Abstract
Familiarity to the mother and the novelty afforded by the postnatal environment are two contrasting sources of neonatal influence. One hypothesis regarding their relationship is the maternal modulation hypothesis, which predicts that the same neonatal stimulation may have different effects depending on the maternal context. Here we tested this hypothesis using physical development, indexed by body weight, as an endpoint and found that, among offspring of mothers with a high initial swim-stress–induced corticosterone (CORT) response, neonatal novelty exposure induced an enhancement in early growth, and among offspring with mothers of a low initial CORT response, the same neonatal stimulation induced an impairment. At an older age, a novelty-induced increase in body weight was also found among offspring of mothers with high postnatal care reliability and a novelty-induced reduction found among offspring of mothers with low care reliability. These results support a maternal modulation of early stimulation effects on physical development and demonstrate that the maternal influence originates from multiple instead of any singular sources. These results (i) significantly extend the findings of maternal modulation from the domain of cognitive development to the domain of physical development; (ii) offer a unifying explanation for a previously inconsistent literature regarding early stimulation effects on body weight; and (iii) highlight the notion that the early experience effect involves no causal primacy but higher order interactions among the initial triggering events and subsequent events involving a multitude of maternal and nonmaternal influences.
Keywords: maternal stress, maternal care, maternal mediation, developmental plasticity, growth enhancement
It seems self-evident that, for a developing infant, both the maternal and nonmaternal environment affects his or her development. Over the past century, ample evidence from both human and animal early-experience studies has confirmed this intuition (1–3). What remains challenging is how these two environmental sources interact to jointly influence offspring development (4–7). Emerging from the rodent developmental literature are two alternative views regarding the relation between maternal and nonmaternal influences. The “maternal mediation view” assumes that nonmaternal environment exerts its effect indirectly through its effect on the mother, who in turn affects the offspring (e.g., see refs. 8 and 9). When taken to the extreme, this view would directly contradict the stress-activation hypothesis (10, 11), which states that the nonmaternal environment has a direct effect on the offspring by activating some aspects of the offspring's hypothalamic-pituitary-adrenal (HPA) function. In contrast, the “maternal modulation view” incorporates the stress-activation hypothesis and assumes that the nonmaternal environment first activates aspects of the HPA axis, and the mother then modulates this effect (12). According to the latter view, the individual mother sets distinct contexts for the same nonmaternal environment to result in different developmental outcomes.
This maternal modulation hypothesis was originally suggested by Smotherman (12) and has been supported by a differential offspring stress response with and without the presence of their mother (13) and by pups’ development of approach or avoidance, two opposite behavioral tendencies, to the same aversive stimulus, an electric shock, according to whether the mother is present or not (14). More recently, the maternal modulation hypothesis has been tested for long-range and continuous (as opposed binary) modulation across the offspring's life span. Family-to-family variations in both maternal-care reliability and maternal self-stress regulation have been shown to account for variations in the effects of neonatal novelty exposure on adult offspring behavior and HPA function (15–18), pointing out an obvious (when thinking human development) (19), yet easily missed possibility (when thinking animal models), that the maternal context itself is multidimensional, including not only her care-giving behaviors (15, 16) but also the mother's self-stress regulation (17, 18).
This notion of multiple maternal influences beyond maternal- care quantity is compatible with a large body of rodent literature. First, differences in offspring's HPA and behavioral functions can be created by prenatal (20) and postnatal (6, 7) manipulation of the mother's stress-response system, including directly adding corticosterone (CORT) to her drinking water (6) and injection of CORT into key brain structures (14). Second, within maternal care behavior, the average amount and variability of maternal care are two distinct aspects of maternal care, playing distinct roles (15, 16). Such a distinction is also supported by findings from human child development, where it is maternal care sensitivity and consistency (i.e., reliability) but not the average amount of care, that has predictive power for infant development (21, 22), particularly when the behavioral outcomes were observed during times of infant distress (23). These findings urge us to consider an integrative view of early experience on the developing organism as a dynamic system in which multiple sources (maternal and nonmaternal) interact to jointly determine the developmental trajectory, with no one source assuming causal primacy (24). Within such a system, one investigates mechanisms of development not by pinpointing one variable and excluding others, but by revealing how one variable interacts with and provides context for another, together accounting for emerging individual differences (25, 26).
By considering how physical development, particularly early growth, might be jointly programmed by neonatal novelty exposure and maternal context, the present study evaluates the usefulness of this theoretical framework. Body weight is a universally monitored index for infant development (27) and a bigger infant is instinctively viewed as a healthy infant. Growth, like cognitive development, is under multiple sources of influences, including nutrition and prenatal maternal health (28), and the function of the HPA axis (29). Early life experience, in general, is known to affect the development of the HPA axis (7, 30, 31), and neonatal novelty exposure in particular is known to affect circulating CORT concentration (5) and sensitivity of hippocampal synaptic plasticity to CORT modulation (32). Therefore, we hypothesized that neonatal experience of novelty may not only affect cognitive (5, 17) but also physical development. As the mother is known to modulate her offspring's HPA function (13), we further predict that physical development will be influenced by neonatal novelty exposure according to the specific maternal physiological and behavioral context.
