Summary
Myeloid-derived suppressor cells (MDSC) play an important role in the cellular network regulating immune responses in cancer, chronic infectious diseases, autoimmunity, and in other pathologic conditions. Morphological, phenotypic and functional heterogeneity is a hallmark of MDSC. This heterogeneity demonstrates the plasticity of this immune suppressive myeloid compartment, and shows how various tumors and infectious agents can have similar biological effects on myeloid cells despite the differences in the factors that they produce to influence the immune system; however, such heterogeneity creates ambiguity in the definition of MDSC as well as confusion regarding the origin and fate of these cells. In this review we will discuss recent findings that help to better clarify these issues and to determine the place of MDSC within the myeloid cell lineage.
Keywords: myeloid-derived suppressor cells, macrophages, dendritic cells, neutrophils
Introduction
Myeloid-derived suppressor cells (MDSC) have become the focus of intense study in recent years. MDSC accumulate in large numbers during many pathologic conditions, including cancer, infectious diseases, trauma, sepsis, etc. They are characterized by their myeloid origin, immature state, and most importantly by their potent ability to suppress different aspects of immune responses, especially T-cell proliferation and cytokine production. Early studies implicated up-regulation of arginase, nitric oxide (NO), and reactive oxygen species (ROS) as the major factors responsible for the immune suppressive activity of MDSC. Since then the important role of these molecules has been convincingly demonstrated and was described in detail in a number of reviews [1–3]. More recently several other mechanisms were added to the mix, including up-regulation of cyclooxigenase-2 and prostaglandin E2 [4], induction of regulatory T cells [5–7], production of TGF-β [8, 9], depletion of cystein [10], and down-regulation of L-selectin expression of T cells [11]. It became apparent that the immune suppressive activity of MDSC is highly pleiotropic and the specific mechanisms used by these cells are dependent on the context of the microenvironment. We will discuss in this review how the variety of immune suppressive mechanisms employed by MDSC is closely connected with heterogeneity of these cells.
Cancer is the predominant pathological condition in which MDSC play an important role. Expansion of MDSC has been detected in practically all studied tumor models and in patients with most tested types of cancer. Elimination of MDSC dramatically improved immune responses in tumor-bearing mice and in cancer patients and in some models it resulted in a direct antitumor effect (see recent reviews [12–14]). In addition, MDSC have been implicated in a whole array of non-immunological functions, such as the promotion of angiogenesis, tumor cell invasion, and metastases [8, 15–17].
Although many underlying mechanisms of MDSC activity and their specific contribution to the pathological processes associated with cancer, infectious diseases and autoimmune abnormalities remain to be elucidated, the overall biological role of MDSC is now widely appreciated. It is clear that MDSC are an important element of a cellular network regulating immune responses; however, the nature of these cells remains controversial. This controversy arises from the fact that MDSC is not a homogenous cell type. The morphological, phenotypic, and functional heterogeneity of MDSC is a hallmark of these cells. This heterogeneity provides MDSC with broad appeal to researchers and clinicians as a potential universal tool developed by nature to control immune responses under various pathological conditions. It has been demonstrated that various tumors and infectious agents can cause similar biological effects on myeloid cells despite the apparent differences in the profile of cytokines and other factors that they produce or employ. However, such a research “blessing” comes at a price. This heterogeneity creates ambiguity in the definition of MDSC, as well as confusion regarding the origin and fate of these cells. Different investigators have contrasting views on the biology of MDSC vis-à-vis monocytes and neutrophils. The biology of MDSC is very complex, and we are under no illusion that in this mini-review we can completely clarify this issue. However, we will try to present our point of view on the functional heterogeneity of MDSC and their place in the hierarchy of myeloid cells.
