Abstract
Individuals with developmental dyslexia (DD) show a disruption in posterior left-hemispheric neural networks during phonological processing. Additionally, compensatory mechanisms in children and adults with DD have been located within frontal brain areas. However, it remains unclear when and how differences in posterior left-hemispheric networks manifest and whether compensatory mechanisms have already started to develop in the prereading brain. Here we investigate functional networks during phonological processing in 36 prereading children with a familial risk for DD (n = 18, average age = 66.50 mo) compared with age and IQ-matched controls (n = 18; average age = 65.61 mo). Functional neuroimaging results reveal reduced activation in prereading children with a family-history of DD (FHD+), compared with those without (FHD−), in bilateral occipitotemporal and left temporoparietal brain regions. This finding corresponds to previously identified hypoactivations in left hemispheric posterior brain regions for school-aged children and adults with a diagnosis of DD. Furthermore, left occipitotemporal and temporoparietal brain activity correlates positively with prereading skills in both groups. Our results suggest that differences in neural correlates of phonological processing in individuals with DD are not a result of reading failure, but are present before literacy acquisition starts. Additionally, no hyperactivation in frontal brain regions was observed, suggesting that compensatory mechanisms for reading failure are not yet present. Future longitudinal studies are needed to determine whether the identified differences may serve as neural premarkers for the early identification of children at risk for DD.
Keywords: functional MRI, pediatric neuroimaging, reading disability, developmental disorder, learning disability
Developmental dyslexia (DD) is a specific learning disability that affects about 5–17% of all children (1, 2). DD is characterized by difficulties with accurate and fluent word recognition and poor spelling and decoding performance. DD cannot be accounted for by poor vision, hearing, or a lack of motivation. Molecular-genetic, twin, and family studies have shown a marked familial risk for DD, with an increasing prevalence in families with one or more members with a diagnosis of DD or reading difficulties (e.g., refs. 3 and 4). In addition, several DD susceptibility genes crucial for early brain development have been reported (5–8). DD can have severe social and psychological consequences (9–11) and may impact a child's life beyond their academic pursuits. Studies have shown that children with learning disabilities are less likely to complete high school (12) and are more likely to enter the juvenile justice system (13).
Most researchers, clinicians, and reading specialists agree that DD typically results from a weakness in the ability to manipulate oral speech sounds of language (2, 14). Individuals with DD are often unable to access the underlying sound structures of words, creating a difficulty in mapping sounds to written language (15–18). Phonological processing skills have been found to be a key predictor of later reading ability in preschool and elementary school-aged children (19–31). In addition to phonological processing deficits, a range of other linguistic impairments have been observed in infants and prereading children who later exhibit weak reading scores, including speech perception (23, 26), syntax production, and comprehension (32–35), language comprehension (26), receptive vocabulary (22, 34), and rapid automatized naming abilities (23, 24, 34, 36–38).
With the advent of modern neuroimaging tools, it is now possible to study the neural substrates of reading and reading-related processes in the conscious human brain. Functional MRI (fMRI) studies have revealed a characteristic network of posterior brain areas typically involved in reading and reading-related tasks in children and adults including: (i) the dorsal or temporoparietal circuit (including lateral extrastriate and left inferior occipital areas) and (ii) the ventral or occipitotemporal circuit [including angular and supramarginal gyrus, inferior parietal lobe, and posterior aspects of the superior temporal gyrus (39–43)]. Cross-sectional studies have demonstrated changes in these highly integrated reading networks depending on reading skill level (e.g., refs. 44 and 45) and converging evidence points toward a characteristic hypoactivation of temporoparietal as well as occipitotemporal brain areas in individuals with DD (44, 46–55).
These functional characteristics in posterior brain regions in children and adults with DD have been complemented by anatomical abnormalities. Voxel-based morphometry reveals differences in gray-matter volume indices in individuals with DD (compared with typical reading controls) in various areas of the brain, including left occipitotemporal and temporoparietal areas (44, 56–61), bilateral fusiform (59), and lingual gyrus (LG) (58) as well as the cerebellum (56–58). Morphological abnormalities in these regions can be identified even before reading skills are present in children as young as 5 to 6 y of age, suggesting atypical early development or even a genetic basis for DD (62).
Furthermore, an increase in activation in left frontal and right lateralized anterior brain areas has been shown in individuals with DD (44, 45, 49, 50, 63). This hyperactivation seen in individuals with DD has been suggested to reflect a compensatory mechanism for the dysfunctional reading system (e.g., refs. 44, 50, and 64). Furthermore, it has been shown that right prefrontal activation in children with a diagnosis of DD can significantly predict reading gains 2.5-y later, indicating that these compensatory mechanisms can function as part of the reading network (64).
Although there is converging evidence suggesting a characteristic structural and functional phenotype of DD, the mechanism by which reading networks fail to develop is poorly understood. It remains unclear whether the characteristic hypoactivation within posterior brain regions is present before reading onset or whether this develops after reading onset and may therefore be a result of reading failure. Moreover, it remains unclear whether compensatory mechanisms in anterior brain regions are a unique characteristic of children with DD that predates reading instruction, or whether these form during literacy acquisition. Cross-sectional and longitudinal electroencephalography studies have reported altered neural correlates in infants and preschoolers with a familial risk for DD during speech perception, and some of these brain measures later predicted reading outcome in elementary school (65–67). These studies strongly suggest that components of a deficient reading network may be observed before reading onset.
To further examine the emergence of reported abnormal brain activations and characteristic behavioral differences in children and adults with a diagnosis of DD, the present study used fMRI in preschool children with (FHD+) and without (FHD−) a familial risk for DD. We hypothesized left-hemispheric hypoactivation in posterior brain regions in children with a familial risk of DD compared with age-matched controls before reading onset. Furthermore, we hypothesized that no differences in anterior brain regions would be seen because we expected compensatory brain regions to form only after repeated reading failure.
