Abstract
Vascular endothelial cells (ECs) are exposed to different flow patterns (i.e., disturbed vs. laminar), and the associated oscillatory shear stress (OSS) or pulsatile shear stress (PSS) lead to differential responses. We investigated the roles of class I and II histone deacetylases (HDAC-1/2/3 and HDAC-5/7, respectively) in regulating NF-E2–related factor-2 (Nrf2) and Krüppel-like factor-2 (KLF2), two transcription factors governing many shear-responsive genes, and the cell cycle in ECs in response to OSS. Application of OSS (0.5 ± 4 dynes/cm2) to cultured ECs sustainably up-regulated class I and II HDACs and their nuclear accumulation, whereas PSS (12 ± 4 dynes/cm2) induced phosphorylation-dependent nuclear export of class II HDACs. En face immunohistochemical examination of rat aortic arch and experimentally stenosed abdominal aorta revealed high HDAC-2/3/5 levels in ECs in areas exposed to disturbed flow. OSS induced the association of HDAC-1/2/3 with Nrf2 and HDAC-3/5/7 with myocyte enhancer factor-2; deacetylation of these factors led to down-regulation of antioxidant gene NAD(P)H quinone oxidoreductase-1 (NQO1) and KLF2. HDAC-1/2/3– and HDAC-3/5/7–specific small interfering RNAs eliminated the OSS-induced down-regulation of NQO1 and KLF2, respectively. OSS up-regulated cyclin A and down-regulated p21CIP1 in ECs and induced their proliferation; these effects were mediated by HDAC-1/2/3. Intraperitoneal administration of the class I-specific HDAC inhibitor valproic acid into bromodeoxyuridine (BrdU)-infused rats inhibited the increased EC uptake of BrdU at poststenotic sites. The OSS-induced HDAC signaling and EC responses are mediated by phosphatidylinositol 3-kinase/Akt. Our findings demonstrate the important roles of different groups of HDACs in regulating the oxidative, inflammatory, and proliferative responses of ECs to disturbed flow with OSS.
Keywords: epigenetics, mechanotransduction, transcriptional regulation
Vascular endothelial cells (ECs) have important homeostatic functions in response to blood flow-induced shear stresses. EC dysfunction is a critical step leading to vascular pathologies, including atherosclerosis, which develops preferentially in arterial branches and curvatures where local flow is disturbed with low and oscillatory shear stress (OSS) (1). Recent evidence indicates that disturbed flow with OSS induces sustained activation of atherogenic genes in ECs to promote their oxidation, inflammation, and proliferation. In contrast, the straight part of the artery, which is exposed to sustained laminar blood flow with pulsatile shear stress (PSS), is generally spared from atherosclerotic lesions, with the associated down-regulation of atherogenic genes and up-regulation of antioxidant, anti-inflammatory, and growth-arrest genes in ECs (1–5). Although there have been numerous studies of the mechanisms involved in modulating EC responses to different flow patterns and shear stresses, there is a lack of information on the role of epigenetic factors in modulating EC gene expression and function in response to these mechanical factors (6).
Histone deacetylases (HDACs), enzymes that remove acetyl groups from ε-N-acetyl lysine amino acids on histones to suppress gene expression in most cases, are important epigenetic factors that also regulate the activation of nonhistone proteins (6, 7). HDACs are classified into four main groups: class I (HDAC-1/2/3 and HDAC-8), class II (HDAC-4/5/6/7 and HDAC-9/10), class III sirtuins (SIRT; i.e., SIRT1-7), and class IV (HDAC-11). Class I and II are considered “classical” HDACs. Exposure of ECs to OSS created by an orbital shaker induces serine/threonine phosphorylation of HDAC-3 to modulate endothelial integrity (8). Laminar shear stress (LSS) at 24 dynes/cm2 induces phosphorylation-dependent nuclear export of HDAC-5 and expression of Krüppel-like factor 2 (KLF2) (9), an important transcription factor that has anti-inflammatory and atheroprotective activity (10). Recent studies indicate that KLF2 and NF-E2–related factor 2 (Nrf2), a transcription factor that can bind to the antioxidant response element (ARE) in the promoter region of many antioxidant genes, including NAD(P)H quinone oxidoreductase-1 (NQO1), to induce their expression (11, 12), govern ∼70% of the shear stress-elicited gene sets in ECs (13). Whether different flow patterns and shear stresses play different roles in modulating the expression and activation of HDACs in ECs to regulate their gene expression and function remains unclear.
In the present study, we investigated the different roles of OSS and PSS in modulating the expression and activation of HDACs in ECs and the consequent modulation in EC gene expression and function. We found that OSS induces the expression of both class I (HDAC-1/2/3) and class II (HDAC-5/7) HDACs and their nuclear accumulation in ECs. In contrast, PSS induced phosphorylation-dependent nuclear export of class II HDACs. These in vitro results were confirmed by en face and immunohistochemical studies of aortic arch and experimentally stenosed abdominal aorta in rats. OSS induced the association of HDAC-1/2/3 with Nrf2 and the association of HDAC-3/5/7 with myocyte enhancer factor-2 (MEF2), leading to down-regulation of NQO1 and KLF2 in ECs. Moreover, our in vitro and in vivo studies showed that HDAC-1/2/3 are involved in OSS-induced EC cell cycle progression and proliferation. Our findings have elucidated the roles of different classes of HDACs in modulating EC responses to different flow patterns and shear stresses, and implicate HDAC-3 as a common epigenetic factor that may concurrently modulate endothelial oxidative, inflammatory, and proliferative responses to disturbed flow with OSS.