Results
We used a split-litter design in which, for 3 min daily during the first 3 wks of life, half of each litter of pups was exposed to a nonhome environment (Novel) and the remaining littermates stayed within the home cage (Home) (Fig. S1). Distinctively different from the neonatal handling procedure (30, 33), our neonatal novelty exposure (NNE) procedure (5) removed the confounding factors in the handling design by matching the amount of experimenter handling, the brief duration of separation from the dam, and the maternal stress associated with the procedure between the Novel and Home rats. The effect of NNE for each given litter is indexed by the novelty effect score [NE-score = (average of Novel offspring) − (average of Home offspring)] (Fig. 1B). The family-specific maternal context is indexed by a measure of maternal stress regulation, the rapid initial rise in the CORT stress response (CORTE) (34), and by two maternal care measures, the quantity (35) and reliability of maternal care (15, 16). Body weights were measured at weaning and adulthood (Fig. S1A and SI Methods).
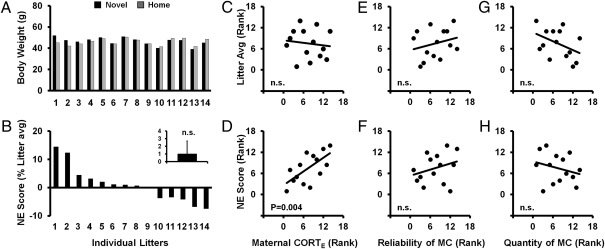
Fig. 1.
Early growth measure/body weight at weaning. (A) Litter-to-litter variations: average body weights of the Novel and Home pups for each of the 14 litters studied. (B) A lack of first-order novelty effect on body weight shown in novelty effect score (NE score) for each litter, defined as the difference between the Novel and Home litter averages shown in A. (C, E, and G) Litter average body weight showing a lack of first-order maternal effect. (D, F, and H) Litter NE score showing second-order interaction effect between maternal characteristics and neonatal novelty exposure. (C and D) Prediction by maternal self-stress regulation. (E and F) Prediction by maternal-care reliability. (G and H) Prediction by maternal-care quantity. Maternal individual differences in self-stress regulation (D), but not care behavior (G and H), account for litter-to-litter variations in the effect of novelty exposure on offspring body weight [significant second-order effect: Novelty×CORTE effect, F(1,12) = 12.279, P = 0.004], with a high and low maternal CORT response predicting a novelty-induced enhancement and impairment in early growth respectively (D). Note that, in B, NE scores are shown in % of average to give relative size of novelty effect and in D, F, and H shown in rank order to correspond to the statistical analysis.
We found that for some rat families, novelty exposure promotes and, in others, impairs early growth (Fig. 1A), resulting in a net zero effect at the level of population (one-sample t test, P > 0.20) (Fig. 1B). This lack of an overall effect on offspring body weight is consistent with early studies reviewed by Daly (36). NNE created variation in body weight, ranging from 7.5% below to 14.4% above the average body weight, well within the normal range of variation in newborn human infant body weight (19.5% below and above population average) (Fig. 1B) (27). Therefore, we consider the effect of novelty exposure on body weight within the range of normal weight variation reflecting individual differences in normal growth. One aspect of maternal self-stress regulation that is germane to the present results is the ability to mount a rapid response to the stressor onset, which we measured 5 min after the onset of a 1-min swim stressor. As this rapid initial rise measure (SI Methods) is not significantly correlated with her pups’ body weights (P > 0.2) (Fig. 1C), the litter-to-litter variations in offspring body weight cannot be accounted for by dam-to-dam variations in this stress-regulation measure.
However, this lack of effect of each of the above individual factors (i.e., a lack of first-order novelty exposure and maternal initial CORT response effects) does not preclude the possibility that the two factors may interact to produce a lawful pattern of influence on early growth. We performed repeated-measures ANCOVA with novelty as a within factor and each of the maternal measures as a covariate to test the interaction effects between postnatal maternal stress regulation measures (factor a) and novelty exposure (factor b). To facilitate understanding and interpretation of the interaction effects, we showed both the influence of maternal self-stress regulation (a) on the novelty effect (b) on body weight (denoted as a > b, as in Figs. 1D and 2D) and the influence of novelty exposure (b) on the effect of maternal self-stress regulation (a) on body weight (denoted as b > a, as in Fig. 3 A and B).
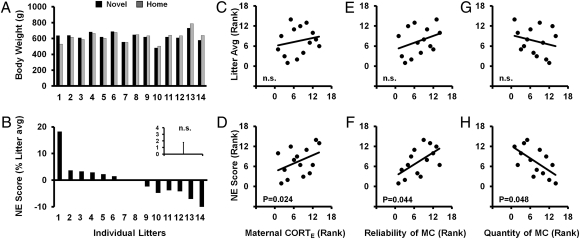
Fig. 2.
