Abstract
CD148 is a receptor-type protein tyrosine phosphatase that is expressed in several cell types, including vascular endothelial cells and duct epithelial cells. Growing evidence demonstrates a prominent role for CD148 in negative regulation of growth factor signals, suppressing cell proliferation and transformation. However, its extracellular ligand(s) remain unknown. To identify the ligand(s) of CD148, we introduced HA-tagged CD148 into cultured endothelial cells and then isolated its interacting extracellular protein(s) by biotin surface labeling and subsequent affinity purifications. The binding proteins were identified by mass spectrometry. Here we report that soluble thrombospondin-1 (TSP1) binds to the extracellular part of CD148 with high affinity and specificity, and its binding increases CD148 catalytic activity, leading to dephosphorylation of the substrate proteins. Consistent with these findings, introduction of CD148 conferred TSP1-mediated inhibition of cell growth to cells which lack CD148 and TSP1 inhibition of growth. Further, we demonstrate that TSP1-mediated inhibition of endothelial cell growth is antagonized by soluble CD148 ectodomain as well as by CD148 gene silencing. These findings provide evidence that CD148 functions as a receptor for TSP1 and mediates its inhibition of cell growth.
Keywords: growth factor signaling, EGF receptor, VEGF receptor, ERK
Tyrosine phosphorylation, which is controlled through the balance and actions of protein tyrosine kinases (PTKs) and protein tyrosine phosphatases (PTPs), plays an essential role in regulating a variety of intracellular signaling, including cell proliferation and differentiation. CD148 (DEP-1/PTPη) is a receptor-type PTP composed of an extracellular segment of fibronectin type Ш (FNШ) repeats, a transmembrane domain, and a single intracellular PTP domain (1). CD148 is expressed in vascular endothelial cells, duct epithelial cells, and hematopoietic lineages (2–5). Growing evidence indicates a prominent role for CD148 in the negative regulation of cell proliferation and transformation. Homozygous-mutant mice in which the CD148 catalytic domain is replaced with GFP die at midgestation because of vascularization failure accompanied by aberrant endothelial cell proliferation and vessel growth (6). Agonistic CD148 antibodies remarkably suppress endothelial cell growth in culture and angiogenesis in corneal assay (7). Furthermore, CD148 is down-regulated in cancer cell lines, in correlation with their malignant phenotype (8, 9), and restoration of CD148 expression suppresses tumor cell growth in culture and in vivo (9–12). Last, loss of heterozygosity at the protein tyrosine phosphatase, receptor type J (CD148) locus is observed frequently in human cancers, implicating CD148 as a tumor suppressor (13). Consistent with its strong growth-inhibitory activity, CD148 dephosphorylates and suppresses growth factor receptors and their signaling proteins, including VEGF receptor 2 (VEGFR2) (14, 15), EGF receptor (EGFR) (16, 17), hepatocyte growth factor receptor (HGFR) (7, 18), FGF receptor (FGFR) (7), PGDF receptor (PDGFR) (19), Erk1/2 (17, 20), phospholipase Cγ1 (21), and p85 (22). Thus, CD148 is thought to function as a suppressor of growth factor signals and to inhibit cell growth and transformation. However, the regulatory mechanisms controlling CD148 activity are largely unknown.
Transmembrane receptor-type PTPs (RPTPs) are composed of an extracellular domain, a single transmembrane domain, and one or two cytoplasmic PTP domains. Structural variability of the extracellular domains suggests that the ectodomain acts as a ligand-binding module to transfer the signals to the inside of the cells. Indeed, it was shown that activity of RPTPβ/ζ is inhibited following binding of pleiotrophin (23). Further, more recent studies showed that heparan or chondroitin sulfate proteoglycans serve as ligands for leukocyte antigen-related PTP (LAR) and a close relative, RPTPσ (24, 25). Syndecan positively regulates LAR activity, whereas Dallylike inhibits LAR function (24). In addition, we and others recently demonstrated that the catalytic activity of CD148 or its close relatives protein tyrosine phosphatase, receptor type O and stomach cancer-associated PTP (SAP-1) is altered by ectodomain events, including antibody binding or forced dimerization (7, 26–28). It is noteworthy that CD148-null mice are viable and show limited phenotype, whereas cytoplasmic GFP knockin mice exhibit lethal vascular defects (6, 29, 30). Although the mechanism currently is unknown, this finding suggests the importance of the interaction between the CD148 ectodomain and its binding partners. However, extracellular ligand(s) for CD148 family RPTPs have not been identified.
Thrombospondin-1 (TSP1) is a trimeric glycoprotein composed of three identical (∼145-kDa) polypeptide chains that contain multiple structural elements (31). In physiological conditions, high expression is restricted to megakaryocytes and platelets, being stored in α-granules, and TSP1 is secreted on platelet activation. Conversely, in pathological conditions and in response to injury and growth factors, TSP1 is synthesized by many cell types including endothelial cells, smooth muscle cells, fibroblasts, and cancer cells (32). It strongly inhibits endothelial cell proliferation and migration (33, 34). TSP1 inhibition of angiogenesis is mediated in part through its endothelial receptors, CD36 and CD47 (35–37). The reported antiangiogenic activities in various tumor models led to current efforts to develop cancer therapeutics based on this molecule (31). TSP1 also exhibits inhibitory activities for epithelial cells and hematopoietic cells (38, 39), but its effects in these cell types are less studied.
