Abstract
Primates depend for their survival on their ability to understand their social environment, and their behavior is often shaped by social circumstances. We report that the orbitofrontal cortex, a brain region involved in motivation and reward, is tuned to social information. Macaque monkeys worked to collect rewards for themselves and two monkey partners. Behaviorally, monkeys discriminated between cues signaling small and large rewards, and between cues signaling rewards to self only and reward to both self and another monkey, with a preference for the former over the latter in both instances. Single neurons recorded during this task encoded the meaning of visual cues that predicted the magnitude of future rewards, as well as the motivational value of rewards obtained in a social context. Furthermore, neuronal activity was found to track momentary social preferences and partner's identity and social rank. The orbitofrontal cortex thus contains key neuronal mechanisms for the evaluation of social information.
Keywords: decision making, social cognition, neurophysiology
Converging sources of evidence from lesion, electrophysiological, and neuroimaging studies show that the orbitofrontal cortex (OFC) plays a key role in motivated behavior (see, for example, refs. 1 and 2). The OFC has been shown to contain a representation of various natural reinforcers, both appetitive (3) and aversive (4), and to be involved in encoding the subjective value of stimuli (5, 6). This finding has led to the conjecture that a common neural-reward currency might exist, which the brain could use to evaluate the cost and benefits of different options during decision making (7, 8).
Obviously, decision making is a process that must integrate a large number of parameters and one such parameter lies in the fact that primates, like many other species, are inherently social beings. Social cues from the environment and internal drives, such as concern for others, social comparison, or perceived status, have a strong impact on our behavior and on the value we assign to objects or actions. Several studies have contributed to defining the contours of a “social brain” dedicated to the processing of socially relevant stimuli and to the regulation of emotional and behavioral responses in a social context (9). Logically, this cerebral network shows considerable overlap with brain structures involved in motivation and value-based decision making, including the OFC. In monkeys, damage to the OFC causes social-interaction deficits, such as a decrease in communicative facial expressions, reduced responses to threatening or affiliative gestures, and impaired development of maternal bonding (10). In humans, damage to the OFC is associated with personality changes and socially inappropriate behavior (11–13), and with impairments in the expression of social emotions, such as attachment, fear, and aggression (14, 15). Notably, it has been suggested that the OFC is involved in valuation mechanisms in social context and contributes to decision making based on social reputation and trust (16), and in the generation of emotions, such as anticipated regret (17, 18).
Somewhat surprisingly, little information is available on the fine-scale neuronal processing of social information and its influence on valuation mechanisms. In the present study we asked the question whether social variables are represented and can modulate value encoding by the OFC.
Results
We recorded single OFC neurons in two monkeys who were trained on a simple visual discrimination task in which they could earn rewards for themselves and for two passive monkey partners physically present in the testing room (Fig. 1). Two main blocks of trials were run (Fig. 2A). In the nonsocial block, only the active monkey earned reward, in the form of a small, medium, or large drop of water. A different visual shape was associated with each reward outcome. These “self-only” trials served to identify reward-value sensitive neurons. In the social block, medium-sized reward trials to self-only were intermixed with “joint” reward trials, where both the monkey and a predesignated partner earned a medium-sized reward. This block served to test the hypothesis that reward value is influenced by the social context and to establish whether this influence is reflected in reward prediction activity by OFC neurons. Because access to fluid was only allowed when in the laboratory and for a fixed period, the best way for the monkey to maximize fluid intake was to respond correctly on all trials for as long as it was allowed to work. Under such circumstances, performances were not expected to show large variations across reward conditions, but where present, were taken as an indicator of the incentive value of the different conditions.
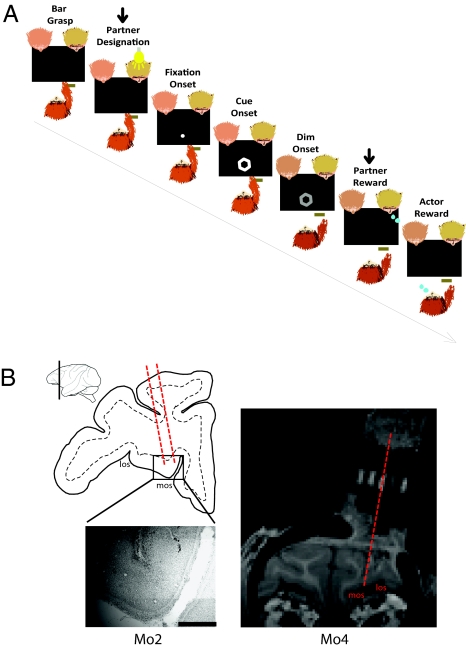
Fig. 1.