Major subsets of MDSC: Appearances matter
Initially, MDSC in mice were defined as cells expressing a Gr-1+CD11b+ phenotype and lacking the expression of markers typical of mature macrophages (MΦ) and dendritic cells (DC) [18, 19]. In humans, MDSC were defined as cells that co-purified with mononuclear cells, lacked markers of lymphocytes, natural killer cells, monocytes, and DC, and expressed the myeloid cell markers CD33 and CD11b, as well as, in some studies, granulocyte markers [20–22]. Morphologically MDSC consists of a mixture of monocytic and granulocytic cells. Recently, specific markers that identify two major subsets of MDSC were described, and currently MDSC in mice and men can be subdivided into two major groups: granulocytic MDSC (G-MDSC) and monocytic MDSC (M-MDSC). As can be discerned from their names G-MDSC have a morphology similar to that of granulocytes, and M-MDSC have a morphology similar to that of monocytes. In mice G-MDSC have a phenotype of CD11b+Ly6G+Ly6Clow, whereas M-MDSC have a phenotype of CD11b+Ly6G−Ly6Chigh [23, 24]. The Ly6G molecule is known to be expressed primarily on granulocytes [25], whereas Ly6C is typically highly expressed on monocytes [26]. Gr-1 antibody (RB6-8C5) can detect both Ly6G and Ly6C epitopes. Interestingly, when Gr-1 antibody is used for the first step of staining for MDSC, subsequent labeling with Ly6G, but not with Ly6C-specific antibody, is significantly decreased [27, 28].
In humans, the phenotype of these cells is less clearly defined, although recent studies have implicated CD15 and CD66b as additional markers allowing for detection of G-MDSC and M-MDSC [29, 30]. G-MDSC and M-MDSC differ not only in morphology and phenotype, but also in the mechanisms by which they suppress immune function.
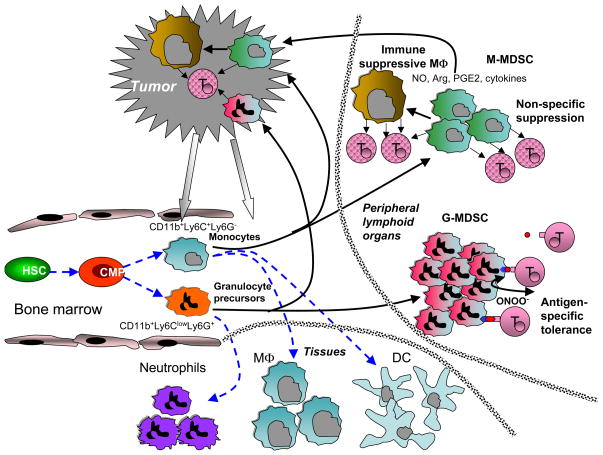
More information about these differences can be found in recent excellent reviews [31, 32]. To summarize, G-MDSC primarily use ROS as the mechanism of immune suppression. In contrast, M-MDSC primarily use up-regulation of inducible nitric oxide synthase (iNOS), arginase and an array of immune suppressive cytokines to suppress various immune functions (Figure 1).
Figure 1. Schematic of possible pathway of MDSC differentiation in cancer.
Blue dashed lines denote myeloid cell differentiation under physiologic conditions. Black solid line shows proposed development of MDSC in tumor-bearing host. HSC – hematopoietic stem cells, CMOP – common myeloid progenitor, MΦ – macrophages, DC –dendritic cells, T – T cells. See detailed explanation in the text.
Early studies of MDSC demonstrated the heterogeneous nature of MDSC and suggested the existence of a small subset of cells within this larger group that could be more suppressive than others. This prompted the search for markers that could be used to identify such a population. Several potential candidates were suggested, such as CD115 (M-CSFR), CD124 (IL-4Rα), CD40, and CD80 [6, 7, 33, 34]. However, further studies indicated that although these markers are undoubtedly expressed on MDSC, they do not define specific immune suppressive populations of MDSC in various tumor models [24]. Two major subsets of MDSC apparently have an important role in the antigen-specific vs. non-specific nature of immune suppression. G-MDSC, which use ROS for their suppressive function, require close cell-cell contact with T cells, which manifests in strong reliance on antigen-specific interaction between MDSC and T cells [35]. M-MDSC, which use up-regulation of NO and arginase, production of immune suppressive cytokines, and other mechanisms, effectively suppress antigen-independent T-cell responses without requiring direct cell-cell contact (Figure 1). Evidence from various laboratories suggests that on a per cell basis M-MDSC are more potent than G-MDSC [16, 23, 28, 36]. However, in most tumor models the vast majority of MDSC in peripheral lymphoid organs are composed of G-MDSC [24].
