Abstract
The ability to induce humoral and cellular immunity via antigen delivery through the unbroken skin (epicutaneous immunization, EPI) has immediate relevance for vaccine development. However, it is unclear which adjuvants induce protective memory CD8 T-cell responses by this route, and the molecular and cellular requirements for priming through intact skin are not defined. We report that cholera toxin (CT) is superior to other adjuvants in its ability to prime memory CD8 T cells that control bacterial and viral challenges. Epicutaneous immunization with CT does not require engagement of classic toll-like receptor (TLR) and inflammasome pathways and, surprisingly, is independent of skin langerin-expressing cells (including Langerhans cells). However, CT adjuvanticity required type-I IFN sensitivity, participation of a Batf3-dependent dendritic cell (DC) population and engagement of CT with suitable gangliosides. Chemoenzymatic generation of CT–antigen fusion proteins led to efficient priming of the CD8 T-cell responses, paving the way for development of this immunization strategy as a therapeutic option.
Most current vaccination methods involve intramuscular or intradermal injection of antigen with suitable adjuvants. However, this approach produces biohazardous needle waste, requires trained personnel for its implementation, and evokes needle phobia, a significant complication that reduces compliance (1). Transdermal or epicutaneous immunization (EPI) strategies aim to avoid these concerns through application of antigen and adjuvants to the unbroken skin. This approach yields both antibody and T-cell responses, including priming of CD8 T cells (2–5). However, little is known about the capacity of EPI to prime memory CD8 T cells capable of protection against pathogen challenge, nor do we understand how adjuvants operate when applied to the intact skin.
Several adjuvants can induce CD8 T-cell priming through EPI. These include toll-like receptor (TLR) agonists such as imiquimod and CpG (6–9), but also the ADP ribosylating bacterial exotoxins such as cholera toxin (CT) and the closely related Escherichia coli heat-labile enterotoxin (LT) (10–12). Application of such toxins to the skin is safe and effective in priming humoral and cellular responses in both mice and humans (4, 10, 13–15). However, previous studies failed to define which adjuvants afford optimal priming of CD8 T-cell responses and durable protective memory. Furthermore, whereas TLR pathways are well characterized, the basis for adjuvanticity of bacterial exotoxins remains mysterious.
Here we compared multiple adjuvants for epicutaneous priming and found that CT was superior in effective induction of CD8 T-cell responses, resulting in protective immunity against pathogen challenge. We find that CT-mediated adjuvanticity occurs in the absence of typical TLR and inflammasome signaling pathways and that langerin-expressing cells (including Langerhans cells) are dispensable for EPI using CT. The adjuvant properties of CT were, however, dependent on host sensitivity to type-I IFN and expression of monosialylated GM1 gangliosides (to which the CT-B subunit binds) and required a Batf3-dependent dendritic cell (DC) population. Using a unique protein engineering approach to efficiently and site-specifically couple peptides to the catalytic domain of a preassembled holotoxin, we show that a nontoxic version of CT can also be used to prime CD8 T-cell responses epicutaneously. Together, our data indicate that CT is a promising adjuvant for priming protective CD8 T-cell responses through the unbroken skin and that this process uses an unconventional adjuvant mechanism.
Results
Epicutaneous Vaccination Using CpG and Cholera Toxin as Adjuvants Induces a Primary CD8 T-Cell Response.
Although several reports describe epicutaneous priming of CD8 T cells (4, 5, 7–9), there is considerable variability in the timing and approach used to determine the T-cell response and the use of skin preconditioning (such as tape stripping and acetone treatment) before immunization. This has made it difficult to compare the efficacy of distinct adjuvants. Hence, we avoided any preconditioning that may disrupt skin barrier function, and simply hydrated the (unshaved) mouse ear skin before brief sequential application of antigen [chicken ovalbumin (OVA) protein or peptide] and a panel of adjuvants (SI Appendix, Fig. S1). As a positive control, we used s.c. immunization with OVA+LPS. To assay early CD8 T-cell responses, we adoptively transferred OVA/Kb-specific T-cell receptor (TCR) transgenic OT-I T cells and monitored the response of these cells 5–6 d following immunization (16).
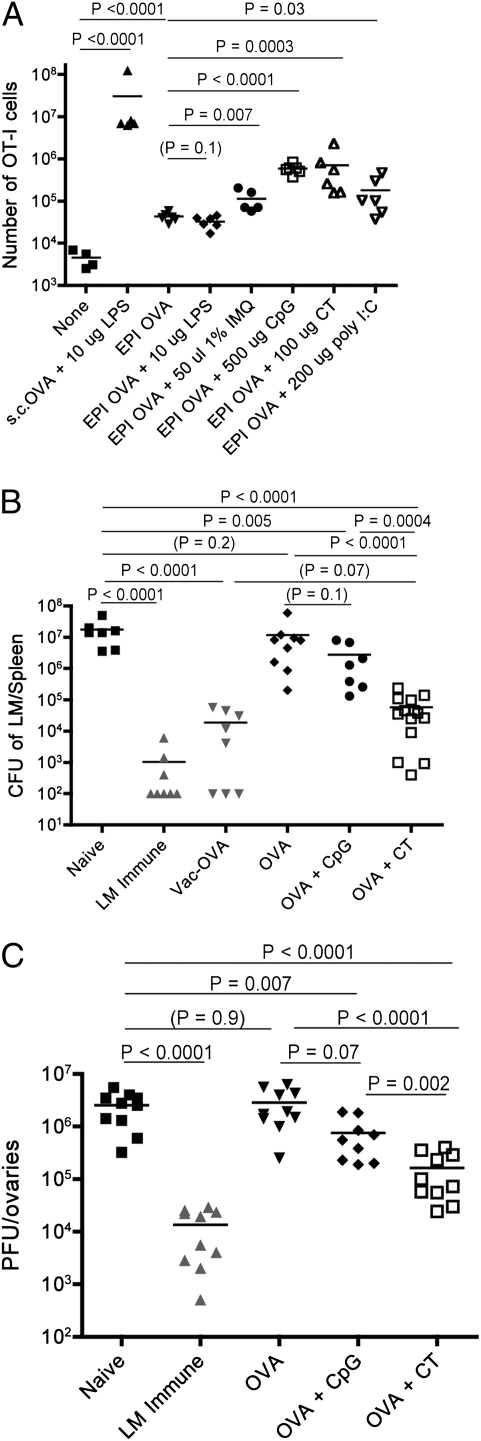
We tested several TLR agonists and the bacterial exotoxin CT as EPI adjuvants. Several TLR ligands enhanced the response by OT-I cells to OVA, but the TLR9 agonist CpG was especially effective (Fig. 1A). Interestingly, CT was similar to CpG in its potency as an EPI adjuvant (Fig. 1A). Consistent with previous studies (16, 17), we observed modest expansion of naïve OT-I T cells to EPI OVA protein alone. Both CT and CpG induced extensive carboxyfluorescein succinimidyl ester dilution and up-regulation of activation markers such as CD44, but epicutaneous priming using CT induced enhanced granzyme B expression levels compared with priming with CpG or other adjuvants (SI Appendix, Fig. S2). Utilization of OVA peptide (SIINFEKL) rather than OVA protein yielded similar results (SI Appendix, Fig. S3A), whereas studies involving EPI on the shaved flank skin revealed CT to be a notably stronger adjuvant than CpG or other TLR adjuvants (SI Appendix, Fig. S3B). Overall, these data demonstrated that EPI using CT yielded more consistent and robust CD8 T-cell priming than other adjuvants.
