Abstract
MitoNEET is a recently identified diabetes drug target that coordinates a transferable 2Fe-2S cluster, and additionally contains an unusual strand swap. In this manuscript, we use a dual basin structure-based model to predict and characterize the folding and functionality of strand swapping in mitoNEET. We demonstrate that a strand unswapped conformation is kinetically accessible and that multiple levels of control are employed to regulate the conformational dynamics of the system. Environmental factors such as temperature can shift route preference toward the unswapped pathway. Additionally we see that a region recently identified as contributing to frustration in folding acts as a regulatory hinge loop that modulates conformational balance. Interestingly, strand unswapping transfers strain specifically to cluster-coordinating residues, opening the cluster-coordinating pocket. Strengthening contacts within the cluster-coordinating pocket opens a new pathway between the swapped and unswapped conformation that utilizes cracking to bypass the unfolded basin. These results suggest that local control within distinct regions affect motions important in regulating mitoNEET’s 2Fe-2S clusters.
Keywords: aging, iron-sulfur cluster, multiple routes, thiazolidinedione, domain swapping
The mitoNEET family of 2Fe-2S proteins are key regulators of health and disease (1–3). Targeting these proteins for drug design has gained considerable interest in the last few years. An important challenge in targeting these proteins is that they do not contain a classical binding pocket and their mechanisms of regulation are only now being uncovered. We recently reported that mitoNEET can act as a cluster transfer protein (4) and that long-range communication within the protein can regulate the cluster-binding domain (5). Uncovering the physical basis for this long-range communication is an important first step toward understanding the structure-function relationships within this protein family.
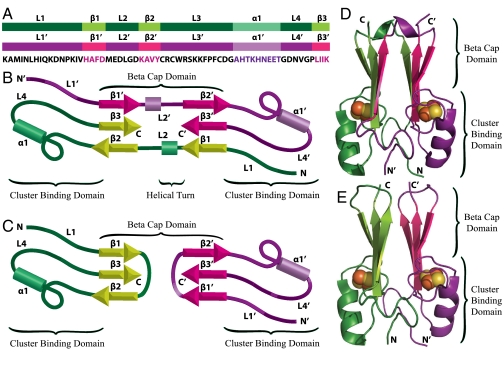
MitoNEET is composed of two protomers (shown in purple and green, Fig. 1) which intertwine via a swapped strand (β1) and loop (L1) to form a β-cap domain and a cluster-binding domain (6–9) (Fig. 1). Loop 2 (L2) leads in to the swapped strand and tethers the two structural halves of the protein together. We reported that the structural elements involved in strand swapping contribute to geometric frustration in folding and mediate communication between the β-cap and cluster-binding domain (5). Specifically, early formation of contacts in loop 2 slows folding. This behavior led us to ask, could loop 2 regulate strand swapping, and is the frustration we observe a consequence of strand swapping in dimeric mitoNEET?
Fig. 1.
Structural organization and domain topology of MitoNEET. Protomers are individually colored in purple and green for clarity. (A) Protomers aligned with the sequence. Highlighted regions correspond to the loops, helices, and β-strands in mitoNEET. (B) Splay diagram of native mitoNEET. (C) Splay diagram of simulated unswapped mitoNEET showing the swapping of strand 1 and loop 1 mediated by the rearrangement of loop 2. Ribbon diagram of native mitoNEET (D) and simulated unswapped mitoNEET (E).
In this system, geometric constraints dominate the landscape over energetic effects, and structure-based models (SBM) capture the overall characteristics of the landscape (5). Multiple basin SBMs are powerful tools that simulate energy landscapes in systems that undergo conformational changes or multimeric association reactions. These models revealed the underlying mechanisms of conformational transitions (10, 11), protein aggregation (12), and domain swapping (13), and additionally have been shown to successfully predict domain swapped structures (14). Recently, we used a dual funneled landscape to explain the unusual kinetic behavior of the rop-dimer system resulting from two distinct and competing conformations (15, 16). Drawing from this success, we undertook a study of the exciting new diabetes drug target mitoNEET that is known to transfer its cluster under oxidative stress conditions. In this study, we test the hypothesis that cluster-binding properties affect quaternary structure via long-range communication. One advantage of dual basin SBMs is that they allow us to construct and examine the behavior of conformations that are functionally important but may not be fully amenable to experimental structural characterization.
