Abstract
Keeping track of various amounts of social cognitive information, including people's mental states, traits, and relationships, is fundamental to navigating social interactions. However, to date, no research has examined which brain regions support variable amounts of social information processing (“social load”). We developed a social working memory paradigm to examine the brain networks sensitive to social load. Two networks showed linear increases in activation as a function of increasing social load: the medial frontoparietal regions implicated in social cognition and the lateral frontoparietal system implicated in nonsocial forms of working memory. Of these networks, only load-dependent medial frontoparietal activity was associated with individual differences in social cognitive ability (trait perspective-taking). Although past studies of nonsocial load have uniformly found medial frontoparietal activity decreases with increasing task demands, the current study demonstrates these regions do support increasing mental effort when such effort engages social cognition. Implications for the etiology of clinical disorders that implicate social functioning and potential interventions are discussed.
Keywords: mentalizing, default-mode network, neuroimaging, cognitive load
The “social brain hypothesis” suggests that the fundamental evolutionary constraint leading to the increase in primate brain size, relative to body size, was the need to keep track of an increasing number of social relationships (1–3). Successful navigation of group living requires not only keeping track of one's own relationships with others, but also other people's relationships with each other, and the particular characteristics of other people and their relationships. The information to be considered grows exponentially with the number of people considered, making it difficult to think about even a handful of people at once.
Although the online maintenance or manipulation of multiple pieces of social information, or “social working memory,” is central to successful functioning in a social context, the brain mechanisms guiding this ability remain elusive. One possibility is that increases in social information processing demands are supported by generic working memory resources. Working memory is the psychological process commonly associated with the holding and flexible updating of multiple pieces of information in mind. As people maintain or manipulate increasing amounts of information, a well-characterized set of brain regions [lateral frontoparietal regions and supplementary motor area (SMA)] become progressively more active (i.e., parametric increases) (4–6). Studies of working memory have focused almost exclusively on cognitive or perceptual information (letters, numbers, and object locations) and have not examined social information that might have been critical in successful primate group living (traits, beliefs, relationship characteristics). Given that social thinking typically includes verbal and visuospatial processing demands, canonical working memory regions may support these basic processes during social working memory.
In addition to recruiting the canonical working memory system, social working memory may also rely on another neurocognitive network to support the processing of increasing social cognitive content. There is a set of brain regions associated with thinking about the mental states or psychological characteristics of other people. This “mentalizing” process reliably recruits activity in medial frontoparietal regions and the tempoparietal junction (TPJ) (7–9). These regions have been observed in numerous studies pitting a social cognition task (i.e., thinking about people's psychological characteristics or mental states) against a cognitive control task (i.e., making judgments about physical objects). However, to date, no studies have examined whether these regions parametrically increase in activity as the amount of social information maintained or manipulated during mentalizing increases.
Increased activation in mentalizing regions in response to parametric increases in social cognitive effort would counter the current understanding of how the brain responds during effortful cognitive processing. Extant research suggests that the canonical working memory system supports effortful processing, whereas regions in the mentalizing system deactivate during effortful cognition, including during traditional working memory tasks (10, 11). Essentially, the relationship between the canonical working memory network and the mentalizing network typically looks like two sides of a seesaw: as the lateral frontoparietal network parametrically increases in activation in response to cognitive effort or task demand, the mentalizing network shows parametric decreases (10–12). In fact, the mentalizing network is virtually identical to a network dubbed the “default-mode network” (13–15), so named because it is more active when individuals are at rest (i.e., by default) than when they engage in a variety of effortful cognitive tasks.
Given the previously identified dynamics between canonical working memory and mentalizing networks, as well as the mentalizing network's tendency to show reduced activity under conditions of increasing effort, it would be surprising if the mentalizing network showed load-dependent parametric increases during a social working memory task. The critical caveat is that previous studies have only examined increases in effortful processing with cognitive and perceptual load. None of the studies linking increased effort with decreased activity in the mentalizing network have examined increased effort associated with increased social task demands (“social load”). Given the importance of managing social information to navigate the social environment, it is possible that the canonical working memory and mentalizing systems each support social working memory rather than showing the inverse relationship commonly observed between the systems. The major goal of the current study, therefore, was to examine whether one or both of these networks increase activation during social load.
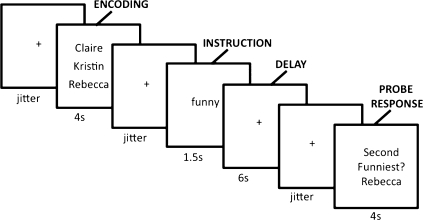
To examine the neurocognitive systems sensitive to social load level, we developed a delayed-response social working memory task that varied working memory load in the social domain on a trial-by-trial basis. During scanning, participants completed trials in which they were presented with the names of two, three, or four of their friends (Fig. 1), mentally ranked their friends along a trait dimension during a delay period, and answered a true/false question about their rank order. Two weeks before the scanning session, we obtained each participant's ranking of 10 friends on each of the trait dimensions we used, allowing accuracy calculations for each trial. First, we explored which brain regions showed parametric increases in activation with increasing levels of social load. Second, we examined whether any activation during social working memory was related to trait perspective-taking, a correlate of social cognitive ability (16). Given that lateral frontoparietal activity during nonsocial working memory tasks is related to general fluid intelligence (17, 18), it is possible that a parallel relationship exists between social working memory and social cognitive ability.