In the present study we used affinity purification and MS to identify the extracellular protein(s) that interact with CD148 in endothelial cells. Here we provide biochemical and functional evidence that soluble TSP1 binds to the extracellular domain of CD148 and acts as an agonistic ligand. Our results identify a pathway of TSP1-mediated inhibition of cell growth and offer a strategy for inhibition of angiogenesis.
Results
Identification of TSP1 as a CD148-Interacting Extracellular Protein in Human Umbilical Vein Endothelial Cells.
To identify the extracellular protein(s) that interact with CD148 in endothelial cells, we introduced HA-tagged CD148 into human umbilical vein endothelial cells (HUVEC) using recombinant adenovirus and then isolated its interacting extracellular protein(s) by biotin surface labeling followed by affinity purifications using anti-HA affinity matrix and avidin beads. Recombinant adenovirus encoding the β-galactosidase gene (Adeno-LacZ) was used as a control. As shown in Fig. S1A, several surface proteins (150, 110, 90, 50, and 30 kDa) were coimmunoprecipitated with CD148/HA, whereas these proteins were not recovered by anti-HA antibodies from Adeno-LacZ–transduced cells. Therefore, we scaled up the experiment, and the proteins were stained with colloidal blue, excised, and subjected to liquid chromatography-tandem MS analysis. By this approach we identified TSP1 (150 kDa) as a CD148-interacting extracellular protein in HUVEC (Fig. S1B).
Interaction of CD148 with TSP1 in Intact Cells.
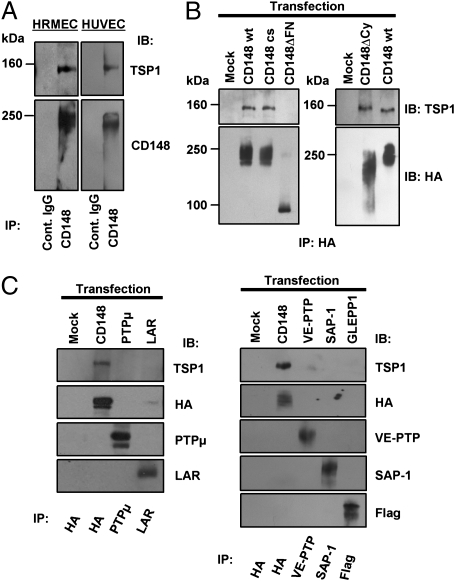
To verify the binding of TSP1 to CD148, we first investigated the interaction between endogenous CD148 and TSP1 by a coimmunoprecipitation experiment using cultured endothelial cells. Second, we assessed the region of CD148 required for its interaction with TSP1. In this experiment, we transfected HA-tagged CD148 forms [WT, catalytically inactive (cs), cytoplasmic domain-deleted (ΔCy), or extracellular FNШ domains-deleted (ΔFN)] to HEK 293 cells, which express low levels of CD148, and assessed their interactions with TSP1 by coimmunoprecipitation and immunoblot analysis. Membrane localization of the CD148 forms was confirmed by anti-HA cell immunostaining (Fig. S2). Last, we evaluated the specificity of CD148 and TSP1 interactions by transfection experiments using other RPTPs. As shown in Fig. 1A, TSP1 was coimmunoprecipitated with endogenous CD148 in cultured human renal microvascular endothelial cells (HRMEC) and HUVEC. Further, the transfection study showed that deletion of the extracellular fibronectin type-III domains (ΔFN), but not the cytoplasmic domain (ΔCy) or catalytic activity (cs mutant), abolishes the CD148 interaction with TSP1 (Fig. 1B). The findings suggest that ectodomain of CD148 is required for its interaction with TSP1. TSP1 did not interact with other RPTPs, including PTPμ, LAR, and the CD148 family RPTPs (vascular endothelial PTP, SAP-1, and glomerular epithelial protein 1) in the same experimental conditions (Fig. 1C). In aggregate, these findings indicate that TSP1 binds with specificity to the extracellular part of CD148.
Fig. 1.
Interaction of CD148 with TSP1 in intact cells. (A) CD148 was immunoprecipitated (IP) from HRMEC or HUVEC using anti-CD148 or control IgG, and the immunocomplexes were immunoblotted (IB) for TSP1 and CD148. (B) HA-tagged CD148 forms (WT, cs, ΔCy, or ΔFN), were transfected to HEK 293 cells. TSP1 (2 μg/mL) was added to the cells before immunoprecipitation. After cross-linking, CD148 was immunoprecipitated using anti-HA, and the immunocomplexes were immunoblotted for TSP1 and HA. (C) RPTPs were transfected to HEK 293 cells, and their interactions with TSP1 were assessed as in B.
Binding of TSP1 to the CD148 Ectodomain.