We used a dual basin SBM to investigate the role of strand swapping in mitoNEET. We demonstrate that although the unswapped conformation is kinetically accessible, it is thermodynamically less stable. The loop responsible for frustration in folding plays a significant role in thermodynamically destabilizing the unswapped structure relative to the native swapped structure, thus regulating strand swapping. Additionally, we see that the unswapped conformation transfers strain to the cluster-binding pocket that results in opening of the cluster-binding pocket and possibly facilitates cluster release. Tightening the contacts between cluster-coordinating residues results in switching from the unswapped conformation to the native swapped structure. These results suggest that not only is a strand unswapped structure accessible, but that it may play an important functional role in cluster transfer and release.
Results
MitoNEET is a homodimer in which the two protomers intertwine to form a β-cap domain and a cluster-binding domain. Each protomer (colored in green and purple for clarity) contains three β-strands, an alpha helix, and four loops (Fig. 1). The cluster-binding domain is composed of L1, L3, α1, and L4, and contains two 2Fe-2S clusters, each with a 3Cys-1His coordination. The β-cap domain consists of two β-sheets sandwiched together. Each β-sheet is composed of three β-strands; two β-strands from one protomer (β2 and β3), and the third β-strand from the second protomer (β1′) leading to a strand swapped configuration. Loop 2 follows the swapped strand (β1′) and loop (L1′) in sequence, tethering the two β-sheets into the β-sandwich, and contributes to frustration in the folding of this protein. This topology raises the question of whether loop 2 plays a regulatory role in strand swapping, and could the frustration we observe be a result of this regulation?
To address these questions, we created a dual funneled landscape comprised of both the native swapped structure and constructed unswapped structure using a SBM. In our model, we introduced additional contacts into strand 1 and loop 1 to allow for an alternate strand unswapped structure. Specifically, residues in strand 1 and loop 1 that make intraprotomer contacts are now allowed to make the corresponding interprotomer contacts, and residues that make interprotomer contacts are allowed to make intraprotomer contacts. In the native state of mitoNEET, strand 1 and loop 1 are swapped between the two structural halves so that they make interprotomer contacts with strand 3′ and loop 4′ (Fig. 1 B and D). The additional contacts allow strand 1 to make intraprotomer contacts with strand 3 and loop 1 to make intraprotomer contacts with loop 4 (Fig. 1 C and E), allowing each β-sheet to form entirely from one protomer, in the order β1, β3, β2. β-Strand 1 is no longer required to swap between the two structural halves, and loop 2 has the option of wrapping underneath a β-sheet instead of stretching between the two β-sheets. In addition, we created a single basin model in which only the unswapped conformation is accessible in order to better examine the folding of this protein.
Mechanism of Assembly.
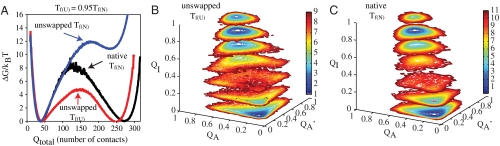
It is informative to first compare the folding of the strand unswapped conformation with that of the native, which we recently reported (5). Results from simulations in which only the strand unswapped conformation is accessible is plotted as the free energy as a function of Qtotal in Fig. 2A. At the folding temperature of the native swapped dimer (Tf(N)), the barrier for unswapped conformation is higher than that for the native swapped conformation, and the folded basin is destabilized. At the folding temperature of the unswapped conformation (Tf(U)), the barrier to folding is significantly lower than that observed for the native structure at Tf(N) and peaks at a higher Qtotal. In Fig. 2B, a free energy surface of assembly for the unswapped conformation is presented. QA represents contact formation in protomer A, QA′ represents contact formation in protomer A′, and QI represents protomer–protomer interface contact formation. At QI = 0.4 interface contact formation, two basins are visible, one corresponding to high QA and low QA′, and the second corresponding to low QA and high QA′. This signature indicates that one protomer may begin folding independently of the other, and the formation of interface contacts between the two protomers helps nucleate the folding of the second protomer. It is only when a number of interface contacts are formed that the folding of the second protomer begins. This folding mechanism differs from that of the native swapped dimer, where dimerization is coupled to protomer formation and no folding of individual protomers is observed. (Fig. 2C).
Fig. 2.
Single basin modeling. (A) Free energy is plotted as a function of Q at Tf(N) for native mitoNEET (black) and unswapped mitoNEET at Tf(N) (blue) and at Tf(U), or 0.95 Tf(N) (red). The unswapped structure is kinetically accessible, but thermodynamically unstable at the folding temperature of the native protein. Free energy surface for the assembly of unswapped mitoNEET at Tf(U) (B) and native mitoNEET at Tf(N) (C). The free energy is projected on to the reaction coordinates Q protomer A, Q protomer A′, and Q interface. A contour of the free energy as a function of QA and QA′ is plotted at seven different values of QI.
Dual Basin Simulations.