Fig. 1.
CT is a potent adjuvant for epicutaneous immunization of protective CD8 T cells. (A) C57BL/6 mice received 2.5 × 105 CD44low OT-I cells 1 d before epicutaneous immunization (EPI), which involved administration of OVA protein with the indicated adjuvants on the ear skin. As a positive control, mice were primed via s.c. priming with OVA and LPS. Six days postimmunization, expansion of OT-I cells was determined by flow cytometric analysis of spleen and lymph nodes. Mice listed as “none” received OT-I cells but no immunization. The dose of each adjuvant is indicated. Each symbol represents an individual mouse. The data are compiled from two experiments and are representative of at least three similar experiments. (B and C) B6 mice were primed via epicutaneous vaccination as in A, except that OT-I cells were not transferred. Thirty days later the animals were boosted with the same immunization approach, and after an additional 30 d were challenged. (B) Mice were challenged with LM-OVA and protective immunity was assayed 5 d later. The data show LM-OVA cfu in the spleen of the indicated animals. As positive controls for protective immunity, mice primed with LM-OVA (“LM immune”) or VV-OVA (“Vac-OVA”) were also challenged. Data are compiled from three to four independent experiments. (C) Mice were challenged with VV-OVA and viral control was measured in the ovaries 3 d later. As a positive control, some mice were infected with LM-OVA at least 1 mo before VV-OVA infection (LM immune). These data are compiled from three individual experiments.
EPI Using Cholera Toxin Induces Protective Memory CD8 T Cells.
To test priming of protective immunity, we eliminated OT-I adoptive transfer and examined the response of endogenous CD8 T cells. Previous studies characterized CD8 T-cell responses soon after immunization (4–6) and did not determine long-term protective immunity. Hence, we tested the capacity of EPI to induce protective immunity against recombinant Listeria monocytogenes (LM) expressing the Kb-restricted OVA epitope (LM-OVA). Immunity against LM depends on CD8 T cells (18), and hence is a rigorous test of functional priming. Protection against LM-OVA was minimal in response to a single round of EPI, but boosting using the same approach elicited potent protective immunity 30 d following the last immunization (SI Appendix, Fig. S4A). Despite the similarities between CpG and CT in priming CD8 T-cell responses (Fig. 1A), EPI involving CT as adjuvant produced significantly better protection against LM-OVA, as measured by pathogen clearance in the spleen (Fig. 1B) and liver (SI Appendix, Fig. S4B). Indeed, protection after EPI using OVA/CT was as potent as that induced by vaccination using a live vaccinia-OVA recombinant (VV-OVA) (Fig. 1B). Potentially the response to CT itself could induce LM immunity; however, priming animals with CT alone induced no protection (SI Appendix, Fig. S5). We also tested whether EPI could elicit protection against vaccinia virus infection. Again, whereas prime/boost using CpG did offer some measure of protection, EPI using CT as adjuvant offered significantly more potent immunity (Fig. 1C). We also explored how EPI priming using different adjuvants affected the size of the antigen-specific memory CD8 T-cell population. Peptide/MHC tetramer enrichment assays were conducted on cells from mice after EPI prime/boost. Whereas EPI using OVA with CT enlarged the OVA/Kb-specific pool, immunization with OVA alone or OVA/CpG failed to do so (SI Appendix, Fig. S6), presenting a plausible explanation for the differences in protective immunity observed for these groups.
Epicutaneous vaccination involving CT thus generates a functional memory CD8 T-cell population, capable of efficient protection against distinct microbes (LM and vaccinia) assessed at different anatomical sites (spleen and liver for LM-OVA; ovaries for VV-OVA), whereas EPI vaccination using CpG was significantly less potent.
Adjuvant Effects of CT in EPI Are Independent of Typical TLR and Inflammasome Signaling Pathways, but Do Require Type-I IFN Responses.
