Abstract
Psychedelic drugs have a long history of use in healing ceremonies, but despite renewed interest in their therapeutic potential, we continue to know very little about how they work in the brain. Here we used psilocybin, a classic psychedelic found in magic mushrooms, and a task-free functional MRI (fMRI) protocol designed to capture the transition from normal waking consciousness to the psychedelic state. Arterial spin labeling perfusion and blood-oxygen level-dependent (BOLD) fMRI were used to map cerebral blood flow and changes in venous oxygenation before and after intravenous infusions of placebo and psilocybin. Fifteen healthy volunteers were scanned with arterial spin labeling and a separate 15 with BOLD. As predicted, profound changes in consciousness were observed after psilocybin, but surprisingly, only decreases in cerebral blood flow and BOLD signal were seen, and these were maximal in hub regions, such as the thalamus and anterior and posterior cingulate cortex (ACC and PCC). Decreased activity in the ACC/medial prefrontal cortex (mPFC) was a consistent finding and the magnitude of this decrease predicted the intensity of the subjective effects. Based on these results, a seed-based pharmaco-physiological interaction/functional connectivity analysis was performed using a medial prefrontal seed. Psilocybin caused a significant decrease in the positive coupling between the mPFC and PCC. These results strongly imply that the subjective effects of psychedelic drugs are caused by decreased activity and connectivity in the brain's key connector hubs, enabling a state of unconstrained cognition.
Keywords: default mode network, hallucinogens, serotonin, depression, 5-HT2A receptor
Psilocybin is the prodrug of psilocin (4-hydroxy-dimethyltryptamine), the primary hallucinogenic component of magic mushrooms, and a classic psychedelic (“mind-manifesting”) drug. Psilocybin has been used for centuries in healing ceremonies (1) and more recently in psychotherapy (2); it is capable of stimulating profound existential experiences (3), which can leave a lasting psychological impression (4). However, despite a wealth of literature on its phenomenology, we currently know very little about how its effects are produced in the brain. The present study sought to address this question using complementary functional MRI (fMRI) techniques and a protocol designed to image the transition from normal waking consciousness to the psychedelic state. Two groups of healthy subjects were scanned using arterial spin labeling (ASL) perfusion and blood-oxygen level-dependent (BOLD) fMRI during intravenous infusion of psilocybin. Infused over 60 s (2 mg in 10-mL saline), psilocybin's subjective effects begin within seconds (5), allowing the capture of the corresponding change in brain state.
Results
ASL Perfusion fMRI.
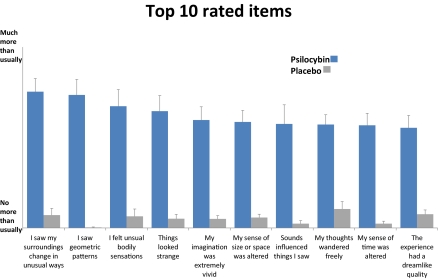
Fifteen healthy, hallucinogen-experienced subjects (five females), mean age 34.1 (SD 8.2) were scanned with ASL. Subjects underwent an anatomical scan followed by two task-free functional scans, each lasting 18 min. Subjects were instructed to relax and a fixation cross was displayed. Solutions were infused manually over 60 s, beginning 6 min after the start of each functional scan. Subjects received placebo (10-mL saline) in the first scan and psilocybin (2 mg in 10-mL saline) in the second. The intensity of the subjective effects was rated via button press on a 0–10 visual analog scale (10 = extremely intense effects) at the start of each functional scan, just before infusion, 5-min postinfusion, and 12-min postinfusion. The average rating 5-min postinfusion was 6.7 (±1.9), and 5.2 (±2.3) 12-min postinfusion. Earlier work showed that the effects of 2 mg i.v. psilocybin are comparable with ∼15 mg of orally administered psilocybin, which is considered a moderate dose (6). Nineteen additional items were rated immediately after each ASL scan. Fig. 1 displays the top 10 rated items from the two studies. Ratings for all of the items used in the ASL and BOLD studies can be found in Table S1.
Fig. 1.
Subjective ratings (n = 30). Displayed are mean values + SEs. Ratings were given shortly after the scans. Subjects were instructed that “no more than usually” refers to normal waking consciousness. All 10 items were scored significantly higher after psilocybin than placebo (P < 0.01).
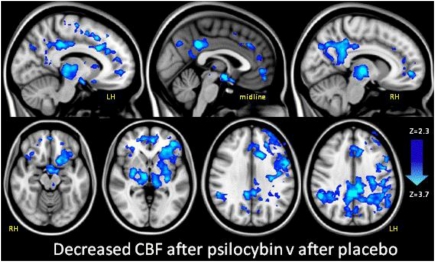
The interaction between cerebral blood flow (CBF) and the infusion event was modeled, contrasting CBF before and after infusion. The subjective effects began toward the end of the infusion period and reached a sustained peak after ∼4 min (5). The first level results were entered into a higher level analysis, contrasting CBF after psilocybin with CBF after placebo for all 15 subjects. Fig. 2 displays these results.