Results
OSS and PSS Play Different Roles in Modulating Expression and Activation of Class I and II HDACs in ECs.
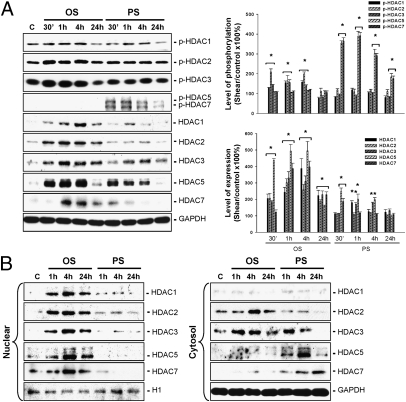
ECs were kept in a static condition as controls or subjected to OSS at 0.5 ± 4 dynes/cm2 or PSS at 12 ± 4 dynes/cm2 for 30 min, 1 h, 4 h, and 24 h, and their expressions and phosphorylations of class I (HDAC-1/2/3) and class II (HDAC-5/7) HDACs were examined by Western blot analysis. OSS induced transient (4 h) increases in the phosphorylation of HDAC-1/2/3, but not of HDAC-5/7, in ECs compared with static control cells (Fig. 1A). In contrast, PSS induced sustained (24 h) increases in phosphorylation of HDAC-5/7, but not of HDAC-1/2/3, in ECs. In addition, OSS induced sustained increases in the expression of all HDAC proteins evaluated, whereas PSS can induce only transient increases in expression of these HDAC proteins. Cellular fractionation assays, involving separation of the cytoplasm and nuclear fractions of cell lysates derived from sheared ECs, demonstrated that OSS induced sustained increases in the protein levels of all examined HDACs in EC nuclei and HDAC-2/3 in EC cytoplasm (Fig. 1B). In contrast, PSS induced a sustained decrease in HDAC-5/7 protein levels in the EC nuclei and a sustained increase in the EC cytoplasm, as well as a transient increase in HDAC-3 expression in the cytoplasm. These results indicate that OSS and PSS play different roles in modulating class I and II HDAC expression and activation, with OSS causing sustained accumulation of all of these HDACs in EC nuclei and PSS causing a sustained translocation of class II HDACs from the nuclei to the cytoplasm.
Fig. 1.
OSS and PSS play differential roles in modulating expression and activation of class I and II HDACs in ECs. (A) ECs were kept under static condition as controls (C) or subjected to OSS at 0.5 ± 4 dynes/cm2 or PSS at 12 ± 4 dynes/cm2 for 30 min (30’), 1 h, 4 h, and 24 h, and their expression and phosphorylation of HDAC-1/2/3/5/7 were determined by Western blot analysis. (B) The subcellular localization of these HDACs was determined by cell fractionation assay. Data in A are mean ± SEM from three independent experiments and are presented as percent change in band density from control cells normalized to internal protein levels. *P < 0.05 vs. static control cells. Results in B are representative of three experiments with similar results.
Induction of HDAC-2/3/5 in Areas of Disturbed Flow in Vivo.
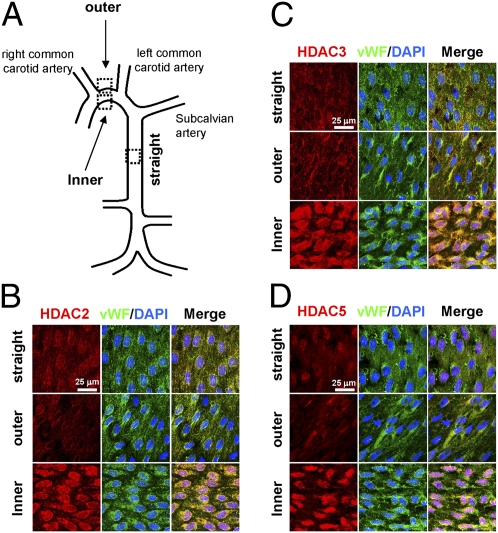
To investigate whether the flow pattern-specific regulation of HDACs found in cultured ECs in vitro also exists in the native circulation in vivo, we examined the aortic arch and the straight segment of thoracic aorta of normal rats (Fig. 2A) by en face coimmunostaining for HDAC-2/3/5 and von Willebrand factor (vWF), with DAPI nuclear counterstaining. High levels of HDAC-2 (Fig. 2B), -3 (Fig. 2C), and -5 (Fig. 2D) were present in ECs in the inner curvature of the aortic arch, where disturbed flow is more likely to occur with relatively low shear stress (1), but not in the outer curvature and the straight segment of thoracic aorta, where the shear stress is high and more laminar (1). The increased HDAC-2/3 expression in the inner curvature of aortic arch was localized in both EC nuclei and EC cytoplasm, whereas the distribution of HDAC-5 was localized mainly in EC nuclei. To further assess the effect of a change from laminar to disturbed flow pattern on these HDAC expressions in vivo, we used a stenosis model in which the rat abdominal aorta was subjected to partial constriction using a U-clip, which produced a 65% constriction in diameter (Fig. S1). Immunohistochemical examination of serial sections along the longitudinal axis of the constricted aortas (n = 5) 3 d later revealed much greater expression of HDAC-2/3/5 in the luminal EC layer at poststenotic sites, where disturbed flow with OSS occurs (1), than upstream and at the midpoint of constriction, where laminar flow occurs with relatively high shear stress. These in vivo results confirm the disturbed flow-specific induction of HDAC-2/3/5 in ECs in the native circulation and at poststenotic sites of the experimentally stenosed aorta.
