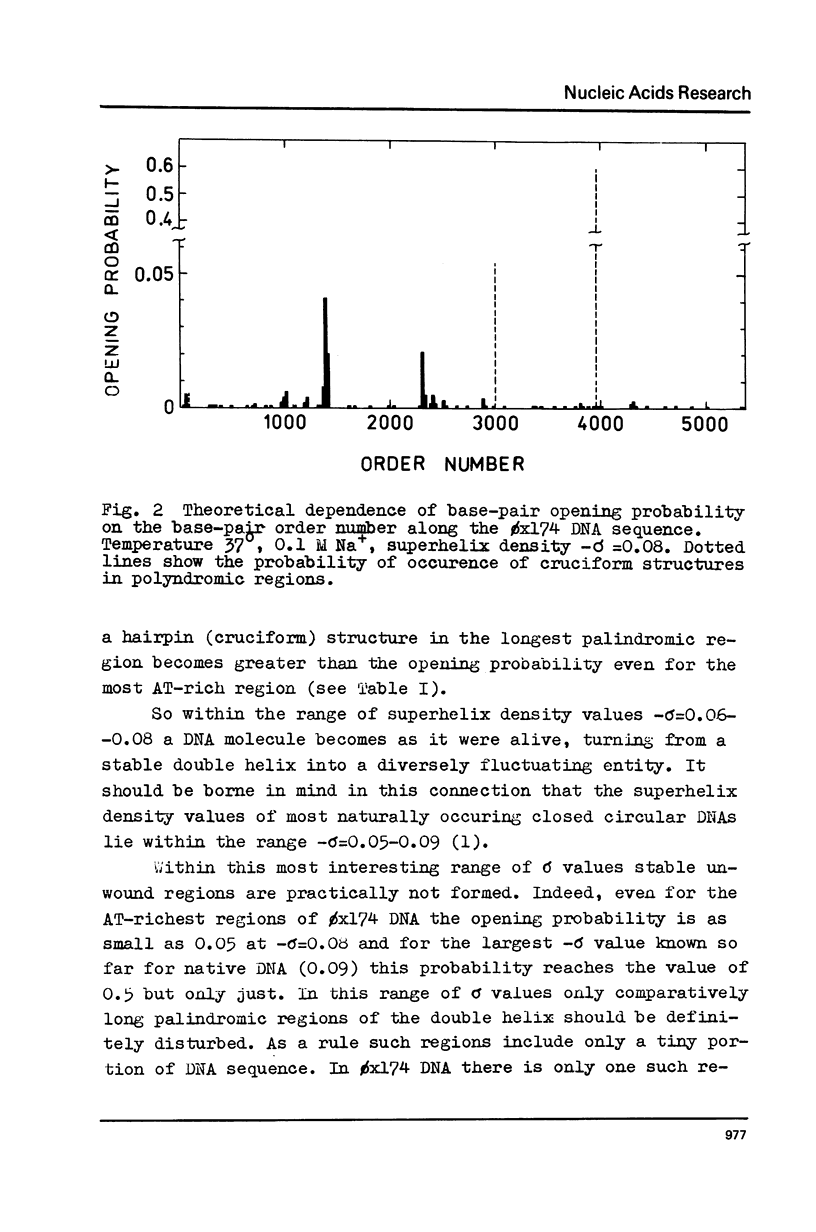
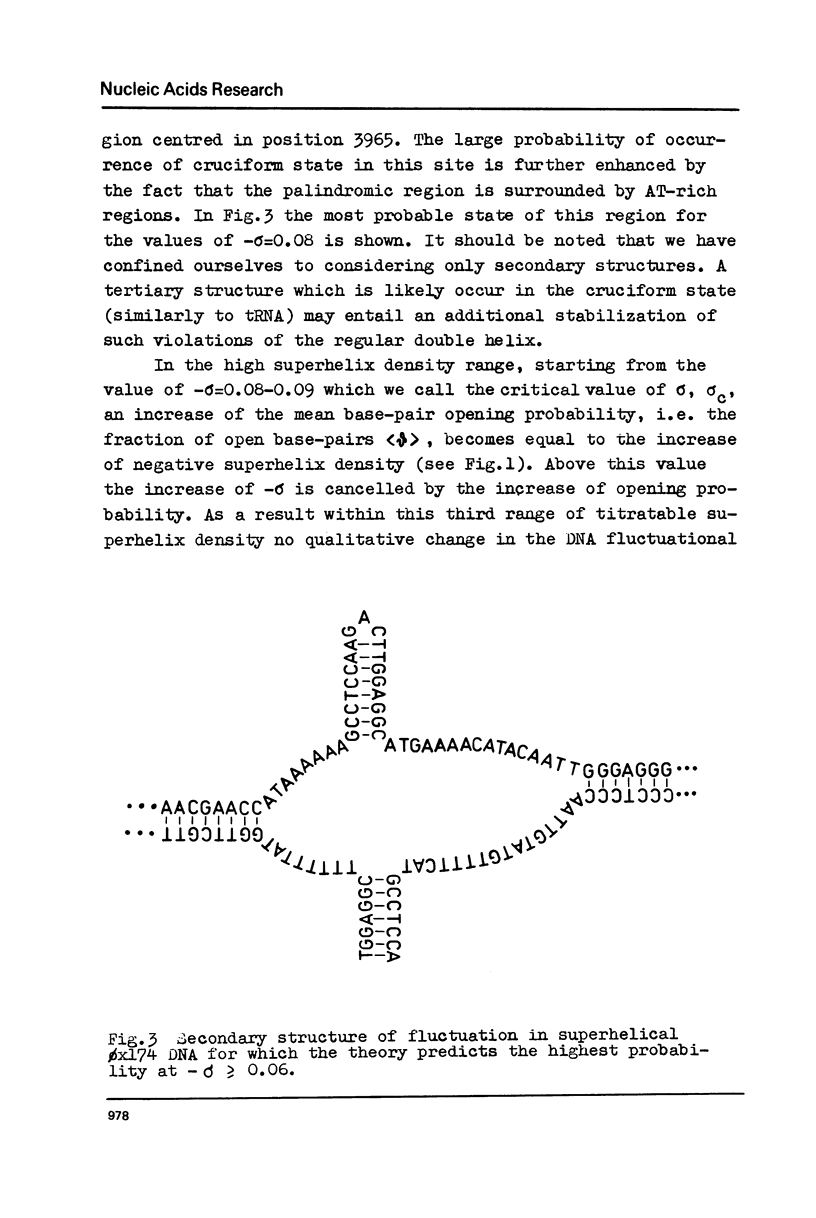
The effect of superhelicity on the base-pair opening probability and on the probability of occurrence of cruciform states in palindromic regions is theoretically treated. The calculations show that below the superhelix density value of -sigma=0.05 superhelicity does not appreciably affect the characteristics of DNA secondary structure fluctuations. In the range of physiological superhelix densities sigma (-sigma=0.05-0.09) the base-pair opening probability markedly increases. However, within this range of sigma the base-pairs are opened only transiently and permanently open regions are not formed. Permanently opened regions appear at higher negative superhelix densities (-sigma greater than 0.10). At the values of -sigma higher than 0.06 a cruciform structure in the palindromic region centred in position 3965 proves to be the most probable fluctuational disturbance in the 0x174 duplex DNA. Different experimental approaches used for probing the fluctuations in superhelical DNA have been analysed. The results suggest that most direct quantitative information can be derived from data on the nicking of closed DNA by single strand-specific endonucleases. Such data (Wang, 1974) accord with the results of theoretical calculations. Calculations show that, due to base-pair opening, the total free energy of superhelical DNA should depend parabolically on sigma only up to some critical value of sigma=sigmac. If negative superhelicity exceeds this critical value, which under physiological conditions proves to be -sigma=0.085, the free energy should increase linearly with -sigma. The biological role of supercoiling is discussed in the light of obtained results.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer W. R. Structure and reactions of closed duplex DNA. Annu Rev Biophys Bioeng. 1978;7:287–313. doi: 10.1146/annurev.bb.07.060178.001443. [DOI] [PubMed] [Google Scholar]
- Bauer W., Vinograd J. Interaction of closed circular DNA with intercalative dyes. II. The free energy of superhelix formation in SV40 DNA. J Mol Biol. 1970 Feb 14;47(3):419–435. doi: 10.1016/0022-2836(70)90312-8. [DOI] [PubMed] [Google Scholar]