Results
Demographics and Behavioral Group Characteristics.
Demographics and behavioral group characteristics are listed in Table S1. FHD+ children scored significantly lower than FHD− children in standardized assessments of core language core language (t(34) = −2.045; P = 0.049), expressive language skills [Clinical Evaluation of Language Fundamentals (CELF) expressive language; (t(34) = −3.037; P = 0.005); VATT (Verb Agreement and Tense Test) repetition (t(30) = −2.412; P = 0.022)], language structure [CELF language structure (t34) = −2.195; P = 0.035)], phonological processing [comprehensive test of phonological processing (CTOPP) Elision (t(33) = −2.422; P = 0.021) and rapid automatized naming (RAN) objects (t(33) = −3.420; P = 0.002) and colors (t(33) = −2.586; P = 0.014)].
fMRI Results.
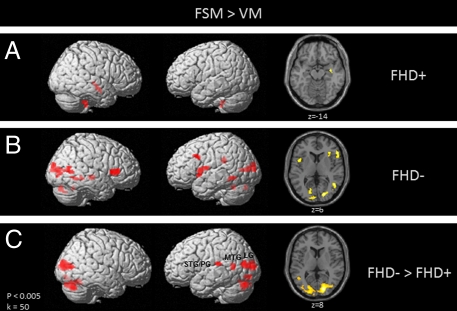
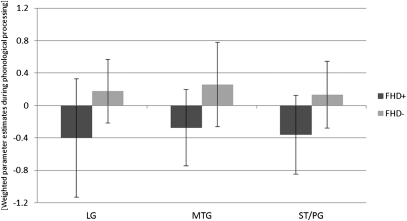
Children were asked to listen to two words and decide whether the target words started with the same initial sound (first-sound matching, FSM). This procedure was contrasted with a voice-matching task (VM), in which children listened to the same word pairs but had to decide whether they were spoken by the same voice (see Methods for a detailed task description). Whole-brain analysis revealed increased activation for FSM > VM in FHD− children in a number of brain regions, including left fusiform gyrus, left inferior frontal/precentral gyrus, bilateral cuneus/middle occipital, left middle frontal gyrus, and right cerebellum (Fig. 1A). FHD+ children activated the right middle temporal gyrus (MTG) and right cerebellum for the same contrast, but failed to show activation in left hemispheric brain regions associated with phonological processing and reading. (Fig. 1B). An independent two-sample t test was used to examine differences in brain activation during FSM vs. VM between the two groups of children. This analysis revealed significantly greater activation for FHD− compared with FHD+ children in bilateral occipitotemporal (left LG and bilateral middle temporal/occipitotemporal gyrus), left temporoparietal [left superior temporal gyrus (STG)/postcentral gyrus (PG) and MTG] brain regions, as well as left and right cerebellum (Fig. 1C and Table 1). The opposite contrast (FHD+ > FHD−) did not yield any significant voxels. Regions of interest analyses, derived from the FSM > VM group comparison, were used to compute correlational analysis with behavioral measures of prereading skills. These results demonstrate a positive correlation of phonological processing skills (CTOPP nonword repetition) with brain activation in left LG (P = 0.003) and STG/PG (P = 0.013) in FHD− children; but no significant correlation was found for left MTG and phonological processing (P > 0.05). In FHD+ children, brain activity within the LG (P = 0.016), MTG (P = 0.003), and STG/PG (P = 0.018) all showed a significant positive correlation with phonological processing. Bar graphs illustrate brain activity in FHD+ and FHD− children within the three regions of interest (LG, MTG, STG/PG) during phonological processing (FSM > VM) (Fig. 2). FHD+ children predominantly show negative parameter estimates for phonological processing (first-sound matching compared with voice matching) in these regions of interest.
Fig. 1.
Statistical parametric maps showing brain activation during phonological processing (FSM > VM) for children with (A) and without (B) a familial risk for DD, as well as group differences between children with compared to without (FHD− > FHD+) a familial risk for DD (C). FHD− show significantly greater activation compared to FHD+ children in bilateral occipitotemporal and left temporoparietal brain regions, as well as left and right cerebellar regions.
Table 1.
Significant differences in brain activation between children with (FHD+) and children without (FHD−) a familial risk for DD during phonological processing (FSM > VM; FHD−>FHD+; at P < 0.005 uncorrected; k = 50)
| Coordinates |
|||||
| Region | x | y | z | z score | Size, voxels |
| Occipitotemporal lobe | |||||
| Middle temporal/occipitotemporal gyrus (R/L) | 18 | −84 | 8 | 4.44 | 1,142 |
| Lingual gyrus (L) | −16 | −86 | −10 | 3.63 | 84 |
| Temporoparietal lobe | |||||
| Superior temporal/postcentral gyrus (L) | −60 | −28 | 14 | 3.19 | 50 |
| Middle temporal gyrus (L) | −48 | −56 | 6 | 2.91 | 83 |
| Other | |||||
| Cerebellum/fusiform gyrus (L) | −28 | −76 | −28 | 3.75 | 206 |
| Cerebellum (R) | 16 | −62 | −18 | 3.86 | 360 |
| Cerebellum (R) | 44 | −70 | −32 | 3.42 | 93 |
Brain regions, which were included in subsequent region of interest analyses, are shown in boldface.
Fig. 2.
Mean brain activation (weighted-parameter estimates) during phonological processing (FSM > VM) in left LG, MTG, and STG/PG for children with (FHD+) and without (FHD−) a familial risk for DD. FHD+ children predominantly show negative parameter estimates, whereas FHD− children predominantly show positive parameter estimates for phonological processing (FSM > VM) in these regions of interest. Error bars represent SDs.
In-Scanner Performance.