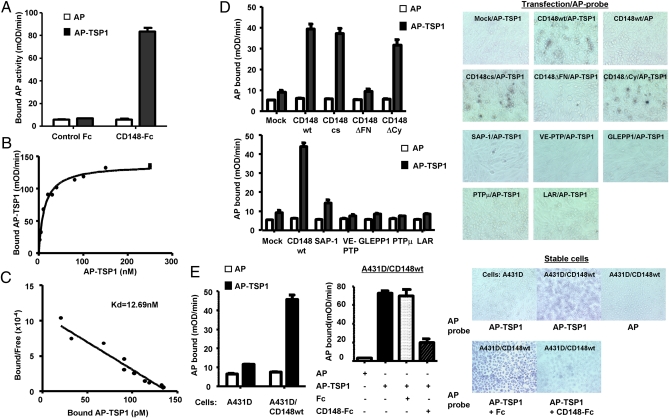
To verify the interaction between TSP1 and the CD148 ectodomain, we next prepared independent fusion proteins, TSP1 linked to an alkaline phosphatase (AP) at its N terminus (AP-TSP1) and CD148 ectodomain fused to human Fc (CD148-Fc) (Fig. S3), and assessed the interaction of these two fusion proteins by in vitro binding assay. As shown in Fig. 2A AP-TSP1, but not AP alone, bound strongly to CD148-Fc but did not bind to Fc alone (control Fc), and the binding was saturated at reasonable doses (Fig. 2B). Scatchard analysis of the binding data revealed a linear plot, indicating a single binding affinity with a dissociation constant (Kd) of 12.69 nM (Fig. 2C). TSP1 showed a binding curve comparable to that of AP-TSP1 (Fig. S4). Further, we investigated the cell-surface interaction between AP-TSP1 and CD148 by an in situ binding assay. Because CD148 is expressed in HEK 293 cells, we used CHO cells, which lack CD148 expression (7). As shown in Fig. 2D, AP-TSP1 bound to CHO cells expressing CD148 WT, CD148ΔCy, or CD148cs, but its binding was limited in mock-transfected cells and in cells transfected with CD148ΔFN or other RPTPs (Fig. 2D). We also assessed the binding of AP-TSP1 to A431D cells stably expressing CD148 WT at levels comparable to levels in cultured endothelial cells (i.e., the physiological level). As shown in Fig. 2E, AP-TSP1 also bound to the A431D/CD148 WT cells, whereas significant binding was not observed in A431D cells that lack CD148 expression. The binding was saturated at reasonable doses, and Scatchard analysis revealed a linear plot with a Kd of 4.2 nM (Fig. S5). Further, CD148-Fc, but not equal molar control Fc, antagonized the AP-TSP1 binding to A431D/CD148 WT cells (Fig. 2E). AP-TSP1 binding to A431D/CD148 WT cells also was suppressed by CD148 gene silencing (Fig. S5). Collectively, these findings indicate that TSP1 binds to the extracellular part of CD148 with high affinity and specificity.
Fig. 2.
Interaction between TSP1 and the CD148 ectodomain. (A) CD148/Fc (5 μg, 22 pmol) or equal molar control Fc (0.6 μg) was added to the medium containing 5 nM of AP or AP-TSP1. Fc proteins were pulled down using protein-G beads, and bound AP proteins were quantified by AP activity assay. Data are means ± SEM of quadruplicate determinations. (B) Saturation binding of AP-TSP1 to CD148-Fc. (C) Scatchard analysis of AP-TSP1 binding to CD148-Fc. (D) CHO cells were transiently transfected with a range of expression plasmids and incubated with 20 nM of AP-TSP1 or AP. The bound AP was assessed by in situ cell staining (Right) and by AP activity measure (Left). (E) Binding of AP-TSP1 to A431D or A431D/CD148 WT stable cells was assessed as in D. Note: AP-TSP1 binding to A431D/CD148 WT cells is antagonized by CD148-Fc (10 μg/mL) but not by control Fc (1.2 μg/mL).
Regulation of CD148 Activity by TSP1.
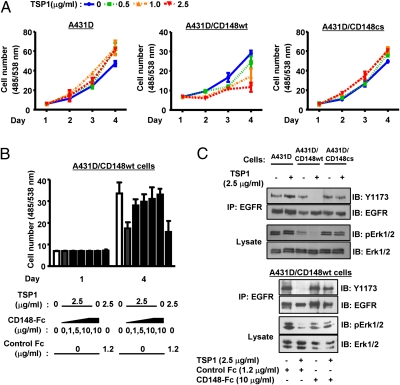
Last, we asked if TSP1 acts as a functional ligand for CD148. For this experiment, we used A431D cells, which lack CD148, CD36, and CD47 expression (Fig. S6). HA-tagged CD148 (WT and cs) was introduced to A431D cells using the LZRS retroviral vector, and the stable cells, whose CD148 level is comparable to that in HRMEC, were sorted by flow cytometry. The CD148− cell fraction also was sorted and used as a control. Physiologically relevant concentrations (<100 nM, 14.5 μg/mL) of TSP1 were added to the cells, and the effects on cell proliferation were assessed. As shown in Fig. 3A, the A431D/CD148 WT cells expressing WT CD148, showed lower cell proliferation than control A431D cells lacking CD148 or A431D/CD148cs cells expressing catalytically inactive CD148, and TSP1 remarkably inhibited cell growth in A431D/CD148 WT cells, whereas it slightly increased cell proliferation in A431D or A431D/CD148cs cells. Further, CD148-Fc antagonized TSP1 inhibition of growth in A431D/CD148 WT cells, whereas equal molar control Fc did not (Fig. 3B). These data suggest that binding of TSP1 to CD148 may increase CD148 catalytic activity and inhibit cell proliferation. Therefore, we next compared the catalytic activity of CD148 in TSP1-treated cells and in control cells. CD148 catalytic activity was assessed by dephosphorylation of its substrates (EGFR, ERK1/2) and by the PTP activity of immunoprecipitable CD148. As shown in Fig. S7, TSP1 treatment significantly increased the catalytic activity of CD148 in A431D/CD148 WT cells, although no difference was observed in the amount of immunoprecipitated CD148 in vehicle- and TSP1-treated cells. Significant CD148 PTP activity was not observed in A431D/CD148cs cells. Consistent with this finding, TSP1 treatment reduced the phosphorylation level of the well-studied CD148 substrates EGFR and ERK1/2 in A431D/CD148 WT cells, whereas significant effects were not observed in A431D or A431D/CD148cs cells (Fig. 3C). Further, CD148-Fc, but not equal molar control Fc, abolished an increase in CD148 catalytic activity and dephosphorylation of its substrates in TSP1-treated A431D/CD148 WT cells (Fig. 3C and Fig. S7). Taken together, these findings indicate that TSP1 can activate CD148, resulting in tyrosine dephosphorylation of defined substrates in intact cells.