Fig. 2.
Decreased CBF after psilocybin (ASL perfusion fMRI). Regions where there was significantly decreased CBF after psilocybin versus after placebo are shown in blue (z: 2.3–3.7). Mixed effects analysis, z > 2.3, P < 0.05 whole-brain cluster-corrected, n = 15. LH, left hemisphere; RH, right hemisphere. Note, we observed no increases in CBF in any region.
The group level results (Fig. 2) revealed significant CBF decreases in subcortical (bilateral thalamus, putamen, and hypothalamus) and cortical regions [the posterior cingulate cortex (PCC), retrosplenial cortex, precuneus, bilateral angular gyrus, supramarginal gyrus, rostral and dorsal anterior cingulate cortex (ACC), paracingulate gyrus, medial prefrontal cortex (mPFC), frontoinsular cortex, lateral orbitofrontal cortex, frontal operculum, precentral gyrus, and superior, middle and inferior frontal gyrus] (Fig. S1). The decreases were localized to high-level association regions (e.g., the PCC and mPFC) and important connector hubs, such as the thalamus, PCC and ACC/mPFC.
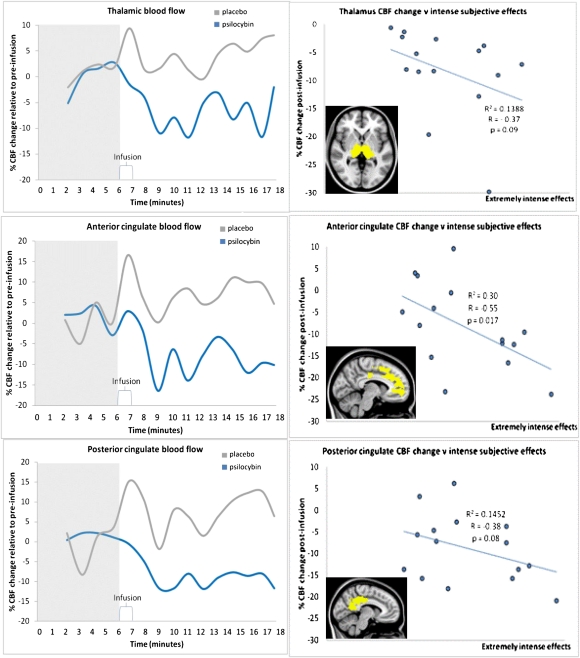
To assess the temporal dynamics of the CBF changes postinfusion, thalamic, ACC, and PCC masks were made; voxels within these were restricted to those that were significantly decreased after psilocybin. For each region of interest (ROI), the percent CBF change postinfusion was plotted against time (Fig. 3). All ROIs showed steep decreases in CBF after psilocybin that were sustained for the duration of the scan.
Fig. 3.
Group CBF changes over time (Left) and CBF vs. subjective effects (Right). Plots on the left show blood flow changes over time for the thalamus, ACC, and PCC. These plots were made by calculating the postinfusion change in CBF as a percentage of the preinfusion CBF. This process was done for each ROI for each individual subject and then the group mean was plotted. Note, these plots are shown for display purposes; error bars are not included because the inclusion of error is implicit in the statistical parametric maps shown in Fig. 2. Plots on the right show the relationship between ROI CBF changes after psilocybin-infusion for each subject and their ratings of the intensity of the subjective effects given 5- and 12-min postinfusion (plotted are the average of these two ratings). There was a significant negative correlation between ACC CBF change postpsilocybin and intense subjective effects (Pearson's correlation, r = −0.55, P = 0.017, one-tailed) and CBF vs. intensity met a trend level significance for the thalamus and PCC (P < 0.01).
To test for a relationship between regional CBF changes and subjective effects, we plotted each subjects’ ROI CBF change postpsilocybin against their ratings of drug effects intensity (Fig. 3D). For each ROI, it was evident that the greater the decreases in CBF, the more intense the subjective effects.
BOLD fMRI.
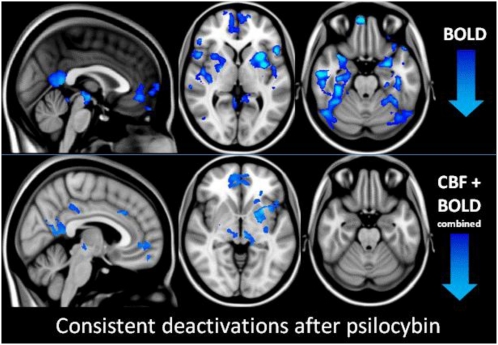
A separate sample of 15 subjects was scanned with BOLD fMRI. The BOLD scans took place ∼6 mo after the ASL. The sample included two females and had a mean age of 32 (SD 8.9). Subjects underwent an anatomical scan followed by an eyes-closed task-free BOLD scan, which lasted 12 min. This process occurred on two visits, ∼14 d apart. Placebo was given on one occasion and psilocybin on the other in a balanced order. Infusions began 6 min after the start of the scan, following the same procedure as the ASL study. The data were high-pass filtered with a cutoff of 300 s and the pharmacodynamics of intravenous psilocybin was used to model changes in the BOLD signal coinciding with the infusion event (5). The first level results were entered into a higher level analysis, contrasting BOLD signal changes before and after psilocybin, and before and after psilocybin versus before and after placebo (Fig. 4, Upper).