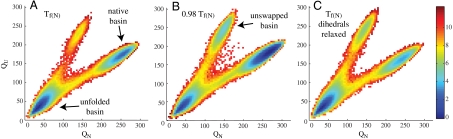
Fig. 3A shows the free energy surface of the dual basin simulations at Tf(N) as a function of two coordinates, QN and QU. QN represents the total number of contacts formed in the native configuration, and QU represents the total number of contacts formed in the unswapped conformation. Two major populations are observed. The basin at low QU and QN represents the unfolded ensemble, and the basin at intermediate QU and high QN corresponds to the native swapped configuration. At high QU and intermediate QN, a small population is present that represents the unstable unswapped configuration. In an effort to explore the formation of the unswapped configuration, we performed simulations at 0.98 Tf(N). The resulting free energy surface is plotted in Fig. 3B. Under these conditions we enhance the population of species at high QU and low QN, corresponding to the unswapped configuration. Below 0.98 Tf(N), it is difficult to get sufficient thermodynamic sampling, however as temperature decreases, the unswapped structure becomes more kinetically accessible. At 0.9 Tf(N), 45% of trajectories starting from the unfolded state transition to the unswapped configuration. Taken together these results indicate that, although the unswapped structure is thermodynamically less stable, it is kinetically accessible.
Fig. 3.
Dual basin modeling. Free energy profiles for dual basin simulations of mitoNEET. (A) Simulations performed at the folding temperature of native mitoNEET with all native contacts and dihedrals. Two basins are present corresponding to native mitoNEET and the unfolded state. The unswapped configuration is accessible, but is unstable at Tf(N). (B) Analysis of folding of native and unswapped mitoNEET with a dual basin simulations performed at 0.98 Tf(N) of native mitoNEET. A third basin is present corresponding to the unswapped configuration. (C) Dual basin simulations performed at Tf(N) with relaxation of contacts and dihedrals in loop 2 allow the formation of the unswapped configuration.
Destabilization of Geometrically Frustrated Loop 2 Increases the Population of the Unswapped Configuration.
Geometric frustration in loop 2 mediates communication between the β-cap and the cluster-binding domain in the native dimer (5). This frustration leads to communication with distal residues that backtrack, or locally unfold, and contribute to regulation of global motions. To examine the effect that frustrated loop 2 has on the landscape of the native and unswapped structures, we removed tertiary contacts and relaxed dihedrals in this region and then repeated dual basin simulations at Tf(N). Our results indicate that the native configuration is still preferred, but relaxing loop 2 increases the accessibility and stability of the unswapped configuration (Fig. 3C).
Strand Unswapping Activates an Alternate Site of Communication in the Cluster-Binding Region.
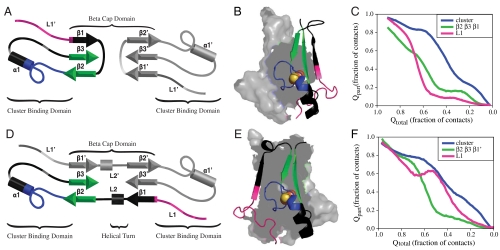
The protein must completely unfold to move between the native swapped and unswapped structures in the SBM where all contacts are uniform, therefore, it is essential to understand the mechanism of unfolding of the respective structures. To address this question, we examined the mechanism of unfolding for the unswapped (Fig. 4 A–C) and native (Fig. 4 D–F) conformation in single basin landscapes. As described previously (5), we analyzed the behavior of individual contacts within one protomer and then partitioned them based on the order in which they unfold. For clarity we show a splay diagram of one protomer colored by groups of contacts to be examined with the second protomer shown in gray (Fig. 4 A and D) and a ribbon diagram colored by groups of contacts to be examined with a surface rendition of the second protomer in gray (Fig. 4 B and E). Regions shown in black are not plotted and are given for reference. We then plot the fractional population of these subsets of contacts (Qpart) as a function of the total number of contacts in the protein during unfolding (Qtotal) (Fig. 4 C and F). Contacts within the cluster-binding pocket and between the two cluster-binding pockets (plotted in blue) are the last to unfold in both models. As reported previously (5), we observe backtracking in loop 1 (plotted in pink) in the native conformation. That is, loop 1 begins to break early in unfolding, then reforms contacts, then subsequently breaks again later in unfolding. The backtracking in the cluster-binding domain is coupled to the unfolding of the β-sheet (plotted in green) in the β-cap domain, demonstrating communication between the two domains. In the unfolding of the unswapped conformation, backtracking is lost in loop 1. Instead, loop 1 appears to initiate unfolding followed closely by the unfolding of the β-sheet.
Fig. 4.
