Abstract
Bifidobacteria comprise a significant proportion of the human gut microbiota. Several bifidobacterial strains are currently used as therapeutic interventions, claiming various health benefits by acting as probiotics. However, the precise mechanisms by which they maintain habitation within their host and consequently provide these benefits are not fully understood. Here we show that Bifidobacterium breve UCC2003 produces a cell surface-associated exopolysaccharide (EPS), the biosynthesis of which is directed by either half of a bidirectional gene cluster, thus leading to production of one of two possible EPSs. Alternate transcription of the two opposing halves of this cluster appears to be the result of promoter reorientation. Surface EPS provided stress tolerance and promoted in vivo persistence, but not initial colonization. Marked differences were observed in host immune response: strains producing surface EPS (EPS+) failed to elicit a strong immune response compared with EPS-deficient variants. Specifically, EPS production was shown to be linked to the evasion of adaptive B-cell responses. Furthermore, presence of EPS+ B. breve reduced colonization levels of the gut pathogen Citrobacter rodentium. Our data thus assigns a pivotal and beneficial role for EPS in modulating various aspects of bifidobacterial–host interaction, including the ability of commensal bacteria to remain immunologically silent and in turn provide pathogen protection. This finding enforces the probiotic concept and provides mechanistic insights into health-promoting benefits for both animal and human hosts.
The human gut is considered one of the most densely colonized ecosystems known, and is estimated to provide residence to 10–100 trillion microorganisms (1). These microbes play an important role in human nutrition and health by promoting nutrient supply, preventing pathogen colonization, and shaping and maintaining normal mucosal immunity. Indeed, these interactions work both ways, as the human gastrointestinal tract also provides the microbiota with access to key nutrients and a stable environment required for growth (2).
Bifidobacteria represent one of the dominant bacterial groups of the human intestinal microbiota (1). Certain members of the genus Bifidobacterium can exert specific health benefits on their host and are therefore considered to be probiotics (3). Consumption of specific bifidobacteria is associated with inhibition or reduction of cancer (4), antimicrobial activity against pathogens (5), and reduction of relapse frequency of ulcerative colitis (6). Despite these reports, molecular mechanisms underlying these health-promoting claims are largely unknown.
One of the proposed mechanisms by which bifidobacteria mediate (some of) these health benefits is the production of exopolysaccharide/capsule (EPS) (7). Bacterial EPS consists of a repeating mono- or oligosaccharide subunit connected by varying glycosidic linkages, thereby generating homo- or heteropolymers, respectively, that are structurally very diverse. Notably, EPSs—particularly in pathogens—are thought to be critical in host–microbe interactions, where they aid in adherence and colonization within the human host (8) and function in immunomodulation (9). Although very little is known about the function of bifidobacterial EPS, it has been suggested to aid in tolerance of the bacterium to bile/acid (10), and has also been shown to serve as a growth substrate for elements of the gut microbiota (7).
We show that the commensal Bifidobacterium breve UCC2003 contains a bidirectional EPS-encoding genetic locus responsible for the production of a surface-attached EPS that provides resistance to both bile and acid in vitro. In mice, this surface EPS aids in long-term persistence and also mediates immune evasion, specifically in avoiding B-cell responses. Colonization of mice with EPS+, but not EPS− B. breve, also provides the host with protection after enteric pathogen challenge. This finding suggests that surface EPS on commensal bacteria can facilitate colonization of their host through evasion of potentially damaging immune responses, and in turn can provide direct health-promoting benefits, in this case via a reduction in pathogen colonization.
Results
Identifying and Characterizing an EPS Locus in B. breve UCC2003.
The genome of B. breve UCC2003 (11) harbors a putative EPS-encoding locus (designated here as eps), which extends from Bbr_0430 to Bbr_451, and encompasses a 25.6-kb region that harbors 20 genes predicted to be involved in EPS biosynthesis (Fig. 1A and Table S1) and two transposase-encoding sequences (Bbr_0432 and Bbr_0433). The majority (i.e., 18 of 22) of these genes are organized as two adjacent but oppositely oriented gene sets, the first encompassing Bbr_0441 to Bbr_0434, designated here as the eps1 operon, the second from Bbr_0442 to Bbr_451, designated as the eps2 operon (Fig. 1A; see also below). The divergence in GC content (Table S1) suggests that the eps locus was acquired by horizontal gene transfer, as indicated also for other EPS-encoding loci (12).
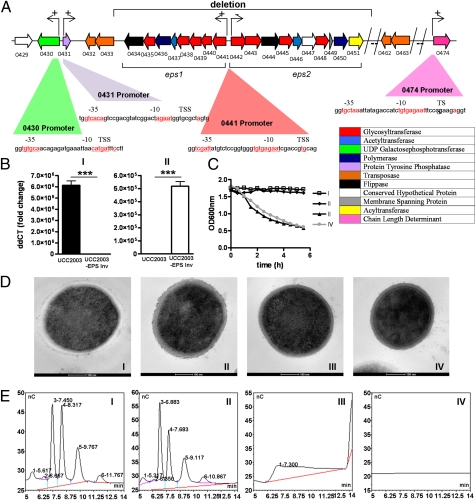
Fig. 1.