Fig. 4.
Brain deactivations after psilocybin. (Upper) Regions where there was a significant decrease in the BOLD signal after psilocybin versus after placebo (z: 1.8–3). Mixed-effects analysis, z > 1.8, P < 0.05 whole brain cluster corrected, n = 15. (Lower) Regions where there was a consistent decrease in CBF and BOLD after psilocybin. For display purposes, significant BOLD decreases were calculated within a mask based on the ASL result (Fig. 2) at an uncorrected voxel level threshold of P = 0.05. Note, we observed no increases in CBF or BOLD signal in any region.
The regional decreases in BOLD signal were similar to the regional decreases in CBF observed with ASL, with consistent decreases in the mPFC, ventral PCC, putamen, and subthalamic nuclei (Fig. 4, Lower). There were, however, additional BOLD signal decreases (e.g., in higher order visual areas) that were not observed with ASL (Fig. 4, Upper, and Fig. S2).
Pharmaco-Physiological Interaction.
These results implicate the ACC/mPFC in the mechanism of action of psilocybin. The ACC/mPFC showed decreased CBF (Fig. 2) and BOLD signal (Fig. 4) after psilocybin and the magnitude of CBF decreases correlated positively with the intensity of the drug's subjective effects (Fig. 3). Based on these results, a ventromedial prefrontal (vmPFC) ROI (Fig. 5, red) was chosen for a psycho-physiological interaction (PPI) analysis. PPIs test for changes in linear coupling under a psychological factor. In our case, this factor was the administration of psilocybin. This finding means that, strictly speaking, we are looking at a pharmaco-physiological interaction. Although commonly referred to as analyses of functional connectivity, PPIs can also be regarded as tests for changes in effective connectivity under a simple linear model of the influence of one region upon another.
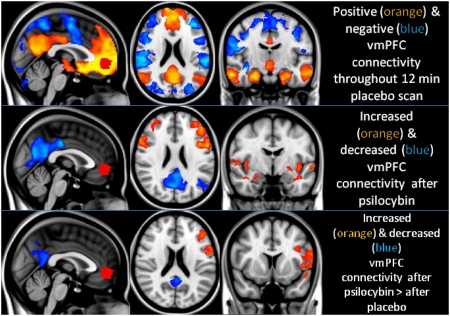
Fig. 5.
Psilocybin-induced changes in vmPFC (red) functional connectivity. (Top) Regions where activity was positively coupled to that of the vmPFC are shown in orange and regions where activity was “negatively” coupled to activity in the vmPFC are shown in blue (it should be noted however, that the appearance of negative connectivity is forced by regression of the global signal). (Middle) Significant increases (orange) and decreases (blue) in functional connectivity after psilocybin infusion. (Bottom) Increases and decreases in functional connectivity after psilocybin that were significantly greater than any connectivity changes after placebo. All analyses were mixed effects, z > 2.3, P < 0.05 whole-brain cluster corrected, n = 15. Note: The significant psycho-physiological interactions in the posterior PCC and left lateral parietal region suggest that the positive coupling (under placebo) has decreased significantly. This finding should not necessarily be interpreted as a negative coupling, simply a significant decrease in a positive coupling.
For each subject, the vmPFC time series was entered into a model that included independent gray matter, white matter, and cerebrospinal fluid time series as regressors of no-interest. Fig. 5 displays regions where activity was positively (yellow/orange) and negatively (blue) coupled to activity in the vmPFC throughout the placebo scan. However, the salient images are those shown as follows in Fig. 5: the middle row displays regions where activity became significantly less (blue) and more (red) coupled to activity in the vmPFC after psilocybin infusion and the bottom row displays these effects relative to those after placebo.
Physiological Correction and Breath-Hold.
BOLD and ASL fMRI measure changes in a vascular signal that is coupled to changes in neural activity. It is therefore important to address factors that may modulate brain vasculature without affecting neural activity. For example, increases in blood carbon dioxide that occur with changes in respiration can drive increases in CBF (7). Thus, to address the possibility that the effects observed in this study were driven by nonneural physiological changes, physiological variance (i.e., heart rate, respiration rate, and respiration depth) was regressed from the functional data before repeating the BOLD analyses described above. This correction step did not significantly alter the statistical parametric maps (Fig. S3), suggesting that changes in physiological parameters were not responsible for the positive outcomes.