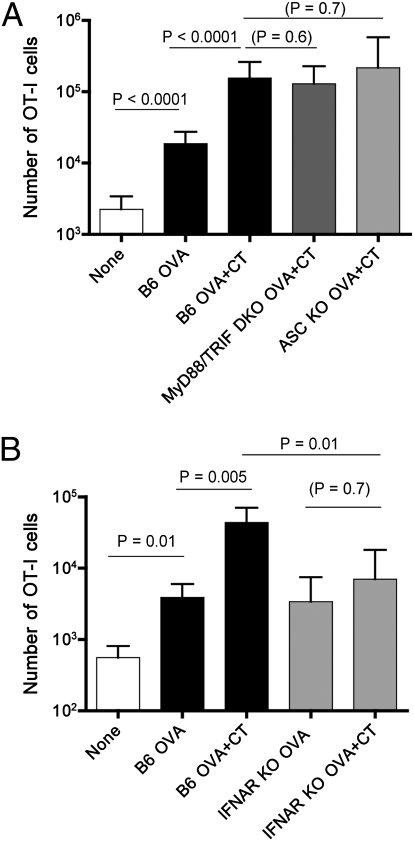
Our data extend previous reports on the potency of CT and the related exotoxin LT in epicutaneous priming of cellular and humoral responses in mice and humans (12–15), but raised the question of how CT confers its adjuvant effects. Most adjuvants induce “danger” signals to activate the innate immune response through pattern recognition receptors (PRRs) (19). TLRs are a major component of this group, and hence we first tested whether CT requires TLR signaling components to act as an adjuvant. All TLRs, and the related IL-1R, depend on one or both of the adapter proteins Myeloid differentiation primary response gene 88 (MyD88) and TIR-domain-containing adapter-inducing IFNβ (TRIF) (19). We therefore tested CT-mediated EPI using MyD88/TRIF double-knockout (dKO) hosts. As expected, MyD88/TRIF deficiency led to drastic reduction in the response of transferred OT-I cells to s.c. OVA/LPS (SI Appendix, Fig. S7A), but did not alter the basal OT-I response to EPI using OVA protein alone (SI Appendix, Fig. S7B). The adjuvant effect of CT was unimpaired by MyD88/TRIF deficiency (Fig. 2A), showing that CT does not require defined TLR or IL-1R signaling pathways for its action (Fig. 2A).
Fig. 2.
The adjuvant effect of CT is independent of typical TLR and inflammasome pathways but requires type-I IFN sensitivity. (A) B6, MyD88/TRIF DKO, and ASC KO mice received 2 × 105 naïve OT-I cells 1 d before indicated epicutaneous immunization on the ear, and expansion of the OT-I population was determined 5 d after priming. These data are pooled from three independent experiments. (B) B6 or IFNAR KO mice were adoptively transferred with naïve OT-I cells 1 d before the indicated immunization (on ear skin), and expansion of donor OT-I cells was monitored 5 d later. The data are compiled from two independent experiments and similar data were obtained in an additional similar experiment.
Other adjuvants activate the immune response through the NLR or inflammasome pathways, most of which operate through the inflammasome pathway, utilizing nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), most of which signal via the apoptosis-associated speck-like protein containing CARD (ASC) adapter protein (20). However, using ASC−/− mice (Fig. 2A) we determined that ASC deficiency did not impede the effects of CT in augmenting the OT-I T-cell response either. Thus, CT adjuvanticity requires neither prototypical inflammasome activation nor TLR signaling.
An earlier study found that the CD8 T-cell response to apoptotic cell-associated antigens was independent of MyD88/TRIF but required responsiveness to type-I IFN (21). To test for an analogous mechanism in our system, we adoptively transferred OT-I cells into IFNAR−/− (lacking the type-I IFN receptor) or WT control mice and primed with OVA (with or without CT) epicutaneously. The adjuvant effect of CT was lost in IFNAR−/− hosts (Fig. 2B). Because we used WT OT-I CD8 T cells, the type-I IFN sensitivity maps to host cells rather than to the responding CD8 T cells themselves. The adjuvant requirements for CT in EPI and cross-presentation of apoptotic cells (21) may therefore involve similar pathways.
Batf3-Dependent but Not Langerin-Expressing DCs Are Required for EPI Using CT as Adjuvant.
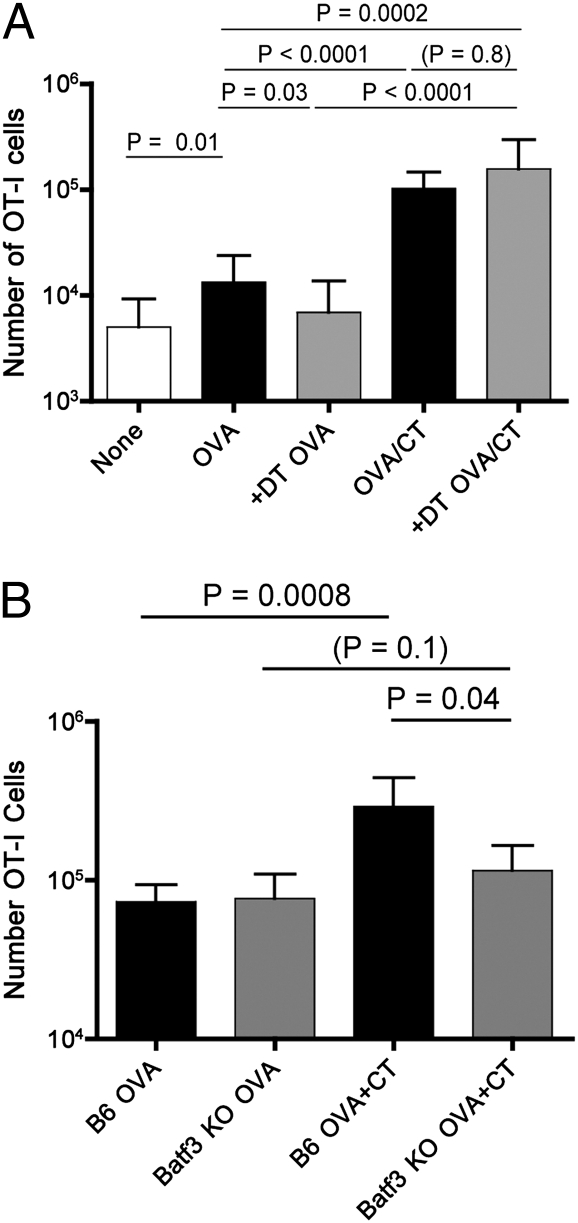
Langerhans cells (LCs) are the predominant antigen presenting cells (APC) in the epidermis, and hence likely candidates to be involved in epicutaneous vaccination (17, 22). Furthermore, dermal CD103+ DCs also express langerin (23) and are critical for CD8 T-cell priming following certain skin infections (24). To explore the requirement for langerin-expressing cells in CT-based EPI, we used mice in which the diptheria toxin receptor (DTR) was knocked into the langerin locus (Lang-DTR), allowing for conditional depletion of Langerhans and langerin+ dermal DC using DT injection (25). Earlier studies revealed that elimination of langerin-expressing cells impairs OT-I T-cell priming when OVA protein (without adjuvants) was applied to flank but not ear skin (16); hence, we immunized on the flank for this experimental series. Surprisingly, DT treatment had no significant impact on EPI when using CT as adjuvant (Fig. 3A), suggesting that neither LC or CD103+ DC are required for the epicutaneous adjuvant effects of CT, in contrast with previous studies using different EPI approaches (26, 27). We therefore sought to extend our analysis to other DC subsets. The Batf3 transcription factor is needed for generation of two major DC populations: CD103+ DC (including those in the dermis) and CD8α+ DC (28, 29). Following adoptive transfer of OT-I cells into Batf3−/− and control animals, we performed EPI using OVA protein, with or without CT. In contrast to wild-type hosts, the adjuvant effect of CT was almost completely lost in Batf3−/− animals, indicating a Batf3-dependent DC population is essential for this form of EPI (Fig. 3B).