Identifying and characterizing the EPS locus in B. breve UCC2003. (A) Schematic diagram of the B. breve UCC2003 EPS locus. For identification of promoters in the EPS: A “+” sign denotes a statistically significant (P < 0.02 by t test) difference in GUS activity between the generated promoter fusion constructs and the pNZ272 control in B. breve UCC2003 at the 12-h growth point, and a “−”sign denotes no statistically significant (P < 0.02 by t test) difference between the promoter fragment and the pNZ272 control in B. breve UCC2003 (n = 3). All cultures had similar growth rates. eps1 and eps2 refer to the two adjacent transcriptional units involved in EPS biosynthesis. 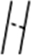 represents a number of genes between Bbr_0451 and Bbr_0462 or between Bbr_463 and Bbr_0474. Transcriptional start sites, −10 and −35 sites of promoters are indicated; the color coding of the genes relates to their predicted function as indicated in the inset. (B) qRT-PCR analysis of the Bbr_0441 and Bbr_0442 transcriptional units. (I) Transcription of Bbr_0441 in strains UCC2003 and UCC2003-EPS Inv; (II) transcription of Bbr_0442 in UCC2003 and UCC2003-EPS Inv. (C) OD measurements (OD600nm) of UCC2003 and its derivatives over a 5.5-h time period grown in batch culture without agitation; the observed drop in OD values for the EPS− derivatives is because of cell sedimentation. (D) Transmission electron microscopy images of B. breve UCC2003 (I) and isogenic derivatives UCC2003-EPSInv (II), UCC2003-EPSdel (III), UCC2003::Bbr_0430 (IV). (Scale bars, 100 nm.) (E) High performance anion-exchange chromatography with pulsed amperometric detection profiles of acid-hydrolyzed surface EPS isolated from UCC2003 and isogenic derivatives (see D for strain coding).
represents a number of genes between Bbr_0451 and Bbr_0462 or between Bbr_463 and Bbr_0474. Transcriptional start sites, −10 and −35 sites of promoters are indicated; the color coding of the genes relates to their predicted function as indicated in the inset. (B) qRT-PCR analysis of the Bbr_0441 and Bbr_0442 transcriptional units. (I) Transcription of Bbr_0441 in strains UCC2003 and UCC2003-EPS Inv; (II) transcription of Bbr_0442 in UCC2003 and UCC2003-EPS Inv. (C) OD measurements (OD600nm) of UCC2003 and its derivatives over a 5.5-h time period grown in batch culture without agitation; the observed drop in OD values for the EPS− derivatives is because of cell sedimentation. (D) Transmission electron microscopy images of B. breve UCC2003 (I) and isogenic derivatives UCC2003-EPSInv (II), UCC2003-EPSdel (III), UCC2003::Bbr_0430 (IV). (Scale bars, 100 nm.) (E) High performance anion-exchange chromatography with pulsed amperometric detection profiles of acid-hydrolyzed surface EPS isolated from UCC2003 and isogenic derivatives (see D for strain coding).
Transcriptional analyses by promoter fusions, quantitative RT-PCR (qRT-PCR) and primer extension delineated the transcriptional units and promoter sequences of this locus, revealing that the gene encoding the only predicted priming glycosyl transferase, Bbr_0430, and a gene (Bbr_0431) putatively encoding a protein involved in EPS chain-length regulation, are each transcribed by a separate promoter and thus, based on their genetic location and orientation, monocistronic (Fig. 1A). Transcriptional analyses further showed that the eps1 operon is constitutively transcribed in UCC2003 from a single promoter, but the eps2 operon is transcriptionally silent (Fig. 1 A and B). Serendipitously, a variant of UCC2003 was isolated, designated UCC2003-EPSInv, which was found to exhibit constitutive transcription of the eps2 operon, but eps1 transcription was undetectable (Fig. 1B). Sequence analysis revealed that the observed transcriptional pattern of UCC2003-EPSInv was the result of an inversion of a 282-bp DNA fragment, located within the intergenic region between eps1 and eps2, thus causing promoter reorientation (Fig. 1A). Because this reoriented fragment is flanked on either side by a 75-bp sequence, which together form a near-perfect (1-bp mismatch) inverted repeat, this promoter reorientation is likely catalyzed by a site-specific DNA recombinase/invertase, reminiscent of the promoter switching phenomenon described for capsule synthesis modulation in Bacteroides fragilis (13).
To demonstrate that the eps locus is responsible for EPS/capsule production, an insertion mutant, designated UCC2003::Bbr_0430, was generated in the monocistronic Bbr_0430 gene, encoding the putative priming glycosyltransferase. In addition, a UCC2003 deletion derivative was fortuitously isolated (designated UCC-EPSdel), which had lost the DNA region between two identical insertion sequence elements (from Bbr_0432/0433 to and including Bbr_0463/0464), which encompassed both eps1 and eps2 (Fig. 1A). Transmission electron microscopy showed that strains UCC2003 and UCC2003-EPSInv produce an outer cell-surface layer, presumed to be a capsule consisting of surface EPS, which is absent in UCC2003-EPSdel and UCC2003::Bbr_0430 (Fig. 1D) (these strains are therefore designated as EPS−). Interestingly, both EPS− strains were found to sediment during growth in liquid medium, but the EPS+ strains remained in suspension (Fig. 1C). Surface EPS isolation and subsequent acid hydrolysis on the presumed EPS− and EPS+ strains showed that no identifiable sugar peaks were present in the chromatograms obtained for the EPS− strains, whereas the chromatogram patterns observed for the EPS+ strains revealed distinct monosaccharide profiles (Fig. 1E). This finding confirms that the two latter strains both produce an EPS surface layer, presumably each having a specific saccharide composition and structure. No detectable monosaccharide peaks were identified when we attempted to isolate EPS from spent growth medium in which B. breve UCC2003 had been cultivated, suggesting that little if any EPS is released from cells during growth in liquid medium.