Fig. 2.
Induction of HDAC-2/3/5 in ECs in the disturbed flow area in the native circulation in vivo. The inner and outer curvatures of the aortic arch and the straight segment of thoracic aorta (A) of normal rats (n = 5) were examined by en face coimmunostaining for HDAC-2 (B), -3 (C), and -5 (D) and vWF using the respective primary andibody, followed by the incubation with fluorescent secondary antibodies, as described in Materials and Methods. Cell nuclei were counterstained with DAPI. Samples were examined and photographed with a Leica TCS SP5 confocal laser scanning microscope with a 60× objective (HCX PLAPO; NA = 1.25, oil immersion). Results are representative of five independent experiments with similar results.
OSS Induces Association of Nrf2 with HDAC-1/2/3 and Its Deacetylation, with Consequential Inhibition of in Vivo Nrf2 Binding to ARE in the Promoter Region of NQO1 and NQO1 Expression.
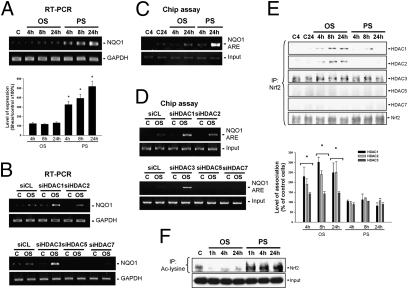
To investigate whether HDACs can modulate Nrf2-ARE binding activity and hence NQO1 expression in ECs in response to OSS, ECs were kept in a static condition as controls or subjected to either OSS or PSS for 4 h, 8 h, and 24 h. RT-PCR analysis showed that PSS, but not OSS, induced sustained (24 h) increases in NQO1 expression in ECs (Fig. 3A). Transfecting ECs with specific small interfering RNAs (siRNAs) of HDAC-1/2/3, but not of HDAC-5/7, induced their NQO1 expression under OSS (Fig. 3B), indicating that knockdown of HDAC-1/2/3, but not of HDAC-5/7, can lead to the inducibility of EC NQO1 in response to OSS. Chromatin immunoprecipitation (ChIP) assays using an antibody against Nrf2 and the NQO1 ARE-specific primers demonstrated that exposure of ECs to PSS, but not to OSS, for 24 h induced the in vivo Nrf2 binding to the NQO1 ARE in ECs (Fig. 3C). The OSS was able to induce in vivo Nrf2-ARE binding activity after transfection of ECs with HDAC-1/2/3–specific siRNAs, but not with HDAC-5/7–specific siRNAs (Fig. 3D). Coimmunoprecipitation assays using an antibody against Nrf2, followed by Western blot analysis with antibodies against different HDACs, demonstrated that OSS, but not PSS, induced sustained increases in the association of Nrf2 with HDAC-1/2/3, but not with HDAC-5/7, in ECs (Fig. 3E). This OSS-induced HDAC–Nrf2 association resulted in deacetylation of Nrf2, whereas PSS induced sustained increases in the levels of acetylated Nrf2 in ECs (Fig. 3F). These results indicate that OSS induces a sustained association of Nrf2 with class I HDACs, which can deacetylase Nrf2 to inhibit its in vivo binding to the NQO1 ARE and thus NQO1 expression in ECs.
Fig. 3.
OSS inhibits in vivo Nrf2 binding to the NQO1 ARE to regulate NQO1 gene expression via enhancement of the HDAC-1/2/3–Nrf2 association in ECs. ECs were kept under static condition as controls (C) or subjected to OSS or PSS for 4 h, 8 h, and 24 h. The expression of NQO1 gene was determined by RT-PCR (A), and the in vivo Nrf2-NQO1 ARE binding activity was analyzed by ChIP assay (C). ECs were transfected with control siRNA (siCL) or HDAC-specific siRNA (siHDAC) (40 nM each) for 48 h before exposure to flow (B and D). In some experiments, the cell lysates were immunoprecipitated with an antibody against Nrf2 (E) or acetylated lysine (F), followed by immunoblotting with antibodies against different HDACs and Nrf2. Data in A and E are mean ± SEM from three independent experiments and are presented as percent changes in band density from control cells normalized to internal protein levels. *P < 0.05 vs. static control cells. Results in B, C, D, and F are representative of three experiments with similar results.
OSS Induces Association of MEF2 with HDAC-3/5/7 and Its Deacetylation to Down-Regulate KLF2, with the Consequential Up-Regulation of Vascular Cell Adhesion Molecule-1 in ECs.