Fig. 3.
The CT adjuvant effect in epicutaneous immunization involves a Batf3-dependent cell, but occurs in the absence of Langerhans cells. (A) Langerin–DTR mice were treated with diphteria toxin to deplete langerin+ cells (“+DT”) or were mock treated. Following adoptive transfer of naïve OT-I cells, the animals were vaccinated, on shaved flank skin, using OVA alone of OVA+CT. Expansion of OT-I cells was determined 6 d later in spleen and lymph nodes. Data were compiled from three independent experiments. (B) B6 and Batf3−/− B6 mice received naïve OT-I cells and 1 d later were EPI primed on the ear skin with OVA alone or OVA+CT, as indicated. The number of OT-I cells was determined 5 d later in the spleen and draining lymph nodes. Data were compiled from three independent experiments.
Adjuvant Effects of CT Require Expression of GM1 Gangliosides and Are Enhanced by Antigen–Adjuvant Conjugation.
Our finding that TLRs and NLRs were dispensable for CT adjuvanticity prompted further exploration of the manner by which CT engages cells during skin priming.
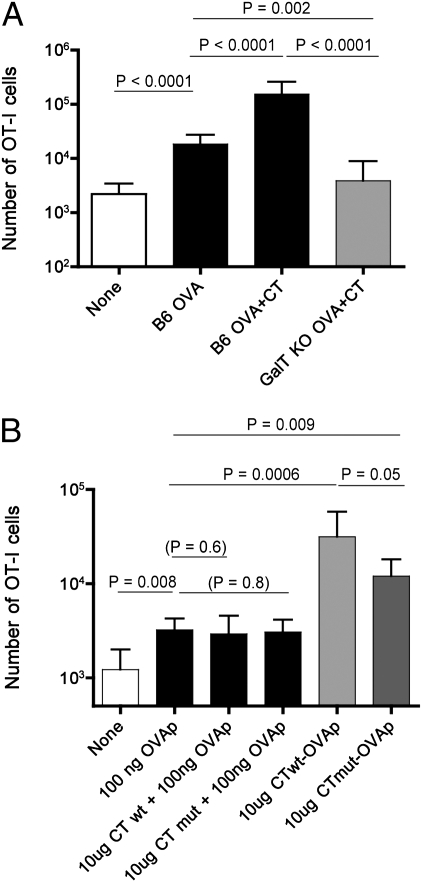
Both LT and CT are AB5-type toxins, composed of an A subunit and five identical B subunits. The A subunit displays ADP ribosylation activity, whereas the pentameric B subunit acts as a receptor for cell entry by high-affinity binding to GM1 ganglioside present at the cell surface (10–12) However, one study reported robust intradermal adjuvant activities of both CT and LT occur in the absence of GM1 binding (30), indicating alternative mechanisms through which CT may provoke the immune response. To determine whether the EPI adjuvant effects of CT require recognition of GM1, we utilized mice (GalT−/−) deficient in the two glycosyltransferases involved in GM1 biosynthesis. Whereas the adjuvant activity of CT was evident in OT-I cells transferred into B6 animals, we saw no adjuvant effect of CT when GalT−/− mice were used as hosts (Fig. 4A). This loss of CT activity might reflect a generalized immune defect in GalT−/− mice; however, when transferred OT-I cells were primed using s.c. LPS/OVA, we observed similar responses in B6 and GalT−/− animals (SI Appendix, Fig. S8), effectively excluding this possibility. The EPI adjuvant properties of CT thus depend on the availability of the gangliosides capable of binding CT-B on host cells, in contrast to studies using intradermal delivery of CT and LT (30).
Fig. 4.
Adjuvant effects of CT in EPI are dependent on GM1 interactions and are enhanced by physical fusion between antigen and adjuvant. (A) B6 and GalT−/− DKO mice were adoptively transferred with naïve OT-I cells 1 d before EPI on ear skin. Five days later, OT-I cells were detected in lymph nodes and spleen by the congeneic marker Thy1.1 in the CD3+ CD8+ population. Data are compiled from two independent experiments and similar data were obtained in an additional experiment. (B) B6 mice were adoptively transferred with naïve OT-I cells 1 d before immunization on the ear. Animals were immunized using sequential administration of free-OVA peptide, followed by either normal (column 3) or enzymatically inactive CT (CTmut) (column 4). Alternatively, mice were immunized by a single application of OVA peptide-tagged CT (column 5) or CTmut (column 6). Compilation of data from two experiments is shown, and analogous results were observed in a similar independent experiment.
For both LT and CT, the B-chain pentamer allows internalization and intracellular trafficking of the holotoxin, ultimately allowing the A1 subunit to reach the cytosol and leading to cellular intoxication (12). Hence, we postulated that superior induction of the CD8 T-cell response could be elicited by combining the adjuvant effect of CT, with concomitant delivery of the antigen to the cytosol for MHC class I presentation. A key element of CT-mediated antigen delivery might derive from the ability of the A1 subunit—and any appended cargo—to be transported across the endoplasmic reticulum (ER) membrane so that it reaches the cytosol and engages the conventional MHC class I processing pathway. To this end, we used a sortase-mediated transpeptidation reaction (31) to couple the OVA peptide (OVAp, SIINFEKL) to the CT-A1 subunit in an otherwise intact and correctly folded holotoxin (32). Furthermore, we were able to use this platform to assess the requirement for CT catalytic activity, by generating a similar construct that contains a CT-A1 chain that was mutated to render it enzymatically inactive (CTmut).