Due to a technical problem, the behavioral responses for the experimental and control tasks could not be recorded in one child and no data for the control task could be recorded for another child. Both children (FHD−) were still included in the analysis because their performance during the training session indicated that the tasks were well understood. In-scanner performance revealed that FHD+ children scored significantly lower than FHD− children during phonological processing [FSM; FHD+/FHD−: 13.83/21.06; t(33) = −4.77; P = 0.000], but no difference was found during VM [FHD+/FHD−: 19.39/20.56; t(32) = −0.47; P = 0.641]. FHD+ children responded faster during VM [FHD+/FHD−: 1,970 ms/2,279 ms; t(32) = −2.26; P = 0.031], but not FSM [FHD+/FHD−: 2,040 ms/2,262 ms; t(33) = −1.324; P = 0.194] compared with FHD− children.
Discussion
The present study provides neuroimaging evidence for the disruption of left hemispheric posterior neural networks during phonological processing in preliterate children with a familial risk for DD. Consistent with previous neuroimaging studies in older children and adults with a diagnosis of DD, prereading children with a familial risk for DD (FHD+) already show reduced activation within a left hemispheric network, including occipitotemporal (LG) and temporoparietal brain areas (STG/PG and MTG) compared with typical controls (FHD−).
Moreover, brain activity within the left LG as well as the STG/PG positively correlates with phonological processing skills in children with and without a family-history of DD. FHD+ children also demonstrate a positive correlation between activation in the MTG and phonological processing. In addition to the altered neural correlates, FHD+ children score significantly lower on standardized tests of phonological processing, expressive language skills, and rapid automatized naming. Because all children were preliterate at the time of testing, the present findings cannot be attributed to reading failure within the at-risk subgroup. Additionally, no differences in home literacy environment or socioeconomic status (SES) were observed. Thus, our results suggest that these behavioral and neural differences in children at risk for DD must reflect a mechanism that develops within the first few years of life or may even be present at birth. However, it is important to note that most children came from households of relatively high SES and also demonstrated relatively strong language skills overall. Future research is needed to determine whether the observed differences can also be found in children from lower SES households and those with poorer language scores.
Converging evidence from many neuroimaging studies point toward a characteristic hypoactivation of left-hemispheric temporoparietal (44, 46, 48–51) and occipitotemporal (44, 46–48, 50) brain regions in children and adults with DD compared with typical reading controls. Furthermore, reduced gray-matter volume indices in temporoparietal and occipitotemporal brain regions have been reported in prereading children at risk for DD compared with their peers (62). The left temporoparietal region of the brain is known to be crucial for the integration of letter and speech sounds (68) and has consistently demonstrated activation during phonological processing tasks in typically reading children and adults (for reviews, see refs. 40–42). In individuals with DD, a hypoactivation of the left temporoparietal region of the brain seems to reflect an inability to map the sounds of languages (phonemes) to its written counterparts (letters/graphemes) (44, 46–51). The left hemispheric occipitotemporal region seems to be involved in the processing of words or pseudowords in typical reading children and adults (for reviews, see refs. 40–42) and has been called the “visual word form area” (69). Several studies suggest that within this region, letters are represented and processed independently of the perceptual dimension of stimulus presentation (69–71). Furthermore, it has been shown that the initial development of visual tuning for print within inferior occipitaltemporal brain regions is delayed in children with DD (72).
In the present study, we found the same characteristic functional atypicality within temporoparietal (STG/PG and MTG) and occipitotemporal brain areas (left LG) during phonological processing when comparing prereading children with a familial risk for DD to those without such a history. It has been hypothesized that hypoactivation in DD within left temporoparietal and occipitotemporal areas of the brain are fundamental to the language disorder itself, as differences in these areas during reading tasks are apparent, even when comparing children with DD to younger, typical readers, who are on the same reading level (44). Our results support this hypothesis and one can further hypothesize that prereading children at risk for DD exhibit reduced gray-matter volume indices in left temporoparietal and occipitotemporal brain regions (62), which then lead to a disruption of the network typically involved in phonological processing and subsequent reading failure.
Our results further show a positive correlation between phonological processing abilities and brain activation in left LG, as well as the STG/PG for children with and without a familial risk for DD. This finding underlines the importance of these posterior brain regions for phonological processing abilities in the preliterate brain. Only children with a familial risk for DD show additional correlations between phonological processing skills and brain activity in left MTG. Therefore, we hypothesize that phonological processing within dorsal and ventral reading networks develop differently for FHD+ and FHD− children. For all children, a specialization in left LG and STG/PG is emerging, visible by a higher skill level of those children with more brain activation in these areas (positive correlation of phonological processing skills and brain activity in left LG and STG/PG). However, most of the children at risk for DD show negative-weighted parameter estimates in left LG and STG/PG, suggesting less specialization than the children with no risk, which show predominately positive-weighted parameter estimates in these regions. In contrast, in the left MTG, children with no risk do show positive-weighted parameter estimates but no correlation with phonological skills, suggesting that this region has been fully developed and increased skill level does not lead to an increase in activation in this region. In children at risk for DD, mostly negative-weighted parameter estimates are observed in the MTG, which again suggests less specialization than the children with no risk (predominately positive-weighted parameter estimates in these regions). However, children at risk show a positive correlation with phonological skills in this region, indicating an emerging specialization depending on the child's skill level.
Our findings are in line with previous research showing that more temporoparietal brain areas are predominantly activated during early reading development, but the more occipitotemporal areas specialize later (73). To summarize, we suggest that a specialization for (auditory) phonological processing within dorsal and ventral brain regions takes place in the prereading brain. However, specialization in dorsal components of the reading network seems to be delayed in children with a higher risk for DD that impacts their phonological processing abilities. Further longitudinal studies are needed to determine how phonological processing develops in these children.