Task description and localization of recording sites in the OFC. (A) Typical joint-reward trial sequence in which a monkey responds to a visual instruction by releasing a manual lever to obtain a reward for itself and for a predesignated partner. The shape of the reward cue specifies the trial's reward contingency. The arrow above the panels indicates the steps that are omitted on self-only reward trials. (B, Left) Drawing of a coronal section through the left frontal lobe of monkey Mo2 at the level indicated on the lateral view. Marks left by two electrode tracks are visible on the enlarged Nissl-stained picture. (Scale bar, 1 mm.) At the level shown in this drawing, area 13 is roughly bounded laterally by the fundus of the lateral orbital sulcus (LOS) and medially by the fundus of the medial orbital sulcus (MOS). (Right) Coronal view of anatomical MRI scans of monkey Mo4 showing the recording chamber position. A special grid with five holes in the central row filled with omega 3 and spaced 3-mm apart was inserted in the chamber, serving as reference to identify the position and angle of electrode tracks with respect to the underlying cortical tissue.
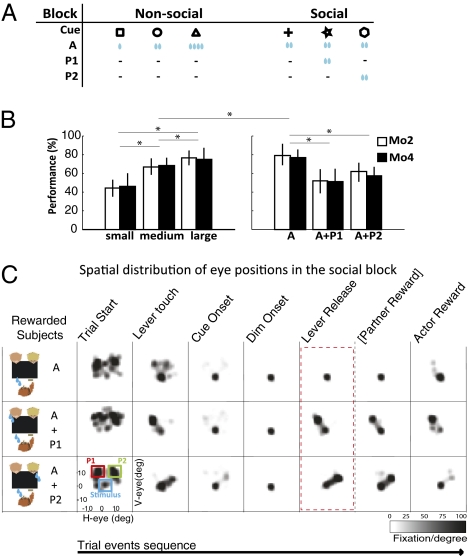
Fig. 2.
Behavioral performance. (A) Reward configurations on nonsocial and social trial blocks. (B) Mean percent correct responses of monkeys Mo2 (n = 47 sessions) and Mo4 (n = 45 sessions) as a function of reward size in the nonsocial block, and for joint and self-only reward conditions in the social block (asterisks and horizontal lines indicate significant pair-wise comparisons, P < 0.01). (C) Distribution of eye fixations over the workspace for different task epochs (combined data from Mo2 and Mo4). On joint-reward trials, monkeys briefly looked at the designated partner's face before fixing their eyes on the visual cue. Right after responding to dimming of the cue, they shifted their gaze back to the partner's face, thereby anticipating the delivery of the partner's reward. On self-only reward trials, the monkeys kept looking at the screen until their own reward was delivered.
Not surprisingly, in the nonsocial block the monkeys worked significantly better for large than for small rewards (P < 0.01 for monkeys Mo2 and Mo4) (Fig. 2B), thus exhibiting a straightforward relation between reward size and motivational value. In the social block, monkeys were on average more willing to work when they were the sole reward recipient than when rewards were granted jointly to the monkey and a partner (P < 0.01 for both monkeys). Note that the amount of water earned by the working monkey was constant across the different conditions of the social block (i.e., the reward was not divided between the monkey and her partner). This finding suggests that the active monkey's perception of its own reward was somehow devalued by the concomitant reward given to the partner (further information on behavioral performance in the social block is presented in SI Experimental Procedures, Behavior and Fig. S1).
To check that, in the social block, the lower performance on joint-reward trials was related to the social context and not merely to the perceived loss of a potential reward, a control experiment was run on one of the monkeys. Self-only and joint-reward trial blocks were conducted with the monkey facing partner 1 on one side and an empty monkey chair equipped with a reward dispenser on the other side. On joint trials with the empty chair as “partner,” after the monkey responded to the dimming of the cue, a drop of water came out of the sipper tube but no one was there to collect it and it simply dripped to the floor. In this task configuration, the proportion of correct responses was significantly reduced on true joint trials, but not on empty-chair trials (Fig. S2), indicating that reward devaluation in the context of this task is specifically induced by the social context through a process of social comparison.
Neural Encoding of Value for Rewards Obtained in a Social Context.
Single-unit recordings were carried out in the OFC. The main criterion that was applied to select a neuron for recording was that it exhibited a clear visual responsiveness. Task-related responses were found in 163 of 213 recorded neurons, mostly located within area 13 of the OFC (see Fig. 1B for verification of recording locations). These cells exhibited statistically significant discharge activity related to at least one task event: partner designation, reward-cue presentation, manual response to cue dimming, reward delivery (Table 1). The present report focuses on cue-related activity. Sixty neurons showed such activity in both nonsocial and social trial blocks and thus allowed us to analyze reward-value signals in both contexts.
Table 1.
Neuronal response counts
| Partner designation | Cue onset | Bar release | Partner reward | Actor reward | Total | |
| Mo 2 | 19 | 53 | 41 | 25 | 15 | 153 |
| Mo 4 | 12 | 34 | 15 | 19 | 10 | 90 |
| Total | 31 | 87 | 56 | 44 | 25 | 243 |
Of 213 total recorded neurons, 163 OFC cells (89 and 74 neurons recorded in Mo2 and Mo4, respectively) had complete datasets and activity associated to at least one task event. A given cell could respond to more than one event. Column totals represent the number of cells that responded to a given event. Row totals correspond to the number of significant responses.