Fig. 1.
Social working memory task.
Results
Behavioral Task Performance.
The purpose of the behavioral analyses was to determine whether our social working memory task produced characteristic accuracy and reaction time (RT) effects observed in prior working memory studies. Replicating previous research suggesting that performance decreases as a function of task demand (working memory load), repeated-measures ANOVA showed a significant effect of task demand on RT [F(2,15) = 11.48; P < 0.001] and accuracy [F(2, 15) = 5.94; P < 0.005]. For RT, post hoc t tests revealed RT was significantly longer for four names compared with three names [t(15) = 2.45; P < 0.05; Table S1] and two names [t(15) = 3.85; P < 0.005]. Similarly, RT was marginally longer for three names compared with two names [t(15) = 1.77, P = 0.096]. Accuracy was significantly higher on two-name trials than three-name trials [t(15) = 3.04; P < 0.01] or four-name trials [t(15) = 3.34; P < 0.005]. However, the difference in accuracy for three-name trials compared with four-name trials was not significant [t(15) = 0.27; P = 0.79].
Functional MRI Results.
Parametric effects during delay.
Parametric analysis of functional fMRI data allows us to see which regions show a linear increase in activity as a function of social load (i.e., trials with two, three, or four friends’ names to be considered along a trait dimension). Our first analyses focused on the delay period beginning after the trait word (e.g., “funny”) was removed until the probe question appeared 6 seconds later (e.g., “second funniest?”). In this statistical model, the first regressor (i.e., average effect) codes the fixed amplitude effect (i.e., the average hemodynamic response, collapsing across all levels of load). The second regressor is the parametric effect, which codes the variable amplitude effect, (i.e., the effect of the hemodynamic response that varies by social load). Thus, effects associated with the parametric load regressor are independent of the basic effects associated with performing a social cognitive task, per se.
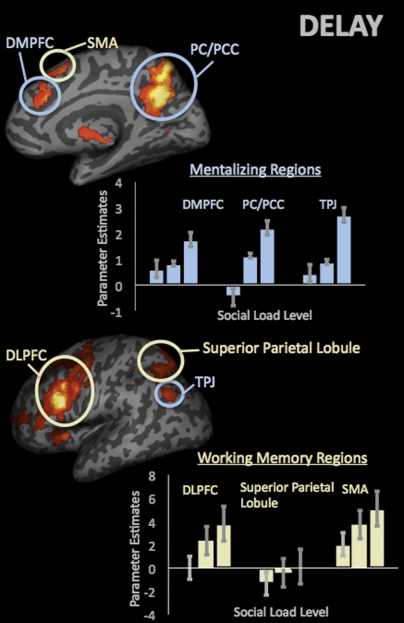
As expected, parametric analyses showed load-dependent increases in canonical working memory regions including dorsolateral prefrontal cortex (DLPFC) (−45, 17, 28), superior parietal lobule (SPL) (30, −67, 58), and SMA (−9, 14, 55). However, in contrast to previous nonsocial studies of working memory, we also observed load-dependent increases in mentalizing regions including dorsomedial prefrontal cortex (DMPFC) (−12, 38, 49) extending into anterior paracingulate cortex (DMPFC/APC) ( 12, 29, 31), precuneus/posterior cingulate cortex (PC/PCC) (0, −61, 46), and TPJ (−42, −70, 40) (Fig. 2 and Table S2).
Fig. 2.
Parametric increases in the mentalizing and canonical working memory regions during the delay period as a function of social load level.
Parametric effects during probe response.
Past working memory studies examine neural responses during the probe response in addition to the delay period, as both are considered component processes of working memory (19). Moreover, the probe response period most closely matches extant social cognitive neuroscience paradigms that do not manipulate social load [i.e., participants make a judgment about others’ traits (20)]. Therefore, we also modeled activation from the onset of the probe question until participants’ button press to determine which regions would show parametric increases as a function of social load. To examine social load effects during the probe response period, a parametric load regressor was entered for each trial to scale the hemodynamic responses expected during the probe period.
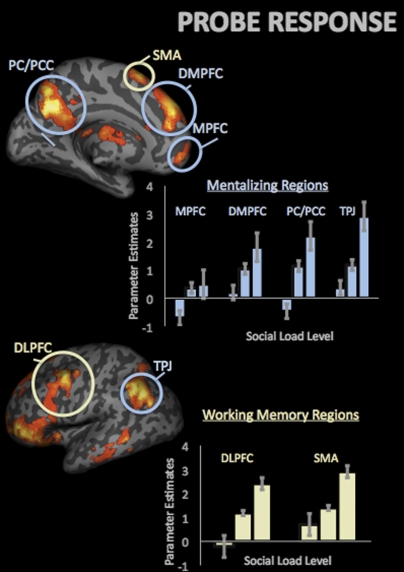
As expected, parametric analysis during the probe response period in canonical working memory regions including DLPFC (−39, 5, 58), SPL (57, –58, 40), and SMA (−6, 20, 64). However, we, once again, also observed load-dependent increases in mentalizing regions including DMPFC extending into anterior paracingulate cortex (9, 47, 52), medial prefrontal cortex (MPFC) (−6, 56, –5), PC/PCC (0, −61, 34), and TPJ (−48, −67, 43) (Fig. 3 and Table S2). As with the delay results, these effects were independent of the average effect associated with performing a social task, per se.