(A–H) Body weight in late adulthood. Organization of the figure is the same as in Fig. 1. Consistent with the early-growth measure, there was no significant first order effect of neonatal novelty exposure on the offspring's body weight at late adulthood (A and B). Nor was there a significant first order effect of maternal context (C, E, and G). Different from the early growth measure, at late adulthood, not only the measure of maternal stress regulation (D), but also the maternal care reliablity (F), can account for litter-to-litter variations in the effect of novelty exposure on offspring body weight [Novelty × CORTE effect: F(1,11) = 6.812, P = 0.024; Novelty × post-NNE maternal care reliability: F(1,11) = 5.173, P = 0.044]. Surprisingly, a higher quantity of average post-NNE maternal care paradoxically predicted less or even negative novelty-induced growth enhancement (G). The association between maternal CORTE and post-NNE maternal care reliability was not statistically significant (P > 0.2).
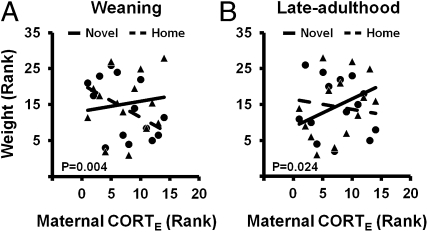
Fig. 3.
Distinct pattern of novelty effect on the relation between the measure of maternal stress regulation and offspring body weight at weaning (A) and late adulthood (B) (same data and statistics as in Figs. 1D and 2D, respectively, but displayed separately for Novel and Home offspring). (A) At weaning, Home (●) siblings’ body weight was more negatively correlated with the maternal CORT measure than the Novel (▲) siblings’ body weight. (B) At late adulthood, Novel siblings’ body weight was more positively correlated with the maternal CORT measure than the Home sibling's body weight. Notice the counter-clockwise rotation of both regression lines from weaning to adulthood, which indicates an increasing positive maternal influence on offspring body weight across development.
In examining the interaction (i.e., a second order effect) at weaning, we found a significant interaction between these two factors on body weight within the normal range [ANCOVA: Novelty × CORTE effect, F(1,12) = 12.279, P = 0.004, f = 1.012] (Fig. 1D). For the mothers who can rapidly mount a large CORT response, the offspring showed novelty induced early growth promotion (positive NE scores, shown as higher ranks); for mothers who cannot, the offspring suffered a novelty-induced early growth impairment (negative NE scores, shown as lower ranks). This interaction effect, first observed at weaning, was replicated twice using two additional variations of body weight measurement at late adulthood (SI Methods) among the same cohort of rat families [13 mo: F(1,11) = 6.812, P = 0.024, f = 0.786 (Fig. 2 A–D); 14 mo: F(1,12) = 4.161, P = 0.032, f = 0.588, one-tailed]. No significant interaction effects were found for weight gain from infancy to early adulthood (weaning to PND 100) and early adulthood weight itself (PND 100) (Ps > 0.2), possibly because of large variation around a time of major hormonal change.
These longitudinal findings, spanning more than half of the rat's expected lifespan, demonstrate that the interaction effect between two salient environmental variables can be demonstrated in the absence of the main effect of novelty exposure or maternal initial CORT response alone. Importantly, it is not the case that pups with novelty exposure will have greater early growth, nor is it the case that a mother with good self-stress regulation will have larger pups. Instead, it is the case that mothers with good self-stress regulation will enable the pups to respond positively to novelty exposure. This sensitivity of the novelty exposure effect to the context of maternal self-stress regulation supports an important notion that the mother can function as both a binary “switch,” setting the direction of the novelty exposure influence on her offspring (14), and a “dial” to set continuous and finer control of the magnitude of this novelty effect (17, 18).
A major source of maternal influence in human development is the support that a mother provides her infant upon the infant's encounter with a stressful situation (37). To capture this aspect, we examined the reliability of maternal care immediately after the nest disturbance and the brief mother-litter separation entailed by the repeated daily novelty exposure (15, 16). Once again, we could not find any evidence that the reliability of postnovelty exposure (PNE) care itself directly affects the weaning and late-adulthood weights of her offspring (Ps > 0.2) (Figs. 1E and 2E). Instead, we found a significant interaction between the reliability of PNE care and NNE, latently expressed at late-adulthood (compare Fig. 1F with Fig. 2F) [F(1,11) = 5.173, P = 0.044, f = 0.686 (Fig. 2F)]. That is, for mothers who showed high reliability in PNE care, the offspring showed a novelty-induced increase in body weight relative to the Home offspring (positive NE score, shown as higher ranks); but for mothers who showed low reliability in her care, the offspring suffered a relative novelty-induced reduction in body weight (negative NE score, shown as lower ranks). Therefore, it is not the case that a mother with high reliability of care will necessarily have larger adult offspring (Figs. 1E and 2E). Instead, it is the case that mothers with high reliability of care will enable the adult offspring to respond to novelty exposure with larger body weight (Figs. 1F and 2F).
In contrast to PNE maternal care reliability, high average quantity of PNE maternal care appears to set an unfavorable condition for novelty-induced weight increase within the normal weight range: offspring of mothers who showed high quantity of care suffered relative weight reduction because of novelty exposure [F(1,11) = 4.922, P = 0.048, f = 0.669] (Fig. 2H). This finding regarding the quantity of PNE care makes sense in the context that the mothers with the higher quantity of PNE care were also the ones who provided lower reliability in care (Rs = −0.916, P < 0.001). Therefore, if a mother cared for her offspring reliably immediately after her offspring experienced each of the repeated stressful events, the offspring can benefit from these stressful events regardless of the absolute quantity of maternal care. The contrasting roles of PNE maternal care reliability and quantity are not only observed in offspring physical development alone, but also in offspring regulation of stress response during social interactions (15) and other forms of learning and plasticity (16).