Furthermore, to test the possibility that psilocybin had acted directly on the cerebral vasculature, we included a blocked breath-hold paradigm at the end of each BOLD scan. Hypercapnia is known to significantly increase the BOLD signal via CO2-induced vasodilation (see ref. 7 and Fig. S4); thus, it was reasoned that a direct effect of psilocybin on brain vasculature would cause an altered BOLD response to breath-hold. However, no difference was found in the BOLD response to breath-hold under psilocybin and placebo, implying that the drug had not acted directly on the vascular system.
Discussion
The fMRI studies reported here revealed significant and consistent outcomes. Psilocybin significantly decreased brain blood flow and venous oxygenation in a manner that correlated with its subjective effects, and significantly decreased the positive coupling of two key structural hubs (the mPFC and the PCC). Our use of fMRI to measure resting-state brain activity after a psychedelic is unique, and because the results are unexpected, they require some explanation.
The effect of psilocybin on resting-state brain activity has been measured before with PET and glucose metabolism (8). This study found a global increase in glucose metabolism after oral psilocybin, which is inconsistent with our fMRI results. One possible explanation for this discrepancy relates to the fact that the radiotracer used to measure glucose metabolism (18F-fluorodeoxyglucose) has a long half-life (110 min). Thus, the effects of psilocybin, as measured by PET, are over much greater timescales than indexed by our fMRI measures. It is therefore possible that phasic or short-term effects of psilocybin show some rebound that is detected by longer-term changes in glucose metabolism. More direct measures of neural activity will help inform this hypothesis, but in support of the inference that psilocybin does decrease neural activity, direct recordings of cortical local field potentials (LFPs) in rats found broadband decreases in resting state LFP power after psilocybin infusion—including γ-power (9)—changes in which are known to correlate with changes in the BOLD signal (10).
It has been commonly assumed that psychedelics work by increasing neural activity; however, our results put this into question. Psilocin is a mixed serotonin receptor agonist, but there is a general consensus that the characteristic subjective and behavioral effects of psychedelics are initiated via stimulation of serotonin (5-Hydroxytryptamine, 5-HT) 2A receptors (11). It is possible that the deactivations observed in the present studies were caused by stimulation of 5-HT receptors other than 5-HT2A; however, this seems unlikely given that the affinity of psychedelics for the 5-HT2A receptor correlates with their potency (12) and 5-HT2A antagonists block the subjective effects of psychedelics (13). There is a large body of preclinical evidence that stimulation of 5-HT2A receptors increases GABAergic transmission and pyramidal cell inhibition (14–21), which may explain the deactivations observed here (Figs. 2 and 4). fMRI studies with serotonergic compounds that stimulate other 5-HT receptors, such as the 5-HT2C (22) or (mainly) the 5-HT1A receptor (23), have not found comparable results to those shown here, and 5-HT2A receptors are present in high concentrations in the cortical regions that were significantly deactivated and decoupled after psilocybin (Table S2).
Stimulation of the 5-HT2A receptor increases excitation in the host cell by reducing outward potassium currents (24). Thus, if the 5-HT2A receptor did mediate the observed deactivations, then it may have been via 5-HT2A-induced excitation of fast-spiking interneurons terminating on pyramidal cells (e.g., ref. 24) or 5-HT2A-induced excitation of pyramidal cells projecting onto interneurons (25).
Regardless of how these effects were initiated at the receptor level, it is necessary for us to offer a functional explanation for them. It is noteworthy that the regions which showed the most consistent deactivations after psilocybin (e.g., the PCC and mPFC) are also those that show disproportionately high activity under normal conditions (26). For example, metabolism in the PCC is ∼20% higher than most other brain regions (27), yet psilocybin decreased its blood flow by up to 20% in some subjects. There is some mystery about the function of the PCC; its large size, buffered location, and rich vasculature means that it is well protected from damage. The high metabolic activity of the PCC and the default-mode network (DMN) with which is it associated (26) has led some to speculate about its functional importance, positing a role in consciousness (28) and high-level constructs, such as the self (29) or “ego” (30, 31). Indeed, the DMN is known to be activated during self-referencing (28) and other high-level functions linked to the self-construct (27). Moreover, DMN regions are also known to host the highest number of cortico-cortical connections in the brain, making them important “connector hubs” (32). These hubs may be critical for efficient information transfer in the brain by allowing communication between different regions via the fewest number of connections (33). However, such an integrative function would confer a significant responsibility on these regions, which may explain why their deactivation has such a profound effect on consciousness, as shown here.
These results may have implications beyond explaining how psilocybin works in the brain by implying that the DMN is crucial for the maintenance of cognitive integration and constraint under normal conditions. This finding is consistent with Aldous Huxley's “reducing valve” metaphor (34) and Karl Friston's “free-energy principle” (35), which propose that the mind/brain works to constrain its experience of the world.