Fig. 3.
CD148 expression confers TSP1 inhibition of cell growth to A431D cells. (A) A431D (lacking CD148) or A431D/CD148 WT or cs stable cells (2 × 103 cells) were plated in 98-well plates and starved; then TSP1 (0, 0.5, 1.0, 2.5 μg/mL) was added to the medium. The medium was replaced at day 2 with fresh TSP1. Cell number was assessed at the indicated time points. Data are means ± SEM of quadruplicate determinations. (B) TSP1 (2.5 μg/mL) was added to A431D/CD148 WT cells with or without CD148-Fc (0, 1, 5, 10 μg/mL) or control Fc (1.2 μg/mL), and cell proliferation was assessed as in A. (C) (Upper) A431D and A431D/CD148 WT or cs cells were plated in 100-mm dishes, serum reduced [2.5% (vol/vol) FBS], and exposed to TSP1 (2.5 μg/mL) or vehicle for 15 min. Phosphorylation levels of EGFR (immunoprecipitated) and ERK1/2 were assessed by immunoblots using the phospho-specific EGFR (Y1173) or ERK1/2 (T202/Y204) antibodies. The membranes were reprobed with antibodies to total EGFR or ERK1/2. (Lower) TSP1 (2.5 μg/mL) was added to A431D/CD148 WT cells with CD148-Fc (10 μg/mL) or control Fc (1.2 μg/mL), and its effects were examined.
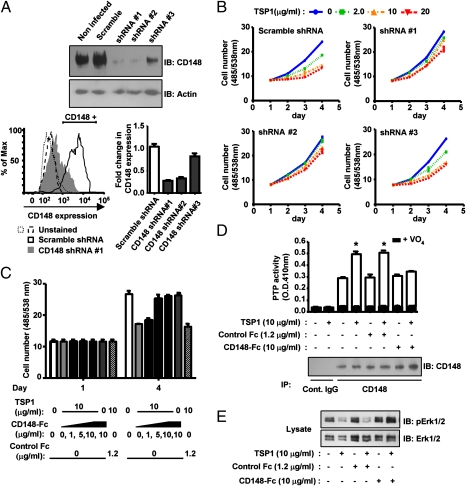
As the final test, we asked whether TSP1 inhibits endothelial cell growth through its interaction with CD148 by antagonizing its binding to CD148 as well as by silencing CD148 expression in endothelial cells. CD148 gene silencing was achieved in HRMEC using lentivirus-mediated shRNA interference, resulting in >80% down-regulation of CD148 (Fig. 4A). Flow cytometric analysis confirmed that CD148 surface expression is knocked down in ∼75% of cells infected with specific shRNA (Fig. 4A). The lentivirus encoding scramble shRNA was used as a control. Reduction of CD148 was not observed in cells infected with scramble shRNA (Fig. 4A). As shown in Fig. 4B, TSP1 treatment remarkably inhibited cell proliferation in HRMEC treated with scramble shRNA, whereas TSP1 inhibition of endothelial growth was largely diminished in the cells treated with CD148-specific shRNA (shRNA#1 or shRNA#2). shRNA#3, which was less effective in CD148 knockdown, showed limited effects. Expression of CD36 and CD47 was not altered by the shRNA treatment (Fig. S8). Further, CD148-Fc antagonized the TSP1 inhibition of endothelial cell growth, whereas equal molar control Fc showed no effects (Fig. 4C). Consistent with these data, TSP1 treatment significantly increased CD148 catalytic activity and decreased VEGFR2 (Fig. S9) and Erk1/2 phosphorylation in HRMEC; these effects were antagonized by CD148-Fc or CD148 gene silencing (Fig. 4 D and E and Fig. S9). Association between CD148 and EGFR or VEGFR2 was not observed in TSP1- or in vehicle-treated cells by a coimmunoprecipitation approach (Fig. S10), perhaps because the CD148 interaction with receptor-type PTKs is a transient reaction (enzyme–substrate interaction) and does not form a stable complex, as demonstrated by a recent study (16). In aggregate, our data demonstrate that TSP1 can act as a functionally important ligand for CD148.