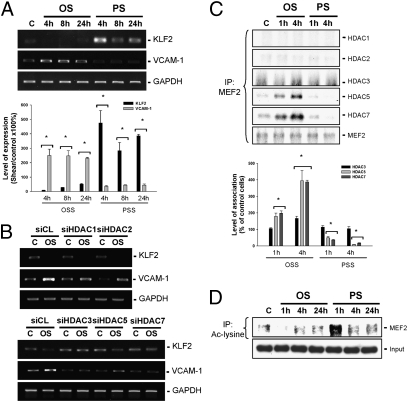
KLF-2 has been shown to negatively regulate EC vascular cell adhesion molecule-1 (VCAM-1) expression to exert anti-inflammatory and atheroprotective effects (10). We investigated whether HDACs can modulate KLF2 and VCAM-1 expression in ECs in response to OSS. OSS induced a sustained (24 h) decrease in KLF2 expression and an increase in VCAM-1 expression in ECs, whereas PSS induced opposite changes in these EC gene expressions (Fig. 4A). Transfecting ECs with HDAC-3/5/7–specific siRNAs, but not with HDAC-1/2–specific siRNAs, resulted in inhibitions of the OSS-mediated down-regulation of KLF2 and up-regulation of VCAM-1 (Fig. 4B). Application of OSS to ECs for 1 h or 4 h induced an association of HDAC-3/5/7, but not of HDAC-1/2, with MEF2, a transcription factor that has been shown to bind to the promoter of KLF2 to repress its gene expression (14) in these ECs (Fig. 4C). In contrast, application of PSS to ECs for 4 h resulted in a diminished HDAC-5/7–MEF2 association in these ECs compared with static control cells. Moreover, OSS induced a transient (4 h) decrease, and PSS induced a transient increase, in the level of acetylated MEF2 in ECs (Fig. 4D). These results indicate that OSS and PSS play different roles in regulating the association of HDAC-3/5/7 with MEF2 and its deacetylation, with the consequent modulation in KLF2 expression in ECs.
Fig. 4.
HDAC-3/5/7 are involved in flow pattern-specific changes in KLF2 and VCAM-1 expression in ECs. (A and B) ECs were kept under static condition as controls or subjected to OSS or PSS for 1 h, 4 h, 8 h, and 24 h, and their KLF2 and VCAM-1 expression was determined by RT-PCR. Before the flow experiments, ECs were transfected with control siRNA (siCL) or HDAC-specific siRNA (siHDAC) for 48 h (B). (C and D) The association of MEF2 with different HDACs (C) and the acetylation of MEF2 (D) were examined by coimmunoprecipitation assays. Data in A and C are mean ± SEM from three independent experiments and are presented as percent changes in band density from control cells normalized to internal protein levels. *P < 0.05 vs. static control cells. Results in B and D are representative of three experiments with similar results.
OSS-Induced Changes in Cell Cycle Regulatory Protein Expression in ECs and Their Proliferation Are Mediated by HDAC-1/2/3.
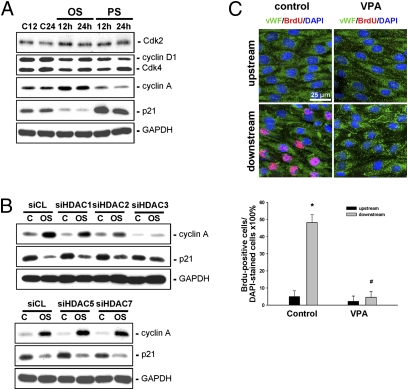
We next investigated the roles of HDACs in modulating cell cycle regulatory protein expression in ECs and their proliferation in response to OSS. Exposure of ECs to OSS for 12 h or 24 h induced up-regulation of cyclin A and down-regulation of p21CIP1, and had no effect on cyclin D1 and Cdk-2 and -4 expression compared with static control cells (Fig. 5A). In contrast, application of PSS to ECs for 12 h or 24 h resulted in down-regulations of cyclins D1 and A and up-regulation of p21CIP1, and had no effect on Cdk-2 and -4 expression in ECs. An incorporation assay of bromodeoxyuridine (BrdU), an analogue of the DNA precursor thymidine that can be incorporated into the DNA of proliferating cells during their synthetic phase, demonstrated that 24 h-shearing of ECs with OSS increases the percentage of BrdU-positive ECs compared with the static cells (Table S1). Transfecting ECs with HDAC-1/2/3–specific siRNAs, but not HDAC-5/7–specific siRNAs, inhibited the OSS-induced up-regulation of cyclin A and down-regulation of p21CIP1 (Fig. 5B), as well as BrdU uptake (Table S1), in ECs.
Fig. 5.
HDAC-1/2/3 are involved in OSS-induced EC proliferation in vitro and in vivo. ECs were kept under static condition as controls or subjected to OSS or PSS for 12 h or 24 h, and their expression of different cell cycle regulatory proteins was determined by Western blot analysis (A). Before exposure to flow, ECs were transfected with control siRNA (siCL) or HDAC-specific siRNA (siHDAC) for 24 h (B). (C) Class I-specific HDAC inhibitor VPA or control saline was injected i.p. into rats (n = 5 each) that had been subjected to stenosis produced using a U-clip. BrdU was injected i.v. into rats 1 d before sacrifice at 2 wk after surgery. En face staining was done on the luminal surfaces of the affected aortas with antibodies against vWF and BrdU, with DAPI counterstaining. Results in A and B are representative of three experiments with similar results. Images shown in C are representative of four rats with similar results. Flow direction is from left to right. Data in C are mean ± SEM of 80–100 cells from four independent experiments for each area.