Fig. 3.
Parametric increases in the mentalizing and canonical working memory regions during the probe response period as a function of social load level.
Because there were significant differences in the reaction times as a function of social load level, we conducted an additional analysis that added the reaction time for each trial as a parametric regressor. We orthogonalized the social load parameter with respect to the RT parameter to examine the unique effect of social load, over and above any effects of RT and the average effect of performing a social cognition task per se. In this analysis, mentalizing regions (DMPFC, MPFC, PC/PCC, and TPJ) and traditional working memory regions (DLPFC, SMA, and SPL) continued to produce activity associated with social load level, independent of reaction time.
Perspective-taking ability.
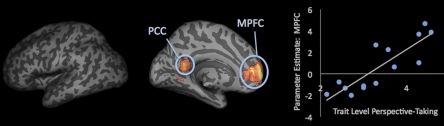
Paralleling findings on nonsocial working memory and fluid intelligence, we examined whether there was a relationship between the parametric recruitment of regions as a function of social load and a measure of trait perspective-taking ability, which has previously been linked to social competence and social reasoning (16, 21). A relation between these assessments would provide further validation of the idea that the brain's ability to manage increasing amounts of social information corresponds with social cognitive ability. Moreover, if the mentalizing regions result in this analysis, it would suggest that social competence may depend on brain regions distinct from those commonly associated with general intelligence. Results from the whole-brain regression of the parametric analysis of the delay period with perspective-taking scale scores entered as a regressor showed significant activation in MPFC (−6, 62, 1) and PC (−9, −49, 13) regions, both of which are central to social cognition (Fig. 4 and Table S3). In contrast, perspective-taking did not correlate with parametric activity in any traditional working memory regions.
Fig. 4.
Regions showing social load dependent increases during the delay period of social working memory trials that correlate with trait-level perspective-taking ability. The lateral view of the brain shows that none of the regions in the frontoparietal canonical working memory network showed parametric activation correlating with perspective-taking scores. The medial view of the brain shows regions in the mentalizing and default-mode network whose parametric activation correlates with perspective-taking scores. This correlation is plotted for MPFC parametric increases by load as a function of perspective-taking scores.
Discussion
Social Load, Default Mode, and Effort.
We identified brain regions involved in maintaining and manipulating increasing amounts of social information that may allow humans to understand complex, multifaceted social interactions. As expected, the canonical working memory system in lateral frontoparietal regions and SMA produced increased activation during delay and probe response periods as social load increased. Similarly, the mentalizing network in medial frontoparietal regions and TPJ also produced increased activation during delay and probe response periods as social load increased. Finally, only mentalizing regions’ parametric increases correlated with trait differences in perspective-taking ability. Prior meta-analyses of nonsocial working memory do not report mentalizing regions increasing with load (6); on the contrary, these regions are typically shown to reduce activation as a function of load in nonsocial working memory tasks (11, 12).
These results are important, in part, because they identify regions that are not only involved in supporting social cognition generally, but regions that are sensitive to the amount of effort needed to support social cognitive processes. That is, this parametric effect cannot be explained simply by the fact that participants are performing a task with social content. The average effect, collapsing across levels of social load, is also included in the model and thus the parametric regressor captures variability in the neural response over and above that which is explained by the average effect. Countless social psychological phenomena have been understood in terms of effortful versus noneffortful social cognition (22), and some studies have suggested that different systems subserve these kinds of processes (23–26). Here, we report neurocognitive evidence of brain regions whose activity scales linearly with increasing task difficulty within the social domain. Furthermore, perspective-taking ability was associated with these load-dependent effects in mentalizing regions, demonstrating that significant variance associated with social cognitive constructs may be explained, in part, by how the mentalizing network scales its response to the level of social load.
The load-dependent increases in the mentalizing network are also compelling because they run counter to the common finding of parametric decreases in these regions as a function of load level (i.e., during effortful processing). Previous studies have uniformly found that increasing levels of cognitive load in working memory and other related tasks produces load-dependent decreases in the default-mode network that is essentially identical to the mentalizing network (10–12). Given past findings, it would have been reasonable to question whether previous mentalizing effects were partly artifacts of lower task difficulty compared with the nonsocial control tasks. As noted earlier, the critical caveat to past load-related findings is that they were all derived from tasks using nonsocial forms of load. The current study suggests that load effects within the mentalizing network are domain-specific and that regions within this network are capable of supporting increasingly effortful cognition, if it is social cognition.