Single basin modeling. Order of unfolding for the unswapped and native configurations of mitoNEET within one protomer. The unswapped configuration of mitoNEET (A and B) and native configuration (D and E) are colored by groups of contacts examined. Pink represents contacts present in loop1, green represents contacts present in the β-sheet (β2, β3, β1, and β2, β3, β1′), blue represents contacts within the cluster-binding pocket and between the two pockets. Regions in black are not plotted and are shown for reference. The average fraction of these subsets of contacts formed as a function of Qtotal for the unswapped configuration (C) and the native configuration (F). In both cases, the cluster-binding pocket is the last to unfold. In the native configuration, contacts in L1 exhibit backtracking. These contacts break early but begin to reform as the β-sheet breaks, and finish breaking late in unfolding. In the unswapped configuration, backtracking is lost. Contacts in L1 are the first to unfold followed by contacts in the β-sheet.
The loss of a backtracking signal in the unswapped conformation does not necessarily suggest that communication between domains has been lost. Although the formation of all individual contacts are possible within our model, we observe that strain from strand unswapping introduces competition between contact formation in different regions. Specifically, the short length of loop 2 prevents the simultaneous formation of all contacts between β1 and β3, and β2 and β3 in the unswapped configuration. In the case that all contacts are formed between β1 and β3, loop 2 pulls on β2, thus destabilizing some contacts between β2 and β3, resulting in destabilization of the β-sheet (plotted in green) in the unswapped simulations, which on average has only 85% contact formation in the folded basin. This strain appears to translate directly in to the cluster-binding pocket. The majority of contacts in the cluster-binding region are able to form completely in the folded state, however, in the unswapped configuration, the two contacts between the cluster-coordinating residues are only formed 80% of the time in the folded basin. These results suggest that strand unswapping shifts the site of communication in the cluster-binding domain from loop 1 to the cluster-coordinating residues.
Stabilizing Cluster-Binding Contacts Triggers Switching.
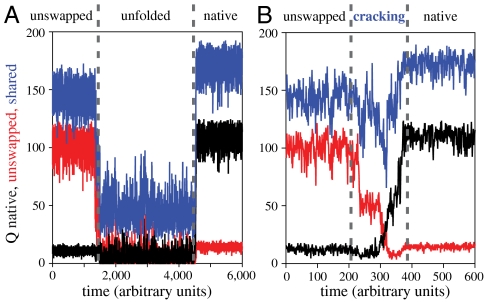
In an effort to address the mechanism of interconversion between the unswapped and the native swapped conformation, we examined the time-dependent evolution of structure within each individual trajectory. Example trajectories plotted as Q as a function of time are shown in Fig. 5. Contacts specific to the unswapped and native swapped structures are plotted in red and black, respectively. Contacts that are not involved in strand swapping and are common between the two structures are given in blue. Plotted in Fig. 5A is an example trajectory in which mitoNEET starts in the unswapped conformation (red), completely unfolds, and then refolds into the native swapped conformation (black). The shared contacts (blue) that describe the global structure must completely unfold to move from the unswapped to the native swapped conformation. That is, only after complete unfolding of the unswapped conformation will the native strand swapped structure form. Therefore complete unfolding is required to move between the native and unswapped basins.
Fig. 5.
Tightening cluster contacts triggers switching by cracking. Sample trajectory of dual basin simulations at Tf(U) (A). Contacts specific to the native configuration are plotted in black, and unswapped contacts are plotted in red. Contacts that define the global fold and are shared between the two configurations are plotted in blue. The unswapped conformation must completely unfold before the native conformation can form. (B) Sample trajectory of dual basin simulations at Tf(U) with cluster-binding contacts tightened. MitoNEET is able to switch from the unswapped conformation to the native without completely unfolding. Cracking in shared contacts relieves strain and preserves the global fold.
In our single basin simulations, we observed communication between the β-cap and the cluster-coordinating residues in the unswapped conformation. The majority of contacts in the cluster-binding region are able to form completely in the folded state, however, two contacts between the coordinating residues are only formed 80% of the time in the folded basin. These residues follow β2 in primary sequence and are positioned perpendicular to β2 in the tertiary structure. Therefore, optimal placement of β2 likely influences the orientation of the cluster-binding residues. Likewise, cluster coordination geometry may influence packing of strands within the β-sheet. To test the role of these cluster-coordinating contacts in strand swapping, we introduce heterogeneity into our dual basin model by strengthening the cluster-coordinating contacts. At the folding temperature of the unswapped conformation Tf(U), or 0.95 Tf(N), we observe switching without fully unfolding between the native and unswapped structures. Specifically, 29% of trajectories that fold in to the unswapped configuration first are now able to switch into the native configuration without completely unfolding. An example of a switching event is shown in Fig. 5B. Contacts shared between the two structures (plotted in blue) do not completely unfold, rather they exhibit a behavior called cracking, or local unfolding in response to local stress in conformational transitions (10, 17, 18). Here cracking preserves the global fold by relieving stress associated with the repositioning of β1 and loop 1. For comparison, low temperature simulations (0.9 Tf(N), 0.95 Tf(N), 0.98 Tf(N)) were run without these contacts strengthened, and switching behavior is not observed at any temperature. Therefore, changes in stability of the 2Fe-2S cluster may be an essential regulator of conformational dynamics within the protein.