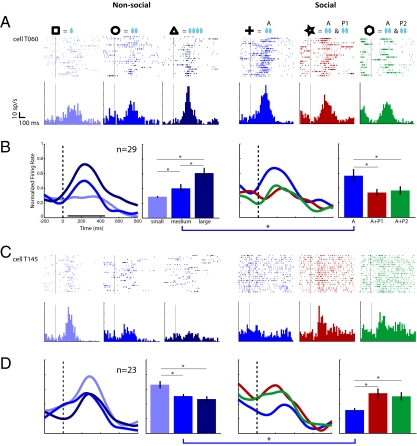
Let us first consider the nonsocial block: the majority of cue-responsive neurons were modulated by reward size and fell in one of two main categories: (i) up-modulated cells, showing a monotonic increase in firing rate with predicted reward size (n = 29/60, 48%) and (ii) down-modulated cells, showing a monotonic decrease in firing rate with predicted reward size (n = 23/60, 38%). Such neurons have been reported consistently in the OFC and are generally interpreted as describing the motivational value of anticipated rewards (5, 19–21). The remaining neurons (n = 8/60, 13%) showed complex, nonlinear firing patterns to the three reward sizes.
Because these OFC neurons also responded to the reward instruction cues in the social block, it was possible to test the hypothesis that cue-related activity encodes the motivational value of expected rewards when motivation is conditioned by the social context. Following the behavioral results, we predicted that if one's own reward is devalued by a partner's concomitant reward, then this diminished value should be reflected in the firing pattern of motivation-coding neurons. This result is indeed what we found. Up-modulated cells responded more strongly to the cue announcing a self-only reward than to cues indicating that rewards would be jointly obtained, in close correspondence to the behavioral pattern (Fig. 3A). Down-modulated cells responded with a weaker discharge for self-only than for joint rewards (Fig. 3C). Population activity for the cells that responded to the reward cue in both the nonsocial and the social blocks are shown in Fig. 3B (up-modulated n = 29, nonsocial block P < 0.04, social block P < 0.002) and Fig. 3D (down-modulated n = 23, social block P < 0.05, nonsocial block P < 0.03). It can be observed that precue activity level is higher in the social block, in particular, in Fig. 3D. This difference is in part because some of these neurons had a visual response to faces at the time of partner designation (see below). However, baseline firing rate of the neurons between trials was also higher in the social than in the nonsocial block both at the single unit (compare e.g., in Fig. 3C, the medium-blue rasters in the nonsocial and in the social blocks) and population levels (Fig. S3). This global activity change might signal a heightened state of arousal in the social block reflecting the monkey's knowledge that the partners would be receiving water, and perhaps also, the perception of some subtle change in the behavior of the partners themselves. To test whether activity levels before the cue accounted for the differences between reward conditions in the social block, we ran separate analyses of covariance on the up-modulated and down-modulated populations, using mean activity during the 500-ms period before cue onset as a covariate. The effect of joint-reward cues remained significant when the variance associated with the precue activity, was accounted for in both neuronal subpopulations [up-modulated: F(2,81) = 4.48, P < 0.02; down-modulated: F(2,63) = 3.80, P < 0.03].
Fig. 3.
Single-unit and population activity of OFC neurons in the nonsocial and social blocks. (Left three panels in A and C) Examples of single units with, respectively, up-modulated and down-modulated responses as a function of reward size; (Right three panels) Activity of the same two neurons in the social block. (B and D) Normalized spike density curves and mean discharge rate for the neuron population from which the corresponding single-unit examples are drawn. The thick horizontal bar below the spike density curves in B indicates the time window used for computing all statistical tests on mean population activity (Right). The asterisks and thin black or blue horizontal lines indicate significant pair-wise comparisons (P < 0.01).
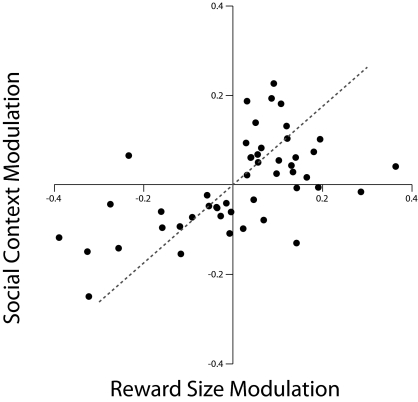
To quantify the relation between cell discharge activity in the two blocks, we computed for each neuron a modulation index for the reward size effect in the nonsocial block and for the reward-sharing effect in the social block (SI Experimental Procedures, Data Analysis). The two indices were significantly correlated (r = 0.57, P < 0.02) (Fig. 4). Therefore, neuronal populations that respond to predicted reward size, through excitatory or inhibitory modulation, do not merely report the expected quantity of reward but the motivational value assigned to it. In keeping with the notion that motivation is a complex multivariate construct (22), the present results suggest that its neural representation in the OFC integrates the influence of socially based factors—in addition to intrinsic properties—on the motivational value of rewards.