It is also worth noting that in our highest load condition, nearly all of the regions within the mentalizing/default-mode network that were observed to increase parametrically were also significantly more active compared with a resting baseline (Figs. 2 and 3). In common social cognition and self-reference paradigms, regions within the mentalizing and default-mode network show increased activity compared with a nonsocial control task (e.g., judgments about the physical world); however they typically show decreased or no activity compared with a resting baseline. Therefore, it is difficult to claim that regions are optimized for social cognition when the tasks used to assess social cognition produce less activity than what is observed during rest. The current data suggest that these prior findings might be attributable to the lower difficulty levels of prior social cognition and self-reference tasks. When performance measures are reported in fMRI studies of social cognition, they are often near ceiling (27–29), implying relative ease. In contrast, average accuracy in our most difficult condition decreased to <60%. Our results, thus, reaffirm the role of these regions in social cognition and suggest the possibility that during rest, individuals are engaged in more complex or challenging kinds of social cognition than what is demanded by most fMRI studies of social cognition. This seems quite reasonable given that five year olds can pass many of the fMRI-based social cognition tasks given to adults (30, 31), and self-reference tasks involve simple global judgments (“Are you talkative?”) (32).
Importantly, parametric increases in the medial frontoparietal and TPJ regions compliment and extend past research implicating these regions in mentalizing. Previous findings show this network reliably engages when participants think about the mental states, traits, and beliefs of others (7–9); however, the operating characteristics of these computations have not been addressed. Our results indicate that at least some components of the mentalizing network are capacity limited and increase activity with effort and social load level. Such findings simultaneously inform and create possible hypotheses for research in social neuroscience, as well as research on the default-mode system, which previously characterized this network as interfering with, rather than supporting, effortful cognition (11, 12).
Social Working Memory and Social Cognitive Ability.
Although there is no agreed upon measure of social cognitive ability, trait perspective-taking has been associated with social competence (16, 21). We examined whether perspective-taking was related to load-dependent neural activity during the social working memory task and found that only regions within the mentalizing network showed this effect. Specifically, individuals higher in trait perspective-taking were more likely to show load-dependent parametric increases in MPFC (Brodmann area 10). This is the only region of the frontal cortex known to be disproportionately larger in humans than other primates after scaling for body size (33). In addition, individual differences in MPFC size correlate with social cognitive competence and social network size (34, 35). Our functional finding and the previous structural findings dovetail nicely with the social brain hypothesis, which emphasizes that social load processing may have been critical in the expansion of prefrontal cortex size in humans.
Assessing Psychopathology, Improving Daily Functioning.
Many psychiatric conditions including schizophrenia, social anxiety, and autism spectrum disorder show dual or differential deficits in social cognition and working memory. Understanding how the medial and lateral frontoparietal networks contribute to social working memory may offer important insights into how these systems contribute to various psychological disorders and the kinds of interventions that might benefit them. For example, both working memory and theory of mind (i.e., the ability to represent other people's mental states) are impaired in patients with schizophrenia (36–38). Individuals with social anxiety show working memory deficits, but enhanced working memory for socially salient words (39). Similarly, a hallmark of autism spectrum disorder is the impaired ability to relate to and take the perspective of others (40, 41). Interestingly, research on working memory capacity in individuals with autism spectrum disorder is mixed (42–45), with some research finding that working memory capacity is relatively intact in high-functioning individuals (42, 43). It is possible that social cognitive deficits in these and other disorders may be better characterized with the inclusion of a social cognition task like social working memory that varies in difficulty level.
Social working memory capacity may also explain variance in healthy individuals’ broader social cognitive ability. Traditional working memory capacity and lateral frontoparietal activity has been linked to cognitive abilities ranging from math and reading to IQ (17, 18, 46, 47). Given that medial frontoparietal activity during our social working memory task was linked to trait perspective-taking, a core social cognitive ability (32), social working memory capacity may be a useful construct for exploring social cognitive ability.
Along these lines, it is also plausible that social working memory training could benefit everyday social competence. Studies show that working memory training not only improves working memory, but these improvements generalize to improved cognitive reasoning and fluid intelligence (48–50). For example, Jaeggi et al. (48) found that in psychologically healthy adults with normal IQ, working memory training led to improvements in fluid intelligence, the ability to reason and solve new problems independent of previously acquired knowledge. Similarly, it is possible that social working memory training would improve both social working memory ability (i.e., how many people can someone effectively think about at once) and other forms of social cognitive reasoning (i.e., perspective-taking) in both atypical and typical populations.
Limitations.
There are a few potential limitations of our study. First, inclusion of a nonsocial working memory task would allow comparison of activation during social working memory trials to activation during cognitive working memory trials, which could identify activations unique to social (rather than nonsocial) working memory. Importantly, this limitation does not detract from our inferences from the current data. That is, our goal was to identify what regions are generally active during social working memory and, in particular, how the medial frontoparietal regions previously associated with decreases during increased mental effort would respond to social cognitive task demand. The responses of both lateral and medial frontoparietal networks across load levels during cognitive working memory have been very clearly characterized in multiple past studies (11, 12). A cognitive comparison, therefore, would not change the pattern observed here in medial frontoparietal regions, which is qualitatively different from what has been observed in cognitive working memory paradigms in the past.