The sortase-linked CT-OVAp and CT(mut)-OVAp were therefore tested for EPI, using the OT-I system. In parallel, we tested sequential administration of OVAp and CT [or CT(mut)], maintaining the same molar ratios of peptide to adjuvant. The fused CT-OVAp was more effective than the fused CT(mut)-OVAp at priming the OT-I response, but both the enzymatically fused complexes were considerably more potent than CT [or CT(mut)] and OVAp added separately (Fig. 4B). When higher doses (i.e., a considerable molar excess) of free peptide was tested, the sortaggable versions of both CT and CT(mut) were able to act as adjuvants (SI Appendix, Fig. S9). This finding is in keeping with our previous findings using commercial CT (SI Appendix, Fig. S3). Hence, these data suggest that optimal sensitivity of EPI priming is achieved with CT–antigen fusion and that CT enzymatic activity enhances, but is not absolutely required for adjuvanticity.
Discussion
We examined the mechanism and efficacy of CD8 T-cell priming via epicutaneous immunization on unbroken skin. Whereas several previous studies demonstrate expansion of antigen-specific CD8 T cells after EPI, most focused on responses early after immunization and/or did not evaluate induction of protective responses against pathogens (4–7). Our finding that EPI using the ADP ribosylating exotoxin CT evokes optimal CD8 T-cell responses (in terms of magnitude, durability, and functional efficacy) parallel work in mice and humans that exploit CT and the related toxin LT (4, 10, 13–15). In agreement with previous studies (6, 7), we found that CpG could also prime CD8 T cells via EPI, although the responses induced with this adjuvant did not persist to generate protective memory. Also, in contrast to earlier reports (7–9, 26, 27), we observed minimal adjuvanticity of the TLR7 agonist imiquimod. These discrepancies may, at least in part, relate to the minimal skin pretreatment we used, because several previous studies employed extensive tape stripping (6, 8, 26, 27) and subsequent acetone treatment (6), which may alter the integrity of the skin and/or properties of APC populations present within it. To ensure eventual applicability, we focused on rapid immunization methods with brief hydration as the only skin pretreatment. With this approach, CT proved superior to other adjuvants tested at elaborating functional CD8 T-cell responses.
Despite numerous reports describing adjuvant activities of CT and LT, the mechanism by which these exotoxins exert such effects was undefined. Here we show that CT adjuvant activity in EPI is independent of major signaling intermediates in the TLR and inflammasome pathways, and that CD8 T-cell priming required sensitivity to type-I IFN by host cells. These findings are reminiscent of the report by Janssen et al., who studied the requirements for CD8 T-cell priming in response to cross-presented apoptotic cells (21). CT induces apoptosis in some epithelial cell lines (33, 34), a trait that may be relevant for adjuvanticity of the toxin and thus account for such mechanistic similarities. However, we observed residual priming activity of enzymatically inactive CT-OVAp, despite the fact that this form of CT has lost its ability to induce cAMP and is correspondingly less toxic (35). Other properties of enzymatically inactive CT, such as its continued ability to direct transfer of the (inactive) A1 subunit from the ER lumen to the cytosol, may be relevant in antigen processing and presentation. Finally, CT binding to GM1 gangliosides induces phenotypic and functional maturation of DCs, suggesting a possible role in APC activation (36). The fact that IFNAR-I is essential for CT-based EPI is intriguing and suggests that TLR-independent pathways of type-I IFN induction (37) are co-opted in CT adjuvant activity. Whereas further resolution of the mechanisms for CT (and other ADP ribosylating exotoxins) adjuvanticity will be needed, our findings suggest that mechanisms distinct from those engaged by “conventional” adjuvants must be at work. This may be especially relevant for adjuvants used in epicutaneous priming, because the skin is routinely exposed to factors (such as various bacterial TLR agonists), which may desensitize these standard adjuvant pathways.
Epicutaneous immunization has long been assumed to involve antigen capture and/or presentation by Langerhans cells or langerin-expressing CD103+ dermal DC (11, 38) and such populations are indeed required for EPI with protein antigens in the absence of adjuvants (16, 26). However, our current studies revealed that EPI involving CT as an adjuvant was unaffected when langerin-expressing DCs were depleted, leading to the unexpected conclusion that LCs—the canonical epidermal DC population—are not required for T-cell priming through the unbroken skin. Such results could arise from redundancy among cross-presenting DC subsets, as has been seen in some models (39). The CT adjuvant effect was lost in Batf3−/− mice, animals that lack both CD8α+ and dermal CD103+ve DCs (28, 29). DT treatment of langerin–DTR mice causes efficient loss of dermal CD103+ve DCs but not CD8α+ DCs (16, 25); hence our studies suggest the CD8α+ DC pool are critical for the CT adjuvant effect (although we cannot rule out redundancy between Batf3-dependent DC subsets). Batf3-dependent DCs have been found critical for cross-presentation of antigen (28, 29) and recent studies indicate that the Batf3-dependent CD8α+ DC pool must respond to type-I IFN to mediate effective cross-presentation in tumor models (40, 41). This finding may suggest a mechanistic basis for the observed requirement for IFNAR expression by host cells in EPI priming using CT (Fig. 2B).
The impressive ability of CT as an EPI adjuvant highlights the potential application of this toxin (or related exotoxins) in development of simplified immunization approaches (“vaccination with a bandaid”). Epicutaneous priming using CT and/or LT in mice and humans shows that the approach is effective (at least in terms of antibody responses) and safe (2, 4, 11, 13–15, 26, 42). Our studies demonstrated efficient priming with short term (20 min) exposure to CT, using natural or recombinant versions of the toxin, and revealed partial priming with nontoxic, enzymatically inactive CT-OVAp. This approach lends itself to implementation of cost-effective and convenient immunization methods, while retaining acceptable safety features. In addition, CT itself is an appealing candidate for an antigen as well as an adjuvant: Given the devastating effects of cholera epidemics (as evidenced by recent outbreaks in Haiti) and renewed calls for application of effective vaccination, it is worthwhile noting that epicutaneous application of CT also primes a potent neutralizing antibody response (2, 3, 10). The opportunity to capitalize on the ability of CT to act as a potent CD8 T-cell adjuvant, while simultaneously inducing protection against the toxin itself, makes this approach attractive.