Fig. 4.
Relationship between reward size and social context modulation. Modulation indices of general form (a − b)/(a + b) were computed for all cells exhibiting cue-related response in the nonsocial and social block (SI Experimental Procedures, Data Analysis). For indicative purposes, indices of 0.11, 0.20, and 0.33 correspond to activity differentials of 25%, 50%, and 100%, respectively (regression r2 = 0.329, P < 0.02).
In studies of choice behavior, OFC neurons are found to show range adaptation and encode rewards in relative terms, their firing rate scaling to the preference between food items (5) and to the range of offered reward values (23, 21). A similar phenomenon is observed here when comparing the response to the identical outcome in the nonsocial and social blocks. The cue response to the medium self-only reward in the social block for the up-modulated cell population is higher (or lower for the down-modulated cell subset) than the response to the same medium reward in the nonsocial block. Thus, the motivational value of a given reward offer is influenced by the social context and this socially adjusted value is reflected in the level of OFC activity, as it is in the monkeys’ behavior (Fig. 1B, middle bars, Left and left bars, Right).
Evidence for Social Preference?
Performance on joint-reward trials did not, on average, depend on who the partner was, whether it was the dominant member of the social group (partner 1, Mo1) or the partner who could procure rewards on the monkey's “day off” (partner 2: Mo2 for Mo4 and Mo4 for Mo2). This finding could suggest that the monkey did not clearly associate the joint-reward cue to a specific partner, but this is clearly not the case. Eye-movement records (Fig. 2C) show that although the monkeys looked exclusively at the center of the screen until the dimming of the cue, following lever response their gaze anticipated the reward outcome. On self-only trials, the monkey kept looking at the screen center until the delivery of its own reward. On joint-reward trials, the monkey gazed at the future reward recipient, well before delivery, and never looked at the other partner. The absence, on average, of a clear social preference, might thus suggest that monkeys were aware of which of the two partners would be rewarded but had no particular incentive to favor one over the other. Another possibility is that social preferences existed but were fluctuating and masked by session-to-session variability. We therefore examined behavioral data from the social block and reassessed the corresponding single-unit activity to test whether neuronal modulations associated with reward prediction tracked the monkeys’ social biases. We identified sessions in which there was a significant difference in the proportion of rewards procured to partner 1 and partner 2 (χ2 test, α-level set at P < 0.05). A social-preference effect was present in one-fourth of the sessions (23 of 92), and in the majority of cases (18 of 23, 78%) we found that the monkeys were selectively biased against partner 1 and in favor of partner 2. The reason for this finding is unclear. It could indicate a greater reluctance to procure rewards to a dominant monkey than to a lower ranking one. Another possibility is that monkeys Mo2 and Mo4, who acted as each other's partner 2, might have been displaying a form of differed reciprocity but were somewhat disinclined to share with the monkey who could never reciprocate. Whichever is the correct explanation, the fact that a systematic behavioral preference was present in some of the sessions allowed us to search for its neural correlates in the OFC.
The neuronal population illustrated in Fig. 3 was therefore sorted according to performance on individual recording sessions, distinguishing between sessions in which partner 2 was preferred over partner 1 and sessions in which no preference or the opposite preference was found. The results are presented in Fig. S4 and show selectively enhanced responses to the partner 2 cues on sessions in which it is the preferred partner, but no such enhancement on sessions in which no behavioral bias is present. Thus, cue-related activity on joint-reward trials reflected the current social preference of the monkeys performing the task.
Neural Activity in the OFC Unrelated to Motivational Value.
In addition to neurons that directly encode motivational value of joint rewards, we found other cells in the OFC that showed cue activity related to the social context of the task. These cells encoded the identity of the rewarded individuals in the social block, and responded selectively or maximally to one of the three conditions: self-only reward (n = 8), joint reward with partner 1 (n = 8), joint reward with partner 2 (n = 10). The remaining cells responded indiscriminately to the three conditions (n = 2) (Fig. S5). However, because these cells showed no reward size-related modulation in the nonsocial block, they cannot be considered as carrying a motivational value signal. It is probably more accurate to interpret the significance of these responses as encoding the identity of the reward recipient specified by the cue. Thus, although one subpopulation of cue-responsive neurons conveys the motivational value of rewards (“what this reward is worth to me”), extending this role to socially influenced value, another subpopulation carries the social context information (“who is getting a reward”), which is needed for value computation.