The pharmaco-physiological interaction results were particularly intriguing, revealing significant decreases in the positive coupling between the PCC and mPFC after psilocybin. This result can be understood in terms of a regression of PCC activity on mPFC activity, in which the regression slope decreases. This finding can either be interpreted as a decrease in the (backward or top-down) connectivity from prefrontal to parietal regions or, equivalently, an increase in the reciprocal (forward or bottom-up) direction from parietal to prefrontal regions. This asymmetrical change in coupling, induced by psilocybin, is consistent with a reduction in the sensitivity of superficial pyramidal cells in the parietal region targeted by prefrontal afferents, which may or may not be associated with a compensatory increase in the influence of parietal regions on prefrontal activity. Whatever the underlying synaptic mechanisms, these results provide clear evidence for a perturbation in reciprocal coupling between these two association areas and speak to a rebalancing of hierarchical activity in distributed high-level modes.
Finally, consistent with their history of use as adjuncts to psychotherapy, the idea has recently re-emerged that psychedelics may be useful in the treatment of certain psychiatric disorders (36). It seems relevant therefore that activity in (37) and connectivity with (38) the mPFC is known to be elevated in depression and normalized after effective treatment (39). The mPFC was consistently deactivated by psilocybin (Fig. 4) and the magnitude of the deactivations correlated with the drug's subjective effects (Fig. 3). Depression has been characterized as an “overstable” state, in which cognition is rigidly pessimistic (39). Trait pessimism has been linked to deficient 5-HT2A receptor stimulation (40, 41), particularly in the mPFC (40), and mPFC hyperactivity has been linked to pathological brooding (42). Recent work has shown that psilocybin can increase subjective well-being (4) and trait openness (43) several months after an acute experience, and depression scores in terminal cancer patients were significantly decreased 6 mo after treatment with psilocybin (2). Our results suggest a biological mechanism for this: decreased mPFC activity via 5-HT2A receptor stimulation. Further work is required to test this hypothesis and the putative utility of psilocybin in depression.
We also observed decreased CBF in the hypothalamus after psilocybin (Fig. 2), which may explain anecdotal reports that psychedelics reduce symptoms of cluster headaches (44). Increased hypothalamic CBF was observed during acute headache in cluster headache sufferers (45) and inhibition of the hypothalamus via direct electrical stimulation can provide therapeutic relief for this condition (46).
To conclude, here we used an advanced and comprehensive fMRI protocol to image the brain effects of psilocybin. These studies offer the most detailed account to date on how the psychedelic state is produced in the brain. The results suggest decreased activity and connectivity in the brain's connector hubs, permitting an unconstrained style of cognition.
Materials and Methods
All subjects gave informed consent to participate in the study. The study was approved by a National Health Service research ethics committee. A physical examination, including electrocardiogram (ECG), routine blood tests, and urine test for drugs of abuse and pregnancy were carried out. A psychiatric assessment was conducted and participants disclosed their drug taking histories. Exclusion criteria were: less than 21 y of age, pregnancy, personal or immediate family history of psychiatric disorder, substance dependence, cardiovascular disease, claustrophobia, blood or needle phobia, or a significant adverse response to a hallucinogenic drug. All subjects had previous experience with a hallucinogenic drug but not within 6 wk of the study.
MRI Anatomical Scan.
All imaging was performed on a 3T GE HDx system. For every functional scan, we obtained an initial 3D FSPGR scan in an axial orientation, with field of view = 256 × 256 × 192 and matrix = 256 × 256 × 192 to yield 1-mm isotropic voxel resolution (TR/TE = 7.9/3.0 ms; inversion time = 450 ms; flip angle = 20°).
MRI Methods.
ASL relative perfusion measurement.
Whole-brain perfusion was measured using single-shot pulsed ASL PICORE (proximal inversion with a control for off-resonance effects) QUIPSSII (quantitative imaging of perfusion using a single subtraction) sequence with GE-EPI readout. The 240 tag-control image pairs with 16 axial slices (3 × 3 × 6-mm voxel resolution, matrix = 64 × 64, with a 1-mm gap in the axial direction) were acquired (TR/TE = 2200/19.8 ms, single-inversion time with TI1 = 700 and TI2 = 1,350 ms for the most proximal slice).
General linear modeling was used to evaluate the drug-induced modulation of the perfusion signal. In the first-level analysis, the difference between tag and control images was explicitly modeled as one regressor representing the voxel-wise mean perfusion per scan per voxel. A second regressor was established from the interaction of the first regressor and the estimated time-course based on the drug pharmacodynamics (5). This represents the within-scan modulation of perfusion by the drug.
The results of fitting this model at the first level were combined in a higher level within-subjects analysis to compare modulation of perfusion during psilocybin infusion with that during saline infusion. A mixed-effects analysis was performed. Z statistical parametric maps were thresholded at a cluster size of z > 2.3 and a whole-brain significance of P < 0.05. To assist with this process, perfusion data underwent unwarping for B0 field distortions and were registered to the T1-weighted high-resolution (1 × 1 × 1 mm) structural scan which was itself registered to a 1-mm resolution Montreal Neurological Institute (MNI) standard brain, using nonlinear registration.
BOLD measurement.