In addition to the characteristic hypoactivation in individuals with DD, some studies have reported hyperactivity primarily in the left frontal and/or right hemispheric regions of the brain in children and adults with DD during reading-related tasks (44, 49, 50, 63). In the present study, however, hyperactivation was not observed in children with a familial risk for DD. However, it has been argued that hyperactivations in DD reflect compensatory strategies to correct for the dysfunction within the left hemispheric reading network (e.g., refs. 44 , 50, and 64), and therefore are most likely to develop after reading acquisition/failure. Our result supports this hypothesis because no compensatory mechanisms were observed in our prereading children. This view is in line with a recent meta-analysis of reading and reading-related tasks in children (mean age 9–11 y) and adults (mean age 18–30 y) with DD, which reported a noticeably smaller number of overactivation foci in pediatric compared with adult neuroimaging studies (55). It has been hypothesized that this finding may reflect an increase in reliance on compensatory mechanisms with age or the presence of more variable compensatory mechanisms in children (55).
DD can have severe psychological and social consequences, potentially negatively impacting a child's life. Negative personal experiences and continued unhappiness about failing in school may lead to frustration, aggression, impulsivity, and antisocial behavior in some children (9, 74). Identifying children at risk for DD at an early age is crucial and offers the chance to eliminate significant personal and social costs. Identifying a learning disability around mid-elementary school is oftentimes too late, as the delayed development may have already affected a child's vocabulary skills (75) and motivation to read (76). Early identification of reading disability offers a chance to implement early remediation programs, which may lead to a normalization of dysfunctional brain patterns, ideally before compensatory mechanisms are needed.
Future research using longitudinal designs is needed to shed light on the development of this neural network in prereading children throughout the development of reading skills. It remains to be determined which of the prereading children with a family-risk for DD will develop a reading disability. Ultimately, the goal will be to determine whether functional differences in prereading children can be used to predict later reading outcome, and perhaps identify DD.
Conclusion
Converging studies illustrate characteristic differences in brain structure and function of children and adults with DD. In the present study, we demonstrate that previously described patterns of hypoactivation in parietotemporal and occipitotemporal brain areas during phonological processing in individuals with DD already exist in prereading children with a familial risk for DD. This discovery suggests that functional and structural brain alterations are fundamental to DD and cannot be solely the result of reading failure itself. However, future studies are needed to address the question of whether phonological deficits, alterations in the brain's reading network, and later reading failure interact with each other in a feedback loop. Furthermore, no compensatory mechanisms in frontal/right hemispheric brain regions were observed in the present sample of preliterate children, suggesting such differences may arise with later reading failure. An advance in the understanding of brain processes in children at risk for DD may lead to strategies that will reduce the severity of DD after reading onset. Most importantly, this research may reduce the clinical, psychological and social impact of DD.
Methods
Participants.
Thirty-six healthy, native English-speaking children with (FHD+, n = 18) and without a familial risk for DD (FHD−, n = 18), were included in the present analyses. All children were enrolled in the Boston Longitudinal Dyslexia study (BOLD). Thirty-two children were right handed, whereas for four children handedness could not be indicated yet (these children have been labeled as ambidextrous: 3 FHD+/1 FHD−). FHD+ children (mean age during imaging session: 68.44 mo) had at least one first-degree relative with a clinical diagnosis of DD; FHD− children (mean age during imaging session 66.72 mo) had no first-degree relatives with DD or reading difficulties. Children with a family history of reading difficulties, but no clinical diagnosis of DD, were excluded from the study. Furthermore, all children were physically healthy and had no history of any neurological or psychological disorder, head injuries or poor vision and hearing. The two groups were matched for age, sex, and nonverbal IQ (77) (Table S1).
All children were screened extensively for prereading status (for a detailed description, see SI Methods). All participating children were tested between May and November of their kindergarten-entry year. This study was approved by the local ethics committee of the Children's Hospital Boston. Verbal assent and informed consent was obtained from each child and guardian, respectively.
Behavioral Group Characteristics.
Participants were characterized with a test battery of standardized assessments examining language and prereading skills, such as expressive and receptive vocabulary (CLEF), phonological processing, and rapid automatized naming (Table S1). Additionally, all participating families completed a socioeconomic background questionnaire (adapted from the MacArthur Research Network: http://www.macses.ucsf.edu/default.php; for a complete overview of SES questions, see Table S2) and a home-literacy questionnaire (78)* (Table S3). Both groups were matched for age (age at psychometric testing, P = 0.570; age at imaging testing, P = 0.264) verbal and nonverbal IQ (Kaufman Brief Intelligence Test verbal IQ, P = 0.739; nonverbal IQ, P = 0.389) and SES (e.g., parental education and total family income over the past 12 mo, P > 0.05). The behavioral assessment was performed on a different day than the imaging session, but the sessions were no more than 6 mo apart (average for FHD+ 1.94 mo; average for FHD− 1.44 mo).
fMRI.
Task procedure.
During the experimental run, children performed a phonological processing task that involved listening to two sequentially presented common-object words spoken in a female or male voice. Pictures of the objects were presented on the screen simultaneously. Children were asked to indicate via button-press whether the two words presented started with the same first sound or not. This task was contrasted with a rest condition. During the rest condition, children were asked to look at a fixation cross for the duration of the block. The control task also involved listening to two common object words spoken in a female or male voice. Mirroring the experimental task, pictures that illustrated the spoken words were presented on the screen simultaneously. Participants were asked to indicate by button-press whether or not the sex of the voice matched for the two words presented. This task was also contrasted with a rest condition. (For a more detailed description of task procedure, including stimulus properties, see SI Methods).
Acquisition and analysis.