A second potential limitation is that our task induces both maintenance and manipulation during the delay period, and hence these component processes cannot be teased apart in our analysis of the delay period. Past research tends to find similar, but more robust, patterns of brain activation for manipulation relative to maintenance (4, 51) in nonsocial working memory tasks. Future research will be necessary to identify whether the same trend is true for activation during social working memory maintenance and manipulation.
Conclusions
Gordon Bower (52), a leading memory researcher, once suggested that the purpose of working memory “is to build up and maintain an internal model of the immediate environment and what has been happening in our world” (p. 54). Past working memory research has focused on the basic building blocks that allow us to handle representations of our immediate environment but has neglected to incorporate relevant social information that makes up much of our mental processing. Our results demonstrate that humans possess mechanisms to support social working memory and that these mechanisms include mentalizing regions in addition to canonical working memory regions. Echoing Bower, we suggest that the purpose of social working memory is to build up and maintain an internal model of the immediate social environment and what has been happening in our social world.
Methods
Participants.
Sixteen right-handed, native English-speaking participants (10 females; mean age, 20 y; SD, 0.89) were recruited from the University of California, Los Angeles (UCLA) community and paid $60 for their participation. All participants provided written informed consent according to the procedures of the UCLA Institutional Review Board.
Procedure.
Two weeks before the scan, participants completed a trait-rating questionnaire for 10 of their close friends. A total of 96 traits were selected from previously rated trait adjectives matched on familiarity, frequency of use, and positive valence (53). For each trait, participants rated how much each of their friends possesses the trait on a 1–100 scale (1 being the least and 100 being the most). These ratings were later used to create social working memory trials (see below Materials). On the day of the scan, participants were instructed for each trial to read the list of the names of friends presented simultaneously on the screen (two, three, or four names; “encoding”; 4 s) and the trait word subsequently displayed for 1.5 s once the names were removed (Fig. 1). For the delay period (6 s), participants were instructed to think about how much each of the previously shown friends possess the given trait and mentally rank them from most to least in terms of the extent to which each friend possesses the trait (e.g., rank them from most funny to least funny). Finally, participants received a true/false probe question regarding the ranked position of a previously encoded friend. For example, a trial with three names (Claire, Kristin, Rebecca) may have shown a probe question such as “second funniest?—Rebecca,” and subjects indicated whether, of the group of friends encoded for that trial, the listed name at the probe question (i.e., Rebecca) was the second funniest of the encoded friends. The ranked position in probe question was randomized across trials to avoid mental set effects. Before the scan, participants completed practice social working memory trials (distinct from those used in the scans) to become familiar with the task. In the scan, participants completed four runs of the social working memory task. Each run had 24 unique trials presented with jittered crosshair fixation periods between and within trial elements. Between each trial, and between each modeled working memory phase (encoding, delay, retrieval), participants saw a fixation crosshair presented for a jittered amount of time [jitter time was randomly chosen and centered around a mean of 1.5 s (54)]. After the scan, participants completed the Empathy Interpersonal Reactivity Index (Empathy IRI) (16).
Materials.
For each social working memory trial, participants encoded the names of 2, 3, or 4 friends selected from a list of their 10 close friends that they provided 2 wk before the scan. To control for rating distance effects on task difficulty, we aimed to select friends that were ranked no more than 25 points apart (on the 100-point scale) and no closer than 5 points apart from one another for each trait word. These distances served as a rule for friend name selection and were adhered to as closely as possible given the distribution of ratings given by the participants (M distance for friend names within a trial, 11.38; SD, 7.24). Each participant was shown their own friends’ names on each trial. Trials were standardized on brightness, contrast, font, and size.
Perspective-taking ability was measured using the perspective-taking (PT) subscale of the Empathy IRI (16). This subscale was used because we wanted an individual difference measure of social cognition that shows variability in scores across healthy adults. The PT scale is a valid and reliable measure of the tendency to adopt the point of view of other people in everyday life (16). A sample item from the PT scale is “I sometimes try to understand my friends better by imagining how things look from their perspective.” Perspective-taking ability is a fundamental component of social cognition, and higher perspective-taking scores on the Empathy IRI are associated with better social functioning (16).
Behavioral Data Acquisition and Analysis.
Working memory is thought to be a limited capacity, given that behavioral performance on working memory tasks worsens as a function of load. During our social working memory scanner trials we collected participants’ reaction time to the probe question as an index of task performance. To compute trial accuracy, we compared participants’ answer to the probe question to their original friend trait ratings. If their answer was consistent with their prescanner ratings about their friends’ traits, the trial was scored as accurate. For example, on the prescan rating questionnaire, for the trait “funny” a subject may have rated Claire an 85, Kristin a 75, and Rebecca a 67. For a trial in which these three friends were encoded, and the probe question was “Second funniest—Rebecca?,” a correct answer would be “false” because Rebecca was actually rated third funniest.
fMRI Data Acquisition and Analysis.