Fig. 4.
TSP1 inhibition of endothelial cell growth is reduced by CD148 knockdown or CD148-Fc. (A) HRMEC were plated in a six-well plate at a density of 50%. Lentivirus (1 × 106 infectious units) encoding CD148-targeting or scramble shRNA was added to the cells with 5 μg/mL Polybrene (Santa Cruz Biotechnology). (Upper) Cells were harvested 72 h after infection, and CD148 expression was assessed by immunoblot analysis. Equal loading was confirmed by reprobing for β-actin. (Lower left) FACS analysis of CD148 expression in HRMEC treated with CD148-targeting (shRNA #1) or scramble shRNA. (Lower right) Fold change of the CD148+ cell fraction, compared with untreated HRMEC. (B) HRMEC treated with CD148-targeting or scramble shRNA lentivirus were plated in 98-well plates (2 × 103 cells per well) 24 h after infection. Cells were starved for 12 h, and then TSP1 (0, 2, 10, 20 μg/mL) was added to the growth medium containing basic FGF (20 ng/mL). The medium was replaced at day 2 with fresh reagents. Cell number was assessed at the indicated time points. Data are means ± SEM of quadruplicate determinations. (C) TSP1 (10 μg/mL) was added to HRMEC with or without CD148-Fc (0, 1, 5, 10 μg/mL) or control Fc (1.2 μg/mL). Cell proliferation was assessed as in B. (D) HRMEC were plated in 100-mm dishes at a density of 30%, serum reduced [2.5% (vol/vol) FBS], and exposed to TSP1 (10 μg/mL) for 15 min with or without CD148-Fc (10 μg/mL) or control Fc (1.2 μg/mL). CD148 was immunoprecipitated using anti-CD148 or control IgG, and the washed immunocomplexes were assayed for PTP activity with or without 1 mM sodium orthovanadate (VO4). The amount of CD148 in the immunocomplexes was assessed by immunoblot analysis. The data show means ± SEM of quadruplicate determinations. *P < 0.01 vs. vehicle-treated cells. (E) HRMEC were treated as in D, and phosphorylation level of Erk1/2 was assessed by immunoblot analysis.
Discussion
A large body of studies has shown that CD148 functions as a suppressor of growth factor signals and strongly inhibits cell proliferation. However, the regulatory mechanisms of CD148 remain to be elucidated. Here, we demonstrate that soluble TSP1 binds to the extracellular part of CD148 with high affinity and specificity and its interaction increases CD148 catalytic activity resulting in inhibition of cell growth. These findings demonstrate that TSP1 can function as a ligand for CD148.
The biological activity of soluble TSP1 is consistent with the reported function of CD148, although multiple receptors and pathways may be involved in TSP1's activity. TSP1 strongly inhibits endothelial cell proliferation, as does CD148 (33, 34). It also suppresses endothelial growth factor signaling, including VEGFR2 (40) and FGFR (41), but the mechanism of this suppression is incompletely understood. Further, the phenotype of TSP1 knockout mice suggests a role of TSP1 in negative regulation of epithelial cell proliferation (38). In this context, it is noteworthy that CD148 is expressed abundantly in megakaryocytes and platelets (3), and its defect impairs platelet aggregation (42), a process in which TSP1 is involved. In addition, TSP1 and CD148 were shown to inhibit T-cell receptor– but not phorbol 12-myristate 13-acetate/ionomycin-mediated T-cell activation and proliferation (21, 39, 43). Thus, a body of evidence indicates that TSP1 acts as a ligand for CD148.
It is known that CD36 and CD47 act as TSP1 receptors and inhibit endothelial cell proliferation and angiogenesis (35–37). Although the interactions between CD148 and these TSP1 receptor pathways currently are unknown, the data for A431D cells (which lack CD36 and CD47) suggest that CD36 or CD47 is not required for the CD148-mediated TSP1 inhibition of cell growth. In this context, it is of note that CD36 is absent in certain vasculature including large arteries, renal endothelium, and umbilical vein endothelial cells (44, 45), whereas CD148 is expressed in these endothelial sites (2). Indeed, CD36 is expressed at low levels in HRMEC and is absent in HUVEC (Fig. S6). The low CD36 levels might be the reason why TSP1 inhibition of cell growth is largely diminished by CD148 silencing in HRMEC. The finding suggests that CD148 may function as a key TSP1 receptor in certain endothelia. On the other hand, it is of interest that ligation of the TSP1 fragment binding CD47 or CD36 suppresses phosphorylation of VEGFR2 at the receptor level (40, 46); the mechanism of this suppression remains to be elucidated. These findings suggest that ligation of CD47 or CD36 may increase CD148 activity, leading to dephosphorylation of VEGFR2. Further studies are required to clarify the interactions between CD148 and CD36 or CD47 pathways.