Furthermore, a contrast between findings at weaning and late-adulthood reveals that the novelty effect on early growth is selectively sensitive to the measure of maternal self-stress regulation but not to characteristics of PNE maternal care, whereas the novelty effect on adult body weight is sensitive to both maternal influences. The influence of maternal self-stress regulation persists from infancy to at least late-adulthood, but the influence of PNE maternal care characteristics only emerges after early infancy. These distinctive time courses, together with a lack of association between the maternal measures of CORT response and care reliability (P > 0.2), suggest that maternal self-stress regulation and PNE maternal care reliability must each play a unique role in setting the context for environmental novelty to affect offspring development.
It is tempting to assume that the persistent interaction between neonatal novelty exposure and maternal self-stress regulation at weaning and late-adulthood (Figs. 1D and 2D) share the same underlying patterns in terms of the relations between the maternal self-stress regulation measures and the body weights of the Novel vs. Home offspring. However, by displaying the same interaction effects differently—that is, separately showing body weight for the Novel and Home rats, instead of showing their difference scores (NE scores)—we observed not only similarity but also differences between the patterns of interactions early and later in life. Distinct between the two ages (compare Fig. 3 A and B), at weaning, Home rats’ body weight, within the normal range, showed a more negative correlation with maternal-evoked CORT than did the Novel rats, with the Novel rats’ trend line nearly flat (compare slope differences in Fig. 3A). However, in late-adulthood (13 mo), Novel rats’ body weight was more positively associated with the same maternal evoked CORT response than the Home rats, with the Home rats’ trend line nearly flat (compare slope differences in Fig. 3B). One interpretation is that for rats with exposures to novelty, they began with early independence from a negative maternal self-stress regulation influence but ended with an emerging positive maternal influence over the course of development (increasing positive slopes from Fig. 3 A to B); for rats with a relatively impoverished home environment (as in the Home rats), the initially negative maternal influence disappeared in the long run with no further positive influence (decreasing negative slopes from Fig. 3 A to B). This apparent age-related difference is supported by a significant three-way interaction effect [ANCOVA: Age × CORTE × Novelty effect: F(1,12) = 17.546, P = 0.001, f = 1.209]. Similar across the two ages, there is a counter-clockwise shift in the slopes of Novel and Home regression lines from Fig. 3 A to B as well as a consistent novelty effect of counter-clockwise shifting at each age. The former implies an increasingly positive influence of maternal self-stress regulation occurring between the two points during development. Therefore, the specific expression of this relationship is dynamically unfolding throughout development, thus arguing against the permanency and supporting a fluidity of early experience effect (38, 39).
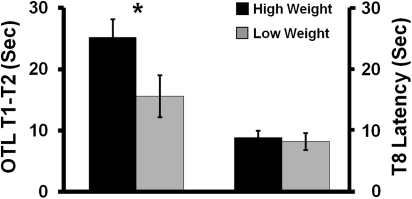
In child development, physical and cognitive development go hand-in-hand (40, 41). Recently, we reported for the same group of rats that neonatal novelty exposure and maternal self-stress regulation also similarly interact to influence offspring cognitive development (17). Therefore, we speculated that the early growth measure may be associated with early cognitive development. We found that the larger pups at weaning showed better working memory in the Morris water task measured at postnatal day (PND) 32–33 than the smaller pups [t(105) = 2.139, P = 0.035, d = 0.418, rats as unit of analysis] (Fig. 4, Left). This cognitive advantage of the larger pups is unlikely to be the result of a weight-related difference in swim speed, because both groups reached a similar performance after overtraining (P > 0.2) (Fig. 4, Right). This finding complements a reliably replicated set of findings in human studies—higher weight at birth and during early infancy are beneficial because infants at the high-end of the early weight distribution ended up as adults with significantly reduced risk factors for major chronic diseases (42)—supporting the importance of promoting normal early growth in the quality of adult life.
Fig. 4.
Offspring whose early growth measure (weaning weight) was within the top 50 percentile showed better spatial working memory (OTL: one trial learning T1-T2) than the bottom 50 percentile. The control measure [i.e., the final trial swim latency (T8 latency)] did not differ after both groups reached asymptote (P > 0.2).
Discussion
The key findings of the present study are (i) during infancy, brief experience of a nonhome environment can increase or decrease offspring body weight depending on the dam's characteristics of self-stress regulation, and this maternal modulation effect persists well into late-adulthood; (ii) independent from this modulation by maternal stress regulation, the reliability of maternal care exerts a delayed and separate modulation effect; (iii) most surprising, a greater amount of maternal care is negatively associated with novelty exposure-induced weight gain; and (iv) a greater early growth as measured by weaning weight is associated with better spatial working memory. Whether similar results can be obtained in females remains to be investigated as major sex differences exist in growth curves (43).