BOLD-weighted fMRI data were acquired using a gradient-echo EPI sequence, TR/TE 3000/35 ms, field-of-view = 192 mm, 64 × 64 acquisition matrix, parallel acceleration factor = 2, 90° flip angle. Fifty-three oblique-axial slices were acquired in an interleaved fashion, each 3 mm thick with zero slice gap (3 × 3 × 3-mm voxels). A total of 240 volumes were acquired.
At the first-level, a basic square function informed by the time-course of intravenous psilocybin's subjective effects (5) was used to model changes in the BOLD signal. A mixed-effects analysis was performed at the group level. Z statistical parametric maps were thresholded using clusters determined by z > 2.3 and a whole-brain cluster significance threshold of P < 0.05. The results were registered to the T1-weighted high-resolution (1 × 1 × 1 mm) structural scan, which was itself nonlinearly registered to a 1-mm MNI standard brain.
Pharmaco-Physiological Interaction/Functional Connectivity Analysis.
A medial prefrontal ROI was drawn on a standard brain over maps of the CBF and BOLD decreases. This mask was then transformed into single-subject functional space and time series were derived for each subject. Gray matter, white matter, and CSF were segmented in each subjects’ anatomical image and these masks were thresholded and transformed into functional space. Their time series were derived and used as regressors of no interest in the general linear model. The primary contrasts of interest within each subjects’ scan was the interaction between vmPFC connectivity and the infusion event (i.e., maps were generated showing regions where activity became significant more and less coupled to activity in the vmPFC ROI after infusion versus before). Maps were generated for the effect of psilocybin infusion alone (Fig. 5, Middle) and psilocybin infusion versus placebo infusion (Fig. 5, Bottom). All maps were generated via mixed effects analyses, thresholded at a cluster size of 2.3, and whole-brain corrected for multiple comparisons with a P value of 0.05.
Physiological Noise Correction.
After infusion of psilocybin, alterations in physiological rhythms, such as the respiration and cardiac cycle, may occur leading to changes in resting state BOLD signal fluctuations, thus biasing connectivity analyses. To adjust for such confounds, physiological correction routines were used. Respiration, cardiac, and end-tidal CO2 traces were recorded during MR acquisitions using a chest bellows, a pulse oximeter, and a nasal canula attached to a capnograph, respectively. After extracting cycle timings, harmonics of the respiration and cardiac cycle (two respiration, two cardiac, and one interaction term) were removed from the data using RETROICOR (47). Variance because of cardiac and respiration rate changes was removed (48, 49) along with end-tidal CO2-related fluctuations (50) using general linear model analyses.
Cerebrovascular Reactivity.
Psilocybin may alter cerebrovascular reactivity (CVR), a blood vessel's ability to respond to a stimulus, thus introducing a bias between conditions. To determine if changes in reactivity occur, MR measurements of CVR were acquired using the approach of Murphy et al. (7). Briefly, participants were asked to perform a breath-hold task (30-s paced breathing, 10-s breath-hold) while MR data with a similar acquisition to above and end-tidal CO2 traces were acquired. BOLD responses to breath-hold were normalized to subject-specific CO2 increases to give percent BOLD/mmHg, a CVR measure. These maps were transformed into MNI space and voxel-wise paired t tests were performed between the placebo and psilocybin conditions.
Supplementary Material
Acknowledgments
The authors thank Alison Diaper, Ann Rich, Sue Wilson, and Karl Friston. These studies received financial and intellectual support from the Beckley Foundation and financial support from the Neuropsychoanalysis Foundation, Multidisplinary Association for Psychedelic Studies, and the Heffter Research Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1119598109/-/DCSupplemental.
See Commentary on page 1820.