Imaging data were collected on a Siemens Trio 3-Tesla MRI scanner at the UCLA Ahmanson-Lovelace Brain Mapping Center. For each participant, we acquired 720 functional T2*-weighted echoplanar image volumes (EPIs) [slice thickness, 4 mm (no gap); 34 slices; repetition time (TR), 2,000 ms; echo time (TE), 30 ms; flip angle, 90°; matrix, 64 × 64; field of view (FOV), 192 mm] divided evenly across four runs. We also acquired a T2-weighted matched-bandwidth anatomical scan (same parameters as for EPIs, except as follows: TR, 5,000 ms; TE, 34 ms; flip angle, 90°; matrix, 128 × 128).
Imaging data were analyzed in SPM8 (Wellcome Department of Cognitive Neurology, Institute for Neurology, London, United Kingdom). Preprocessing for each participant's images included skull-stripping using Brain Extraction Tool (BET) (55), spatial realignment to correct for head motion, normalization into a standard stereotactic space as defined by the Montreal Neurological Institute, and spatial smoothing using an 8-mm Gaussian kernel, full width at half-maximum.
The data were modeled as an event-related design. Each trial comprised separately modeled events for encoding (names), delay (6-s crosshair fixation), and retrieval (probe question about an encoded friends’ trait ranking). Based on previous working memory research (56–61), our fMRI analyses focused only on trials answered correctly at retrieval. Encoding and delay periods were modeled as a boxcar spanning their duration. Retrieval was modeled as a boxcar from probe onset to the subject's response. Each event type (encoding, delay, and retrieval) also had an associated parametric modulator (regressor) coding for the trial load (two names, three names, or four names). We orthogonalized the social load parameter with respect to the main effect to examine the unique effect of social load, over and above any effects of performing the task collapsing across load level. We also created a model in which we orthogonalized the social load parameter with respect to the average effect and any effect attributable to RT (Results).
For all analyses, linear contrasts were computed for each participant as a measure of differential BOLD activation and then entered into random effects analyses at the group level for statistical inference. All whole-brain analyses were conducted using a statistical criterion of at least 43 contiguous voxels exceeding a voxel-wise threshold of P < 0.005. This joint voxel-wise and cluster-size threshold corresponds to a false-positive discovery rate of 5% across the whole brain, as estimated by a Monte Carlo simulation implemented using AlphaSim in AFNI (62). For visual presentation, thresholded t-statistic maps were surface rendered using the SPM Surfrend toolbox version 1.0.2 (I. Kahn, Israel Institute of Technology).
Supplementary Material
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1121077109/-/DCSupplemental.
References
- 1.Dunbar R. The social brain hypothesis. Evol Anthropol. 1998;6:178–190. [Google Scholar]
- 2.Byrne R, Whiten A. Machiavellian Intelligence: Social Expertise and the Evolution of Intellect in Monkeys, Apes, and Humans. UK: Clarendon Press; 1988. [Google Scholar]
- 3.Humphrey NK. The Social Function of Intellect. Cambridge: Cambridge University Press; 1976. [Google Scholar]
- 4.D'Esposito M, Postle BR, Ballard D, Lease J. Maintenance versus manipulation of information held in working memory: An event-related fMRI study. Brain Cogn. 1999;41:66–86. doi: 10.1006/brcg.1999.1096. [DOI] [PubMed] [Google Scholar]
- 5.Rypma B, Prabhakaran V, Desmond JE, Glover GH, Gabrieli JD. Load-dependent roles of frontal brain regions in the maintenance of working memory. Neuroimage. 1999;9:216–226. doi: 10.1006/nimg.1998.0404. [DOI] [PubMed] [Google Scholar]
- 6.Wager TD, Smith EE. Neuroimaging studies of working memory: A meta-analysis. Cogn Affect Behav Neurosci. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- 7.Kampe KK, Frith CD, Frith U. “Hey John”: Signals conveying communicative intention toward the self activate brain regions associated with “mentalizing,” regardless of modality. J Neurosci. 2003;23:5258–5263. doi: 10.1523/JNEUROSCI.23-12-05258.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchell JP, Neil Macrae C, Banaji MR. Forming impressions of people versus inanimate objects: Social-cognitive processing in the medial prefrontal cortex. Neuroimage. 2005;26:251–257. doi: 10.1016/j.neuroimage.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 9.Saxe R, Wexler A. Making sense of another mind: The role of the right temporo-parietal junction. Neuropsychologia. 2005;43:1391–1399. doi: 10.1016/j.neuropsychologia.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Greicius MD, Menon V. Default-mode activity during a passive sensory task: Uncoupled from deactivation but impacting activation. J Cogn Neurosci. 2004;16:1484–1492. doi: 10.1162/0898929042568532. [DOI] [PubMed] [Google Scholar]