B. breve UCC2003 Surface EPS Is Linked to Acid and Bile Resistance.
To investigate the potential relationship between UCC2003s ability to produce EPS and tolerance of low pH and bile salt-containing environments, growth profiles of UCC2003 and EPS− derivatives were monitored. When the two EPS− strains were grown at a pH of 5.0 or pH 4.0, they exhibited a significantly reduced (P < 0.001) growth rate and reached a lower end-point OD600nm, compared with their EPS+ counterparts (Fig. 2A). When these strains were grown in 0.3% bovine bile the EPS− strains exhibited significantly (P < 0.01) lower growth rates and lower end-point OD600nm than the EPS+ strain UCC2003 (Fig. 2A). These results show that the EPS layer has a protective effect under low pH and bile conditions.
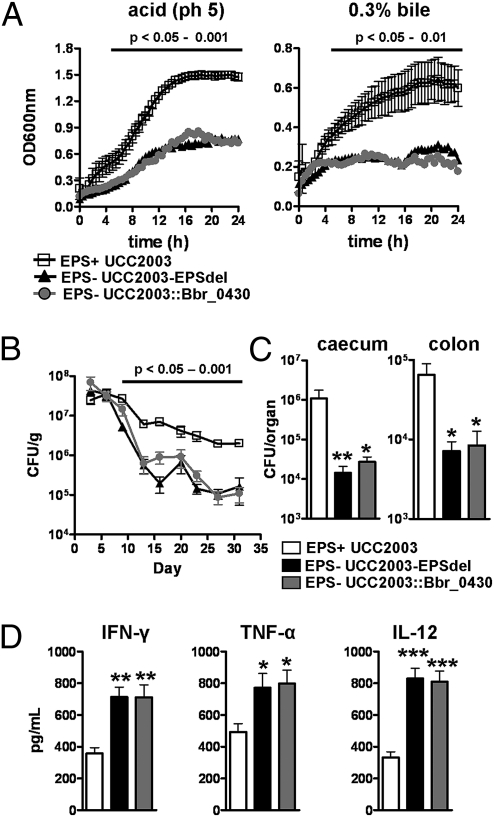
Fig. 2.
B. breve surface EPS protects against acid and bile, facilitates in vivo persistence, and modulates cytokine expression from stimulated splenocytes. (A) Growth curve of UCC2003 (EPS+), UCC2003-EPSdel (EPS−), and UCC2003::Bbr_0430 (EPS−) in de Man-Rogosa-Sharpe (MRS) pH5.0 or in MRS supplemented with 0.3% bile over 24 h at 37 °C. Data represent mean ± SD (B) BALB/c mice were treated orally with ∼1 × 109 UCC2003, UCC2003-EPSdel, or UCC2003::Bbr_0430 on 3 consecutive days and bacterial numbers (CFU) in feces determined (data represent log10 CFU/g feces ± SD). (C) Organs were also removed on day 31 to determine CFU; columns show log10 CFU/organ (± SD). (D) Spleens were harvested from naïve BALB/c mice and stimulated with wild-type (EPS+), deletion, and insertion mutants (EPS−) B. breve strains at 1:1 ratio for ∼20 h. Cells were also stimulated with ConA (dotted line). Columns represent the mean ± SD stimulation indices of splenocytes from 10 mice from two independent experiments. Significance was determined relative to mice treated with UCC2003 (EPS+) at the same time point using the Kruskal–Wallis test followed by Dunn's multiple comparison test; *P < 0.05; **P < 0.01; ***P < 0.001.
B. breve Surface EPS Impacts on Persistence, but Not Initial Colonization, in Mice.
To establish if bifidobacterial EPS production impacts on colonization ability and persistence patterns in BALB/c mice, viable counts of B. breve UCC2003 and various derivatives were determined from fecal samples over a 31-d period following a 3-d treatment period. First, a comparison was made between B. breve UCC2003 and B. breve UCC2003-EPSInv to establish whether expression of different EPS impacts on colonization or persistence of B. breve. No differences were observed between these two EPS+ strains in fecal counts. We next compared EPS+ B. breve UCC2003 with EPS− B. breve strains and noted that from as early as 9 d posttreatment, and up until the end of the study, significantly lower (P < 0.05–0.001) bacterial numbers were recovered from mice treated with EPS− strains compared with EPS+-treated animals (Fig. 2B). On day 31, the viable counts of the EPS− strains within the murine caeca and colons were also significantly reduced (P < 0.05) compared with those of EPS+ strains (Fig. 2C).
B. breve Surface EPS Modulates Cytokine Levels in Stimulated Naïve Splenocytes.
To determine if B. breve EPS expression has an effect on host immune responses, we isolated splenocytes from naïve mice and stimulated them with isogenic EPS+ or EPS− B. breve strains (Fig. 2D). All B. breve strains induced higher cytokine levels compared with unstimulated negative controls. However, cells stimulated with EPS+ had significantly (P < 0.01) lower levels of the proinflammatory cytokines IFN-γ, TNF-α, and IL-12 compared with UCC2003-EPSdel and UCC2003::Bbr_0430 (EPS−)-stimulated cells.