This may explain why in many tumor models antigen-specific tolerance of T cells in peripheral lymphoid organs was detected, although T cells retained the ability to respond to non-specific stimuli [37, 38]. However, the proportion of G-MDSC to M-MDSC in different tumor models is highly variable and depends on factors that are not yet well understood. This may also explain some inconsistent results from different laboratories regarding the antigen-specificity of MDSC-mediated suppression, which further contributes to the complex role of these cells in cancer. In addition, in tumor site the ratio between G-MDSC and M-MDSC is much lower than in lymphoid organs, which may influence the nature of immune suppression observed in tumor site. The mechanism of preferential accumulation of M-MDSC in tumor site is currently not clear. It is possible that the nature of chemokines produced by tumor cells is responsible for preferential migration of M-MDSC to the tumor site. Alternatively, tumor microenvironment due to hypoxia, low pH, etc may not support survival of G-MDSC. It is important to point out that it is likely that the morphological and functional heterogeneity of MDSC is not limited to these two major subsets. There are likely other intermediate groups of cells with distinct phenotype reflecting different stages of cell differentiation. Some of them have been recently described [39] but more subsets will probably be described in the future.
Subsets of MDSC and the Gr-1 molecule: A new role for an old molecule or an identity crisis
Recently, several studies pointed out the possibility that the immune suppressive functions of MDSC can be identified by the level of expression of Gr-1. In the spleens of healthy mice Gr-1lowCD11bhighLy-6ChighSSClow M-MDSC and Gr-1highCD11blow G-MDSC were described as having potent suppressive activity, whereas Gr-1highCD11bhigh and Gr-1lowCD11bhighSSClow cells were not suppressive [40]. Dolcetty et al. separated Gr-1high and Gr-1int from spleens of tumor-bearing mice by means of immunomagnetic sorting, optimized in a multistep separation protocol [28]. CD11b+Gr-1int cells, mainly comprised M-MDSC and myeloid precursors, had potent suppressive activity against CD8+ T-cells. However, CD11b+Gr-1high cells, represented mostly by G-MDSC, exerted appreciable suppression only in some tumor models and when present in high numbers. The CD11b+Gr-1int but not CD11b+Gr-1high cells were also immunosuppressive in vivo following adoptive transfer [28]. Freshly isolated splenic Gr-1int but not Gr-1high MDSC from tumor-bearing mice reduced production of IFN-γ in CD8+ T cells. In addition, splenic Gr-1int MDSC from tumor-bearing mice differentiated into CD11c+F4/80+ cells in the presence of GM-CSF [41].
These results make a case in favor of Gr-1 as an important marker of immune suppressive activity. However, it has been known that expression of the Gr-1 molecule is increased with granulocyte maturation, whereas expression is transient on cells in the monocytic lineage. Gr-1high cells were enriched for end-stage neutrophils, whereas Gr-1low cells contained more immature myeloid cells and myelocytes [42]. As was noted in the studies referenced above and from our own experience, at least in tumor models Gr-1high and Gr-1int cells closely corresponded to G-MDSC and M-MDSC subsets. In support of this view, inhibition of M-CSFR, which is highly expressed on M-MDSC, strongly reduced the recruitment of Gr-1lowLy6Chigh M-MDSC but had no effect on Gr-1high Ly6Clow G-MDSC in the tumor [16]. As we discussed above, M-MDSC had more potent suppressive activity than G-MDSC, which may explain the difference in suppressive activity between Gr-1high and Gr-1int MDSC. The data regarding potential signaling via Gr-1 raises an interesting paradox regarding the possible role of this molecule in MDSC function.
Ribechini et al. [27] recently demonstrated that in bone marrow cells Gr-1-specific antibody induced signaling via STAT-1, STAT-3 and STAT-5, similar to the effect of GM-CSF. Gr-1 antibody induced myeloid cell expansion and upregulation of macrophage markers. Suppressive activity of both Gr-1high and Gr-1low MDSC was transiently ablated by Gr-1 antibody injection. These results suggest a functional role of the Gr-1 molecule in both MDSC function and differentiation. However, they also imply that if the Gr-1 receptor has a natural ligand (which is likely), then Gr-1high MDSC should have more prominent immune suppressive activity. This further suggests that the differences in functional activity between MDSC with different levels of Gr-1 expression could be a reflection of differences in the abundance of this molecule on G-MDSC and M-MDSC, rather than an indication that Gr-1 acts as a direct marker of cells with immune suppressive activity.