A social dimension was also represented by a class of OFC neurons that discharged when the monkeys gazed at their partner's face at the onset of joint-reward trials. These cells were modulated by facial identity (Fig. 5A). Surprisingly, the distribution of these face-sensitive neurons was strongly biased, with a large majority of cells responding preferentially to the face of partner 1 (partner 1 n = 22, partner 2 n = 4, no difference n = 5, χ2 = 19.9, P < 0.0001), a phenomenon that accounts for the large difference visible in the population responses (Fig. 5B). It is highly unlikely that such a difference could result from a sampling bias. One possible explanation is that the monkeys attended more and directed their gaze longer to partner 1, the dominant monkey, than to partner 2. We therefore computed the amount of time the monkeys spent looking at each face in the 500-ms interval between partner designation and fixation of the central target. Monkey Mo2 showed no gazing bias (respectively 122 and 110 ms for partner 1 and partner 2, t test P = 0.60) but monkey Mo4 showed a nonsignificant preference for looking at partner 2 (respectively 120 and 154 ms for partner 1 and partner 2, t test P = 0.06).
Fig. 5.
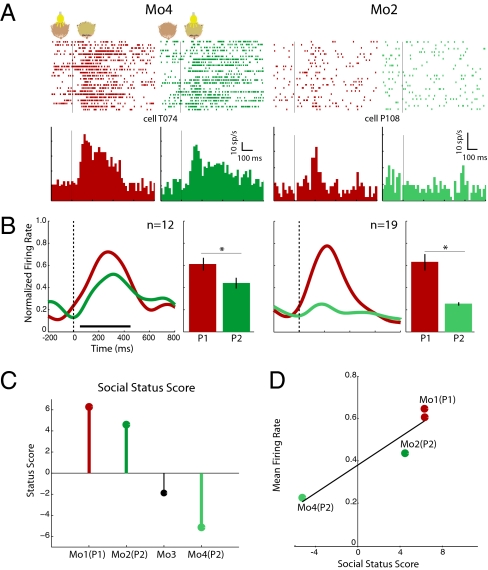
Face-selective responses of OFC neurons. (A) Single unit from monkeys Mo4 (Left) and Mo2 (Right). Both cells respond more strongly to P1 than P2 (Left: P1= 62.4 sp/s and P2= 52.5 sp/s; Right: P1= 24.8 sp/s and P2= 9.5 sp/s). (B) Respective normalized population spike density curves and mean discharge rate. (C) Social status score of the four members of the colony (SI Experimental Procedures, Animals). (D) Relationship between face-selective mean population activity and social status score (regression r2 = 0.937, P < 0.002). Color codes correspond to the same individuals in the different panels. (Conventions as in Fig. 3, asterisk and horizontal lines indicate significant pair-wise comparisons, P < 0.05 or better).
An alternative explanation for the stronger representation of partner 1 is that face neurons in the OFC encode social status. We approached this issue using our limited dataset by quantifying the hierarchical position of each monkey in the colony using observations of their natural social interactions (SI Experimental Procedures, Animals). We then examined cell-population activity separately for each of the recorded monkeys and related it to the social rank of their respective partners (Fig. 5B). Monkey Mo2's partners were Mo1, the highest-ranked member of the minicolony, and Mo4, the lowest-ranked member. Population activity for cells recorded from monkey Mo2 showed a large difference between the high- and low-status partners (P < 0.001). In contrast, monkey Mo4's partners were Mo1, the highest-status monkey, and Mo2, who was ranked lower but close to Mo1. In this monkey, population activity showed only a small difference between the two partners (P < 0.04). Pooling the mean population responses from the two monkeys and plotting these against measured social rank suggests that the activity of OFC face-selective neurons encode the perceived social rank of an individual relative to the observer (Fig. 5 C and D). Obviously, a more rigorous test of this hypothesis would necessitate a broader set of exemplars of faces of monkeys with different social ranks.
Discussion
Several brain areas contain neurons that carry reward information, including the midbrain dopaminergic nuclei, basal ganglia, and several cortical areas (24, 25). It has been proposed that one characteristic of the OFC is its capacity to encode reward value in a common neural currency (23). This capacity is a useful mechanism in decision making when one needs to choose between disparate alternatives, like different kinds of food, and between alternatives that are complex and require combining several positively and negatively weighted attributes, as in most real-life situations. Social information is one such attribute and it can possess a finite value, as shown in a behavioral study in which monkey observers could “pay” for access to social information by renouncing to a certain amount of juice reward (26). In the present study, we show that social information weighs on the valuation process that takes place in the OFC, and that it is expressed in neurons describing the value assigned to joint and self-only rewards.