Materials and Methods
Mice.
C57BL/6 mice were purchased from The Jackson Laboratory or the National Cancer Institute and were used at 6–8 wk of age. OT-1.PL (Thy1.1+) mice were generated and maintained at the University of Minnesota. IFNAR KO mice (43) were a kind gift of Matt Mescher (University of Minnesota, Minneapolis). GalnT1/2 (GalT−/−) DKO mice, lacking expression of GalNAcT (N-acetylgalactosaminyltransferase) 1 and 2, were obtained from the Mouse Model Core of the Consortium for Functional Glycomics (National Institute of General Medical Sciences) and were maintained by breeding mice deficient for GalnT2 (44) and heterozygous for GalnT1 (45). Typing of double KO mice was confirmed by peripheral blood cells failing to stain with FITC-CTB (B subunit of CT) (Sigma Aldrich) (35, 46). MyD88/TRIF DKO mice (47) and ASC KO mice (20) were obtained from Shizuo Akira (Osaka University, Osaka, Japan) via Marc Jenkins (University of Minnesota). Lang–DTR mice (25) were obtained from Bernard Malissen (Centre d'immunologie de Marseille Luminy, Marseille), whereas Batf3−/− mice (29) were generously provided by Ken Murphy (Washington University, St. Louis) via Dan Kaplan (University of Minnesota). Animals were maintained under specific pathogen-free conditions, and experimental procedures were approved by the University of Minnesota Institutional Animal Care and Use Committee.
T-Cell Adoptive Transfer.
CD44 low CD8+ cells were purified from Thy-1.1+ve OT-I mice by negative selection using magnetic cell sorting MACS (Miltenyi Biotec) as previously described (16) using MACS microbeads. Cell purity (>90%) was confirmed by flow cytometry. Purified cells (2.5 × 105) were injected into the tail vein of recipient mice.
Epicutaneous Immunization.
Immunization was typically on the ear skin, without prior treatment. In some experiments (specified in the text) the flank skin was used, in which case mice were shaved (under anesthetic) at least 2 d before immunization (16). Immunization followed the scheme described in SI Appendix, Fig. S1. Briefly, mice were anesthetized and the skin site hydrated with water (15 min), then OVA protein (500 μg in 25 μL of PBS; Sigma-Aldrich) applied topically for 20 min. After washing with water and air drying, the indicated adjuvants were applied to the same site for 20 min. Unless otherwise indicated, the following doses of adjuvants were used: CT (List Biological Laboratories), 100 μg; CpG 1826 (Invivogen) 500 μg; Poly I:C (Amersham) 200 μg; LPS (Sigma) 10 μg; and imiquimod (3M) 50 μL of 1% cream. The immunization site was extensively washed with water and allowed to dry before the animals recovered. In the case of recombinant CT and CT(mut) used for the OVA–CT fusion studies, 10 μg of CT [or CT(mut)] was used. For sortase fused OVA–CT/CT(mut), the immunization involved a single application step. In titration experiments we found similar adjuvant effects using either 10 or 100 μg of commercial CT for EPI on ear skin, but for experimental consistency data with 100 μg CT are shown throughout. The control mice received PBS in place of antigen or adjuvants, as indicated.
CT Fusion Preparation.
Details on the preparation of CT fusions with peptides using sortase-mediated transpeptidation reactions have been described elsewhere (32). Briefly, the sortase-recognition motif (LPETG) was cloned between residues Arg192 and Ser193 using site-directed mutagenesis following the manufacturer instructions (Stratagene). This engineered version of CT was expressed and purified as described (32). CT was labeled with a GGGSIINFEKL peptide using sortase A from Staphylococcus aureus as described (31). The catalytic inactive mutant of CT (E110D/E112D in subunit A) (48) used in these studies was created by site-directed mutagenesis using engineered CT containing the LPETG motif as template. No difference in expression or efficiency of labeling was detected between the two versions of CTx. The yield of the transpeptidation reactions was higher than 90% as inferred by SDS/PAGE and Coomassie-blue staining.
Flow Cytometry.
For adoptive transfer experiments, mice were killed 5–6 d postimmunization. A single-cell suspension from lymph node and spleen was stained with anti-Thy-1.1, anti-CD8, and anti-CD3 or OVA/Kb tetramer to detect transferred OT-I cells. In some studies, cells were stained for CD44 and CD62L or were prepared with Cytofix/Cytoperm (BD Pharmigen), and stained with anti-Granzyme B (Caltag). Antibodies for flow cytometry were obtained from eBioscience or Biolegend. Cells were analyzed using a BD Pharmigen LSR II flow cytometer and data analyzed using FlowJo (TreeStar) software.
Pathogen Protection Assays.
Typically, C57BL/6 mice were vaccinated as indicated and received a boost at 30 d later. After an additional 30 d, protective immunity to LM-OVA was monitored, essentially as described previously (49). Briefly, mice were challenge with 105 LM-OVA bacteria via retroorbital injection. At day 5 postinfection, mice were killed and spleens and livers harvested and homogenized to obtain bacterial counts, determined by plating the suspension on LB-streptomycin. The limit of detection was ∼100 microorganisms.
For protection against vaccinia virus, vaccinated mice received a challenge with 5 × 106 pfu (plaque forming units) of VV-OVA injected i.v. At day 3 postinfection, ovaries were harvested in PBS and frozen as a single-cell suspension. After two freeze–thaw cycles, the ovary homogenate was incubated at 37 °C for 45 min with 0.25 mg/mL trypsin (Sigma). 143B cells (ATCC) were grown to confluence. Dilutions of the ovary homogenate were added in duplicate to the cellular monolayer and left for 2 d. Plaques were counted and total viral load per ovaries was calculated.
Tetramer Enrichment Assays.