References
- 1.Wasson GR. Seeking the magic mushroom. Life Magazine. 1957 May 15:109–120. [Google Scholar]
- 2.Grob CS, et al. Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer. Arch Gen Psychiatry. 2011;68:71–78. doi: 10.1001/archgenpsychiatry.2010.116. [DOI] [PubMed] [Google Scholar]
- 3.Griffiths RR, Richards WA, McCann U, Jesse R. Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology (Berl) 2006;187:268–283. doi: 10.1007/s00213-006-0457-5. discussion 284–292. [DOI] [PubMed] [Google Scholar]
- 4.Griffiths R, Richards W, Johnson M, McCann U, Jesse R. Mystical-type experiences occasioned by psilocybin mediate the attribution of personal meaning and spiritual significance 14 months later. J Psychopharmacol. 2008;22:621–632. doi: 10.1177/0269881108094300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carhart-Harris RL, et al. The administration of psilocybin to healthy, hallucinogen-experienced volunteers in a mock-functional magnetic resonance imaging environment: A preliminary investigation of tolerability. J Psychopharmacol. 2010;25:1562–1567. doi: 10.1177/0269881110367445. [DOI] [PubMed] [Google Scholar]
- 6.Hasler F, Grimberg U, Benz MA, Huber T, Vollenweider FX. Acute psychological and physiological effects of psilocybin in healthy humans: A double-blind, placebo-controlled dose-effect study. Psychopharmacology (Berl) 2004;172:145–156. doi: 10.1007/s00213-003-1640-6. [DOI] [PubMed] [Google Scholar]
- 7.Murphy K, Harris AD, Wise RG. Robustly measuring vascular reactivity differences with breath-hold: normalising stimulus-evoked and resting state BOLD fMRI data. Neuroimage. 2011;54:369–379. doi: 10.1016/j.neuroimage.2010.07.059. [DOI] [PubMed] [Google Scholar]
- 8.Vollenweider FX, et al. Positron emission tomography and fluorodeoxyglucose studies of metabolic hyperfrontality and psychopathology in the psilocybin model of psychosis. Neuropsychopharmacology. 1997;16:357–372. doi: 10.1016/S0893-133X(96)00246-1. [DOI] [PubMed] [Google Scholar]
- 9.Páleníček T, et al. Comparison of the effects of hallucinogens psilocin and mescaline on quantitative EEG and sensorimotor gating \x{2013} an animal model of psychosis. Psychiatrie. 2011;15:44–48. [Google Scholar]
- 10.Shmuel A, Leopold DA. Neuronal correlates of spontaneous fluctuations in fMRI signals in monkey visual cortex: Implications for functional connectivity at rest. Hum Brain Mapp. 2008;29:751–761. doi: 10.1002/hbm.20580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nichols DE. Hallucinogens. Pharmacol Ther. 2004;101:131–181. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Glennon RA, Titeler M, McKenney JD. Evidence for 5-HT2 involvement in the mechanism of action of hallucinogenic agents. Life Sci. 1984;35:2505–2511. doi: 10.1016/0024-3205(84)90436-3. [DOI] [PubMed] [Google Scholar]
- 13.Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Bäbler A, Vogel H, Hell D. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport. 1998;9:3897–3902. doi: 10.1097/00001756-199812010-00024. [DOI] [PubMed] [Google Scholar]
- 14.Shen RY, Andrade R. 5-Hydroxytryptamine2 receptor facilitates GABAergic neurotransmission in rat hippocampus. J Pharmacol Exp Ther. 1998;285:805–812. [PubMed] [Google Scholar]
- 15.Scruggs JL, Schmidt D, Deutch AY. The hallucinogen 1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropane (DOI) increases cortical extracellular glutamate levels in rats. Neurosci Lett. 2003;346:137–140. doi: 10.1016/s0304-3940(03)00547-0. [DOI] [PubMed] [Google Scholar]
- 16.Abi-Saab WM, Bubser M, Roth RH, Deutch AY. 5-HT2 receptor regulation of extracellular GABA levels in the prefrontal cortex. Neuropsychopharmacology. 1999;20:92–96. doi: 10.1016/S0893-133X(98)00046-3. [DOI] [PubMed] [Google Scholar]
- 17.Zhang QJ, et al. Unilateral lesion of the nigrostriatal pathway decreases the response of interneurons in medial prefrontal cortex to 5-HT 2A/2C receptor stimulation in the rat. Brain Res. 2010;1312:127–137. doi: 10.1016/j.brainres.2009.11.052. [DOI] [PubMed] [Google Scholar]
- 18.Ashby CR, Jr, Jiang LH, Kasser RJ, Wang RY. Electrophysiological characterization of 5-hydroxytryptamine2 receptors in the rat medial prefrontal cortex. J Pharmacol Exp Ther. 1990;252:171–178. [PubMed] [Google Scholar]
- 19.Marek GJ, Aghajanian GK. LSD and the phenethylamine hallucinogen DOI are potent partial agonists at 5-HT2A receptors on interneurons in rat piriform cortex. J Pharmacol Exp Ther. 1996;278:1373–1382. [PubMed] [Google Scholar]
- 20.el Mansari M, Blier P. In vivo electrophysiological characterization of 5-HT receptors in the guinea pig head of caudate nucleus and orbitofrontal cortex. Neuropharmacology. 1997;36:577–588. doi: 10.1016/s0028-3908(97)00035-x. [DOI] [PubMed] [Google Scholar]
- 21.Bergqvist PB, Dong J, Blier P. Effect of atypical antipsychotic drugs on 5-HT2 receptors in the rat orbito-frontal cortex: An in vivo electrophysiological study. Psychopharmacology (Berl) 1999;143:89–96. doi: 10.1007/s002130050923. [DOI] [PubMed] [Google Scholar]
- 22.Anderson IM, et al. 5-HT(2C) receptor activation by m-chlorophenylpiperazine detected in humans with fMRI. Neuroreport. 2002;13:1547–1551. doi: 10.1097/00001756-200208270-00012. [DOI] [PubMed] [Google Scholar]