Treatment of Mice with EPS+ B. breve Causes Reduced Levels of Proinflammatory Immune Cells Compared with EPS− Strains.
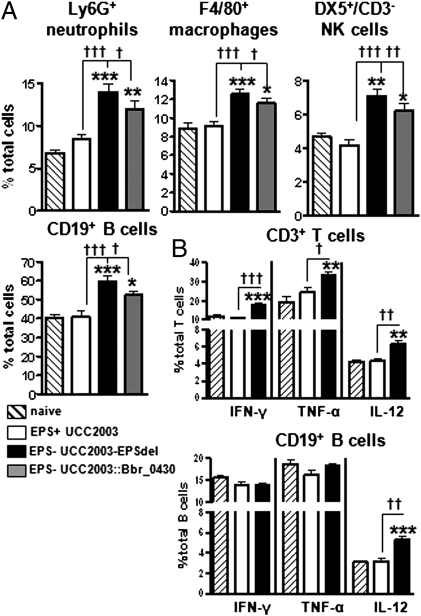
To determine if murine treatment with EPS+ or EPS− B. breve impacts on in vivo immune responses, spleens from untreated (naïve) mice or from mice given three oral doses of UCC2003 (EPS+), UCC2003-EPSdel (EPS−), or UCC2003::Bbr_0430 (EPS−) were examined by flow cytometry. Mice treated with UCC2003 (EPS+) did not exhibit any differences in either percentage or total cell number of any of the immune cells monitored compared with untreated mice. In contrast, mice treated with either of the EPS− strains had significantly increased (P < 0.01) percentages and total cell numbers of Ly6G+ neutrophils, F4/80+ macrophages, DX5+/CD3− NK cells, and CD19+ B cells compared with naïve mice (Fig. 3A and Table S2). Mice treated with EPS− B. breve strains also had increased percentages and total immune cell numbers compared with EPS+-treated mice. Analysis of intracellular cytokine expression (Fig. 3B and Table S3) revealed that although the numbers of T cells did not change, their cytokine profile was significantly altered. Concentrating on the cytokines that were altered in the stimulated naïve splenocyte studies (Fig. 2D), we observed that the percentage of IL-12, IFN-γ, and TNF-α–positive T cells, was significantly higher (P < 0.01) in mice treated with a EPS− B. breve strain compared with both naïve or EPS+-treated mice. Analysis of B-cell populations revealed significant increases (P < 0.01) in the IL-12–producing subpopulation in EPS−-treated mice compared with naïve and UCC2003 (EPS+)-treated mice. Total cell-number examination revealed more IFN-γ– and TNF-α–producing B cells in mice treated with the EPS− B. breve strain compared with either naïve or UCC2003-treated mice. Innate populations were also examined and demonstrated significantly lower (P < 0.05) proinflammatory cytokine profiles in EPS+ compared with EPS− B. breve-treated mice (Fig. S1).
Fig. 3.
B. breve surface EPS modulates recruitment and cytokine profile of immune cell populations in mice. (A) Cells were isolated from spleens of BALB/c mice 31 d after initial treatment, stained with fluorochrome-labeled mAb, and analyzed by flow cytometry. Columns represent the mean percentage ± SD of at least eight mice from two independent experiments. (B) Isolated cells were stimulated for 6 h with BD Leukocyte Activation Mixture plus GolgiPlug in vitro, stained with surface mAb to determine CD3+ and CD19+ populations, and then permeabilized and stained with anticytokine fluorochrome-labeled mAb. Data represent percent of cytokine-positive cells out of total specific cell population ± SD. *P < 0.05, **P < 0.01, and ***P < 0.001 between naïve and B. breve-treated mice; †P < 0.05; ††P < 0.01, and †††P < 0.001 between EPS+ and EPS− using one-way ANOVA followed by Bonferroni's multiple comparison test.
Absence of Surface EPS on B. breve UCC2003 Alters Its Antibody Responses.
Mice treated with EPS− B. breve strains had significantly higher (P < 0.01) total numbers of CD134+/CD19low/B220low plasma B cells compared with animals treated with the EPS+ strain or naïve mice on day 31 (Fig. 4A). Analysis of serum antibody titers revealed that although mice treated with EPS+ B. breve did mount a modest antigen-specific total Ig response, a much higher response was observed in mice treated with the EPS− strain (Fig. 4B). This result was also the case for the serum antibody subtypes IgG3, IgG1, and IgG2a. Mice treated with the B. breve EPS+ strain had no detectable serum IgG3 titers above preimmune levels. Although overall fecal IgA titers were low, we again observed a significantly higher (P < 0.01) level in mice treated with the EPS− B. breve strain compared with those treated with UCC2003.
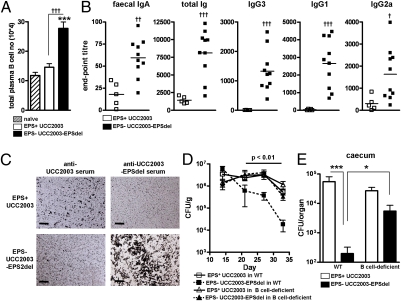
Fig. 4.