Discussion
Frustration in Folding a Necessary Consequence of Regulating Conformational Balance.
In a previous study, we identified loop 1 and loop 2 as interesting regions of frustration in mitoNEET. We reported that, for folding directly into the native structure of mitoNEET, a subset of contacts exhibit backtracking (5). That is, these contacts form early in folding, but to complete folding they break to relieve geometric frustration, and reform later. More importantly, we demonstrated that loop 2 in the β-cap domain controls the backtracking behavior of loop 1 in the cluster-binding domain, thus mediating communication between the two domains. By relaxing loop 2, we were able to remove backtracking in loop 1 and decrease the barrier to folding. An important question is why has mitoNEET evolved this frustration in loop 2?
In our present study, we introduce the same relaxation of contacts and dihedrals in loop 2 that previously decreased the barrier to folding, however, we do this in a dual basin landscape. Relaxing this loop destabilizes the native configuration and allows the unswapped conformation to be significantly populated at Tf(N) (Fig. 3C). In contrast, when loop 2 is geometrically constrained, the unswapped configuration is significantly destabilized relative to the native conformation (Fig. 3A), suggesting that loop 2 functions as a regulatory hinge loop for the strand swapping of β1 and loop 1, thus controlling the association of β1 with β3, and β1 with β3′. A more relaxed loop would result in a faster folding protein, but the additional geometric constraint imposed by loop 2 is necessary for regulating the balance between strand swapped conformers.
Although the structuring of loop 2 biases the folding of mitoNEET toward the native structure, the unswapped structure is not forbidden. At 0.98 Tf(N), we see that the unswapped conformation becomes accessible, and in low-temperature kinetic runs we demonstrate that route preference can be significantly shifted toward the unswapped folding pathway, suggesting that the route preference and conformational balance are dynamic and sensitive to environmental control.
Tightening Cluster-Binding Pocket in Unswapped Conformation Opens Switching Pathway.
In contrast to the native folding pathway, which requires coupled folding and dimerization, the unswapped folding pathway allows protomers to form more independently of each other prior to dimerization (Fig. 2 B and C). Because proteins are monomeric emerging from the ribosome, and unfolded proteins are more vulnerable to degradation, prefolding of the unswapped monomer may provide a degree of protection until a partner is produced. In our perfectly homogenous SBM, if the unswapped dimer is formed first, the protein must fully unfold before it can find the native swapped configuration (Fig. 5A). However real proteins have heterogeneity in contact strength, and mitoNEET likely experiences dynamic heterogeneity near the 2Fe-2S cluster. In vivo tightening in the cluster pocket may arise as a result of the formation of a disulfide bond between C72 and C83, protein or drug binding, or cluster assembly and insertion. Tightening cluster-binding contacts in our model opens a switching pathway previously unavailable. This route provides an additional level of conformational control by facilitating strand swapping without complete unfolding. As strand 1 and loop 1 switch from the unswapped position in to the native swapped position, the global fold exhibits cracking but remains intact (Fig. 5B). An intriguing possibility is that the strain in loop 2 facilitates cluster assembly or insertion into the unswapped structure by pulling open the cluster-binding pocket via β-strand 2, thus increasing the accessibility of the cluster-coordinating residues. Switching may then provide a convenient mechanism for preserving the coordination of the 2Fe-2S cluster while moving from the unswapped structure to the more thermodynamically stable native structure (Fig. 2A). Alternatively, the preferred pathway between the unswapped and the native conformation may be through complete unfolding, and switching may be a fail-safe mechanism activated in response to accidental cluster insertion into the unswapped conformation. In this case, switching would be a better alternative to complete unfolding which could release toxic free iron into the cell.
Evolution of Functional Control Through Geometric Frustration.