Acquisition parameters and stimuli software are specified in SI Methods. Image processing and analyses were carried out using SPM5 (www.fil.ion.ucl.ac.uk/spm) executed in MATLAB (Mathworks). Before statistical analysis, all images were preprocessed using realignment, normalization, and smoothing modules in SPM5. Because of the age of participants, a rigorous procedure for artifact detection was chosen. Upon visual inspection of all raw images, preprocessed images were used to create an explicit mask excluding potential artifactual time points through the art-imaging toolbox (http://spnl.stanford.edu/tools/ArtRepair/ArtRepair.htm) for each child. In addition, movement regressors were added. Artifactual time points and movement regressors were identified using a movement threshold of 3 mm and a rotation threshold of 0.05 mm. The resulting images were visually inspected and only those images containing artifacts were removed from further analysis. Subjects were only included in the analysis when more than 80% of the pictures were artifact-free. The general linear approach implemented in SPM5 was used to analyze the data in a block design for each subject. Contrast images for experimental > control condition (“first-sound matching > voice matching”) were obtained. Contrasts comparing with the rest condition were not computed. Finally, a second-level analysis using a two-sample t test was performed to examine functional differences between children with and without a family history of DD. Results are reported at a significance level of P < 0.005, uncorrected, and extent threshold of 50 voxels for each group separately and for those regions that showed significantly more activation in FHD− compared with FHD+ children.
Previous research has shown an involvement of left hemispheric brain regions, including occipitotemporal and temporoparietal areas, during reading and reading-related tasks in typical reading individuals (e.g., refs. 40–42). These regions have shown to be hypoactivated in children and adults with a diagnosis of dyslexia (44, 46–51, 79). Therefore, we chose to investigate the following left-hemispheric regions of interest: LG, STG/PG, and MTG. Regions of interest were extracted from the second level T-contrast (FSM > VM) using MARSBAR.† Correlation analysis within each group separately (FHD+/FHD− children) was used to relate brain function in these regions of interest with phonological processing skills (CTOPP, nonword repetition) usingthe SPSS software package, version 19.0 (80). False-discovery rate-corrected results with a P value below 0.05 are reported as significant.
In-Scanner Performance.
Button-presses were recorded during the experimental and control tasks. The participants’ in-scanner performance was closely monitored (for details see ref. 81). To ensure that the participants were engaged in the tasks, participants with more than 40% of trials unanswered were excluded from the imaging analyses. Children were instructed to indicate their answer as soon as they saw a question mark appear on the screen (after the presentation of the second word; for task design and figure., see Fig. S1 and SI Methods). Children were allowed to correct their response. During the training session, the research team provided verbal feedback on trial performance; no feedback was given during actual neuroimaging. Response correction was taken into account in consequent analysis, if it occurred before the first word of the consequent trial was presented. Task accuracy and reaction time were compared between children with and without a family history of DD using paired two-sample t-tests implemented by the SPSS software package, version 19.0 (80). Results (significant two-tailed) with a P value less than 0.05 are reported as significant.
Supplementary Material
Acknowledgments
We thank all participating families. This research was funded by the Charles H. Hood Foundation, a Children's Hospital Boston pilot grant, the Swiss National Foundation, National Institute of Child Health and Human Development Grant R01 HD65762-01, and the Janggen-Pöhn Stiftung (N.M.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
*Denney MK, English JP, Gerber M, Leafstedt J, Rutz M, Family and home literacy practices: Mediating factors for preliterate English learners at risk, Annual Meeting of the American Educational Research Associations, April 10–14, 2001, Seattle, WA.
†Matthew Brett, Jean-Luc Anton, Romain Valabregue, Jean-Baptiste Poline, Region of interest analysis using an SPM toolbox, 8th International Conference on Functional Mapping of the Human Brain, June 2-6, 2002, Sendai, Japan. Available on CD-ROM in NeuroImage, Vol 16, No 2.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1107721109/-/DCSupplemental.
References
- 1.World Health Organization . The ICD-10 Classification of Mental and Behavioral Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva: WHO; 1992. [Google Scholar]
- 2.Lyon GR, Shaywitz SE, Shaywitz BA. Defining dyslexia, comorbidity, teachers’ knowledge of language and reading. A definition of dyslexia. Ann Dyslexia. 2003;53(1):1–14. [Google Scholar]
- 3.Childs B, Finucci JM. Genetics, Epidemiology, and Specific Reading Disability. New York: Guilford; 1983. [Google Scholar]
- 4.Pennington BF. Annotation: The genetics of dyslexia. J Child Psychol Psychiatry. 1991;31:193–201. doi: 10.1111/j.1469-7610.1990.tb01561.x. [DOI] [PubMed] [Google Scholar]