- Beard P., Morrow J. F., Berg P. Cleavage of circular, superhelical simian virus 40 DNA to a linear duplex by S1 nuclease. J Virol. 1973 Dec;12(6):1303–1313. doi: 10.1128/jvi.12.6.1303-1313.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchan P., Wang J. C., Echols H. Effect of circularity and superhelicity on transcription from bacteriophagelambda DNA. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3077–3081. doi: 10.1073/pnas.70.11.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brack C., Bickle T. A., Yuan R. The relation of single-stranded regions in bacteriophage PM2 supercoiled DNA to the early melting sequences. J Mol Biol. 1975 Aug 25;96(4):693–702. doi: 10.1016/0022-2836(75)90146-1. [DOI] [PubMed] [Google Scholar]
- Dean W. W., Lebowitz J. Partial alteration of secondary structure in native superhelical DNA. Nat New Biol. 1971 May 5;231(18):5–8. [PubMed] [Google Scholar]
- Delius H., Mantell N. J., Alberts B. Characterization by electron microscopy of the complex formed between T4 bacteriophage gene 32-protein and DNA. J Mol Biol. 1972 Jun 28;67(3):341–350. doi: 10.1016/0022-2836(72)90454-8. [DOI] [PubMed] [Google Scholar]
- Depew D. E., Wang J. C. Conformational fluctuations of DNA helix. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4275–4279. doi: 10.1073/pnas.72.11.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M., Mizuuchi K., O'Dea M. H., Nash H. A. DNA gyrase: an enzyme that introduces superhelical turns into DNA. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3872–3876. doi: 10.1073/pnas.73.11.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M., O'Dea M. H., Itoh T., Tomizawa J. Novobiocin and coumermycin inhibit DNA supercoiling catalyzed by DNA gyrase. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4474–4478. doi: 10.1073/pnas.73.12.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germond J. E., Vogt V. M., Hirt B. Characterization of the single-strand-specific nuclease S1 activity on double-stranded supercoiled polyoma DNA. Eur J Biochem. 1974 Apr 16;43(3):591–600. doi: 10.1111/j.1432-1033.1974.tb03446.x. [DOI] [PubMed] [Google Scholar]