Distinct Contributions from Maternal Physiology and Maternal Care.
Maternal individual differences in self-stress regulation and in PNE maternal care consistency are two maternal contexts potentially influencing offspring physical development. Maternal self-stress regulation may directly influence the amount of maternal CORT exposure experienced by the developing infants, and maternal care consistency, evidently important in human (2) and rodent (15, 16) development, may indirectly influence the amount of circulating CORT generated by the developing infants by facilitating or impeding the recovery of the offspring's stress response to any stressor. Both sources could influence the amount of offspring exposure to circulating CORT, which in turn affects processes involved in the infant's energy metabolism and growth (29). If maternal modulation via stress regulation is mediated by maternal modulation via maternal-care reliability or vice versa, then one would expect that these two maternal variables also show significant correlations and that the predictions by the two variables should be temporally coupled. We found no significant correlations between the two maternal measures, nor did we find temporally coupled modulation effects via these two maternal predictors. Instead, we found that although the modulation effect via maternal self-stress regulation was detectable at weaning and remained detectable at late-adulthood, the modulation effect via day-to-day postdisturbance maternal care was absent during infancy and emerged later during late-adulthood. Therefore, these dissociations between the two modulation effects are consistent with the conclusion that maternal stress regulation and maternal care reliability are likely exerting their modulation via two distinct underlying mechanisms.
Distinct Contribution of Care Reliability and Quantity.
In human developmental studies, consistency instead of quantity of care is the most predictive for offspring development (44, 45). Our measure of care reliability captures, in part, the essence of the consistency concept, thus offering a more direct connection to the construct of attachment security via mother–child interaction (2, 44). In the rat, maternal care reliability has been shown to positively predict novelty-induced enhancement in both spatial memory (16) and HPA plasticity (15). Here we provide converging evidence, from the domain of physical development, that postnatal-care reliability, specifically reliability measured after a brief dam-pup separation and nest disturbance (<15 min), is also a critical contextual variable that regulates whether a particular rat family will experience novelty-induced enhancement or impairment. In contrast, the quantity of such care negatively predicts novelty-related growth enhancement. Similar negative associations have been reported for novelty-induced enhancement in offspring spatial memory (16) and HPA plasticity (15). These opposite patterns of prediction by care reliability and quantity can be reconciled because mothers who showed higher average care behavior happened to have provided their care with lower day-to-day reliability (i.e., a negative association between the quantity and the reliability of postdisturbance of maternal care). Lack of association between greater maternal care and offspring body weight is also consistent with other studies supporting the general notion that higher maternal care quantity does not always lead to beneficial effects (46, 47).
Unifying Explanation for Opposite Effects of Early Stimulation.
Literature on the effects of early life stimulation (mostly via neonatal handling) on body weight appears to be inconsistent. Based on a mixture of findings (8, 6, and 15 studies showing increase, decrease, and no change, respectively), Daly (36) concluded that there was no evidence supporting a handling effect on growth. An alternative interpretation of these seemingly conflicting results is that early stimulation can have opposite effects depending on the specific maternal context. Such a maternal modulation model not only offers a unifying explanation for both a novelty exposure-induced enhancement and impairment in early growth across different rat families within one study, but also offers a possible explanation for a mixture of findings across different studies by different laboratories. Just as within the present study—where positive, near zero, and negative NE scores are associated with variations in maternal behavioral and stress response characteristics—a wide range of handling effects across different studies may have been associated with similar sources of variations in maternal context across different laboratories.
Context and Second-Order Effect.
Although the critical importance of context has been recognized by pioneers in human personality, developmental, and evolutionary psychology (25, 48–50), such awareness has not yet been echoed by researchers working with animal models, whose work is nevertheless frequently interpreted in the context of human development. As has been shown here, without considering relevant contextual variables, one may falsely conclude that an experimental manipulation or intervention has no affect on the intended target behavioral or biological processes (36). Our findings demonstrate how, on the one hand, such conclusions can be drawn if one examines only the main effect of an experimental manipulation, and on the other hand, how lawful patterns of observations can be made if one takes into consideration critical contextual variables. Therefore, the inclusion of a contextual variable may be beneficial not only to the investigation of psychological processes (49), but also scientific investigation more broadly defined. These findings indicate a need for more studies that use experimental designs with hypothesis-driven inclusion of critical covariates to catch the potential influence of a natural context (24, 25).
Fluidity of Early Experience Effects.
Early life experience is known to have lasting effects, often referred to as “permanent alteration” (e.g., see ref. 35). However, long-lastingness may not imply permanency because long-lasting effect can be demonstrated by the presence of any effect long after early experience, without showing that this very effect is fixed across time, hence permanency (38, 39). Here, the distinct patterns of relations between offspring body weight and maternal characteristics (Fig. 3) at weaning and late adulthood provide data from yet another domain supporting fluidity, instead of permanency, of early experience effects. Consistent with this finding, we previously found that neonatal brief experience of novelty led to enhanced spatial memory performance during adulthood—a long-lasting effect—yet additional novelty experience during early adulthood can modify this effect of neonatal novelty exposure (39), and that combined neonatal and adulthood novelty exposure produced one pattern of interaction at 5 mo of age but a distinct new pattern at 15 mo of age (38). These observations indicate that the effect of an earlier experience is subject to continuing modification via dynamic interactions among a multitude of events that make up the full context of life, and conclusions drawn from observations made at one point during development may be different from conclusions drawn from observations made at another point, when embedded in a richer developmental context.