- 23.McKie S, et al. Neuronal effects of acute citalopram detected by pharmacoMRI. Psychopharmacology (Berl) 2005;180:680–686. doi: 10.1007/s00213-005-2270-y. [DOI] [PubMed] [Google Scholar]
- 24.Andrade R. Serotonergic regulation of neuronal excitability in the prefrontal cortex. Neuropharmacology. 2011;61:382–386. doi: 10.1016/j.neuropharm.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alexander GM, et al. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron. 2009;63:27–39. doi: 10.1016/j.neuron.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raichle ME, et al. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 28.Raichle ME. The neural correlates of consciousness: An analysis of cognitive skill learning. Philos Trans R Soc Lond B Biol Sci. 1998;353:1889–1901. doi: 10.1098/rstb.1998.0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proc Natl Acad Sci USA. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carhart-Harris RL, Mayberg HS, Malizia AL, Nutt D. Mourning and melancholia revisited: Correspondences between principles of Freudian metapsychology and empirical findings in neuropsychiatry. Ann Gen Psychiatry. 2008;7:9. doi: 10.1186/1744-859X-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carhart-Harris RL, Friston KJ. The default-mode, ego-functions and free-energy: A neurobiological account of Freudian ideas. Brain. 2010;133:1265–1283. doi: 10.1093/brain/awq010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hagmann P, et al. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6:e159. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bullmore E, et al. Generic aspects of complexity in brain imaging data and other biological systems. Neuroimage. 2009;47:1125–1134. doi: 10.1016/j.neuroimage.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 34.Huxley A. The Doors of Perception and Heaven and Hell. London: Harper & Brothers; 1954. [Google Scholar]
- 35.Friston K. The free-energy principle: A unified brain theory? Nat Rev Neurosci. 2010;11:127–138. doi: 10.1038/nrn2787. [DOI] [PubMed] [Google Scholar]
- 36.Sessa B. Can psychedelics have a role in psychiatry once again? Br J Psychiatry. 2005;186:457–458. doi: 10.1192/bjp.186.6.457. [DOI] [PubMed] [Google Scholar]
- 37.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: Implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci USA. 2010;107:11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holtzheimer PE, Mayberg HS. Stuck in a rut: Rethinking depression and its treatment. Trends Neurosci. 2011;34:1–9. doi: 10.1016/j.tins.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhagwagar Z, et al. Increased 5-HT(2A) receptor binding in euthymic, medication-free patients recovered from depression: A positron emission study with [(11)C]MDL 100,907. Am J Psychiatry. 2006;163:1580–1587. doi: 10.1176/ajp.2006.163.9.1580. [DOI] [PubMed] [Google Scholar]
- 41.Meyer JH, et al. Dysfunctional attitudes and 5-HT2 receptors during depression and self-harm. Am J Psychiatry. 2003;160:90–99. doi: 10.1176/appi.ajp.160.1.90. [DOI] [PubMed] [Google Scholar]
- 42.Farb NA, Anderson AK, Bloch RT, Segal ZV. Mood-linked responses in medial prefrontal cortex predict relapse in patients with recurrent unipolar depression. Biol Psychiatry. 2011;70:366–372. doi: 10.1016/j.biopsych.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Griffiths RR, et al. Mystical experiences occasioned by the hallucinogen psilocybin lead to increases in the personality domain of openness. J Psychopharmacol. 2011;25:1453–1461. doi: 10.1177/0269881111420188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sewell RA, Halpern JH, Pope HG., Jr Response of cluster headache to psilocybin and LSD. Neurology. 2006;66:1920–1922. doi: 10.1212/01.wnl.0000219761.05466.43. [DOI] [PubMed] [Google Scholar]
- 45.May A, Bahra A, Büchel C, Frackowiak RS, Goadsby PJ. Hypothalamic activation in cluster headache attacks. Lancet. 1998;352:275–278. doi: 10.1016/S0140-6736(98)02470-2. [DOI] [PubMed] [Google Scholar]
- 46.Franzini A, Messina G, Cordella R, Marras C, Broggi G. Deep brain stimulation of the posteromedial hypothalamus: Indications, long-term results, and neurophysiological considerations. Neurosurg Focus. 2010;29:E13. doi: 10.3171/2010.5.FOCUS1094. [DOI] [PubMed] [Google Scholar]
- 47.Glover GH, Li T-Q, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med. 2000;44:162–167. doi: 10.1002/1522-2594(200007)44:1<162::aid-mrm23>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 48.Shmueli K, et al. Low-frequency fluctuations in the cardiac rate as a source of variance in the resting-state fMRI BOLD signal. Neuroimage. 2007;38:306–320. doi: 10.1016/j.neuroimage.2007.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Birn RM, Diamond JB, Smith MA, Bandettini PA. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. Neuroimage. 2006;31:1536–1548. doi: 10.1016/j.neuroimage.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 50.Wise RG, Ide K, Poulin MJ, Tracey I. Resting fluctuations in arterial carbon dioxide induce significant low frequency variations in BOLD signal. Neuroimage. 2004;21:1652–1664. doi: 10.1016/j.neuroimage.2003.11.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.