B. breve EPS influences B-cell responses. (A) Cells were isolated from spleens of B. breve-treated mice on day 31 and data represent total cell number of CD19low/B220low/CD138high plasma cells. Columns represent means ± SD. Significant differences were determined by the one-way ANOVA followed by Bonferroni's multiple comparison test. ***P < 0.001. (B) Antibody titers were measured following treatment of BALB/c mice with UCC2003 (EPS+) and UCC2003-EPSdel (EPS−). Graphs show titers from individual serum and fecal samples (minus naïve titers) collected on day 31. Bars represent means from at least eight individual mice from two independent experiments. (C) Visualization of slide agglutination performed with the indicated antiserum and cultures of B. breve. Slides were counterstained with India ink and agglutination visualized by light microscopy (Original magnification, 20×). (Scale bars, 200 μm.) (D) B-cell–deficient mice and C57BL/6 controls were treated orally with ∼1 × 109 EPS+ or EPS− B. breve strains on 3 consecutive days and fecal CFU determined. (E) On day 14, organ counts were also determined. Data represent log10 CFU (± SD) from eight individual mice. Significant differences determined by the Kruskal–Wallis test followed by Dunn's multiple comparison test. *P < 0.05; ***P < 0.001.
We next wanted to determine if the presence of surface EPS would impact on agglutination with serum raised in mice treated with either EPS+ or EPS− B. breve strains. From the images presented in Fig. 4C, B. breve UCC2003 (EPS+) was only weakly agglutinated with anti-UCC2003 serum, but not anti-EPS− serum, but the EPS− B. breve strain was shown to be strongly agglutinated with serum obtained from EPS−-treated mice, but not with anti-UCC2003 serum. Thus, it appears that EPS elicits only weak antibody responses, as well as potentially masking other B. breve UCC2003 surface antigens from exposure to antibody responses.
Efficient Colonization of B. breve Requires Evasion of B-Cell Responses.
Given the apparent role of surface EPS in controlling host immune responses, we wanted to define the key adaptive host effector mechanism that is modulated by EPS. We focused on the role of B cells, because mice treated with EPS+ compared with EPS− B. breve had reduced B-cell numbers together with diminished cytokine and antibody profiles (Figs. 3 and 4). Treatment of B-cell–deficient mice (14) with UCC2003 (EPS+) led to similar bacterial numbers in the feces throughout the study compared with WT C57BL/6 controls. (Fig. 4D). However, B-cell–deficient mice failed to reduce the number of EPS− B. breve several weeks following colonization, which was observed in B-cell–proficient mice (Fig. 4D). We also observed this profile within the caeca at the end of the study (Fig. 4E). Colonization and immune responses in control C57BL/6 mice after treatment with isogenic EPS+ and EPS− B. breve strains were similar to those observed in BALB/c animals. Taken together, these data indicate that B. breve surface EPS modulates B cells and is critical for long-term colonization.
Presence of Surface EPS on B. breve Directly Impacts on the Colonization and Persistence of the Gut Pathogen Citrobacter rodentium.
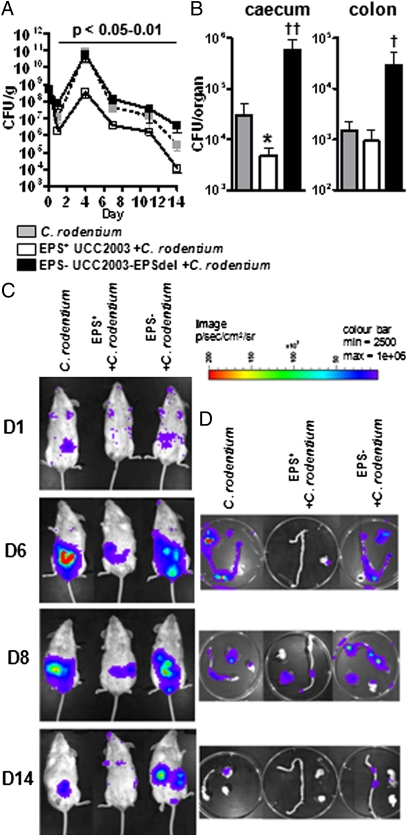
To investigate if surface EPS impacts on host protection against an invading pathogen, mice were treated with B. breve EPS+ or EPS− strains before oral gavage of bioluminescent C. rodentium (15). As early as 24 h postinfection, we observed significantly lower (P < 0.05) C. rodentium fecal counts from mice treated with EPS+ B. breve compared with EPS− B. breve-treated mice, which continued throughout the study up until day 14 (Fig. 5A). By day 3, and up until the study end, we observed that EPS+ B. breve-treated mice had reduced numbers of C. rodentium compared with C. rodentium-infected mice not treated with B. breve. Whole-body bioluminescent data confirmed these observations (Fig. 5C). When organs were excised and imaged on days 6 and 8, we observed that mice treated with EPS− B. breve and infected with C. rodentium, as well as untreated mice, showed strong luminescent signals from both the cecum and colon, but infected mice treated with EPS+ UCC2003 showed either undetectable or lower bioluminescence. At the end of the study (day 14), mice treated with EPS− B. breve had detectable signals in both organs, with untreated mice no longer having any signal from the colon. Importantly, mice treated with EPS+ B. breve UCC2003 and infected with C. rodentium no longer had detectable luminescence in any of the organs (Fig. 5D), as also confirmed by viable count determination (Fig. 5B). Throughout the trial the colonization levels of the B. breve strains were similar to those described in our animal studies described above.