- Gralla J., Crothers D. M. Free energy of imperfect nucleic acid helices. II. Small hairpin loops. J Mol Biol. 1973 Feb 5;73(4):497–511. doi: 10.1016/0022-2836(73)90096-x. [DOI] [PubMed] [Google Scholar]
- Hayashi Y., Hayashi M. Template activities of the phi X-174 replicative allomorphic deoxyribonucleic acids. Biochemistry. 1971 Nov;10(23):4212–4218. doi: 10.1021/bi00799a009. [DOI] [PubMed] [Google Scholar]
- Kato A. C., Bartok K., Fraser M. J., Denhardt D. T. Sensitivity of superhelical DNA to a single-strand specific endonuclease. Biochim Biophys Acta. 1973 Apr 21;308(7):68–78. doi: 10.1016/0005-2787(73)90123-8. [DOI] [PubMed] [Google Scholar]
- Keller W. Determination of the number of superhelical turns in simian virus 40 DNA by gel electrophoresis. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4876–4880. doi: 10.1073/pnas.72.12.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebowitz J., Chaudhuri A. K., Gonenne A., Kitos G. Carbodiimide modification of superhelical PM2 DNA: considerations regarding reaction at unpaired bases and the unwinding of superhelical DNA with chemical probes. Nucleic Acids Res. 1977 Jun;4(6):1695–1711. doi: 10.1093/nar/4.6.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebowitz J., Garon C. G., Chen M. C., Salzman N. P. Chemical modification of simian virus 40 DNA by reaction with a water-soluble carbodiimide. J Virol. 1976 Apr;18(1):205–210. doi: 10.1128/jvi.18.1.205-210.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukashin A. V., Vologodskii A. V., Frank-Kamenetskii M. D., Lyubchenko Y. L. Fluctuational opening of the double helix as revealed by theoretical and experimental study of DNA interaction with formaldehyde. J Mol Biol. 1976 Dec 25;108(4):665–682. doi: 10.1016/s0022-2836(76)80111-8. [DOI] [PubMed] [Google Scholar]
- Lyubchenko Y. L., Vologodskii A. V., Frank-Kamenetskii M. D. Direct comparison of theoretical and experimental melting profiles for RF II phiX174 DNA. Nature. 1978 Jan 5;271(5640):28–31. doi: 10.1038/271028a0. [DOI] [PubMed] [Google Scholar]