Social comparison provided the monkey with a means to evaluate its own reward. When it expected to acquire a given reward size for itself but also that a monkey partner would receive an identical reward, the monkeys’ motivation, as measured through its correct response rate, was reduced to a level corresponding to a smaller reward size in the nonsocial block. This finding suggests that the knowledge that the partner would obtain a reward had a value that was in a sense “subtracted” from the intrinsic value of the monkey's reward. One possible explanation for such behavior is an adversity to the inequity inherent in the temporal order of rewards. The delay between lever release and delivery of reward to the active monkey was the same for all reward conditions; hence, the temporal discounting of the rewards was objectively constant. However, the fact that the partner received its reward first might have generated a perceived inequity. Our results are nevertheless consistent with other studies, where this explanation may not apply. A behavioral experiment related to our own, but using a direct preference response, also found that monkeys more often choose a reward to self-only than a reward to both self and another monkey (27). Furthermore, observations made on a large animal colony showed that when a macaque can choose, by pulling on one of two slides, between granting food access to itself only or to both itself and a partner sitting in an adjacent compartment, altruistic behavior is not exhibited systematically (28). Prosocial choices are generally directed by high-ranking individuals toward low ranking ones, and almost exclusively in favor of kin partners. The lower in the social hierarchy and the more distant the kinship, the more egoistical monkeys behave toward others (see SI Experimental Procedures, Working for No Reward? for further evidence of lack of altruism in this type of experimental paradigm).
The monkeys’ lower motivation to work for joint rewards is encoded through reduced activity in one class of neurons, the firing rate of which is a direct function of reward value, and through enhanced activity in another class of neurons, the firing rate of which is an inverse function of reward value. A further analysis indicates that OFC neurons may also track social preference by showing enhanced activity when joint rewards are obtained with the session's preferred partner. This finding argues for a motivational-value representation in the OFC that integrates the influence of social context on goal-directed actions. Such mechanisms would be well suited to play a role in evaluation processes taking place during social comparison (29, 30), and in emotions such as regret, envy, or gloating, which influence our decision making (31, 32).
In the present study, the animal's choice was between accepting and declining a reward offer. Declining an offer had a cost in terms of access to fluid and should happen only if the expected benefit does not justify the expenditure of attentional and motor resources needed to generate the operant response. The relationship between the behavior and the neuronal responses that we measured in the OFC thus reflect the motivational value of individual reward outcomes. Subjective preferences were inferred indirectly by comparing the proportion of accepted offers for each individual option. This aspect is a limitation of our study, compared with experiments that use forced-choice paradigms, and thus allow computations of neural functions of decision value (5, 23, 27). Nevertheless, the fact that the reward-size effects that we found in the OFC are qualitatively similar to those reported in decision-making studies suggests that the present results do capture some aspect of subjective value. In fact, there is good evidence that identical conclusions about the effects of reward value on behavior can be drawn from a single cue or a choice task (21).
Although value-encoding is a salient feature of OFC function (3), this is by no means its only function and we found other classes of neurons that were intermingled with reward-value sensitive neurons. Macaque monkeys have a cognitive representation of identity and spontaneously associate corresponding faces and voices of familiar conspecifics (33). Visual responses to faces have been reported in the macaque OFC (19) and a prefrontal face-specific zone has been identified in macaques at a location consistent with our recording sites using functional MRI (34). Here we report that neurons responding when monkeys look at the face of other group members are not merely selective to facial features, but describe the identity of individual faces. The fact that an enhanced response to the high-status partner's face was found in both of the recorded monkeys is consistent with a possible neuronal representation of social rank. Although this conclusion must await further evidence, because the social group we studied was quite small, it is quite plausible given that one well-known characteristic of social organization in macaque societies is the matrilineal hierarchy that exists between families and is inherited from mothers to daughters (35). Awareness of one's social rank and of the rank of other members of the group has important adaptive value (access to shared resources, protection against competitors), and it is not surprising that female monkeys in captivity also exhibit strict dominance relationships. Computation of social status by the OFC might be a prerequisite for strategic adaptation of behavior to the social context, a process in which the more lateral portion of the prefrontal cortex has been implicated (36).
In conclusion, we show that the activity of area 13 neurons is modulated in a manner that could mediate social comparison and decision-making in a social context. How pervasive such properties are within the network of areas that have been linked to the processing of reward, motivation, and emotions is unclear. Neuroimaging data suggest a possible topography related to the degree of abstractness and to the valence of reinforcers within in the human OFC (37). Although social modulations of neuronal activity were observed throughout the portion of area 13 that was explored in the present study, it would be interesting in the future to explore possible functional homologies between the two species. Other regions that are functionally related to the OFC, particularly the insula (38) and the anterior cingulate cortex (39, 40), have also been implicated in these functions. Further work is needed to characterize more precisely the nature of social information processing in these different brain structures.
Experimental Procedures
The monkeys who participated in the experiments were all females, housed in a hierarchically organized colony of four animals. Monkey Mo1, the dominant member of the group, served as one of the passive partners. Monkeys Mo2 and Mo4 performed the task and also served as each other's passive partner (designated as partner 2) on alternate sessions. Briefly, rewards could be earned by fixating a visual cue appearing on a horizontal screen placed in full view of the three monkeys, and releasing a hand-held lever within 400 ms of cue-dimming. No reward was distributed if the monkey broke fixation or released the lever too early or too late. The cue's shape specified the reward condition. In the nonsocial block, only the active monkey was rewarded and the cue predicted the size of the upcoming reward. In the social block, the cue specifies whether the current trial was a “self-only” trial, in which the active monkey earned a medium-sized reward (33%), or a “joint” trial, in which both the active monkey and either partner 1 (33%) or partner 2 (33%) earned a medium-sized reward.