Fig. 5.
Presence of surface EPS-producing B. breve impacts on the colonization and persistence of the gut pathogen C. rodentium. BALB/c mice were treated with ∼1 × 109 EPS+ or EPS− B. breve strains on 3 consecutive days, before oral infection with ∼5 × 108 C. rodentium. (A) Fecal CFU were determined every 3–4 d and data represent log10 CFU/g feces (± SD). (B) Organs were also removed on day 14 to determine CFU. Significance was determined relative to mice infected with C. rodentium alone or mice treated with EPS− B. breve and infected with C. rodentium at the same time point using the Kruskal–Wallis test followed by Dunn's multiple comparison test. n = 8 mice per group. *P < 0.05; †P < 0.05; ††P < 0.001. (C) Images were acquired with the IVIS50 camera and are displayed as pseudocolor images, with variations in color representing light intensity at a given location. Red represents the most intense light emission, and blue corresponds to the weakest signal. For each time point three mice were imaged (whole body) and (D) the open colon and cecum of one representative mouse is shown.
Discussion
There are numerous pathogenic roles described for EPS-producing bacteria. However, the precise biological role of EPS produced by commensal bacteria, such as bifidobacteria, has yet to be determined. In this study, we characterized EPS production and localization of UCC2003, and provide evidence that B. breve surface EPS plays a key role in in vivo persistence, evasion of immune responses, and is involved in reducing infection levels of a gut pathogen.
The genetic components responsible for surface EPS biosynthesis in B. breve UCC2003 are represented by two adjacent, but oppositely oriented operons, eps1 and eps2, predicted to direct the production of structurally different polysaccharides. The intergenic region between eps1 and eps2 contains a single promoter that only transcribes eps1 in B. breve UCC2003, although it can invert its orientation via a mechanism that probably involves site-specific DNA inversion, thus allowing transcription of eps2. Interestingly, the gene predicted to encode the priming glycosyl transferase, which is responsible for the first step in oligosaccharide subunit formation (16), is contained within a separate, monocistronic transcriptional unit, indicating that biosynthesis of these two surface EPS molecules employs a shared initiation step.
Production of surface EPS by B. breve UCC2003 was shown to provide tolerance to the adverse environmental conditions that are found in the gastrointestinal tract of its human host. From these findings we hypothesize that the ability to resist environmental stresses may correlate with colonization efficacy, although this was not apparent from our colonization data. The latter finding suggests that surface EPS is not involved in initial establishment, or at least not at the very high levels at which these B. breve were administered to mice. Examination at later time points following colonization showed that defined isogenic derivatives of B. breve UCC2003, that are unable to produce surface EPS, are 100-fold less abundant compared with the EPS+ UCC2003. The reduction in EPS− B. breve numbers in the murine gut compared with UCC2003 points to a modulating role for surface EPS toward the immune response. In vitro levels of proinflammatory cytokines were highly elevated in EPS− B. breve cocultures, whereas the presence of EPS markedly reduced these cytokine levels. An immune-modulatory role for other bacterial EPS has been proposed previously, also based on observations of the effect of the expression of polysaccharide on cytokine responses in cells in tissue culture and bone marrow-derived macrophages and dendritic cells (17). However, we wanted to determine if this modulatory effect by surface EPS was also apparent in vivo. We concentrated on later time points, as levels of EPS− B. breve only begin to fall significantly, in relation to EPS+ UCC2003, after 1 wk, suggesting the adaptive response is most influenced by surface EPS. Indeed, we observed impairment in immune cell trafficking of B cells, in addition to a number of innate cellular populations, when comparing EPS+ and EPS− treated mice. IL-12 is a known stimulator of T cells in the production of both IFN-γ and TNF-α, both of which are critical chemo-attractants for immune populations. Notably, we found that IFN-γ–, TNF-α–, and IL-12–positive T cells, and IL-12–positive B cells were significantly attenuated in mice treated with B. breve UCC2003 (EPS+) compared with EPS− B. breve-treated mice.
Because B. breve resides mostly in the intestinal lumen and at the epithelial surface, B-cell responses would be expected to be important for any immune clearance of this bacterium. When we examined B-cell responses, we observed significantly lower numbers of splenic plasma cells and correspondingly observed diminished antibody responses in mice treated with UCC2003 compared with mice treated with EPS− B. breve. Notably, polysaccharide immunogens are typically T-cell–independent B-cell antigens. Previous studies have shown that capsular polysaccharides, through lack of T-cell help, induce only weak antibody responses (18). The polysaccharide structure of surface EPS therefore correlates with the weakly agglutinating anti-UCC2003 antibodies, enabling EPS+ B. breve to evade host expulsion through avoidance of the complement cascade or other activated immune cells. Indeed, IL-12 has been shown to enhance humoral immunity, through both IFN-γ–dependent and independent mechanisms, specifically to T-cell–independent antigens (19). Therefore, an EPS-mediated reduction in these cytokines also correlates with the reduction in overall antibody levels and affinity. It also appears that surface EPS masks other important B. breve UCC2003 surface antigens, which are exposed in its EPS− derivatives, leading to detection and consequent generation of stronger immune responses. Moreover, binding of antibodies to the surface of bacteria may also prevent binding to host cells, and this may additionally explain why we observe the gradual reduction of EPS− levels relative to levels of EPS+ B. breve in similarly treated mice. Using a B-cell–deficient mouse strain, we confirmed that B. breve UCC2003 surface EPS is crucial for persistence within the murine host, through subversion of B-cell responses, similar to that used by many pathogenic bacteria (20). However, as far as we are aware, this study is unique in looking at EPS-dependent modulation of B-cell responses in a commensal bacterium.