- Pulleyblank D. E., Shure M., Tang D., Vinograd J., Vosberg H. P. Action of nicking-closing enzyme on supercoiled and nonsupercoiled closed circular DNA: formation of a Boltzmann distribution of topological isomers. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4280–4284. doi: 10.1073/pnas.72.11.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed S. I., Ferguson J., Davis R. W., Stark G. R. T antigen binds to simian virus 40 DNA at the origin of replication. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1605–1609. doi: 10.1073/pnas.72.4.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson J. P. Effects of supercoiling on transcription from bacteriophage PM2 deoxyribonucleic acid. Biochemistry. 1974 Jul 16;13(15):3164–3169. doi: 10.1021/bi00712a025. [DOI] [PubMed] [Google Scholar]
- Scheffler I. E., Elson E. L., Baldwin R. L. Helix formation by d(TA) oligomers. II. Analysis of the helix-coli transitions of linear and circular oligomers. J Mol Biol. 1970 Feb 28;48(1):145–171. doi: 10.1016/0022-2836(70)90225-1. [DOI] [PubMed] [Google Scholar]
- Sobell H. M. Molecular mechanism for genetic recombination. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2483–2487. doi: 10.1073/pnas.69.9.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C. L., Chay T. R., Loga S. Rupture of base pairing in double-stranded poly(riboadenylic acid)-poly(ribouridylic acid) by formaldehyde: medium chain lenghts. Biochemistry. 1977 Aug 23;16(17):3727–3739. doi: 10.1021/bi00636a001. [DOI] [PubMed] [Google Scholar]
- Uhlenbeck O. C., Borer P. N., Dengler B., Tinoco I., Jr Stability of RNA hairpin loops: A 6 -C m -U 6 . J Mol Biol. 1973 Feb 5;73(4):483–496. doi: 10.1016/0022-2836(73)90095-8. [DOI] [PubMed] [Google Scholar]
- Vologodskii A. V., Frank-Kamenetskii M. D. Theoretical melting profiles and denaturation maps of DNA with known sequence: fdDNA. Nucleic Acids Res. 1978 Jul;5(7):2547–2556. doi: 10.1093/nar/5.7.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. C. Interactions between twisted DNAs and enzymes: the effects of superhelical turns. J Mol Biol. 1974 Aug 25;87(4):797–816. doi: 10.1016/0022-2836(74)90085-0. [DOI] [PubMed] [Google Scholar]
- Woodworth-Gutai M., Lebowitz J. Introduction of interrupted secondary structure in supercoiled DNA as a function of superhelix density: consideration of hairpin structures in superhelical DNA. J Virol. 1976 Apr;18(1):195–204. doi: 10.1128/jvi.18.1.195-204.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]