MitoNEET and miner1 are distinct drug targets (2, 19, 20), and their crystal structures reveal a dimeric and strand swapped topology in which each protomer contains one CDGSH domain that coordinates a 2Fe-2S cluster (6–9, 21). Recently, homologous structures from archaea and bacteria have been released that show significant permutations in the β-cap domain. These structures include a monomeric structure containing tandem CDGSH domains and two strands per β-sheet, a dimeric strand unswapped structure with four strands per β-sheet, and a dimeric strand swapped structure with five strands per β-sheet (22). These permutations raise the question of how and why dimerization and strand swapping evolved in this class of proteins. It has been proposed that frustration in folding is the result of conservation of functional residues (23–27). Local energetic frustration is present near the binding sites of many proteins (28), and the functional regions of some proteins have been shown to be responsible for decreased stability (29–31), folding traps (25, 26), long-lived partially folded intermediates (32), and misfolding (33). An intriguing idea is that frustration has been engineered into proteins throughout evolution. The NEET family of proteins may have evolved different strand swapped folds to maintain careful control of 2Fe-2S cluster release and conformational balance in the presence of changing environmental conditions.
Conclusion
We used a dual basin SBM to predict and characterize the folding and functionality of strand swapping in mitoNEET. Our results show that multiple levels of control regulate the conformational dynamics of this system. A loop which was previously identified as contributing frustration to folding acts as a regulatory loop destabilizing the unswapped conformation relative to the native conformation. Local tuning of contacts in this loop reverses this effect, increasing the accessibility of the unswapped conformation. Additionally, route preference is shifted toward the unswapped folding pathway by modulating temperature. We demonstrate that not only is a strand unswapped structure accessible, but also that strand unswapping preferentially destabilizes contacts opening the cluster-binding pocket. Tightening these local contacts opens an alternate route between the unswapped conformation and the native swapped conformation that bypasses the unfolded basin. This local control of global structure and dynamics offers a powerful mechanism to evolve multiple functional states with a single protein sequence.
Methods
A coarse-grained structure-based model was used to model the protein as described previously (34), in which each residue is represented by its Cα atom. Our model consists of native interactions in addition to some nonnative interactions to model domain swapping as described below. These nonnative interactions introduce some additional frustration into the system not normally present in purely structure-based models. In our coarse-grained studies, the iron-sulfur cluster is implicitly included in the contacts present between cluster-binding residues. Simulations were performed using version 3.3.3 of the Groningen Machine for Chemical Simulations (GROMACS) software package (35). The integrator used was stochastic dynamics. The Berendsen algorithm was used with the coupling constant of two. The time step τ was 0.0005. Each protomer was temperature coupled separately. A harmonic potential with an offset of 20 Å was applied to the center of mass of each protomer to hold the two protomers together.
Native contacts were identified using the Structure-Based Models in Gromacs (SMOG) Web server (36) with a Contacts of Structural Unit contact map (37) and were assigned interaction distances based on the crystal structure of mitoNEET stored in Protein Data Bank ID 2QH7 (9). Slight asymmetry in the crystal structure results in 14 unsymmetric contacts between the two protomers. To simplify our model, we removed these 14 contacts from the set of native contacts. To model a dual funneled landscape with domain swapping, additional contacts were introduced in strand 1 and loop 1 in both chains. For each intraprotomer contact present between residues i in B1 and L1 and j in chain A (i′ in B1′ and L1′ and j′ in chain B), the identical interprotomer contacts between residues i in B1 and L1 of chain A and j′ in chain B were created using the original intraprotomer interaction distances. Likewise, for all interprotomer contacts present between residues i in B1 and L1 of chain A and j′ in chain B, the corresponding intraprotomer contacts between residues i in B1 and L1 of chain A and j in chain A were added. The resulting contact map consisted of 92 interprotomer contacts and 34 intraprotomer contacts specific to strand unswapping, 92 interprotomer contacts and 34 intraprotomer contacts specific to native strand swapping, and 40 interprotomer contacts and 158 intraprotomer contacts common to both the native strand swapped and strand unswapped structure.
The symmetry in multimeric systems such as mitoNEET can create duplicate pathways distinguishable only through naming of the individual protomers, and averaging over these pathways can obscure the actual order of folding events. To address this problem, we used Cytoscape (38) to cluster transition state structures as described previously (5) and to examine the order of folding events within a cluster.
Acknowledgments.