- 11.McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 2003;15:394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- 12.Metzak P, et al. Constrained principal component analysis reveals functionally connected load-dependent networks involved in multiple stages of working memory. Hum Brain Mapp. 2011;32:856–871. doi: 10.1002/hbm.21072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raichle M, Snyder A. A default mode of brain function: A brief history of an evolving idea. Neuroimage. 2007;37:1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 14.Raichle ME, et al. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: A quantitative meta-analysis. J Cogn Neurosci. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- 16.Davis M. Measuring individual differences in empathy: Evidence for a multidimensional approach. J Pers Soc Psychol. 1983;44:113–126. [Google Scholar]
- 17.Conway AR, Kane MJ, Engle RW. Working memory capacity and its relation to general intelligence. Trends Cogn Sci. 2003;7:547–552. doi: 10.1016/j.tics.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Gray JR, Chabris CF, Braver TS. Neural mechanisms of general fluid intelligence. Nat Neurosci. 2003;6:316–322. doi: 10.1038/nn1014. [DOI] [PubMed] [Google Scholar]
- 19.Rypma B, D'Esposito M. The roles of prefrontal brain regions in components of working memory: Effects of memory load and individual differences. Proc Natl Acad Sci USA. 1999;96:6558–6563. doi: 10.1073/pnas.96.11.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitchell JP, Heatherton TF, Macrae CN. Distinct neural systems subserve person and object knowledge. Proc Natl Acad Sci USA. 2002;99:15238–15243. doi: 10.1073/pnas.232395699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Underwood B, Moore B. Perspective-taking and altruism. Psychol Bull. 1982;91:143–173. [Google Scholar]
- 22.Chaiken S, Trope Y. Dual-Process Theories in Social Psychology. New York: Guilford Press; 1999. [Google Scholar]
- 23.Evans JS. Dual-processing accounts of reasoning, judgment, and social cognition. Annu Rev Psychol. 2008;59:255–278. doi: 10.1146/annurev.psych.59.103006.093629. [DOI] [PubMed] [Google Scholar]
- 24.Smith E, DeCoster J. Dual process models in social and cognitive psychology: Conceptual integration and links to underlying memory systems. Pers Soc Psychol Rev. 2000;4:108–131. [Google Scholar]
- 25.Lieberman MD, Gaunt R, Gilbert D, Trope Y. Reflection and reflexion: A social cognitive neuroscience approach to attributional inference. Adv Exp Soc Psychol. 2002;34:199–249. [Google Scholar]
- 26.Strack F, Deutsch R. Reflective and impulsive determinants of social behavior. Pers Soc Psychol Rev. 2004;8:220–247. doi: 10.1207/s15327957pspr0803_1. [DOI] [PubMed] [Google Scholar]
- 27.Brunet E, Sarfati Y, Hardy-Baylé MC, Decety J. A PET investigation of the attribution of intentions with a nonverbal task. Neuroimage. 2000;11:157–166. doi: 10.1006/nimg.1999.0525. [DOI] [PubMed] [Google Scholar]
- 28.Fletcher PC, et al. Other minds in the brain: A functional imaging study of “theory of mind” in story comprehension. Cognition. 1995;57:109–128. doi: 10.1016/0010-0277(95)00692-r. [DOI] [PubMed] [Google Scholar]
- 29.Walter H, et al. Understanding intentions in social interaction: The role of the anterior paracingulate cortex. J Cogn Neurosci. 2004;16:1854–1863. doi: 10.1162/0898929042947838. [DOI] [PubMed] [Google Scholar]
- 30.Wimmer H, Perner J. Beliefs about beliefs: Representation and constraining function of wrong beliefs in young children's understanding of deception. Cognition. 1983;13:103–128. doi: 10.1016/0010-0277(83)90004-5. [DOI] [PubMed] [Google Scholar]
- 31.Sommer M, et al. Neural correlates of true and false belief reasoning. Neuroimage. 2007;35:1378–1384. doi: 10.1016/j.neuroimage.2007.01.042. [DOI] [PubMed] [Google Scholar]
- 32.Kelley WM, et al. Finding the self? An event-related fMRI study. J Cogn Neurosci. 2002;14:785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- 33.Semendeferi K, Armstrong E, Schleicher A, Zilles K, Van Hoesen GW. Prefrontal cortex in humans and apes: A comparative study of area 10. Am J Phys Anthropol. 2001;114:224–241. doi: 10.1002/1096-8644(200103)114:3<224::AID-AJPA1022>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 34.Powell JL, Lewis PA, Dunbar RI, García-Fiñana M, Roberts N. Orbital prefrontal cortex volume correlates with social cognitive competence. Neuropsychologia. 2010;48:3554–3562. doi: 10.1016/j.neuropsychologia.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 35.Lewis PA, Rezaie R, Brown R, Roberts N, Dunbar RI. Ventromedial prefrontal volume predicts understanding of others and social network size. Neuroimage. 2011;57:1624–1629. doi: 10.1016/j.neuroimage.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Couture SM, Penn DL, Roberts DL. The functional significance of social cognition in schizophrenia: A review. Schizophr Bull. 2006;32(Suppl 1):S44–S63. doi: 10.1093/schbul/sbl029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldman-Rakic PS. Working memory dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci. 1994;6:348–357. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- 38.Pickup GJ, Frith CD. Theory of mind impairments in schizophrenia: Symptomatology, severity and specificity. Psychol Med. 2001;31:207–220. doi: 10.1017/s0033291701003385. [DOI] [PubMed] [Google Scholar]