- 5.Hannula-Jouppi K, et al. The axon guidance receptor gene ROBO1 is a candidate gene for developmental dyslexia. PLoS Genet. 2005;1:e50. doi: 10.1371/journal.pgen.0010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meng H, et al. DCDC2 is associated with reading disability and modulates neuronal development in the brain. Proc Natl Acad Sci USA. 2005;102:17053–17058. doi: 10.1073/pnas.0508591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paracchini S, et al. The chromosome 6p22 haplotype associated with dyslexia reduces the expression of KIAA0319, a novel gene involved in neuronal migration. Hum Mol Genet. 2006;15:1659–1666. doi: 10.1093/hmg/ddl089. [DOI] [PubMed] [Google Scholar]
- 8.Galaburda AM, LoTurco J, Ramus F, Fitch RH, Rosen GD. From genes to behavior in developmental dyslexia. Nat Neurosci. 2006;9:1213–1217. doi: 10.1038/nn1772. [DOI] [PubMed] [Google Scholar]
- 9.Humphrey N, Mullins PM. Self-concept and self-esteem in developmental dyslexia. J Res Spec Educ Needs. 2004;2:1–13. [Google Scholar]
- 10.Saracoglu B, Minden H, Wilchesky M. The adjustment of students with learning disabilities to university and its relationship to self-esteem and self-efficacy. J Learn Disabil. 1989;22:590–592. doi: 10.1177/002221948902200913. [DOI] [PubMed] [Google Scholar]
- 11.Valas H. Students with learning disabilities and low-achieving students: Peer acceptance, loneliness, self-esteem, and depression. Soc Psychol Educ. 1999;3(3):173–192. [Google Scholar]
- 12.Marder C, D'Amico R. How well are youth with disabilities really doing? A comparison of youth with disabilities and youth in general. In: Programs OoSE, editor. US Department of Education. Menlo Park, CA: SRI International; 1992. [Google Scholar]
- 13.Wagner M, Blackorby J, Cameto R, Hebbeler K, Newman L. The Transition Experiences of Young People with Disabilities. A Summary of Findings from the National Longitudinal Transition Study of Special Education Students. Menlo Park, CA: SRI International; 1993. [Google Scholar]
- 14.Reason R. Educational practice and dyslexia. Psychologist. 2001;4:298–301. [Google Scholar]
- 15.Liberman IY, Shankweiler D, Liberman AM. The alphabetic principle and learning to read. In: Shankweiler D, Liberman IY, editors. Phonology and Reading Disability: Solving the Reading Puzzle. Ann Arbor, MI: University of Michigan Press; 1989. pp. 1–33. [Google Scholar]
- 16.Stein J. The magnocellular theory of developmental dyslexia. Dyslexia. 2001;7:12–36. doi: 10.1002/dys.186. [DOI] [PubMed] [Google Scholar]
- 17.Wagner RK, Torgesen JK. The nature of phonological processing and its causal role in the aquisition of reading skills. Psychol Bull. 1987;101:192–212. [Google Scholar]
- 18.Ramus F. Developmental dyslexia: Specific phonological deficit or general sensorimotor dysfunction? Curr Opin Neurobiol. 2003;13:212–218. doi: 10.1016/s0959-4388(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 19.Juel C. Learning to read and write: A longitudinal study of 54 children from first through fourth grades. J Educ Psychol. 1988;80:437–447. [Google Scholar]
- 20.Vaessen A, Gerretsen P, Blomert L. Naming problems do not reflect a second independent core deficit in dyslexia: Double deficits explored. J Exp Child Psychol. 2009;103:202–221. doi: 10.1016/j.jecp.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Scarborough H. Predicting the future achievement of second graders with reading disabilities: Contributions of phonemic awareness, verbal memory, rapid naming, and IQ. Ann Dyslexia. 1998;48(1):115–136. [Google Scholar]
- 22.Stanovich KE, Seigel LS. Phenotypic performance profile of children with reading disabilities: A regression-based test of the phonological-core variable-difference model. J Educ Psychol. 1994;86(1):24–53. [Google Scholar]
- 23.Pennington BF, Lefly DL. Early reading development in children at family risk for dyslexia. Child Dev. 2001;72:816–833. doi: 10.1111/1467-8624.00317. [DOI] [PubMed] [Google Scholar]
- 24.Snowling MJ, Gallagher A, Frith U. Family risk of dyslexia is continuous: Individual differences in the precursors of reading skill. Child Dev. 2003;74:358–373. doi: 10.1111/1467-8624.7402003. [DOI] [PubMed] [Google Scholar]
- 25.Burgess SR, Lonigan CJ. Bidirectional relations of phonological sensitivity and prereading abilities: Evidence from a preschool sample. J Exp Child Psychol. 1998;70:117–141. doi: 10.1006/jecp.1998.2450. [DOI] [PubMed] [Google Scholar]
- 26.Flax JF, Realpe-Bonilla T, Roesler C, Choudhury N, Benasich A. Using early standardized language measures to predict later language and early reading outcomes in children at high risk for language-learning impairments. J Learn Disabil. 2009;42:61–75. doi: 10.1177/0022219408326215. [DOI] [PubMed] [Google Scholar]
- 27.Lundberg I, Olofsson A, Wall S. Reading and spelling skills in the first school years predicted from phonemic awareness skills in the kindergarten. Scand J Psychol. 1980;21(1):159–173. [Google Scholar]
- 28.Mann V, Ditunno P. Phonological deficiencies: Effective predictors of future reading. In: Pavlides G, editor. Perspectives on Dyslexia. Vol 2. UK: Wiley, Chichester; 1990. pp. 105–131. [Google Scholar]
- 29.Mann VA, Liberman IY. Phonological awareness and verbal short-term memory. J Learn Disabil. 1984;17:592–599. doi: 10.1177/002221948401701005. [DOI] [PubMed] [Google Scholar]