The authors thank Jeff Noel, Michael Jamros, and Mark Paddock for helpful discussions. This work was supported in part by the Center for Theoretical Biological Physics sponsored by the National Science Foundation (NSF, Grant PHY-0822283) and by NSF MCB-1051438, and by National Institutes of Health Grant GM-54038. E.L.B. was also supported by a San Diego Fellowship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Wiley SE, Murphy AN, Ross SA, van der Geer P, Dixon JE. MitoNEET is an iron-containing outer mitochondrial membrane protein that regulates oxidative capacity. Proc Natl Acad Sci USA. 2007;104:5318–5323. doi: 10.1073/pnas.0701078104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colca JR, et al. Identification of a novel mitochondrial protein (“mitoNEET”) cross-linked specifically by a thiazolidinedione photoprobe. Am J Physiol Endocrinol Metab. 2004;286:252E–260E. doi: 10.1152/ajpendo.00424.2003. [DOI] [PubMed] [Google Scholar]
- 3.Wiley SE, et al. The outer mitochondrial membrane protein mitoNEET contains a novel redox-active 2Fe-2S cluster. J Biol Chem. 2007;282:23745–23749. doi: 10.1074/jbc.C700107200. [DOI] [PubMed] [Google Scholar]
- 4.Zuris JA, et al. Facile transfer of [2Fe-2S] clusters from the diabetes drug target mitoNEET to an apo-acceptor protein. Proc Natl Acad Sci USA. 2011;108:13047–13052. doi: 10.1073/pnas.1109986108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baxter EL, Jennings PA, Onuchic JN. Interdomain communication revealed in the diabetes drug target mitoNEET. Proc Natl Acad Sci USA. 2011;108:5266–5271. doi: 10.1073/pnas.1017604108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conlan AR, et al. Mutation of the His ligand in mitoNEET stabilizes the 2Fe–2S cluster despite conformational heterogeneity in the ligand environment. Acta Crystallogr D Biol Crystallogr. 2011;67:516–523. doi: 10.1107/S0907444911011577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hou X, et al. Crystallographic studies of human MitoNEET. J Biol Chem. 2007;282:33242–33246. doi: 10.1074/jbc.C700172200. [DOI] [PubMed] [Google Scholar]
- 8.Lin J, Zhou T, Ye K, Wang J. Crystal structure of human mitoNEET reveals distinct groups of iron-sulfur proteins. Proc Natl Acad Sci USA. 2007;104:14640–14645. doi: 10.1073/pnas.0702426104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paddock ML, et al. MitoNEET is a uniquely folded 2Fe-2S outer mitochondrial membrane protein stabilized by pioglitazone. Proc Natl Acad Sci USA. 2007;104:14342–14347. doi: 10.1073/pnas.0707189104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitford PC, Miyashita O, Levy Y, Onuchic JN. Conformational transitions of adenylate kinase: Switching by cracking. J Mol Biol. 2007;366:1661–1671. doi: 10.1016/j.jmb.2006.11.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okazaki K-i, Koga N, Takada S, Onuchic JN, Wolynes PG. Multiple-basin energy landscapes for large-amplitude conformational motions of proteins: Structure-based molecular dynamics simulations. Proc Natl Acad Sci USA. 2006;103:11844–11849. doi: 10.1073/pnas.0604375103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dima RI, Thirumalai D. Probing the instabilities in the dynamics of helical fragments from mouse PrPC. Proc Natl Acad Sci USA. 2004;101:15335–15340. doi: 10.1073/pnas.0404235101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho SS, Levy Y, Onuchic JN, Wolynes PG. Overcoming residual frustration in domain-swapping: the roles of disulfide bonds in dimerization and aggregation. Phys Biol. 2005;2:S44–S55. doi: 10.1088/1478-3975/2/2/S05. [DOI] [PubMed] [Google Scholar]
- 14.Yang S, et al. Domain swapping is a consequence of minimal frustration. Proc Natl Acad Sci USA. 2004;101:13786–13791. doi: 10.1073/pnas.0403724101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levy Y, Cho SS, Shen T, Onuchic JN, Wolynes PG. Symmetry and frustration in protein energy landscapes: A near degeneracy resolves the Rop dimer-folding mystery. Proc Natl Acad Sci USA. 2005;102:2373–2378. doi: 10.1073/pnas.0409572102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schug A, Whitford PC, Levy Y, Onuchic JN. Mutations as trapdoors to two competing native conformations of the Rop-dimer. Proc Natl Acad Sci USA. 2007;104:17674–17679. doi: 10.1073/pnas.0706077104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whitford PC, Onuchic JN, Wolynes PG. Energy landscape along an enzymatic reaction trajectory: Hinges or cracks? HFSP J. 2008;2:61–64. doi: 10.2976/1.2894846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyashita O, Wolynes PG, Onuchic JN. Simple energy landscape model for the kinetics of functional transitions in proteins. J Phys Chem B. 2005;109:1959–1969. doi: 10.1021/jp046736q. [DOI] [PubMed] [Google Scholar]