Several studies have shown that CD148 distributes dominantly to endothelial or epithelial cell–cell contacts, and its catalytic activity is increased with cell density (2, 7, 14, 18), suggesting a role for CD148 in contact inhibition. Further, CD148 is expressed in normal endothelium or duct epithelial cells (2–4), whereas TSP1 is expressed at relatively low levels in normal tissues, and its junctional distribution has not been described. Although recent work showed that functionally significant levels of TSP1 are present in normal tissues even if they are not detectable by immunohistochemistry (47), there seems to be a discrepancy between the localization of CD148 and TSP1 expression in normal tissues. Because TSP1 is produced by many types of cells in pathological settings (32), it is likely that TSP1 or its proteolytic products act as a ligand for CD148 in pathological conditions. In this context, it is of note that A431D/CD148 WT cells showed lower cell proliferation without the addition of TSP1. Although the mechanism is currently unknown, this lower level of proliferation might result from CD148 activation by cell–cell contacts or by an intracellular (ligand-independent) mechanism such as intracellular tyrosine phosphorylation. (While this manuscript was in review, Whiteford et al. (48) reported that syndecan-2 may act as a ligand for CD148. It would be of interest to investigate the involvement of syndecan-2 in TSP1-independent CD148 activity.) Alternatively, TSP1 is known to be present in plasma; thus it may engage CD148 at the surface of endothelial cells, inhibiting endothelial cell growth, and maintaining vascular homeostasis; however, standard detection methods place circulating concentrations at low levels (100–200 pM) compared with the levels (1–100 nM) in pathological tissues (49). Further investigation is required to clarify the details of local, regional, and systemic TSP1 actions mediated through CD148.
In conclusion, the present study identifies TSP1 as a functional ligand for CD148 and defines a pathway of TSP1 inhibition of cell growth. Given the fact that CD148 has a strong activity to inhibit endothelial or epithelial cell growth, further investigation of this pathway, including isolation of the CD148-specific TSP1 fragment (or peptide sequence), should provide a approach to antiangiogenesis and anticancer therapy.
Materials and Methods
Antibodies, plasmids, reagents, and methods for recombinant adenovirus, affinity purification and mass spectrometry, cell culture and transfection, fusion proteins, stable cell preparation, shRNA knockdown, in vitro and in situ binding assays, cell proliferation assay, PTP activity assay, immunoprecipitation and immunoblot analysis, and statistics used in this study can be found in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Drs. Takashi Matozaki, Susan M. Brady-Kalnay, Takuji Shirasawa, Michel Streuli, and Albert Reynolds for constructs, antibodies, and cells; Rosie Jiang, Jing Hao, and Colette Hunt for technical assistance; and Dr. Tom Daniel for critical reading of the manuscript. We also thank the Vanderbilt Mass Spectrometry Research Center (supported by Academic Venture Capitol Fund); the Monoclonal Antibody Core [supported by National Institutes of Health (NIH) Grant CA68485] for protein preparation; the Molecular Genetics Core (supported by NIH Grant AR041943) for adenovirus preparation; and the Vanderbilt University Medical Center (VMC) Flow Cytometry Shared Resource (supported by NIH Grants CA68485 and DK058404). This work was supported by NIH Grant DK38517 (to T.T.), American Heart Association Grant GRNT7700069 (to T.T.), and in part by Clinical and Translational Sciences Award Grant UL1 RR024975-01 to VMC from the National Center for Research Resources/NIH.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1106171109/-/DCSupplemental.
References
- 1.Ostman A, Yang Q, Tonks NK. Expression of DEP-1, a receptor-like protein-tyrosine-phosphatase, is enhanced with increasing cell density. Proc Natl Acad Sci USA. 1994;91:9680–9684. doi: 10.1073/pnas.91.21.9680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takahashi T, et al. Endothelial localization of receptor tyrosine phosphatase, ECRTP/DEP-1, in developing and mature renal vasculature. J Am Soc Nephrol. 1999;10:2135–2145. doi: 10.1681/ASN.V10102135. [DOI] [PubMed] [Google Scholar]
- 3.Borges LG, et al. Cloning and characterization of rat density-enhanced phosphatase-1, a protein tyrosine phosphatase expressed by vascular cells. Circ Res. 1996;79:570–580. doi: 10.1161/01.res.79.3.570. [DOI] [PubMed] [Google Scholar]
- 4.Autschbach F, et al. Expression of the membrane protein tyrosine phosphatase CD148 in human tissues. Tissue Antigens. 1999;54:485–498. doi: 10.1034/j.1399-0039.1999.540506.x. [DOI] [PubMed] [Google Scholar]
- 5.de la Fuente-García MA, et al. CD148 is a membrane protein tyrosine phosphatase present in all hematopoietic lineages and is involved in signal transduction on lymphocytes. Blood. 1998;91:2800–2809. [PubMed] [Google Scholar]