It is important to point out that separation protocols used in referenced above studies did not allow for complete distinction between Ly6G positive and Gr-1int cells. Therefore, some Ly6G+ cells had intermediate level of Gr-1 expression, which may contribute to some discrepancies in functional activity of these cells observed by different groups. This issue could probably be resolved using direct experiments with regulated Gr-1 expression on different MDSC subsets. It is likely that more specific markers of cell populations are needed to narrow down the more potently suppressive sub-population of cells. In a recent study a novel marker of MDSC, CD49d, was suggested to be such a marker [43]. The CD49d+ subset of MDSC was mainly monocytic and strongly suppressed antigen-specific T-cell proliferation in an NO-dependent mechanism similarly to Gr-1dull/int MDSC. CD11b+CD49d+Gr-1+ MDSC were more potent suppressors of antigen-specific T-cell responses than CD11b+CD49d−Gr1+ MDSC [43]. It is likely that in the next few years more markers of suppressive subsets of MDSC will be determined, which would help to clarify the biology of MDSC in pathologic conditions.
The origin of MDSC: A new tale for old cells or an old tale for new cells
Since the first characterization of MDSC, the nature of these cells has been a subject of debate. MDSC are very similar to monocytes and granulocytes and share common morphologic features. The CD11b+Ly6G+Ly6Clow phenotype of G-MDSC is identical to that of neutrophils, and the CD11b+Ly6G−Ly6Chigh phenotype of M-MDSC is the same as that of the so-called inflammatory monocytes [44, 45]. What then sets these cells apart? Why even use the term MDSC instead of granulocytes and monocytes? We believe these questions are at the core of the unique biological role of these cells.
MDSC are not simply the result of myeloid precursor cells expanding under pathological conditions. MDSC is a functional definition of immature myeloid cells that have acquired potent immune suppressive activity and other non-immunological functions. They also have impaired ability to differentiate into mature myeloid cells, and as a result accumulate in peripheral lymphoid organs. It became apparent that these cells do not really exist in healthy individuals or control mice. Precursors of mature myeloid cells with the same phenotype present in physiologic conditions do not share the functional characteristics of MDSC, and probably should not be called as such. Moreover, acute bacterial infection or stress does not necessarily result in the generation of MDSC. These cells accumulate only during long-term unresolved pathological conditions such as chronic infection, inflammation, or cancer. Large numbers of factors are involved in the expansion of MDSC, including different cytokines, complement, toll-like receptor agonists, etc. and have been reviewed in detail elsewhere [2, 12, 46]. These factors not only cause the expansion of cells with MDSC phenotype, but more importantly also result in their activation, which manifests in the up-regulation of many intermediates with potential immune suppressive activity, such as ROS, iNOS, COX2, and arginase. These two processes (cell expansion and activation) are closely connected, co-dependent, and are regulated by the same set of transcription factors i.e. STAT3, STAT1, STAT6, NF-κB [2, 12, 46]. This may provide an explanation of some unique biological activities of these cells. M-MDSC can persist without differentiating into mature macrophages or DC in vitro and in vivo and have high levels of both iNOS and arginase, two proteins that are not usually up-regulated together, and separately are often considered to be hallmarks of different subsets of polarized classical (M1) and alternatively activated (M2) MΦ, respectively [47]. G-MDSC retain high levels of ROS for an extended period of time, whereas neutrophils die within hours after the induction of respiratory burst by various stimuli [48]. The question remains, however, are there any markers that would allow for distinguishing MDSC from monocytes and neutrophils? Based on recent developments in the field we believe that the identification of such markers is just a matter of time. This would represent a major advance in our ability to better characterize these cells.
Can MDSC be generated ex vivo from normal myeloid progenitors? This question is important not only for better comprehension of the biology of these cells, but also for potential therapeutic use as cell therapy in autoimmune diseases and transplantation. Recently several groups have reported interesting observations addressing this possibility. Marigo et al. [49] have shown that the combination of GM-CSF and IL-6 allowed for a rapid generation of MDSC from precursors present in mouse and human bone marrow. In mice these cells were able to impair the priming of CD8+ T cells, and enabled long term acceptance of pancreatic islet allografts [49]. Consistent with these data Lechner et al [50] reported that GM-CSF and IL-6 were able to generate suppressive human CD33+ myeloid cells ex vivo. Zhou et al [39] reported generation of MDSC from mouse embryonic stem cells and bone marrow hematopoietic progenitor cells. Adoptive transfer of these MDSC prevented alloreactive T-cell-mediated graft-versus-host disease [39]. Bone marrow progenitor cells could be induced by LPS to develop into CD11b+Gr1intF4/80+ cells that when adoptively transferred suppressed allergen-induced airway inflammation in recipient mice [51]. Altogether these encouraging results suggest that MDSC-based therapy is feasible and needs to be further developed.