- 19.Geldenhuys WJ, Funk MO, Barnes KF, Carroll RT. Structure-based design of a thiazolidinedione which targets the mitochondrial protein mitoNEET. Bioorg Med Chem Lett. 2010;20:819–823. doi: 10.1016/j.bmcl.2009.12.088. [DOI] [PubMed] [Google Scholar]
- 20.Amr S, et al. A Homozygous mutation in a novel zinc-finger protein, ERIS, is responsible for Wolfram Syndrome 2. Am J Hum Genet. 2007;81:673–683. doi: 10.1086/520961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conlan AR, et al. Crystal structure of miner1: The redox-active 2Fe-2S protein causative in Wolfram Syndrome 2. J Mol Biol. 2009;392:143–153. doi: 10.1016/j.jmb.2009.06.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin J, Zhang L, Lai S, Ye K. Structure and molecular evolution of CDGSH iron-sulfur domains. PLoS One. 2011;6:e24790. doi: 10.1371/journal.pone.0024790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jäger M, et al. Structure-function-folding relationship in a WW domain. Proc Natl Acad Sci USA. 2006;103:10648–10653. doi: 10.1073/pnas.0600511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karanicolas J, Brooks CL. Integrating folding kinetics and protein function: Biphasic kinetics and dual binding specificity in a WW domain. Proc Natl Acad Sci USA. 2004;101:3432–3437. doi: 10.1073/pnas.0304825101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gosavi S, Chavez LL, Jennings PA, Onuchic JN. Topological frustration and the folding of interleukin-1β. J Mol Biol. 2006;357:986–996. doi: 10.1016/j.jmb.2005.11.074. [DOI] [PubMed] [Google Scholar]
- 26.Gosavi S, Whitford PC, Jennings PA, Onuchic JN. Extracting function from a β-trefoil folding motif. Proc Natl Acad Sci USA. 2008;105:10384–10389. doi: 10.1073/pnas.0801343105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin G. Downhill protein folding: Evolution meets physics. C R Biol. 2005;328:701–712. doi: 10.1016/j.crvi.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 28.Ferreiro DU, Hegler JA, Komives EA, Wolynes PG. Localizing frustration in native proteins and protein assemblies. Proc Natl Acad Sci USA. 2007;104:19819–19824. doi: 10.1073/pnas.0709915104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schreiber G, Buckle AM, Fersht AR. Stability and function: Two constraints in the evolution of barstar and other proteins. Structure. 1994;2:945–951. doi: 10.1016/s0969-2126(94)00096-4. [DOI] [PubMed] [Google Scholar]
- 30.Zhang X-j, Baase WA, Shoichet BK, Wilson KP, Matthews BW. Enhancement of protein stability by the combination of point mutations in T4 lysozyme is additive. Protein Eng. 1995;8:1017–1022. doi: 10.1093/protein/8.10.1017. [DOI] [PubMed] [Google Scholar]
- 31.Shoichet BK, Baase WA, Kuroki R, Matthews BW. A relationship between protein stability and protein function. Proc Natl Acad Sci USA. 1995;92:452–456. doi: 10.1073/pnas.92.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Friel CT, Alastair Smith D, Vendruscolo M, Gsponer J, Radford SE. The mechanism of folding of Im7 reveals competition between functional and kinetic evolutionary constraints. Nat Struct Mol Biol. 2009;16:318–324. doi: 10.1038/nsmb.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nordlund A, et al. Functional features cause misfolding of the ALS-provoking enzyme SOD1. Proc Natl Acad Sci USA. 2009;106:9667–9672. doi: 10.1073/pnas.0812046106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clementi C, Nymeyer H, Onuchic JN. Topological and energetic factors: What determines the structural details of the transition state ensemble and “en-route” intermediates for protein folding? An investigation for small globular proteins. J Mol Biol. 2000;298:937–953. doi: 10.1006/jmbi.2000.3693. [DOI] [PubMed] [Google Scholar]
- 35.Hess B, Kutzner C, van der Spoel D, Lindahl E. GROMACS 4: Algorithms for highly efficient, load-balanced, and scalable molecular simulation. J Chem Theory Comput. 2008;4:435–447. doi: 10.1021/ct700301q. [DOI] [PubMed] [Google Scholar]
- 36.Noel JK, Whitford PC, Sanbonmatsu KY, Onuchic JN. SMOG@ctbp: Simplified deployment of structure-based models in GROMACS. Nucleic Acids Res. 2010;38:W657–W661. doi: 10.1093/nar/gkq498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sobolev V, Sorokine A, Prilusky J, Abola EE, Edelman M. Automated analysis of interatomic contacts in proteins. Bioinformatics. 1999;15:327–332. doi: 10.1093/bioinformatics/15.4.327. [DOI] [PubMed] [Google Scholar]
- 38.Shannon P, et al. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]