Mice were primed and boosted by EPI, as described in Fig. 2. At 30 d following boosting, spleen and lymph nodes were combined and subjected to OVA/Kb tetramer enrichment, as described previously (50). Absolute numbers of OVA/Kb tetramer binding CD8 T cells were determined.
In Vivo Depletion of LCs.
Depletion of langerin+ cells was performed in Lang–DTR mice (25) by i.p. injection of 1 μg DT (List Biological Laboratories) at day −1 or, in some experiments, days −4 and −1, relative to the day of vaccination, as described previously (16).
Statistics.
Data were transformed to log10, and an unpaired two-tailed Student´s t test was used to determine significance with Prism software (GraphPad).
Supplementary Material
Acknowledgments
We thank Shizuo Akira, Bernard Malissen, Kenneth Murphy, Dan Kaplan, Matt Mescher, and Marc Jenkins for providing mouse strains; Laura Bursch for advice and assistance; and the past and present S.C.J. and K.A.H. laboratory members for input. These studies were supported by Grant U01 AI070380 from the National Institutes of Health (to S.C.J. and K.A.H.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1105771109/-/DCSupplemental.
References
- 1.Jacobson RM, et al. Vaccine Research Group Making vaccines more acceptable—methods to prevent and minimize pain and other common adverse events associated with vaccines. Vaccine. 2001;19:2418–2427. doi: 10.1016/s0264-410x(00)00466-7. [DOI] [PubMed] [Google Scholar]
- 2.Glenn GM, Rao M, Matyas GR, Alving CR. Skin immunization made possible by cholera toxin. Nature. 1998;391:851. doi: 10.1038/36014. [DOI] [PubMed] [Google Scholar]
- 3.Godefroy S, et al. Immunization onto shaved skin with a bacterial enterotoxin adjuvant protects mice against respiratory syncytial virus (RSV) Vaccine. 2003;21:1665–1671. doi: 10.1016/s0264-410x(02)00733-8. [DOI] [PubMed] [Google Scholar]
- 4.Kahlon R, et al. Optimization of epicutaneous immunization for the induction of CTL. Vaccine. 2003;21:2890–2899. doi: 10.1016/s0264-410x(03)00141-5. [DOI] [PubMed] [Google Scholar]
- 5.Belyakov IM, Hammond SA, Ahlers JD, Glenn GM, Berzofsky JA. Transcutaneous immunization induces mucosal CTLs and protective immunity by migration of primed skin dendritic cells. J Clin Invest. 2004;113:998–1007. doi: 10.1172/JCI20261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klimuk SK, Najar HM, Semple SC, Aslanian S, Dutz JP. Epicutaneous application of CpG oligodeoxynucleotides with peptide or protein antigen promotes the generation of CTL. J Invest Dermatol. 2004;122:1042–1049. doi: 10.1111/j.0022-202X.2004.22411.x. [DOI] [PubMed] [Google Scholar]
- 7.Wang LF, et al. Cross-priming with an epicutaneously introduced soluble protein antigen generates Tc1 cells. Eur J Immunol. 2006;36:2904–2911. doi: 10.1002/eji.200535770. [DOI] [PubMed] [Google Scholar]
- 8.Itoh T, Celis E. Transcutaneous immunization with cytotoxic T-cell peptide epitopes provides effective antitumor immunity in mice. J Immunother. 2005;28:430–437. doi: 10.1097/01.cji.0000171289.78495.b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rechtsteiner G, Warger T, Osterloh P, Schild H, Radsak MP. Cutting edge: Priming of CTL by transcutaneous peptide immunization with imiquimod. J Immunol. 2005;174:2476–2480. doi: 10.4049/jimmunol.174.5.2476. [DOI] [PubMed] [Google Scholar]
- 10.Glenn GM, et al. Transcutaneous immunization with cholera toxin protects mice against lethal mucosal toxin challenge. J Immunol. 1998;161:3211–3214. [PubMed] [Google Scholar]
- 11.Glenn GM, et al. Transcutaneous immunization: A human vaccine delivery strategy using a patch. Nat Med. 2000;6:1403–1406. doi: 10.1038/82225. [DOI] [PubMed] [Google Scholar]
- 12.Partidos CD, Beignon AS, Briand JP, Muller S. Modulation of immune responses with transcutaneously deliverable adjuvants. Vaccine. 2004;22:2385–2390. doi: 10.1016/j.vaccine.2003.11.063. [DOI] [PubMed] [Google Scholar]
- 13.Frerichs DM, et al. Controlled, single-step, stratum corneum disruption as a pretreatment for immunization via a patch. Vaccine. 2008;26:2782–2787. doi: 10.1016/j.vaccine.2008.02.070. [DOI] [PubMed] [Google Scholar]
- 14.Yu J, et al. Transcutaneous immunization using colonization factor and heat-labile enterotoxin induces correlates of protective immunity for enterotoxigenic Escherichia coli. Infect Immun. 2002;70:1056–1068. doi: 10.1128/IAI.70.3.1056-1068.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Güereña-Burgueño F, et al. Safety and immunogenicity of a prototype enterotoxigenic Escherichia coli vaccine administered transcutaneously. Infect Immun. 2002;70:1874–1880. doi: 10.1128/IAI.70.4.1874-1880.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang L, et al. Langerin expressing cells promote skin immune responses under defined conditions. J Immunol. 2008;180:4722–4727. doi: 10.4049/jimmunol.180.7.4722. [DOI] [PubMed] [Google Scholar]
- 17.Stoitzner P, et al. Langerhans cells cross-present antigen derived from skin. Proc Natl Acad Sci USA. 2006;103:7783–7788. doi: 10.1073/pnas.0509307103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pamer EG. Immune responses to Listeria monocytogenes. Nat Rev Immunol. 2004;4:812–823. doi: 10.1038/nri1461. [DOI] [PubMed] [Google Scholar]
- 19.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto M, et al. ASC is essential for LPS-induced activation of procaspase-1 independently of TLR-associated signal adaptor molecules. Genes Cells. 2004;9:1055–1067. doi: 10.1111/j.1365-2443.2004.00789.x. [DOI] [PubMed] [Google Scholar]