Using the murine pathogen C. rodentium as a model for human enteropathogenic Escherichia coli and enterohemorrhagic E. coli (which also occupy the same environmental niche as B. breve) (21), we found that surface EPS expression had a profound effect on initial pathogen colonization, as well as burden. EPS+ and EPS− B. breve had similar colonization levels at early time points. Therefore, the mechanism by which UCC2003 reduces C. rodentium levels does appear to be solely because of total B. breve load. One of the mechanisms by which UCC2003 may “curb” pathogen levels within its host, compared with EPS− derivatives, may be through the ability to form biofilms. Furthermore, the absence of surface EPS may lead to this otherwise immunological silent commensal being detected as a foreign bacterium, and therefore stimulating a strong immune response. This finding may explain the significantly higher levels of C. rodentium observed in EPS− treated mice, compared with C. rodentium-infected mice not treated with B. breve, as coinfection can result in increased pathogen burden and associated pathology (22). More studies are required to properly dissect this protective mechanism; however, this surface EPS-dependent pathogen defense represents an exciting new avenue for probiotic research.
In conclusion, we have comprehensively characterized the biological functions of surface EPS in the human commensal B. breve UCC2003. Our findings are consistent with an EPS-mediated strategy that prevents a commensal bacterium from evoking a strong adaptive immune response within the local environment. Furthermore, surface EPS production by UCC2003 provides protection against infection of a murine pathogen, thus representing a mode of action to elicit positive health benefits.
Materials and Methods
Bacterial Techniques.
For detailed information on bacterial strains, culturing conditions, and analysis techniques used in this study see SI Materials and Methods and Table S4.
Animals and Colonization by B. breve.
Male and female BALB/c mice (6–8 wk old) were purchased from Harlan. Both wild-type and B-cell–deficient B6.129S2-Igh-6tm1Cgn/J C57BL/6 mice, were obtained from the Jackson Laboratory. Animal husbandry and experimental procedures were approved by the University College Cork ethics committee. Groups of mice were orally gavaged with ∼1 × 109 CFU per mouse for 3 consecutive days with B. breve strains. Fresh fecal samples were plated at several time points for 31 d posttreatment. At the end of the studies mice were killed, and small intestine, cecum, and colons were excised and organ homogenates plated.
Flow Cytometry.
Single-cell suspensions were prepared and stained as previously described (23), with monoclonal antibodies laid out in Table S5. For intracellular staining, cells were incubated with BD Leukocyte Activation Mixture with BD GolgiPlug or GolgiPlug alone (BD Biosciences) for 6 h. Samples were acquired on a FACSLSRII and data were analyzed using DIVA software.
Evaluation of Antibody Responses.
Serum samples from mice were obtained at the end of the study (day 31) and analyzed for the presence of total Ig, IgG1, IgG2a, and IgG3, as previously described (23) and in SI Materials and Methods. For agglutination assays, B. breve suspensions were incubated with appropriate serum and visualized with India ink at a magnification of 20× using an Olympus microscope.
C. rodentium Infections and Bioluminescent Imaging of Mice.
Groups of BALB/c mice were treated with the appropriate B. breve strains as described above. A group of untreated mice was also included as a control. C. rodentium was grown and administered to mice. At selected time points postinfection, assessment of bioluminescence from living animals was measured by the use of an IVIS50 system (Xenogen), as previously described (15) and in SI Materials and Methods.
Statistical Analysis.
Experimental results were plotted and analyzed for statistical significance with Prism4 software (GraphPad) using Student t test, one-way ANOVA followed by Bonferroni post hoc correction and ANOVA Kruskal–Wallis test with Dunn's multiple comparison test. A P value of < 0.05 was used as significant in all cases.
Supplementary Material
Acknowledgments
We thank Collette Manly, Breda Kearney, and Therese Uniacke for technical assistance; Aaron Mitchell for his comments; and Pablo Alvarez-Martin for isolating Bifidobacterium breve UCC2003-EPSInv. This study was supported by Science Foundation Ireland Grant 07/CE/B1368, The Wellcome Trust, an Embark Postgraduate scholarship, and Travelling Studentship in the Sciences from the National University of Ireland.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1115621109/-/DCSupplemental.
References
- 1.Turnbaugh PJ, Gordon JI. The core gut microbiome, energy balance and obesity. J Physiol. 2009;587:4153–4158. doi: 10.1113/jphysiol.2009.174136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelly D, Conway S, Aminov R. Commensal gut bacteria: Mechanisms of immune modulation. Trends Immunol. 2005;26:326–333. doi: 10.1016/j.it.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 3.WHO 2001. Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria (World Health Organization, Geneva). www.who.int/entity/foodsafety/publications/fs_management/en/probiotics.pdf. Accessed January 10, 2012.