- 30.Nation K, Hulme C. Phonemic segmentation, not onset-rime segmentation, predicts early reading and spelling skills. Read Res Q. 1997;32(2):154–167. [Google Scholar]
- 31.Stuart M, Coltheart M. Does reading develop in a sequence of stages? Cognition. 1988;30(2):139–181. doi: 10.1016/0010-0277(88)90038-8. [DOI] [PubMed] [Google Scholar]
- 32.Tunmer WE. The role of language related factors in reading disability. In: Shankweiler D, Liberman IY, editors. Phonology and reading disability: Solving the reading puzzle. Ann Arbor, MI: University of Michigan Press; 1989. pp. 91–131. [Google Scholar]
- 33.Butler PD, et al. Dysfunction of early-stage visual processing in schizophrenia. Am J Psychiatry. 2001;158:1126–1133. doi: 10.1176/appi.ajp.158.7.1126. [DOI] [PubMed] [Google Scholar]
- 34.Share DL, McGee R, Silva PA. IQ and reading progress: A test of the capacity notion of IQ. J Am Acad Child Adolesc Psychiatry. 1989;28:97–100. doi: 10.1097/00004583-198901000-00018. [DOI] [PubMed] [Google Scholar]
- 35.Silva PA, McGee R, Williams S. Some characteristics of 9-year-old boys with general reading backwardness or specific reading retardation. J Child Psychol Psychiatry. 1985;26:407–421. doi: 10.1111/j.1469-7610.1985.tb01942.x. [DOI] [PubMed] [Google Scholar]
- 36.Wolf M, Goodglass H. Dyslexia, dysnomia, and lexical retrieval: A longitudinal investigation. Brain Lang. 1986;28:154–168. doi: 10.1016/0093-934x(86)90098-2. [DOI] [PubMed] [Google Scholar]
- 37.Badian NA. Nonverbal learning disability, school behavior, and dyslexia. Ann Dyslexia. 1992;42(1):159–178. doi: 10.1007/BF02654944. [DOI] [PubMed] [Google Scholar]
- 38.Gallagher A, Frith U, Snowling MJ. Precursors of literacy delay among children at genetic risk of dyslexia. J Child Psychol Psychiatry. 2000;41:203–213. [PubMed] [Google Scholar]
- 39.McCandliss BD, Noble KG. The development of reading impairment: A cognitive neuroscience model. Ment Retard Dev Disabil Res Rev. 2003;9:196–204. doi: 10.1002/mrdd.10080. [DOI] [PubMed] [Google Scholar]
- 40.Pugh KR, et al. Neurobiological studies of reading and reading disability. J Commun Disord. 2001;34:479–492. doi: 10.1016/s0021-9924(01)00060-0. [DOI] [PubMed] [Google Scholar]
- 41.Sandak R, et al. The neurobiology of adaptive learning in reading: A contrast of different training conditions. Cogn Affect Behav Neurosci. 2004;4:67–88. doi: 10.3758/cabn.4.1.67. [DOI] [PubMed] [Google Scholar]
- 42.Schlaggar BL, McCandliss BD. Development of neural systems for reading. Annu Rev Neurosci. 2007;30:475–503. doi: 10.1146/annurev.neuro.28.061604.135645. [DOI] [PubMed] [Google Scholar]
- 43.Temple E. Brain mechanisms in normal and dyslexic readers. Curr Opin Neurobiol. 2002;12:178–183. doi: 10.1016/s0959-4388(02)00303-3. [DOI] [PubMed] [Google Scholar]
- 44.Hoeft F, et al. Functional and morphometric brain dissociation between dyslexia and reading ability. Proc Natl Acad Sci USA. 2007;104:4234–4239. doi: 10.1073/pnas.0609399104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turkeltaub PE, Gareau L, Flowers DL, Zeffiro TA, Eden GF. Development of neural mechanisms for reading. Nat Neurosci. 2003;6:767–773. doi: 10.1038/nn1065. [DOI] [PubMed] [Google Scholar]
- 46.Gaillard WD, Balsamo LM, Ibrahim Z, Sachs BC, Xu B. fMRI identifies regional specialization of neural networks for reading in young children. Neurology. 2003;60:94–100. doi: 10.1212/wnl.60.1.94. [DOI] [PubMed] [Google Scholar]
- 47.McCrory EJ, Mechelli A, Frith U, Price CJ. More than words: A common neural basis for reading and naming deficits in developmental dyslexia? Brain. 2005;128:261–267. doi: 10.1093/brain/awh340. [DOI] [PubMed] [Google Scholar]
- 48.Paulesu E, et al. Dyslexia: Cultural diversity and biological unity. Science. 2001;291:2165–2167. doi: 10.1126/science.1057179. [DOI] [PubMed] [Google Scholar]
- 49.Shaywitz BA, et al. Disruption of posterior brain systems for reading in children with developmental dyslexia. Biol Psychiatry. 2002;52:101–110. doi: 10.1016/s0006-3223(02)01365-3. [DOI] [PubMed] [Google Scholar]
- 50.Shaywitz SE, et al. Functional disruption in the organization of the brain for reading in dyslexia. Proc Natl Acad Sci USA. 1998;95:2636–2641. doi: 10.1073/pnas.95.5.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Temple E, et al. Disrupted neural responses to phonological and orthographic processing in dyslexic children: An fMRI study. Neuroreport. 2001;12:299–307. doi: 10.1097/00001756-200102120-00024. [DOI] [PubMed] [Google Scholar]
- 52.Houdé O, Rossi S, Lubin A, Joliot M. Mapping numerical processing, reading, and executive functions in the developing brain: An fMRI meta-analysis of 52 studies including 842 children. Dev Sci. 2010;13:876–885. doi: 10.1111/j.1467-7687.2009.00938.x. [DOI] [PubMed] [Google Scholar]
- 53.Jobard G, Crivello F, Tzourio-Mazoyer N. Evaluation of the dual route theory of reading: A metanalysis of 35 neuroimaging studies. Neuroimage. 2003;20:693–712. doi: 10.1016/S1053-8119(03)00343-4. [DOI] [PubMed] [Google Scholar]
- 54.Richlan F, Kronbichler M, Wimmer H. Functional abnormalities in the dyslexic brain: A quantitative meta-analysis of neuroimaging studies. Hum Brain Mapp. 2009;30:3299–3308. doi: 10.1002/hbm.20752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Richlan F, Kronbichler M, Wimmer H. Meta-analyzing brain dysfunctions in dyslexic children and adults. Neuroimage. 2011;56:1735–1742. doi: 10.1016/j.neuroimage.2011.02.040. [DOI] [PubMed] [Google Scholar]