To further explore the role of class I HDACs in EC proliferation in response to OSS in vivo, the class I-specific HDAC inhibitor valproic acid (VPA) was injected i.p. into the rats for 2 wk (n = 5), followed by i.v. injection of BrdU 24 h before sacrifice. Control rats were injected with saline (n = 5). En face staining of the affected aortas in rats receiving saline injection with anti-BrdU antibody revealed that ∼50% of ECs displayed BrdU uptake at poststenotic sites (Fig. 5C). In contrast, virtually no BrdU uptake was detected in ECs in the area upstream of the constriction. This increased BrdU uptake in ECs at poststenotic sites was inhibited by VPA injection, confirming the inhibitory role of class I HDACs in OSS-induced EC proliferation in vivo.
Phosphatidylinositol 3-Kinase/Akt Regulates NQO1 and KLF2 Expression in ECs and Their Proliferation Through Its Regulation of HDAC Expression and Activity in Response to OSS.
To investigate whether phosphatidylinositol 3-kinase (PI3K)/Akt is involved in OSS regulation of the examined HDACs in ECs, ECs were pretreated with specific PI3K inhibitor LY294002 (10 μM) or vehicle control for 1 h, then kept under static condition or subjected to OSS for 1 h. The OSS-induced expression of HDAC-1/2/3/7 and phosphorylation of HDAC-1/2/3 were inhibited by pretreatment of ECs with LY294002 (Fig. S2A). LY294002 pretreatment did not inhibit OSS-induced HDAC-5 expression, however. Coimmunoprecipitation assays demonstrated that LY294002 pretreatment inhibited the OSS-induced association of HDAC-1/2/3 with Nrf2 and its deacetylation in ECs (Fig. S2B). This LY294002-inhibition of an OSS-induced HDAC–Nrf2 association and Nrf2 deacetylation led to the inducibility of NQO1 in ECs in response to OSS (Fig. S2D). As expected, the OSS-induced association of HDAC-3/5/7 with MEF2 and its deacetylation also was inhibited by pretreating ECs with LY294002 (Fig. S2C), which led to the OSS induction of KLF2 and inhibition of VCAM-1 expression in ECs (Fig. S2D). Moreover, pretreatment of ECs with LY294002 inhibited the OSS-induced increases in the percentage of BrdU-positive ECs compared with the vehicle control-treated cells (Table S1). These results indicate that PI3K/Akt regulates HDAC expression and activation and thus NQO1 and KLF2 expression in ECs and their proliferation in response to OSS.
Discussion
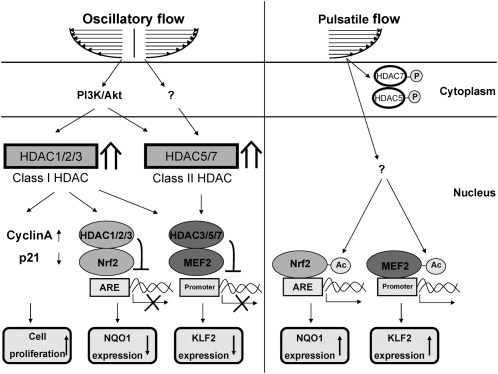
Our present study has identified the mechanisms by which HDACs serve as important mechanosensitive molecules to modulate vascular endothelial oxidative, inflammatory, and proliferative responses to disturbed flow with OSS (summarized in Fig. 6). Several lines of evidence support this conclusion. First, OSS induces sustained expression and nuclear accumulation of class I HDAC-1/2/3 and class II HDAC-5/7 in ECs, whereas PSS induces phosphorylation-dependent nuclear export of HDAC-5/7 in ECs. The OSS induction of EC HDAC-2/3/5 in vitro was confirmed by en face and immunohistochemical studies on rat aortic arch and experimentally stenosed abdominal aorta in vivo. Second, OSS induces sustained associations of HDAC-1/2/3 with Nrf2, which deacetylate Nrf2 and inhibit its binding to the NQO1 ARE and thus NQO1 expression in ECs. Third, OSS and PSS play different roles in modulating EC KLF2 expression through their differential regulation in HDAC-3/5/7–MEF2 associations and MEF2 deacetylation. These responses are accompanied by the corresponding regulation of VCAM-1. Fourth, HDAC-1/2/3 regulate OSS-induced EC proliferation in vitro and in vivo. Finally, the OSS-induced HDAC signaling and consequent EC responses are mediated by PI3K/Akt. Thus, our findings provide mechanistic insight into the roles of different classes of HDACs in modulating EC signaling, gene expression, and function in response to different flow patterns and shear stresses.
Fig. 6.
Schematic diagram of HDAC signaling and its modulation in gene expression and function in ECs in response to OSS and PSS.