The fate of MDSC in tumor-bearing hosts: How bad may become worse
What happens with MDSC in the body? Do they die as immature cells or differentiate into other cells? Early studies showed that if tumor-derived MDSC were placed in culture conditions without tumor-derived factors or transferred into tumor-free recipients they differentiated into mature functionally competent MΦ and DC (rev. in [2]). Consistent with those observations were data demonstrating that surgical removal of tumors resulted in elimination of MDSC, although the fate of those MDSC was not clear. A combination of different cytokines allowed for the generation of functionally competent DC from MDSC in vitro [52, 53]. These data further underscore the fact that MDSC include precursors of MΦ and DC, and in vitro manipulation in the absence of tumor-derived factors can successfully differentiate these cells into mature myeloid cells. The situation in the presence of tumor-derived factors and in tumor-bearing hosts is quite different, however. MDSC isolated from the spleens of tumor-bearing mice differentiated in the presence of tumor-derived factors into immune suppressive MΦ [53]. After adoptive transfer into tumor-bearing recipients, MDSC freshly isolated from the spleens of tumor-bearing mice differentiated into tumor-associated MΦ with potent immune suppressive activity [54]. Recently we have studied the fate of spleen MDSC from tumor-bearing mice after adoptive transfer directly into the tumor site, using the model of ascitis. Within several hours after transfer, donor MDSC had up-regulated expression of iNOS and arginase and were able to suppress non-specific T-cell activation. Within 48 hr these cells acquired the morphology and phenotype of MΦ. Very few donor CD11c+ DC were observed in the tumor site. This was in sharp contrast with the spleen, where donor MDSC persisted for much longer after adoptive transfer, and differentiated equally into MΦ and DC [55]. This effect was recapitulated in vitro by hypoxia, suggesting that hypoxia could be involved in MDSC differentiation into tumor-associated MΦ. Interestingly, MΦ differentiated from MDSC in hypoxic conditions did not show preferential polarization to either the M1 or M2 type; although these MDSC expressed high levels of genes associated with both types [55]. This was consistent with the reportfrom Umemura et al. [56] that immune suppressive CD11b+F4/80+ monocytes/macrophages that infiltrated murine colon carcinoma and glioma simultaneously expressed CXCL10 and CD206 proteins, which are typical M1 and M2 MΦ activation markers, respectively. In our study [55], HIF-1α apparently was one of the major factors regulating differentiation of MDSC to MΦ in the tumor microenvironment, since HIF-1α-deficient MDSC had substantially reduced ability to differentiate into MΦ. Although data on the fate of MDSC requires further development, it appears that in tumor-bearing hosts MDSC may differentiate preferentially into immune suppressive tumor-associated MΦ, thus forming the immune suppressive axis focused on inhibition of antitumor immune responses (Figure 1).
When a conclusion is simply the place where you got tired of thinking
Recent years have witnessed high interest for MDSC. Their biological role has become evident. Their contribution to pathological processes goes far beyond immune suppression. In this review we focused on only one aspect of MDSC biology: their heterogenic nature and its functional significance. Heterogeneity is a key characteristic of these cells. In the long run this is a blessing for researchers and physicians, for it opens opportunities to better understand the complex nature of myeloid cell involvement in regulation of immune responses. It also provides a conceptual bridge linking hematopoietic progenitor cells and suppressive myeloid cells in many pathologic conditions. However, in the short run MDSC heterogeneity is a curse, since it complicates comprehension of the place occupied by these cells in the hierarchy of myeloid cells. We believe that evidence accumulated so far indicates that MDSC are not just activated inflammatory monocytes or neutrophils. However, the molecular mechanisms directly responsible for abnormal differentiation and function of myeloid cell precursors that convert them into MDSC remain to be elucidated. Identification of markers allowing for discrimination between MDSC and classic neutrophils or monocytes in tissues would help to advance our understanding of the biological role of MDSC in various pathologic conditions. MDSC heterogeneity suggests that it would indeed be possible to find phenotypic markers allowing for better identification of specific immune suppressive subsets of these cells. Finally, identification of factors that would allow stable conversion of monocytes into MDSC for the purpose of cell therapy would open exciting new therapeutic opportunities.
Acknowledgments
We thank H. Weber for help in preparation of the manuscript. The work described in this paper was supported in part by NIH grants CA84488 and CA100062 to DIG.