- 4.Coakley M, et al. Inhibitory effect of conjugated alpha-linolenic acid from Bifidobacteria of intestinal origin on SW480 cancer cells. Lipids. 2009;44:249–256. doi: 10.1007/s11745-008-3269-z. [DOI] [PubMed] [Google Scholar]
- 5.Fukuda S, et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469:543–547. doi: 10.1038/nature09646. [DOI] [PubMed] [Google Scholar]
- 6.Ishikawa H, et al. Randomized controlled trial of the effect of bifidobacteria-fermented milk on ulcerative colitis. J Am Coll Nutr. 2003;22:56–63. doi: 10.1080/07315724.2003.10719276. [DOI] [PubMed] [Google Scholar]
- 7.Salazar N, et al. Exopolysaccharides produced by Bifidobacterium longum IPLA E44 and Bifidobacterium animalis subsp. lactis IPLA R1 modify the composition and metabolic activity of human faecal microbiota in pH-controlled batch cultures. Int J Food Microbiol. 2009;135:260–267. doi: 10.1016/j.ijfoodmicro.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 8.Conover MS, Sloan GP, Love CF, Sukumar N, Deora R. The Bps polysaccharide of Bordetella pertussis promotes colonization and biofilm formation in the nose by functioning as an adhesin. Mol Microbiol. 2010;77:1439–1455. doi: 10.1111/j.1365-2958.2010.07297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu CL, Wang YZ, Jin ML, Yang XQ. Preparation, characterization and immunomodulatory activity of selenium-enriched exopolysaccharide produced by bacterium Enterobacter cloacae Z0206. Bioresour Technol. 2009;100:2095–2097. doi: 10.1016/j.biortech.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 10.Alp G, Aslim B. Relationship between the resistance to bile salts and low pH with exopolysaccharide (EPS) production of Bifidobacterium spp. isolated from infants feces and breast milk. Anaerobe. 2010;16:101–105. doi: 10.1016/j.anaerobe.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Motherway MO, et al. Functional genome analysis of Bifidobacterium breve UCC2003 reveals type IVb tight adherence (Tad) pili as an essential and conserved host-colonization factor. Proc Nat Acad Sci USA. 2011;108:11217–11222. doi: 10.1073/pnas.1105380108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bourgoin F, Pluvinet A, Gintz B, Decaris B, Guédon G. Are horizontal transfers involved in the evolution of the Streptococcus thermophilus exopolysaccharide synthesis loci? Gene. 1999;233:151–161. doi: 10.1016/s0378-1119(99)00144-4. [DOI] [PubMed] [Google Scholar]
- 13.Coyne MJ, Weinacht KG, Krinos CM, Comstock LE. Mpi recombinase globally modulates the surface architecture of a human commensal bacterium. Proc Natl Acad Sci USA. 2003;100:10446–10451. doi: 10.1073/pnas.1832655100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kitamura D, Roes J, Kühn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 15.Wiles S, et al. Organ specificity, colonization and clearance dynamics in vivo following oral challenges with the murine pathogen Citrobacter rodentium. Cell Microbiol. 2004;6:963–972. doi: 10.1111/j.1462-5822.2004.00414.x. [DOI] [PubMed] [Google Scholar]
- 16.van Kranenburg R, Vos HR, van Swam II, Kleerebezem M, de Vos WM. Functional analysis of glycosyltransferase genes from Lactococcus lactis and other Gram-positive cocci: Complementation, expression, and diversity. J Bacteriol. 1999;181:6347–6353. doi: 10.1128/jb.181.20.6347-6353.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu MH, et al. Exopolysaccharide activities from probiotic Bifidobacterium: Immunomodulatory effects (on J774A.1 macrophages) and antimicrobial properties. Int J Food Microbiol. 2010;144:104–110. doi: 10.1016/j.ijfoodmicro.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Weintraub A. Immunology of bacterial polysaccharide antigens. Carbohydr Res. 2003;338:2539–2547. doi: 10.1016/j.carres.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 19.Buchanan RM, Arulanandam BP, Metzger DW. IL-12 enhances antibody responses to T-independent polysaccharide vaccines in the absence of T and NK cells. J Immunol. 1998;161:5525–5533. [PubMed] [Google Scholar]
- 20.Merino S, Tomás JM. Bacterial Capsules and Evasion of Immune Responses. NJ: John Wiley & Sons, Ltd; 2010. [Google Scholar]
- 21.Schauer DB, Falkow S. The eae gene of Citrobacter freundii biotype 4280 is necessary for colonization in transmissible murine colonic hyperplasia. Infect Immun. 1993;61:4654–4661. doi: 10.1128/iai.61.11.4654-4661.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karp CL, Auwaerter PG. Coinfection with HIV and tropical infectious diseases. II. Helminthic, fungal, bacterial, and viral pathogens. Clin Infect Dis. 2007;45:1214–1220. doi: 10.1086/522180. [DOI] [PubMed] [Google Scholar]
- 23.Hall LJ, Clare S, Dougan G. NK cells influence both innate and adaptive immune responses after mucosal immunization with antigen and mucosal adjuvant. J Immunol. 2010;184:4327–4337. doi: 10.4049/jimmunol.0903357. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.