HDACs are enzymes that remove Lys acetylation of histone and nonhistone proteins to regulate a variety of biological processes (6, 7). Mounting evidence indicates that HDACs are involved in the control of EC gene expression and function (6, 7); however, their role in modulating EC responses to different flow patterns and shear stresses remains poorly understood. LSS at 24 dynes/cm2 was shown to induce phosphorylation-dependent nuclear export of HDAC5 and its dissociation from MEF2, leading to inductions of KLF2 and endothelial nitric oxide (NO) synthase (eNOS) (9). OSS induces HDAC-3 phosphorylation in ECs to modulate their survival and integrity (8). Our present results indicated that different flow patterns and shear stresses play different roles in modulating EC HDAC signaling, with increased expression and nuclear accumulation of all examined HDACs in ECs in response to OSS. This OSS induction of EC HDACs was confirmed by our in vivo findings of high expression of HDAC-2/3/5 in ECs in the disturbed flow-prone areas, such as the inner curvature of the aortic arch and the poststenotic sites compared with the outer curvature and the straight part of the thoracic aorta, as well as the area upstream of the constriction, where laminar flow with high shear stress occurs. Thus, our findings suggest that OSS can increase the levels of HDACs and their interaction with transcription factors in EC nuclei to suppress transcriptional activity and downstream gene expression, whereas PSS can induce phosphorylation-dependent nuclear export of class II HDACs to decrease their interaction with transcription factors and thus increase their transcriptional activity and downstream gene expression in EC nuclei.
Previous studies have demonstrated that both OSS and LSS can induce nuclear accumulation of Nrf2 in ECs, but only LSS can enhance Nrf2 binding to the NQO1 ARE in EC nuclei (15). This finding suggests the presence of certain mediator(s) that could be induced by OSS to prevent Nrf2 binding to the NQO1 ARE. Our present findings provide several lines of evidence to demonstrate that these mediators may be class I HDACs. First, OSS cannot induce the in vivo Nrf2 binding to the NQO1 ARE and NQO1 expression in ECs. Second, OSS induces nuclear accumulation of HDAC-1/2/3 in ECs. Third, knockdown of HDAC-1/2/3 leads to the induction of Nrf2-ARE binding activity and thus NQO1 expression in ECs in response to OSS. Fourth, only OSS, and not PSS, can induce the association of HDAC-1/2/3 with Nrf2 to cause its deacetylation. Thus, OSS may possibly induce the formation of class I HDAC–Nrf2 heterocomplexes in EC nuclei to deacetylate Nrf2, thereby leading to decreases in Nrf2 ARE-binding activity and NQO1 expression in ECs. This notion is supported by the results of Sun et al. (16), who reported that acetylation of Nrf2 augments its DNA-binding activity during the antioxidant response. Our findings indicate that class I HDACs may modulate OSS-mediated repression of the Nrf2-dependent antioxidant response in ECs through their association with and deacetylation of Nrf2.
An additional transcription factor that is critical for the modulation of shear-responsive genes in ECs is KLF2, which has atheroprotective and anti-inflammatory effects (10, 13). Inhibition of VCAM-1 and leukocyte adhesion by induction of KLF2 in ECs has been reported (10). KLF2 has been shown to be up-regulated in ECs by prolonged LSS in vitro and highly expressed in ECs of atherosclerosis-resistant regions of human aortas in vivo (17). A recent study by Wang et al. (9) demonstrated that LSS can stimulate phosphorylation-dependent nuclear export of HDAC5 to induce KLF2 expression through the dissociation of HDAC-5 from MEF2. Our findings demonstrate that, along with HDAC-5, exposure of ECs to PSS can also stimulate the phosphorylation and nuclear export of HDAC-7 and its dissociation from and acetylation of MEF2 in these ECs. In contrast to PSS, OSS induces the expression and accumulation of HDAC-3/5/7 in EC nuclei and their association with and deacetylation of MEF2, leading to inhibited KLF2 expression. This HDAC-mediated down-regulation of KLF2 in OSS-stimulated ECs is accompanied by the up-regulation of VCAM-1. Thus, OSS and PSS play opposing roles in modulating KLF2 expression and inflammatory responses in ECs by differentially modulating the levels of HDACs and their association with MEF2 in EC nuclei.
Recent evidence suggests that class I HDACs may play important roles in modulating cellular survival (18). Genetic deletion of HDAC-1 in mice is embryonic lethal, and HDAC-2 knockout causes perinatal lethality due to cardiac defects (19, 20). Lentivirus-mediated HDAC-3 silencing results in mice lethality due to disruption of basement and rupture of blood vessels (8). In concert with these previous findings, our present findings demonstrate that HDAC-1/2/3 play important roles in modulating cell cycle progression and proliferation in ECs in response to OSS through their regulation of cyclin A and p21CIP1. Knockdown of HDAC-1/2/3 in ECs resulted in inhibited BrdU uptake in response to OSS. This inhibitory role of class I HDACs in OSS-induced EC proliferation was confirmed by i.p. injection with the class I-specific HDAC inhibitor VPA into BrdU-infused rats, which demonstrated decreased BrdU uptake in ECs at poststenotic sites. Our findings suggest that class I HDACs may serve as important molecular targets for therapeutic interventions against EC proliferation-associated vascular disorders.