References
- 1.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5:641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 2.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodriguez PC, Ochoa AC. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives. Immunol Rev. 2008;222:180–191. doi: 10.1111/j.1600-065X.2008.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodriguez PC, Hernandez CP, Quiceno D, Dubinett SM, Zabaleta J, Ochoa JB, Gilbert J, Ochoa AC. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J Exp Med. 2005;202:931–939. doi: 10.1084/jem.20050715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Serafini P, Mgebroff S, Noonan K, Borrello I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res. 2008;68:5439–5449. doi: 10.1158/0008-5472.CAN-07-6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, Divino CM, Chen SH. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–1131. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 7.Pan PY, Ma G, Weber KJ, Ozao-Choy J, Wang G, Yin B, Divino CM, Chen SH. Immune stimulatory receptor CD40 is required for T-cell suppression and T regulatory cell activation mediated by myeloid-derived suppressor cells in cancer. Cancer Res. 2010;70:99–108. doi: 10.1158/0008-5472.CAN-09-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M, Carbone DP, Matrisian LM, Richmond A, Lin PC, Moses HL. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13:23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li H, Han Y, Guo Q, Zhang M, Cao X. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1. J Immunol. 2009;182:240–249. doi: 10.4049/jimmunol.182.1.240. [DOI] [PubMed] [Google Scholar]
- 10.Srivastava MK, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 2010;70:68–77. doi: 10.1158/0008-5472.CAN-09-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanson EM, Clements VK, Sinha P, Ilkovitch D, Ostrand-Rosenberg S. Myeloid-derived suppressor cells down-regulate L-selectin expression on CD4+ and CD8+ T cells. J Immunol. 2009;183:937–944. doi: 10.4049/jimmunol.0804253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Talmadge JE. Pathways mediating the expansion and immunosuppressive activity of myeloid-derived suppressor cells and their relevance to cancer therapy. Clin Cancer Res. 2007;13:5243–5248. doi: 10.1158/1078-0432.CCR-07-0182. [DOI] [PubMed] [Google Scholar]
- 14.Ko JS, Bukowski RM, Fincke JH. Myeloid-derived suppressor cells: a novel therapeutic target. Curr Oncol Rep. 2009;11:87–93. doi: 10.1007/s11912-009-0014-6. [DOI] [PubMed] [Google Scholar]
- 15.Yang L, DeBusk L, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, Matrisian L, Carbone D, Lin P. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6:409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 16.Priceman SJ, Sung JL, Shaposhnik Z, Burton JB, Torres-Collado AX, Moughon DL, Johnson M, Lusis AJ, Cohen DA, Iruela-Arispe ML, Wu L. Targeting distinct tumor-infiltrating myeloid cells by inhibiting CSF-1 receptor: combating tumor evasion of antiangiogenic therapy. Blood. 2010;115:1461–1471. doi: 10.1182/blood-2009-08-237412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shojaei F, Wu X, Malik AK, Zhong C, Baldwin ME, Schanz S, Fuh G, Gerber HP, Ferrara N. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b(+)Gr1(+) myeloid cells. Nat Biotechnol. 2007;25:911–920. doi: 10.1038/nbt1323. [DOI] [PubMed] [Google Scholar]
- 18.Bronte V, Wang M, Overwijk W, Surman D, Pericle F, Rosenberg SA, Restifo NP. Apoptotic death of CD8+ T lymphocytes after immunization: induction of a suppressive population of Mac-1+/Gr-1+ cells. J Immunol. 1998;161:5313–5320. [PMC free article] [PubMed] [Google Scholar]
- 19.Gabrilovich D, Ishida T, Oyama T, Ran S, Kravtsov V, Nadaf S, Carbone DP. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood. 1998;92:4150–4166. [PubMed] [Google Scholar]
- 20.Almand B, Clark JI, Nikitina E, English NR, Knight SC, Carbone DP, Gabrilovich DI. Increased production of immature myeloid cells in cancer patients. A mechanism of immunosuppression in cancer. J Immunol. 2001;166:678–689. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 21.Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, McDermott D, Quiceno D, Youmans A, O’Neill A, Mier J, Ochoa AC. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 2005;65:3044–3048. doi: 10.1158/0008-5472.CAN-04-4505. [DOI] [PubMed] [Google Scholar]