Further details on the behavioral and electrophysiological methods are presented in SI Experimental Procedures.
Supplementary Material
Acknowledgments
We thank Jean-Luc Charieau and Fabrice Hérant for expert animal care; Véronique Sgambato-Faure for histological assistance; and Léon Tremblay for helpful discussion. This study was funded by the Centre National de la Recherche Scientifique; Human Frontier Science Program RGP0056/2005-C (to A.S. and J.-R.D.); and Agence Nationale de la Recherche BLAN-SVSE4-023-01 (to J.-R.D.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1111715109/-/DCSupplemental.
References
- 1.Rolls ET, Hornak J, Wade D, McGrath J. Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage. J Neurol Neurosurg Psychiatry. 1994;57:1518–1524. doi: 10.1136/jnnp.57.12.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plassmann H, O'Doherty J, Rangel A. Orbitofrontal cortex encodes willingness to pay in everyday economic transactions. J Neurosci. 2007;27:9984–9988. doi: 10.1523/JNEUROSCI.2131-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rolls ET. The orbitofrontal cortex and reward. Cereb Cortex. 2000;10:284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- 4.Morrison SE, Salzman CD. The convergence of information about rewarding and aversive stimuli in single neurons. J Neurosci. 2009;29:11471–11483. doi: 10.1523/JNEUROSCI.1815-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tremblay L, Schultz W. Relative reward preference in primate orbitofrontal cortex. Nature. 1999;398:704–708. doi: 10.1038/19525. [DOI] [PubMed] [Google Scholar]
- 6.Sescousse G, Redouté J, Dreher J-C. The architecture of reward value coding in the human orbitofrontal cortex. J Neurosci. 2010;30:13095–13104. doi: 10.1523/JNEUROSCI.3501-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montague PR, Berns GS. Neural economics and the biological substrates of valuation. Neuron. 2002;36:265–284. doi: 10.1016/s0896-6273(02)00974-1. [DOI] [PubMed] [Google Scholar]
- 8.Kim H, Shimojo S, O'Doherty JP. Overlapping responses for the expectation of juice and money rewards in human ventromedial prefrontal cortex. Cereb Cortex. 2010;21:769–776. doi: 10.1093/cercor/bhq145. [DOI] [PubMed] [Google Scholar]
- 9.Blakemore S-J. The social brain in adolescence. Nat Rev Neurosci. 2008;9:267–277. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- 10.Machado CJ, Bachevalier J. The impact of selective amygdala, orbital frontal cortex, or hippocampal formation lesions on established social relationships in rhesus monkeys (Macaca mulatta) Behav Neurosci. 2006;120:761–786. doi: 10.1037/0735-7044.120.4.761. [DOI] [PubMed] [Google Scholar]
- 11.Bechara A, Tranel D, Damasio H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain. 2000;123:2189–2202. doi: 10.1093/brain/123.11.2189. [DOI] [PubMed] [Google Scholar]
- 12.Moll J, Zahn R, de Oliveira-Souza R, Krueger F, Grafman J. Opinion: The neural basis of human moral cognition. Nat Rev Neurosci. 2005;6:799–809. doi: 10.1038/nrn1768. [DOI] [PubMed] [Google Scholar]
- 13.Forbes CE, Grafman J. The role of the human prefrontal cortex in social cognition and moral judgment. Annu Rev Neurosci. 2010;33:299–324. doi: 10.1146/annurev-neuro-060909-153230. [DOI] [PubMed] [Google Scholar]
- 14.Strenziok M, et al. Developmental effects of aggressive behavior in male adolescents assessed with structural and functional brain imaging. Soc Cogn Affect Neurosci. 2009;6:2–11. doi: 10.1093/scan/nsp036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willis ML, Palermo R, Burke D, McGrillen K, Miller L. Orbitofrontal cortex lesions result in abnormal social judgements to emotional faces. Neuropsychologia. 2010;48:2182–2187. doi: 10.1016/j.neuropsychologia.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 16.Shamay-Tsoory SG, Tibi-Elhanany Y, Aharon-Peretz J. The green-eyed monster and malicious joy: The neuroanatomical bases of envy and gloating (schadenfreude) Brain. 2007;130:1663–1678. doi: 10.1093/brain/awm093. [DOI] [PubMed] [Google Scholar]
- 17.Camille N, et al. The involvement of the orbitofrontal cortex in the experience of regret. Science. 2004;304:1167–1170. doi: 10.1126/science.1094550. [DOI] [PubMed] [Google Scholar]
- 18.Coricelli G, Dolan RJ, Sirigu A. Brain, emotion and decision making: The paradigmatic example of regret. Trends Cogn Sci. 2007;11:258–265. doi: 10.1016/j.tics.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Thorpe SJ, Rolls ET, Maddison S. The orbitofrontal cortex: Neuronal activity in the behaving monkey. Exp Brain Res. 1983;49:93–115. doi: 10.1007/BF00235545. [DOI] [PubMed] [Google Scholar]