Several molecular signals have been found to modulate HDAC activity in ECs in response to shear stress. LSS at 10 dynes/cm2 induces NO-mediated nuclear shuttling of EC HDAC-4/5, which requires the association of protein phosphatase 2A with the phosphorylated calcium-calmodulin–dependent kinase-IV/HDAC complex (21). LSS at 24 dynes/cm2 also stimulates HDAC-5 phosphorylation through a calcium-calmodulin–dependent pathway and induces HDAC-5 nuclear export by creating binding sites for the 14–3-3 chaperon protein (9). Chen et al. (22) demonstrated that LSS at 12 dynes/cm2 strengthens the association of SIRT1 with eNOS to enhance NO production in ECs, which requires AMP-activated protein kinase phosphorylation of eNOS. Moreover, Zeng et al. (23) demonstrated that LSS at 12 dynes/cm2 modulates the differentiation of embryonic stem cell-derived progenitor cells into ECs through the PI3K-Akt-HDAC3-p53 pathway. Zampetaki et al. (8) recently demonstrated that Akt can associate with HDAC-3 to regulate EC survival in response to disturbed flow with OSS. These results suggest that ECs may use the same signaling pathway (e.g., PI3K/Akt) for responses to different shear stresses. In concert with the study of Zampetaki et al. (8), our present findings demonstrate that PI3K/Akt can regulate the signaling of all examined HDACs and their consequent modulation of gene expression and function in ECs in response to OSS. Although pretreating ECs with the specific PI3K inhibitor LY294002 cannot inhibit OSS-induced HDAC-5 expression, it does inhibit OSS-induced association of HDAC-5 with MEF2 and its deacetylation, with a consequent increase in the expression of KLF2 in ECs. The precise mechanisms by which PI3K/Akt modulates HDAC signaling in ECs and the consequent modulations of EC responses to OSS warrant further investigation.
In summary, the present study demonstrates that different flow patterns and shear stresses play different roles in modulating the expression and activation of class I and II HDACs in ECs, with increased expression and accumulation of these HDACs in EC nuclei in response to OSS. HDAC-3 may serve as a common epigenetic factor to modulate signaling and gene expression involved in EC oxidation, inflammation, and proliferation in response to OSS, and thus may be a promising molecular target for therapeutic intervention against vascular disorders associated with EC dysfunction, such as atherosclerosis.
Materials and Methods
Cell Cultures.
Human umbilical vein ECs were isolated from fresh human umbilical cords using the collagenase perfusion technique. Cell pellets were resuspended in a culture medium consisting of Medium 199 supplemented with 20% (vol/vol) FBS and 1% (vol/vol) penicillin/streptomycin (all from Gibco). Details of the procedure are provided in SI Materials and Methods.
Flow Apparatus.
The cultured ECs were subjected to PSS at 12 ± 4 dynes/cm2 or to OSS at 0.5 ± 4 dynes/cm2 in a parallel-plate flow chamber. Detailed information on the flow apparatus is provided in SI Materials and Methods.
RNA Isolation and RT-PCR.
The following primer sequences were used in this study: NQO1: sense, 5′-CCTGTAGCTGAAGGTTTGCTGG-3′; antisense, 5′-CCTACCTGTGATGTCCTTTCTGG-3′; KLF2: sense, 5′-CCTCCCAAACTGTGACTGGT-3′; antisense, 5′-ACTCGTCAAGGAGGATCGTG-3′; GAPDH sense, 5′-CAACTACATGGTTTACATGTTCC-3′; antisense, 5′-GGACTGTGGTCATGAGTCCT-3′. The RNA isolation and RT-PCR procedures are described in detail in SI Materials and Methods.
ChIP Assay.
ChIP assays were performed with the EZ ChIP Assay Kit (Upstate Biologicals) in accordance with the manufacturer's protocol. Primer sequences for human NQO1 ARE were designed as sense, 5′-AAGTGTGTTGTATGGGCCCC-3′; antisense, 5′-TCGTCCCAAGAGAGTCCAGG-3′. Details are provided in SI Materials and Methods.
Animals.
Twenty adult male (SD) IGS rats (Biolasco) weighing 400–450 g were used for the aortic stenosis experiments, with 5 rats in the control group and 15 rats in the stenosis group. For the 15 rats in the stenosis group, U-shaped titanium clips (Ethicon) were surgically applied to the abdominal aortas to create local stenosis, in which 10 sham control rats received normal saline by i.p. injection, 5 rats were injected i.p. with the class I-specific HDAC inhibitor VPA. All animal experiments were performed and maintained in accordance with National Institutes of Health guidelines and with the approval of the Animal Research Committee of National Health Research Institutes.
Animal Model of Aortic Stenosis.
Stenosis of the rat abdominal aorta was produced using a U-shaped titanium clip, as described previously (24). In brief, after anesthetization with isoflurane, the rat abdominal aorta (segment between the renal artery and iliac artery) was exposed, and the clip was held with a pair of forceps and placed around the isolated segment (∼1 cm proximal from the iliac arterial bifurcation) to partially constrict the abdominal aorta. The rat was euthanized at 3 d after surgery, and the aorta was perfusion-fixed with 4% paraformaldehyde at 120 mm Hg. The fixed aorta was embedded in paraffin blocks for immunohistochemical studies.
Supplementary Material
Acknowledgments
This work was supported by NSC-99-2321-B-400-002/NSC-100-2325-B-400-011 and NHRI-ME-100-PP06 (to J.-J. C.), HL-106579/HL-104402 (to S.C.), and NSC-99-2911-I-009-101 to the University System of Taiwan-University of California San Diego International Center of Excellence in Advanced Bioengineering (to S.C. and J.-J.C.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1121214109/-/DCSupplemental.