Potential Relevance to Human Health.
In humans, low body weight early in life, even within the normal range of weight variation, is associated with increased risk of death because of coronary heart disease, hypertension, and type 2 diabetes across the Western and Eastern worlds (42). Therefore, understanding factors that influence body weight at birth and early infancy may have a high payoff given the high cost of health care associated with these diseases. Given the recognized challenges in retrospective epidemiological analyses of causal factors and in designing studies of the potential impact of development on later disease outcomes across most of a lifetime (51), animal models offer the needed opportunity to build a possible causal model and to design interventions based on such a causal model. The current study suggests that small systematic variations in environmental novelty, combined with maternal physiological and behavioral context, can account for variations in the offspring's early growth, thus suggesting two early environmental variables as entry points for designing human intervention studies to influence early growth, thereby altering long-range health outcomes.
Conclusions
The present study offers findings regarding two early life determinants of physical growth in a single cohort of male rats followed from birth to late-adulthood. The two most surprising findings are: (i) a psychological manipulation, as simple as 3 min daily away from the familiarity of home environment, for the first 3 wk of life can have a maternal-context–dependent effect on physical growth during infancy—we refer to this finding as maternal modulation of novelty-related growth; (ii) this second-order interaction effect can exist in the absence of the first-order novelty exposure and maternal effects. These findings (i) offer a unifying explanation for the seemingly contradicting early growth literature; (ii) expand previous findings of maternal modulation of novelty effects from the psychological and physiological domains to the physical domain; (iii) offer a previously unexplored alternative view that multiple sources of maternal influences—her physiology and her behavior—can operate separately and in parallel in modulating the impact of other salient environmental factors; (iv) offer a positive story of early experience-dependent plasticity that identifies some of the conditions for growth enhancement; (v) offer observations that point to potential adaptive values associated with higher body weight; (vi) support the dynamic systems view that emphasizes the interaction among multiple factors instead of pinpointing a single causal factor; and (vii) provide evidence for ways in which young animals make differential responses to environmental events according to differential maternal signals (52, 53).
Methods
Sixty-six male pups born from 14 Long-Evans hooded dams (Harlan) were included in the study. Rats were maintained with a 12-h light/dark cycle (lights on at 0700 hours) and with food and water, unless otherwise specified during weight measurement. Using the NNE procedure based on a split-litter design (5), we exposed approximately half of the siblings from each litter to a relatively novel nonhome environment and kept the remaining half in only the home cage during PND 1–21. Offspring body weight was measured at weaning (PND 22), early (PND 100) and late adulthood (13 mo and 14 mo of age). To maximize the sensitivity of detecting weight difference between Novel and Home offspring, rats within the same litters were measured one immediately after another and the order of measurement between the Novel and Home rats was counter-balanced. Offspring spatial working memory performance was measured by one-trial learning, defined as the decrease in swim latency between trial 1 and trial 2 on the testing day. Maternal context were characterized using two measures of her HPA functions, basal CORT (CORTB), and swim-stress evoked CORT (CORTE) responses, and two measures of maternal care behavior: amount and variability of care across PND 1–10. ANCOVAs were performed on ranked weight data because of the existence of outliers. Because significant results were found only for CORTE, only CORTE-related findings are shown. For further details, see SI Methods.
Supplementary Material
Acknowledgments
The authors thank K. Akers and M. Nakazawa for their assistance and Drs. C. Kinsley and D. Smith for commenting on an earlier version of this manuscript.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1121056109/-/DCSupplemental.