- 56.Brambati SM, et al. Regional reductions of gray matter volume in familial dyslexia. Neurology. 2004;63:742–745. doi: 10.1212/01.wnl.0000134673.95020.ee. [DOI] [PubMed] [Google Scholar]
- 57.Brown WE, et al. Preliminary evidence of widespread morphological variations of the brain in dyslexia. Neurology. 2001;56:781–783. doi: 10.1212/wnl.56.6.781. [DOI] [PubMed] [Google Scholar]
- 58.Eckert MA, et al. Anatomical signatures of dyslexia in children: Unique information from manual and voxel based morphometry brain measures. Cortex. 2005;41:304–315. doi: 10.1016/s0010-9452(08)70268-5. [DOI] [PubMed] [Google Scholar]
- 59.Kronbichler M, et al. Developmental dyslexia: Gray matter abnormalities in the occipitotemporal cortex. Hum Brain Mapp. 2008;29:613–625. doi: 10.1002/hbm.20425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pernet C, Andersson J, Paulesu E, Demonet JF. When all hypotheses are right: A multifocal account of dyslexia. Hum Brain Mapp. 2009;30:2278–2292. doi: 10.1002/hbm.20670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Silani G, et al. Brain abnormalities underlying altered activation in dyslexia: A voxel based morphometry study. Brain. 2005;128:2453–2461. doi: 10.1093/brain/awh579. [DOI] [PubMed] [Google Scholar]
- 62.Raschle NM, Chang M, Gaab N. Structural brain alterations associated with dyslexia predate reading onset. Neuroimage. 2010;57:742–749. doi: 10.1016/j.neuroimage.2010.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Georgiewa P, et al. Phonological processing in dyslexic children: A study combining functional imaging and event related potentials. Neurosci Lett. 2002;318:5–8. doi: 10.1016/s0304-3940(01)02236-4. [DOI] [PubMed] [Google Scholar]
- 64.Hoeft F, et al. Neural systems predicting long-term outcome in dyslexia. Proc Natl Acad Sci USA. 2011;108:361–366. doi: 10.1073/pnas.1008950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maurer U, et al. Neurophysiology in preschool improves behavioral prediction of reading ability throughout primary school. Biol Psychiatry. 2009;66:341–348. doi: 10.1016/j.biopsych.2009.02.031. [DOI] [PubMed] [Google Scholar]
- 66.Guttorm TK, Leppänen PH, Richardson U, Lyytinen H. Event-related potentials and consonant differentiation in newborns with familial risk for dyslexia. J Learn Disabil. 2001;34:534–544. doi: 10.1177/002221940103400606. [DOI] [PubMed] [Google Scholar]
- 67.Maassen B, Pasman J, Nijland L, Rotteveel J. Clinical use of AEVP- and AERP-measures in childhood speech disorders. Clin Linguist Phon. 2006;20:125–134. doi: 10.1080/02699200400026819. [DOI] [PubMed] [Google Scholar]
- 68.van Atteveldt N, Formisano E, Goebel R, Blomert L. Integration of letters and speech sounds in the human brain. Neuron. 2004;43:271–282. doi: 10.1016/j.neuron.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 69.Cohen L, et al. Language-specific tuning of visual cortex? Functional properties of the Visual Word Form Area. Brain. 2002;125:1054–1069. doi: 10.1093/brain/awf094. [DOI] [PubMed] [Google Scholar]
- 70.Riesenhuber M, Poggio T. Hierarchical models of object recognition in cortex. Nat Neurosci. 1999;2:1019–1025. doi: 10.1038/14819. [DOI] [PubMed] [Google Scholar]
- 71.Cantlon JF, Pinel P, Dehaene S, Pelphrey KA. Cortical representations of symbols, objects, and faces are pruned back during early childhood. Cereb Cortex. 2011;21:191–199. doi: 10.1093/cercor/bhq078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maurer U, et al. Impaired tuning of a fast occipito-temporal response for print in dyslexic children learning to read. Brain. 2007;130:3200–3210. doi: 10.1093/brain/awm193. [DOI] [PubMed] [Google Scholar]
- 73.Sandak R, Mencl WE, Frost SJ, Pugh KR. The neurobiological basis of skilled and impaired reading: Recent findings and new directions. Sci Stud Read. 2004;8:273–292. [Google Scholar]
- 74.Baker SF, Ireland JL. The link between dyslexic traits, executive functioning, impulsivity and social self-esteem among an offender and non-offender sample. Int J Law Psychiatry. 2007;30:492–503. doi: 10.1016/j.ijlp.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 75.Cunningham AE, Stanovich KE. Tracking the unique effects of print exposure in children: Association with vocabulary, general knowledge, and spelling. J Educ Psychol. 1991;83:264–274. [Google Scholar]
- 76.Oka E, Paris S. Patterns of motivation and reading skills in underachieving children. In: Ceci S, editor. Handbook of Cognitive, Social, and Neuropsychological Aspects of Learning Disabilities. Vol 2. Hillsdale, NJ: Lawrence-Erlbaum; 1986. pp. 220–237. [Google Scholar]
- 77.Kaufman AS, Kaufman NL. KBIT-2: Kaufman Brief Intelligence Test. Minneapolis, MN: NCS Pearson, Inc.; 1997. [Google Scholar]
- 78.Katzir T, Lesaux NK, Kim Y-S. The role of reading self-concept and home literacy practices in fourth grade reading comprehension. Read Writ. 2009;22:261–276. [Google Scholar]
- 79.Simos PG, et al. Dyslexia-specific brain activation profile becomes normal following successful remedial training. Neurology. 2002;58:1203–1213. doi: 10.1212/wnl.58.8.1203. [DOI] [PubMed] [Google Scholar]
- 80.SPSS Inc. SPSS Base 10.0 for Windows User's Guide. Chicago IL: SPSS Inc.; 1999. [Google Scholar]
- 81.Raschle NM, et al. Making MR imaging child's play—Pediatric neuroimaging protocol, guidelines and procedure. J Vis Exp. 2009;29:p11, 1309. doi: 10.3791/1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.