- 6.Takahashi T, et al. A mutant receptor tyrosine phosphatase, CD148, causes defects in vascular development. Mol Cell Biol. 2003;23:1817–1831. doi: 10.1128/MCB.23.5.1817-1831.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takahashi T, et al. A monoclonal antibody against CD148, a receptor-like tyrosine phosphatase, inhibits endothelial-cell growth and angiogenesis. Blood. 2006;108:1234–1242. doi: 10.1182/blood-2005-10-4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang L, et al. Thyroid cell transformation inhibits the expression of a novel rat protein tyrosine phosphatase. Exp Cell Res. 1997;235:62–70. doi: 10.1006/excr.1997.3659. [DOI] [PubMed] [Google Scholar]
- 9.Trapasso F, et al. Restoration of receptor-type protein tyrosine phosphatase eta function inhibits human pancreatic carcinoma cell growth in vitro and in vivo. Carcinogenesis. 2004;25:2107–2114. doi: 10.1093/carcin/bgh224. [DOI] [PubMed] [Google Scholar]
- 10.Trapasso F, et al. Rat protein tyrosine phosphatase eta suppresses the neoplastic phenotype of retrovirally transformed thyroid cells through the stabilization of p27(Kip1) Mol Cell Biol. 2000;20:9236–9246. doi: 10.1128/mcb.20.24.9236-9246.2000. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Iuliano R, et al. An adenovirus carrying the rat protein tyrosine phosphatase eta suppresses the growth of human thyroid carcinoma cell lines in vitro and in vivo. Cancer Res. 2003;63:882–886. [PubMed] [Google Scholar]
- 12.Balavenkatraman KK, et al. DEP-1 protein tyrosine phosphatase inhibits proliferation and migration of colon carcinoma cells and is upregulated by protective nutrients. Oncogene. 2006;25:6319–6324. doi: 10.1038/sj.onc.1209647. [DOI] [PubMed] [Google Scholar]
- 13.Ruivenkamp CA, et al. Ptprj is a candidate for the mouse colon-cancer susceptibility locus Scc1 and is frequently deleted in human cancers. Nat Genet. 2002;31:295–300. doi: 10.1038/ng903. [DOI] [PubMed] [Google Scholar]
- 14.Grazia Lampugnani M, et al. Contact inhibition of VEGF-induced proliferation requires vascular endothelial cadherin, beta-catenin, and the phosphatase DEP-1/CD148. J Cell Biol. 2003;161:793–804. doi: 10.1083/jcb.200209019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chabot C, Spring K, Gratton JP, Elchebly M, Royal I. New role for the protein tyrosine phosphatase DEP-1 in Akt activation and endothelial cell survival. Mol Cell Biol. 2009;29:241–253. doi: 10.1128/MCB.01374-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tarcic G, et al. An unbiased screen identifies DEP-1 tumor suppressor as a phosphatase controlling EGFR endocytosis. Curr Biol. 2009;19:1788–1798. doi: 10.1016/j.cub.2009.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sacco F, et al. Tumor suppressor density-enhanced phosphatase-1 (DEP-1) inhibits the RAS pathway by direct dephosphorylation of ERK1/2 kinases. J Biol Chem. 2009;284:22048–22058. doi: 10.1074/jbc.M109.002758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palka HL, Park M, Tonks NK. Hepatocyte growth factor receptor tyrosine kinase met is a substrate of the receptor protein-tyrosine phosphatase DEP-1. J Biol Chem. 2003;278:5728–5735. doi: 10.1074/jbc.M210656200. [DOI] [PubMed] [Google Scholar]
- 19.Kovalenko M, et al. Site-selective dephosphorylation of the platelet-derived growth factor beta-receptor by the receptor-like protein-tyrosine phosphatase DEP-1. J Biol Chem. 2000;275:16219–16226. doi: 10.1074/jbc.275.21.16219. [DOI] [PubMed] [Google Scholar]
- 20.Massa A, et al. The phosphotyrosine phosphatase eta mediates somatostatin inhibition of glioma proliferation via the dephosphorylation of ERK1/2. Ann N Y Acad Sci. 2004;1030:264–274. doi: 10.1196/annals.1329.033. [DOI] [PubMed] [Google Scholar]
- 21.Baker JE, Majeti R, Tangye SG, Weiss A. Protein tyrosine phosphatase CD148-mediated inhibition of T-cell receptor signal transduction is associated with reduced LAT and phospholipase Cgamma1 phosphorylation. Mol Cell Biol. 2001;21:2393–2403. doi: 10.1128/MCB.21.7.2393-2403.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsuboi N, et al. The tyrosine phosphatase CD148 interacts with the p85 regulatory subunit of phosphoinositide 3-kinase. Biochem J. 2008;413:193–200. doi: 10.1042/BJ20071317. [DOI] [PubMed] [Google Scholar]
- 23.Meng K, et al. Pleiotrophin signals increased tyrosine phosphorylation of beta beta-catenin through inactivation of the intrinsic catalytic activity of the receptor-type protein tyrosine phosphatase beta/zeta. Proc Natl Acad Sci USA. 2000;97:2603–2608. doi: 10.1073/pnas.020487997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson KG, et al. The HSPGs Syndecan and Dallylike bind the receptor phosphatase LAR and exert distinct effects on synaptic development. Neuron. 2006;49:517–531. doi: 10.1016/j.neuron.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 25.Shen Y, et al. PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science. 2009;326:592–596. doi: 10.1126/science.1178310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dave RK, Hume DA, Elsegood C, Kellie S. CD148/DEP-1 association with areas of cytoskeletal organisation in macrophages. Exp Cell Res. 2009;315:1734–1744. doi: 10.1016/j.yexcr.2009.02.023. [DOI] [PubMed] [Google Scholar]