- 20.Hikosaka K, Watanabe M. Delay activity of orbital and lateral prefrontal neurons of the monkey varying with different rewards. Cereb Cortex. 2000;10:263–271. doi: 10.1093/cercor/10.3.263. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi S, Pinto de Carvalho O, Schultz W. Adaptation of reward sensitivity in orbitofrontal neurons. J Neurosci. 2010;30:534–544. doi: 10.1523/JNEUROSCI.4009-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berridge KC. Motivation concepts in behavioral neuroscience. Physiol Behav. 2004;81:179–209. doi: 10.1016/j.physbeh.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Padoa-Schioppa C, Assad JA. The representation of economic value in the orbitofrontal cortex is invariant for changes of menu. Nat Neurosci. 2008;11:95–102. doi: 10.1038/nn2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin-Soelch C, et al. Reward mechanisms in the brain and their role in dependence: Evidence from neurophysiological and neuroimaging studies. Brain Res Brain Res Rev. 2001;36:139–149. doi: 10.1016/s0165-0173(01)00089-3. [DOI] [PubMed] [Google Scholar]
- 25.Salzman CD, Paton JJ, Belova MA, Morrison SE. Flexible neural representations of value in the primate brain. Ann N Y Acad Sci. 2007;1121:336–354. doi: 10.1196/annals.1401.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deaner RO, Khera AV, Platt ML. Monkeys pay per view: Adaptive valuation of social images by rhesus macaques. Curr Biol. 2005;15:543–548. doi: 10.1016/j.cub.2005.01.044. [DOI] [PubMed] [Google Scholar]
- 27.Chang SWC, Winecoff AA, Platt ML. Vicarious reinforcement in rhesus macaques (Macaca mulatta) Front Neurosci. 2011;5:27. doi: 10.3389/fnins.2011.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Massen JJM, van den Berg LM, Spruijt BM, Sterck EHM. Generous leaders and selfish underdogs: Pro-sociality in despotic macaques. PLoS ONE. 2010;5:e9734. doi: 10.1371/journal.pone.0009734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brosnan SF, De Waal FBM. Monkeys reject unequal pay. Nature. 2003;425:297–299. doi: 10.1038/nature01963. [DOI] [PubMed] [Google Scholar]
- 30.Yamagishi T, et al. The private rejection of unfair offers and emotional commitment. Proc Natl Acad Sci USA. 2009;106:11520–11523. doi: 10.1073/pnas.0900636106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shamay-Tsoory SG, Tomer R, Goldsher D, Berger BD, Aharon-Peretz J. Impairment in cognitive and affective empathy in patients with brain lesions: Anatomical and cognitive correlates. J Clin Exp Neuropsychol. 2004;26:1113–1127. doi: 10.1080/13803390490515531. [DOI] [PubMed] [Google Scholar]
- 32.Bault N, Coricelli G, Rustichini A. Interdependent utilities: How social ranking affects choice behavior. PLoS ONE. 2008;3:e3477. doi: 10.1371/journal.pone.0003477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sliwa J, Duhamel J-R, Pascalis O, Wirth S. Spontaneous voice-face identity matching by rhesus monkeys for familiar conspecifics and humans. Proc Natl Acad Sci USA. 2011;108:1735–1740. doi: 10.1073/pnas.1008169108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsao DY, Schweers N, Moeller S, Freiwald WA. Patches of face-selective cortex in the macaque frontal lobe. Nat Neurosci. 2008;11:877–879. doi: 10.1038/nn.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waal F. Peacemaking Among Primates. Cambridge, MA: Harvard University Press; 1989. [Google Scholar]
- 36.Fujii N, Hihara S, Nagasaka Y, Iriki A. Social state representation in prefrontal cortex. Soc Neurosci. 2009;4:73–84. doi: 10.1080/17470910802046230. [DOI] [PubMed] [Google Scholar]
- 37.Kringelbach ML, Berridge KC. Towards a functional neuroanatomy of pleasure and happiness. Trends Cogn Sci. 2009;13:479–487. doi: 10.1016/j.tics.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanfey AG, Rilling JK, Aronson JA, Nystrom LE, Cohen JD. The neural basis of economic decision-making in the Ultimatum Game. Science. 2003;300:1755–1758. doi: 10.1126/science.1082976. [DOI] [PubMed] [Google Scholar]
- 39.Behrens TEJ, Hunt LT, Rushworth MFS. The computation of social behavior. Science. 2009;324:1160–1164. doi: 10.1126/science.1169694. [DOI] [PubMed] [Google Scholar]
- 40.Rudebeck PH, Bannerman DM, Rushworth MFS. The contribution of distinct subregions of the ventromedial frontal cortex to emotion, social behavior, and decision making. Cogn Affect Behav Neurosci. 2008;8:485–497. doi: 10.3758/CABN.8.4.485. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.