References
- 1.Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: Pathophysiological basis and clinical perspectives. Physiol Rev. 2011;91:327–387. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hahn C, Schwartz MA. Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol. 2009;10:53–62. doi: 10.1038/nrm2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiu JJ, Usami S, Chien S. Vascular endothelial responses to altered shear stress: Pathologic implications for atherosclerosis. Ann Med. 2009;41:19–28. doi: 10.1080/07853890802186921. [DOI] [PubMed] [Google Scholar]
- 4.Traub O, Berk BC. Laminar shear stress: Mechanisms by which endothelial cells transduce an atheroprotective force. Arterioscler Thromb Vasc Biol. 1998;18:677–685. doi: 10.1161/01.atv.18.5.677. [DOI] [PubMed] [Google Scholar]
- 5.Resnick N, Gimbrone MA., Jr Hemodynamic forces are complex regulators of endothelial gene expression. FASEB J. 1995;9:874–882. doi: 10.1096/fasebj.9.10.7615157. [DOI] [PubMed] [Google Scholar]
- 6.Zhou J, Lim SH, Chiu JJ. Epigenetic regulation of vascular endothelial biology/pathobiology and response to fluid shear stress. Cell Mol Bioeng. 2011;4:560–578. [Google Scholar]
- 7.Zhou B, Margariti A, Zeng L, Xu Q. Role of histone deacetylases in vascular cell homeostasis and arteriosclerosis. Cardiovasc Res. 2011;90:413–420. doi: 10.1093/cvr/cvr003. [DOI] [PubMed] [Google Scholar]
- 8.Zampetaki A, et al. Histone deacetylase 3 is critical in endothelial survival and atherosclerosis development in response to disturbed flow. Circulation. 2010;121:132–142. doi: 10.1161/CIRCULATIONAHA.109.890491. [DOI] [PubMed] [Google Scholar]
- 9.Wang W, et al. Fluid shear stress stimulates phosphorylation-dependent nuclear export of HDAC5 and mediates expression of KLF2 and eNOS. Blood. 2010;115:2971–2979. doi: 10.1182/blood-2009-05-224824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.SenBanerjee S, et al. KLF2 Is a novel transcriptional regulator of endothelial proinflammatory activation. J Exp Med. 2004;199:1305–1315. doi: 10.1084/jem.20031132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wasserman WW, Fahl WE. Functional antioxidant responsive elements. Proc Natl Acad Sci USA. 1997;94:5361–5366. doi: 10.1073/pnas.94.10.5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nioi P, McMahon M, Itoh K, Yamamoto M, Hayes JD. Identification of a novel Nrf2-regulated antioxidant response element (ARE) in the mouse NAD(P)H:quinone oxidoreductase 1 gene: Reassessment of the ARE consensus sequence. Biochem J. 2003;374:337–348. doi: 10.1042/BJ20030754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boon RA, Horrevoets AJ. Key transcriptional regulators of the vasoprotective effects of shear stress. Hamostaseologie. 2009;29:39–40, 41–43. [PubMed] [Google Scholar]
- 14.Parmar KM, et al. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J Clin Invest. 2006;116:49–58. doi: 10.1172/JCI24787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hosoya T, et al. Differential responses of the Nrf2-Keap1 system to laminar and oscillatory shear stresses in endothelial cells. J Biol Chem. 2005;280:27244–27250. doi: 10.1074/jbc.M502551200. [DOI] [PubMed] [Google Scholar]
- 16.Sun Z, Chin YE, Zhang DD. Acetylation of Nrf2 by p300/CBP augments promoter-specific DNA binding of Nrf2 during the antioxidant response. Mol Cell Biol. 2009;29:2658–2672. doi: 10.1128/MCB.01639-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dekker RJ, et al. KLF2 provokes a gene expression pattern that establishes functional quiescent differentiation of the endothelium. Blood. 2006;107:4354–4363. doi: 10.1182/blood-2005-08-3465. [DOI] [PubMed] [Google Scholar]
- 18.Walkinshaw DR, Tahmasebi S, Bertos NR, Yang XJ. Histone deacetylases as transducers and targets of nuclear signaling. J Cell Biochem. 2008;104:1541–1552. doi: 10.1002/jcb.21746. [DOI] [PubMed] [Google Scholar]
- 19.Lagger G, et al. Essential function of histone deacetylase 1 in proliferation control and CDK inhibitor repression. EMBO J. 2002;21:2672–2681. doi: 10.1093/emboj/21.11.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montgomery RL, et al. Histone deacetylases 1 and 2 redundantly regulate cardiac morphogenesis, growth, and contractility. Genes Dev. 2007;21:1790–1802. doi: 10.1101/gad.1563807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Illi BC, et al. Nitric oxide modulates chromatin folding in human endothelial cells via protein phosphatase 2A activation and class II histone deacetylases nuclear shuttling. Circ Res. 2008;102:51–58. doi: 10.1161/CIRCRESAHA.107.157305. [DOI] [PubMed] [Google Scholar]
- 22.Chen Z, et al. Shear stress, SIRT1, and vascular homeostasis. Proc Natl Acad Sci USA. 2010;107:10268–10273. doi: 10.1073/pnas.1003833107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeng L, et al. HDAC3 is crucial in shear- and VEGF-induced stem cell differentiation toward endothelial cells. J Cell Biol. 2006;174:1059–1069. doi: 10.1083/jcb.200605113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miao H, et al. Effects of flow patterns on the localization and expression of VE-cadherin at vascular endothelial cell junctions: In vivo and in vitro investigations. J Vasc Res. 2005;42:77–89. doi: 10.1159/000083094. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.