- 27.Hower AE, Beltran PJ, Bixby JL. Dimerization of tyrosine phosphatase PTPRO decreases its activity and ability to inactivate TrkC. J Neurochem. 2009;110:1635–1647. doi: 10.1111/j.1471-4159.2009.06261.x. [DOI] [PubMed] [Google Scholar]
- 28.Wälchli S, Espanel X, Hooft van Huijsduijnen R. Sap-1/PTPRH activity is regulated by reversible dimerization. Biochem Biophys Res Commun. 2005;331:497–502. doi: 10.1016/j.bbrc.2005.03.196. [DOI] [PubMed] [Google Scholar]
- 29.Zhu JW, Brdicka T, Katsumoto TR, Lin J, Weiss A. Structurally distinct phosphatases CD45 and CD148 both regulate B cell and macrophage immunoreceptor signaling. Immunity. 2008;28:183–196. doi: 10.1016/j.immuni.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trapasso F, et al. Genetic ablation of Ptprj, a mouse cancer susceptibility gene, results in normal growth and development and does not predispose to spontaneous tumorigenesis. DNA Cell Biol. 2006;25:376–382. doi: 10.1089/dna.2006.25.376. [DOI] [PubMed] [Google Scholar]
- 31.Zhang X, Lawler J. Thrombospondin-based antiangiogenic therapy. Microvasc Res. 2007;74:90–99. doi: 10.1016/j.mvr.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bornstein P. Thrombospondins: Structure and regulation of expression. FASEB J. 1992;6:3290–3299. doi: 10.1096/fasebj.6.14.1426766. [DOI] [PubMed] [Google Scholar]
- 33.Good DJ, et al. A tumor suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable from a fragment of thrombospondin. Proc Natl Acad Sci USA. 1990;87:6624–6628. doi: 10.1073/pnas.87.17.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taraboletti G, Roberts D, Liotta LA, Giavazzi R. Platelet thrombospondin modulates endothelial cell adhesion, motility, and growth: A potential angiogenesis regulatory factor. J Cell Biol. 1990;111:765–772. doi: 10.1083/jcb.111.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dawson DW, et al. CD36 mediates the In vitro inhibitory effects of thrombospondin-1 on endothelial cells. J Cell Biol. 1997;138:707–717. doi: 10.1083/jcb.138.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiménez B, et al. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat Med. 2000;6:41–48. doi: 10.1038/71517. [DOI] [PubMed] [Google Scholar]
- 37.Isenberg JS, et al. CD47 is necessary for inhibition of nitric oxide-stimulated vascular cell responses by thrombospondin-1. J Biol Chem. 2006;281:26069–26080. doi: 10.1074/jbc.M605040200. [DOI] [PubMed] [Google Scholar]
- 38.Crawford SE, et al. Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell. 1998;93:1159–1170. doi: 10.1016/s0092-8674(00)81460-9. [DOI] [PubMed] [Google Scholar]
- 39.Li Z, He L, Wilson K, Roberts D. Thrombospondin-1 inhibits TCR-mediated T lymphocyte early activation. J Immunol. 2001;166:2427–2436. doi: 10.4049/jimmunol.166.4.2427. [DOI] [PubMed] [Google Scholar]
- 40.Zhang X, et al. Thrombospondin-1 modulates vascular endothelial growth factor activity at the receptor level. FASEB J. 2009;23:3368–3376. doi: 10.1096/fj.09-131649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kanda S, Shono T, Tomasini-Johansson B, Klint P, Saito Y. Role of thrombospondin-1-derived peptide, 4N1K, in FGF-2-induced angiogenesis. Exp Cell Res. 1999;252:262–272. doi: 10.1006/excr.1999.4622. [DOI] [PubMed] [Google Scholar]
- 42.Senis YA, et al. The tyrosine phosphatase CD148 is an essential positive regulator of platelet activation and thrombosis. Blood. 2009;113:4942–4954. doi: 10.1182/blood-2008-08-174318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tangye SG, et al. Negative regulation of human T cell activation by the receptor-type protein tyrosine phosphatase CD148. J Immunol. 1998;161:3803–3807. [PubMed] [Google Scholar]
- 44.Knowles DM, 2nd, et al. Monoclonal anti-human monocyte antibodies OKM1 and OKM5 possess distinctive tissue distributions including differential reactivity with vascular endothelium. J Immunol. 1984;132:2170–2173. [PubMed] [Google Scholar]
- 45.Swerlick RA, Lee KH, Wick TM, Lawley TJ. Human dermal microvascular endothelial but not human umbilical vein endothelial cells express CD36 in vivo and in vitro. J Immunol. 1992;148:78–83. [PubMed] [Google Scholar]
- 46.Kaur S, et al. Thrombospondin-1 inhibits VEGF receptor-2 signaling by disrupting its association with CD47. J Biol Chem. 2010;285:38923–38932. doi: 10.1074/jbc.M110.172304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Isenberg JS, et al. Thrombospondin-1 limits ischemic tissue survival by inhibiting nitric oxide-mediated vascular smooth muscle relaxation. Blood. 2007;109:1945–1952. doi: 10.1182/blood-2006-08-041368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whiteford JR, et al. Syndecan-2 is a novel ligand for the protein tyrosine phosphatase receptor CD148. Mol Biol Cell. 2011;22:3609–3624. doi: 10.1091/mbc.E11-02-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Isenberg JS, Frazier WA, Roberts DD. Thrombospondin-1: A physiological regulator of nitric oxide signaling. Cell Mol Life Sci. 2008;65:728–742. doi: 10.1007/s00018-007-7488-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.