- 21.Janssen E, et al. Efficient T cell activation via a Toll-Interleukin 1 Receptor-independent pathway. Immunity. 2006;24:787–799. doi: 10.1016/j.immuni.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 22.Sparber F, Tripp CH, Hermann M, Romani N, Stoitzner P. Langerhans cells and dermal dendritic cells capture protein antigens in the skin: Possible targets for vaccination through the skin. Immunobiology. 2010;215:770–779. doi: 10.1016/j.imbio.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bursch LS, et al. Identification of a novel population of Langerin+ dendritic cells. J Exp Med. 2007;204:3147–3156. doi: 10.1084/jem.20071966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bedoui S, et al. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat Immunol. 2009;10:488–495. doi: 10.1038/ni.1724. [DOI] [PubMed] [Google Scholar]
- 25.Kissenpfennig A, et al. Dynamics and function of Langerhans cells in vivo: Dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22:643–654. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Stoitzner P, et al. Tumor immunotherapy by epicutaneous immunization requires langerhans cells. J Immunol. 2008;180:1991–1998. doi: 10.4049/jimmunol.180.3.1991. [DOI] [PubMed] [Google Scholar]
- 27.Stein P, et al. UV exposure boosts transcutaneous immunization and improves tumor immunity: Cytotoxic T-cell priming through the skin. J Invest Dermatol. 2011;131:211–219. doi: 10.1038/jid.2010.254. [DOI] [PubMed] [Google Scholar]
- 28.Edelson BT, et al. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8alpha+ conventional dendritic cells. J Exp Med. 2010;207:823–836. doi: 10.1084/jem.20091627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hildner K, et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zoeteweij JP, et al. GM1 binding-deficient exotoxin is a potent noninflammatory broad spectrum intradermal immunoadjuvant. J Immunol. 2006;177:1197–1207. doi: 10.4049/jimmunol.177.2.1197. [DOI] [PubMed] [Google Scholar]
- 31.Popp MW, Antos JM, Ploegh HL. Site-specific protein labeling via sortase-mediated transpeptidation. Curr Protoc Protein Sci. 2009;Chapter 15 doi: 10.1002/0471140864.ps1503s56. Unit 15.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guimaraes CP, et al. Identification of host cell factors required for intoxication through use of modified cholera toxin. J Cell Biol. 2011;195:751–764. doi: 10.1083/jcb.201108103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allam M, Bertrand R, Zhang-Sun G, Pappas J, Viallet J. Cholera toxin triggers apoptosis in human lung cancer cell lines. Cancer Res. 1997;57:2615–2618. [PubMed] [Google Scholar]
- 34.Pessina A, et al. Bcl-2 down modulation in WEHI-3B/CTRES cells resistant to Cholera Toxin (CT)-induced apoptosis. Cell Res. 2006;16:306–312. doi: 10.1038/sj.cr.7310038. [DOI] [PubMed] [Google Scholar]
- 35.Jobling MG, Holmes RK. Biological and biochemical characterization of variant A subunits of cholera toxin constructed by site-directed mutagenesis. J Bacteriol. 2001;183:4024–4032. doi: 10.1128/JB.183.13.4024-4032.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawamura YI, et al. Cholera toxin activates dendritic cells through dependence on GM1-ganglioside which is mediated by NF-kappaB translocation. Eur J Immunol. 2003;33:3205–3212. doi: 10.1002/eji.200324135. [DOI] [PubMed] [Google Scholar]
- 37.Baccala R, Hoebe K, Kono DH, Beutler B, Theofilopoulos AN. TLR-dependent and TLR-independent pathways of type I interferon induction in systemic autoimmunity. Nat Med. 2007;13:543–551. doi: 10.1038/nm1590. [DOI] [PubMed] [Google Scholar]
- 38.Romani N, Clausen BE, Stoitzner P. Langerhans cells and more: Langerin-expressing dendritic cell subsets in the skin. Immunol Rev. 2010;234:120–141. doi: 10.1111/j.0105-2896.2009.00886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kastenmüller K, et al. Protective T cell immunity in mice following protein-TLR7/8 agonist-conjugate immunization requires aggregation, type I IFN, and multiple DC subsets. J Clin Invest. 2011;121:1782–1796. doi: 10.1172/JCI45416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diamond MS, et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med. 2011;208:1989–2003. doi: 10.1084/jem.20101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fuertes MB, et al. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8alpha+ dendritic cells. J Exp Med. 2011;208:2005–2016. doi: 10.1084/jem.20101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giudice EL, Campbell JD. Needle-free vaccine delivery. Adv Drug Deliv Rev. 2006;58:68–89. doi: 10.1016/j.addr.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 43.Müller U, et al. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 44.Mohlke KL, et al. Mvwf, a dominant modifier of murine von Willebrand factor, results from altered lineage-specific expression of a glycosyltransferase. Cell. 1999;96:111–120. doi: 10.1016/s0092-8674(00)80964-2. [DOI] [PubMed] [Google Scholar]
- 45.Takamiya K, et al. Mice with disrupted GM2/GD2 synthase gene lack complex gangliosides but exhibit only subtle defects in their nervous system. Proc Natl Acad Sci USA. 1996;93:10662–10667. doi: 10.1073/pnas.93.20.10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Mello Coelho V, et al. Quantitative differences in lipid raft components between murine CD4+ and CD8+ T cells. BMC Immunol. 2004;5:2. doi: 10.1186/1471-2172-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamamoto M, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 48.Teter K, Jobling MG, Sentz D, Holmes RK. The cholera toxin A1(3) subdomain is essential for interaction with ADP-ribosylation factor 6 and full toxic activity but is not required for translocation from the endoplasmic reticulum to the cytosol. Infect Immun. 2006;74:2259–2267. doi: 10.1128/IAI.74.4.2259-2267.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hamilton SE, Wolkers MC, Schoenberger SP, Jameson SC. The generation of protective memory-like CD8+ T cells during homeostatic proliferation requires CD4+ T cells. Nat Immunol. 2006;7:475–481. doi: 10.1038/ni1326. [DOI] [PubMed] [Google Scholar]
- 50.Haluszczak C, et al. The antigen-specific CD8+ T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion. J Exp Med. 2009;206:435–448. doi: 10.1084/jem.20081829. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.