- 22.Schmielau J, Finn OJ. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of T-cell function in advanced cancer patients. Cancer Res. 2001;61:4756–4760. [PubMed] [Google Scholar]
- 23.Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P, Van Ginderachter JA. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T-cell suppressive activity. Blood. 2008;111:4233–4244. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- 24.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fleming TJ, Fleming ML, Malek TR. Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow. RB6-8C5 mAb to granulocyte-differentiation antigen (Gr-1) detects members of the Ly-6 family. J Immunol. 1993;151:2399–2408. [PubMed] [Google Scholar]
- 26.Sunderkotter C, Nikolic T, Dillon MJ, Van Rooijen N, Stehling M, Drevets DA, Leenen PJ. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172:4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- 27.Ribechini E, Leenen PJ, Lutz MB. Gr-1 antibody induces STAT signaling, macrophage marker expression and abrogation of myeloid-derived suppressor cell activity in BM cells. Eur J Immunol. 2009;39:3538–3551. doi: 10.1002/eji.200939530. [DOI] [PubMed] [Google Scholar]
- 28.Dolcetti L, Peranzoni E, Ugel S, Marigo I, Fernandez Gomez A, Mesa C, Geilich M, Winkels G, Traggiai E, Casati A, Grassi F, Bronte V. Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by GM-CSF. Eur J Immunol. 2010;40:22–35. doi: 10.1002/eji.200939903. [DOI] [PubMed] [Google Scholar]
- 29.Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, Golshayan A, Rayman PA, Wood L, Garcia J, Dreicer R, Bukowski R, Finke JH. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15:2148–2157. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez PC, Ernstoff MS, Hernandez C, Atkins M, Zabaleta J, Sierra R, Ochoa AC. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 2009;69:1553–1560. doi: 10.1158/0008-5472.CAN-08-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peranzoni E, Zilio S, Marigo I, Dolcetti L, Zanovello P, Mandruzzato S, Bronte V. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr Opin Immunol. 2010 doi: 10.1016/j.coi.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 32.Ribechini E, Greifenberg V, Sandwick S, Lutz MB. Subsets, expansion and activation of myeloid-derived suppressor cells. Med Microbiol Immunol. 2010;199:273–281. doi: 10.1007/s00430-010-0151-4. [DOI] [PubMed] [Google Scholar]
- 33.Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, Basso G, Brombacher F, Borrello I, Zanovello P, Bicciato S, Bronte V. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest. 2006;116:2777–2790. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang R, Cai Z, Zhang Y, Yutzy WHt, Roby KF, Roden RB. CD80 in immune suppression by mouse ovarian carcinoma-associated Gr-1+CD11b+ myeloid cells. Cancer Res. 2006;66:6807–6815. doi: 10.1158/0008-5472.CAN-05-3755. [DOI] [PubMed] [Google Scholar]
- 35.Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, Herber D, Schneck J, Gabrilovich D. Altered recognition of antigen is a novel mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007;13:828–835. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nausch N, Galani IE, Schlecker E, Cerwenka A. Mononuclear myeloid-derived “suppressor” cells express RAE-1 and activate natural killer cells. Blood. 2008;112:4080–4089. doi: 10.1182/blood-2008-03-143776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monu N, Frey AB. Suppression of proximal T cell receptor signaling and lytic function in CD8+ tumor-infiltrating T cells. Cancer Res. 2007;67:11447–11454. doi: 10.1158/0008-5472.CAN-07-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fricke I, Mirza N, Dupont J, Lockhart G, Jackson A, Lee JH, Sosman JA, Gabrilovich DI. Treatment of cancer patients with VEGF-Trap overcomes defects in DC differentiation but is insufficient to improve antigen-specific immune responses. Clin Cancer Res. 2007;13:4840–4848. doi: 10.1158/1078-0432.CCR-07-0409. [DOI] [PubMed] [Google Scholar]