- 39.Amir N, Bomyea J. Working memory capacity in generalized social phobia. J Abnorm Psychol. 2011;120:504–509. doi: 10.1037/a0022849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baron-Cohen S, Leslie AM, Frith U. Does the autistic child have a “theory of mind”? Cognition. 1985;21:37–46. doi: 10.1016/0010-0277(85)90022-8. [DOI] [PubMed] [Google Scholar]
- 41.Dawson G, Fernald M. Perspective-taking ability and its relationship to the social behavior of autistic children. J Autism Dev Disord. 1987;17:487–498. doi: 10.1007/BF01486965. [DOI] [PubMed] [Google Scholar]
- 42.Bennetto L, Pennington BF, Rogers SJ. Intact and impaired memory functions in autism. Child Dev. 1996;67:1816–1835. [PubMed] [Google Scholar]
- 43.Ozonoff S, Strayer DL. Further evidence of intact working memory in autism. J Autism Dev Disord. 2001;31:257–263. doi: 10.1023/a:1010794902139. [DOI] [PubMed] [Google Scholar]
- 44.Russell J, Jarrold C, Henry L. Working memory in children with autism and with moderate learning difficulties. J Child Psychol Psychiatry. 1996;37:673–686. doi: 10.1111/j.1469-7610.1996.tb01459.x. [DOI] [PubMed] [Google Scholar]
- 45.Williams DL, Goldstein G, Minshew NJ. The profile of memory function in children with autism. Neuropsychology. 2006;20:21–29. doi: 10.1037/0894-4105.20.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Daneman M, Carpenter P. Individual differences in working memory and reading. J Verbal Learn Verbal Behav. 1980;19:450–466. [Google Scholar]
- 47.Geary DC, Hoard MK, Byrd-Craven J, DeSoto MC. Strategy choices in simple and complex addition: Contributions of working memory and counting knowledge for children with mathematical disability. J Exp Child Psychol. 2004;88:121–151. doi: 10.1016/j.jecp.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 48.Jaeggi SM, Buschkuehl M, Jonides J, Perrig WJ. Improving fluid intelligence with training on working memory. Proc Natl Acad Sci USA. 2008;105:6829–6833. doi: 10.1073/pnas.0801268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jaeggi SM, Buschkuehl M, Jonides J, Shah P. Short- and long-term benefits of cognitive training. Proc Natl Acad Sci USA. 2011;108:10081–10086. doi: 10.1073/pnas.1103228108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klingberg T, et al. Computerized training of working memory in children with ADHD—a randomized, controlled trial. J Am Acad Child Adolesc Psychiatry. 2005;44:177–186. doi: 10.1097/00004583-200502000-00010. [DOI] [PubMed] [Google Scholar]
- 51.Veltman DJ, Rombouts SA, Dolan RJ. Maintenance versus manipulation in verbal working memory revisited: An fMRI study. Neuroimage. 2003;18:247–256. doi: 10.1016/s1053-8119(02)00049-6. [DOI] [PubMed] [Google Scholar]
- 52.Bower G. Cognitive psychology: An introduction. In: Estes W, editor. Handbook of Learning and Cognitive Processes. Vol 1. Hillsdale, NJ: Erlbaum; 1975. pp. 25–80. [Google Scholar]
- 53.Dumas J, Johnson M, Lynch A. Likableness, familiarity, and frequency of 844 person-descriptive words. Pers Individ Dif. 2002;32:523–531. [Google Scholar]
- 54.Wager TD, Nichols TE. Optimization of experimental design in fMRI: A general framework using a genetic algorithm. Neuroimage. 2003;18:293–309. doi: 10.1016/s1053-8119(02)00046-0. [DOI] [PubMed] [Google Scholar]
- 55.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Funahashi S, Bruce S, Goldman-Rakic P. Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. J Neurophysiol. 1989;61:1–19. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- 57.Fuster JM. Unit activity in prefrontal cortex during delayed-response performance: Neuronal correlates of transient memory. J Neurophysiol. 1973;36:61–78. doi: 10.1152/jn.1973.36.1.61. [DOI] [PubMed] [Google Scholar]
- 58.Huijbers W, Pennartz CM, Rubin DC, Daselaar SM. Imagery and retrieval of auditory and visual information: Neural correlates of successful and unsuccessful performance. Neuropsychologia. 2011;49:1730–1740. doi: 10.1016/j.neuropsychologia.2011.02.051. [DOI] [PubMed] [Google Scholar]
- 59.Pessoa L, Gutierrez E, Bandettini P, Ungerleider L. Neural correlates of visual working memory: fMRI amplitude predicts task performance. Neuron. 2002;35:975–987. doi: 10.1016/s0896-6273(02)00817-6. [DOI] [PubMed] [Google Scholar]
- 60.Rosenkilde CE, Bauer RH, Fuster JM. Single cell activity in ventral prefrontal cortex of behaving monkeys. Brain Res. 1981;209:375–394. doi: 10.1016/0006-8993(81)90160-8. [DOI] [PubMed] [Google Scholar]
- 61.Watanabe M. Prefrontal unit activity during delayed conditional Go/No-Go discrimination in the monkey. I. Relation to the stimulus. Brain Res. 1986;382:1–14. doi: 10.1016/0006-8993(86)90104-6. [DOI] [PubMed] [Google Scholar]
- 62.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.