References
- 1.Bowlby J. Attachment and Loss. New York: Basic Books; 1969. [Google Scholar]
- 2.Ainsworth MDS, Blehar MC, Waters E, Wall S. Patterns of Attachment: A Psychological Study of the Strange Situation. Hillsdale, NJ: Lawrence Erlbaum; 1978. [Google Scholar]
- 3.Rutter M. Maternal Deprivation Reassessed. 2nd Ed. England: Penguin books; 1981. [Google Scholar]
- 4.Pryce CR, Feldon J. Long-term neurobehavioural impact of the postnatal environment in rats: Manipulations, effects and mediating mechanisms. Neurosci Biobehav Rev. 2003;27:57–71. doi: 10.1016/s0149-7634(03)00009-5. [DOI] [PubMed] [Google Scholar]
- 5.Tang AC, Akers KG, Reeb BC, Romeo RD, McEwen BS. Programming social, cognitive, and neuroendocrine development by early exposure to novelty. Proc Natl Acad Sci USA. 2006;103:15716–15721. doi: 10.1073/pnas.0607374103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catalani A, Alemà GS, Cinque C, Zuena AR, Casolini P. Maternal corticosterone effects on hypothalamus-pituitary-adrenal axis regulation and behavior of the offspring in rodents. Neurosci Biobehav Rev. 2011;35:1502–1517. doi: 10.1016/j.neubiorev.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 7.Macrì S, Zoratto F, Laviola G. Early-stress regulates resilience, vulnerability and experimental validity in laboratory rodents through mother-offspring hormonal transfer. Neurosci Biobehav Rev. 2011;35:1534–1543. doi: 10.1016/j.neubiorev.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 8.Liu D, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 9.Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- 10.Russell P. Infantile stimulation in rodents: A consideration of possible mechanisms. Psychol Bull. 1971;75:192–202. [Google Scholar]
- 11.Parker KJ, Buckmaster CL, Sundlass K, Schatzberg AF, Lyons DM. Maternal mediation, stress inoculation, and the development of neuroendocrine stress resistance in primates. Proc Natl Acad Sci USA. 2006;103:3000–3005. doi: 10.1073/pnas.0506571103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smotherman WP. Mother-infant interaction and the modulation of pituitary-adrenal activity in rat pups after early stimulation. Dev Psychobiol. 1983;16:169–176. doi: 10.1002/dev.420160303. [DOI] [PubMed] [Google Scholar]
- 13.Stanton ME, Levine S. Inhibition of infant glucocorticoid stress response: Specific role of maternal cues. Dev Psychobiol. 1990;23:411–426. doi: 10.1002/dev.420230504. [DOI] [PubMed] [Google Scholar]
- 14.Moriceau S, Sullivan RM. Maternal presence serves as a switch between learning fear and attraction in infancy. Nat Neurosci. 2006;9:1004–1006. doi: 10.1038/nn1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akers KG, et al. Social competitiveness and plasticity of neuroendocrine function in old age: Influence of neonatal novelty exposure and maternal care reliability. PLoS ONE. 2008;3:e2840. doi: 10.1371/journal.pone.0002840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reeb-Sutherland BC, Tang AC. Functional specificity in the modulation of novelty exposure effects by reliability of maternal care. Behav Brain Res. 2012;226:345–350. doi: 10.1016/j.bbr.2011.08.047. [DOI] [PubMed] [Google Scholar]
- 17.Tang AC, Reeb-Sutherland BC, Yang Z, Romeo RD, McEwen BS. Neonatal novelty-induced persistent enhancement in offspring spatial memory and the modulatory role of maternal self-stress regulation. J Neurosci. 2011;31:5348–5352. doi: 10.1523/JNEUROSCI.6808-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang AC, et al. Converging influence of neonatal novelty experience and maternal self-stress regulation on the plasticity of offspring acoustic startle response latency. Behav Brain Res. 2011;221:253–260. doi: 10.1016/j.bbr.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 19.Essex MJ, et al. Exploring risk factors for the emergence of children's mental health problems. Arch Gen Psychiatry. 2006;63:1246–1256. doi: 10.1001/archpsyc.63.11.1246. [DOI] [PubMed] [Google Scholar]
- 20.Glover V, O'Connor TG, O'Donnell K. Prenatal stress and the programming of the HPA axis. Neurosci Biobehav Rev. 2010;35:17–22. doi: 10.1016/j.neubiorev.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 21.Gunnar MR, Brodersen L, Nachmias M, Buss K, Rigatuso J. Stress reactivity and attachment security. Dev Psychobiol. 1996;29:191–204. doi: 10.1002/(SICI)1098-2302(199604)29:3<191::AID-DEV1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 22.Nachmias M, Gunnar M, Mangelsdorf S, Parritz RH, Buss K. Behavioral inhibition and stress reactivity: The moderating role of attachment security. Child Dev. 1996;67:508–522. [PubMed] [Google Scholar]
- 23.Leerkes EM. Maternal sensitivity during distressing tasks: A unique predictor of attachment security. Infant Behav Dev. 2011;34:443–446. doi: 10.1016/j.infbeh.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thelen E, Smith LB. A Dynamic Systems Approach to the Development of Cognition and Action. Cambridge MA: MIT Press; 1994. [Google Scholar]
- 25.Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Dev Psychopathol. 2005;17:271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- 26.Phillips DA, Fox NA, Gunnar MR. Same place, different experiences: Bringing individual differences to research in child care. Child Dev Perspect. 2011;5:44–49. [Google Scholar]
- 27.World Health Organization WHO child growth standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. Geneva: WHO; 2006. p. 312. [Google Scholar]