- 39.Zhou Z, French DL, Ma G, Eisenstein S, Chen Y, Divino CM, Keller G, Chen SH, Pan PY. Development and function of myeloid-derived suppressor cells generated from mouse embryonic and hematopoietic stem cells. Stem Cells. 2010;28:620–632. doi: 10.1002/stem.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greifenberg V, Ribechini E, Rossner S, Lutz MB. Myeloid-derived suppressor cell activation by combined LPS and IFN-gamma treatment impairs DC development. Eur J Immunol. 2009;39:2865–2876. doi: 10.1002/eji.200939486. [DOI] [PubMed] [Google Scholar]
- 41.Yamamoto Y, Ishigaki H, Ishida H, Itoh Y, Noda Y, Ogasawara K. Analysis of splenic Gr-1(int) immature myeloid cells in tumor-bearing mice. Microbiol Immunol. 2008;52:47–53. doi: 10.1111/j.1348-0421.2008.00009.x. [DOI] [PubMed] [Google Scholar]
- 42.Hestdal K, Ruscetti F, Ihle J, Jacobsen S, Dubois C, Kopp W, Longo D, Keller J. Characterization and regulation of RB6-8C5 antigen expression on murine bone marrow cells. J Immunol. 1991;147:22–28. [PubMed] [Google Scholar]
- 43.Haile LA, Gamrekelashvili J, Manns MP, Korangy F, Greten TF. CD49d is a new marker for distinct myeloid-derived suppressor cell subpopulations in mice. J Immunol. 2010;185:203–210. doi: 10.4049/jimmunol.0903573. [DOI] [PubMed] [Google Scholar]
- 44.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mantovani A, Sica A, Allavena P, Garlanda C, Locati M. Tumor-associated macrophages and the related myeloid-derived suppressor cells as a paradigm of the diversity of macrophage activation. Hum Immunol. 2009;70:325–330. doi: 10.1016/j.humimm.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 46.Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest. 2007;117:1155–1166. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 48.Quinn MT, Gauss KA. Structure and regulation of the neutrophil respiratory burst oxidase: comparison with nonphagocyte oxidases. J Leukoc Biol. 2004;76:760–781. doi: 10.1189/jlb.0404216. [DOI] [PubMed] [Google Scholar]
- 49.Marigo I, Bosio E, Solito S, Mesa C, Fernandez A, Dolcetti L, Ugel S, Sonda N, Bicciato S, Falisi E, Calabrese F, Basso G, Zanovello P, Cozzi E, Mandruzzato S, Bronte V. Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity. 2010;32:790–802. doi: 10.1016/j.immuni.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 50.Lechner MG, Liebertz DJ, Epstein AL. Characterization of Cytokine-Induced Myeloid-Derived Suppressor Cells from Normal Human Peripheral Blood Mononuclear Cells. J Immunol. 2010 doi: 10.4049/jimmunol.1000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arora M, Poe SL, Oriss TB, Krishnamoorthy N, Yarlagadda M, Wenzel SE, Billiar TR, Ray A, Ray P. TLR4/MyD88-induced CD11b(+)Gr-1(int)F4/80(+) non-migratory myeloid cells suppress Th2 effector function in the lung. Mucosal Immunol. 2010 doi: 10.1038/mi.2010.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Q, Pan PY, Gu P, Xu D, Chen SH. Role of immature myeloid Gr-1+ cells in the development of antitumor immunity. Cancer Res. 2004;64:1130–1139. doi: 10.1158/0008-5472.can-03-1715. [DOI] [PubMed] [Google Scholar]
- 53.Narita Y, Wakita D, Ohkur T, Chamoto K, Nishimura T. Potential differentiation of tumor bearing mouse CD11b+Gr-1+ immature myeloid cells into both suppressor macrophages and immunostimulatory dendritic cells. Biomed Res. 2009;30:7–15. doi: 10.2220/biomedres.30.7. [DOI] [PubMed] [Google Scholar]
- 54.Kusmartsev S, Gabrilovich D. STAT1 signaling regulates tumor-associated macrophage-mediated T cell deletion. J Immunol. 2005;174:4880–4891. doi: 10.4049/jimmunol.174.8.4880. [DOI] [PubMed] [Google Scholar]
- 55.Corzo CA, Condamine T, Lu L, Cotter MJ, Youn J-I, Cheng P, Cho H-I, Celis E, Quiceno DG, Padhya T, McCaffrey TV, McCaffrey JC, Gabrilovich DI. HIF-1α regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010 doi: 10.1084/jem.20100587. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Umemura N, Saio M, Suwa T, Kitoh Y, Bai J, Nonaka K, Ouyang GF, Okada M, Balazs M, Adany R, Shibata T, Takami T. Tumor-infiltrating myeloid-derived suppressor cells are pleiotropic-inflamed monocytes/macrophages that bear M1- and M2-type characteristics. J Leukoc Biol. 2008;83:1136–1144. doi: 10.1189/jlb.0907611. [DOI] [PubMed] [Google Scholar]