- 28.UNICEF. WHO Low Birthweight: Country, Regional and Global Estimates. Geneva: WHO; 2004. p. 27. [Google Scholar]
- 29.Dallman MF. Stress-induced obesity and the emotional nervous system. Trends Endocrinol Metab. 2010;21:159–165. doi: 10.1016/j.tem.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levine S. Stimulation in infancy. Sci Am. 1960;202:81–86. [PubMed] [Google Scholar]
- 31.Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- 32.Zou B, Golarai G, Connor JA, Tang AC. Neonatal exposure to a novel environment enhances the effects of corticosterone on neuronal excitability and plasticity in adult hippocampus. Brain Res Dev Brain Res. 2001;130:1–7. doi: 10.1016/s0165-3806(01)00173-0. [DOI] [PubMed] [Google Scholar]
- 33.Denenberg VH. Critical periods, stimulus input, and emotional reactivity: A theory of infantile stimulation. Psychol Rev. 1964;71:335–351. doi: 10.1037/h0042567. [DOI] [PubMed] [Google Scholar]
- 34.Levine S. Plasma-free corticosteroid response to electric shock in rats stimulated in infancy. Science. 1962;135:795–796. doi: 10.1126/science.135.3506.795-a. [DOI] [PubMed] [Google Scholar]
- 35.Champagne FA, Francis DD, Mar A, Meaney MJ. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol Behav. 2003;79:359–371. doi: 10.1016/s0031-9384(03)00149-5. [DOI] [PubMed] [Google Scholar]
- 36.Daly M. Early stimulation of rodents: A critical review of present interpretations. Br J Psychol. 1973;64:435–460. doi: 10.1111/j.2044-8295.1973.tb01370.x. [DOI] [PubMed] [Google Scholar]
- 37.Gunnar MR, Talge NM, Herrera A. Stressor paradigms in developmental studies: What does and does not work to produce mean increases in salivary cortisol. Psychoneuroendocrinology. 2009;34:953–967. doi: 10.1016/j.psyneuen.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang AC, Reeb-Sutherland BC, Yang Z. Functional brain asymmetry in adult novelty response: On fluidity of neonatal novelty exposure effects. Behav Brain Res. 2011;221:91–97. doi: 10.1016/j.bbr.2011.02.047. [DOI] [PubMed] [Google Scholar]
- 39.Yang Z, Tang AC. Novelty-induced enhancement in spatial memory: Is infancy a critical period? Behav Brain Res. 2011;219:47–54. doi: 10.1016/j.bbr.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 40.Rutter M, English and Romanian Adoptees (ERA) Study Team Developmental catch-up, and deficit, following adoption after severe global early privation. J Child Psychol Psychiatry. 1998;39:465–476. [PubMed] [Google Scholar]
- 41.van Ijzendoorn MH, Juffer F. The Emanuel Miller Memorial Lecture 2006: Adoption as intervention. Meta-analytic evidence for massive catch-up and plasticity in physical, socio-emotional, and cognitive development. J Child Psychol Psychiatry. 2006;47:1228–1245. doi: 10.1111/j.1469-7610.2006.01675.x. [DOI] [PubMed] [Google Scholar]
- 42.Barker D. The developmental origins of obesity. In: Bouchard C, editor. Obesity: Epidemiology, Etiology, Consequences and Treatment. London: Henry Stewart Talks Ltd; 2007. [Google Scholar]
- 43.Hughes PCR, Tanner JM. A longitudinal study of the growth of the black-hooded rat: Methods of measurement and rates of growth for skull, limbs, pelvis, nose-rump and tail lengths. J Anat. 1970;106:349–370. [PMC free article] [PubMed] [Google Scholar]
- 44.Ainsworth MDS. The development of infant-mother interaction among the Ganda. In: Foss BM, editor. Determinants of Infant Behavior. New York: Wiley; 1963. pp. 67–104. [Google Scholar]
- 45.Hane AA, Fox NA. Ordinary variations in maternal caregiving influence human infants’ stress reactivity. Psychol Sci. 2006;17:550–556. doi: 10.1111/j.1467-9280.2006.01742.x. [DOI] [PubMed] [Google Scholar]
- 46.Macrí S, Mason GJ, Würbel H. Dissociation in the effects of neonatal maternal separations on maternal care and the offspring's HPA and fear responses in rats. Eur J Neurosci. 2004;20:1017–1024. doi: 10.1111/j.1460-9568.2004.03541.x. [DOI] [PubMed] [Google Scholar]
- 47.Ragan CM, Loken E, Stifter CA, Cavigelli SA. Within-litter variance in early pup-mother interactions and adult offspring responses to novelty. Dev Psychobiol. 2012 doi: 10.1002/dev.20581. 10.1002/dev.20581. [DOI] [PubMed] [Google Scholar]
- 48.Mischel W. From personality and assessment (1968) to personality science. J Res Pers. 2009;43:282–290. [Google Scholar]
- 49.Pluess M, Belsky J. Differential susceptibility to rearing experience: The case of childcare. J Child Psychol Psychiatry. 2009;50:396–404. doi: 10.1111/j.1469-7610.2008.01992.x. [DOI] [PubMed] [Google Scholar]
- 50.Smith E, Collins E. Situated cognition. In: Mesquita B, Barrett LF, Smith E, editors. The Mind in Context. New York: Guilford; 2010. [Google Scholar]
- 51.Gluckman PD, Hanson MA. Living with the past: Evolution, development, and patterns of disease. Science. 2004;305:1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- 52.Hinde RA. Some implications of evolutionary theory and comparative data for the study of human prosocial and aggressive behavior. In: Olweus D, Block J, Radke-Yarrow M, editors. Development of Anti-Social and Prosocial Behaviour. Orlando, FL: Academic Press; 1986. [Google Scholar]
- 53.Rossiter MC. The role of environmental variation in parental effects expression. In: Mousseau TA, Fox CW, editors. Maternal Effects as Adaptations. London: Oxford University Press; 1999. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.