At early times during viral infections, natural killer (NK) cells can contribute to direct antiviral defense (1, 2). The effect is most dramatic when they are stimulated to release the contents of their cytolytic granules for lysis of virus-infected target cells. Type 1 interferons (IFNs), produced during innate responses to infections, induce elevated NK cell cytotoxicity. Killing, however, is ultimately dependent on signaling through NK activating receptors by ligands expressed on target cell surfaces. A complex repertoire of germ-line genes encodes NK receptors (2). Some of these are conserved, whereas others are polygenic and polymorphic. At later times, adaptive immunity, such as that delivered by cytotoxic CD8 T cells, mediates protection. The kinetics and magnitudes of viral replication compared with developing T-cell responses can shift the balance between the virus and an infected host through three states: viral clearance with long-lived immunity, immune-mediated pathology with life-threatening conditions, and functional exhaustion of T cells with resulting chronic infection. The report by Lang et al. in PNAS (3) provides evidence of a role for NK cell cytotoxicity in limiting CD8 T-cell responses, to result in persistent viral infection as well as elevated infection-induced disease. This immunoregulatory function suggests that NK cells direct the balance between the virus and the host, but the results must be considered in the context of the targets of NK cell-mediated lysis, the race in establishing states of infection, and the complexities associated with the experimental systems used to identify the effects.
A potential role for NK cell-mediated cytotoxicity in immunoregulation was first considered because although NK cells are induced to have elevated killing whenever type 1 IFNs are induced, they are important in early direct defense against some but not all viruses (1, 4). The best evidence for their direct antiviral function comes from studies of murine cytomegalovirus (MCMV) infections of mice. Here, their maximal protective effect depends on cytotoxicity and on an NK cell-activating receptor present in MCMV-resistant but not -sensitive strains of mice, Ly49H, with a virus-induced ligand, m157, expressed on infected target cells (2). In the case of infections of mice with the lymphocytic choriomeningitis virus (LCMV), however, type 1 IFNs are induced and stimulate NK cell cytotoxic functions, but an NK cell contribution to early resistance is difficult to detect (4). The absence of an NK-activating receptor and/or a virus-induced ligand for an activating receptor may account for an inability to access NK cell killing for direct LCMV defense. Why, however, would a pathway inducing NK cell-mediated cytotoxicity be preserved without benefit to antiviral defense? Studies aimed at addressing this issue have shown that the presence of NK cells can limit T-cell responses to either MCMV or LCMV infection (5–7) and adaptive memory responses to antigen delivered by a nonreplicating adenovirus vector (8). Conversely, NK cell lysis of target cells has been reported to support T-cell responses by providing antigens for presentation (9), and direct NK cell antiviral defense has been shown to promote early CD8 T-cell responses to MCMV by limiting the magnitudes of other innate responses (10). Thus, data are accumulating for immunoregulatory functions mediated through NK cell cytotoxicity, but the results suggest effects both inhibiting and enhancing adaptive immunity, and the pathways to their delivery remain poorly defined.
Lang et al. (3) now investigate LCMV infection in C57BL/6 mice rendered NK cell deficient throughout the infection. Challenge viruses were different LCMV strains, including relatively mild as well as more aggressive variants that can establish chronic infections. The most remarkable aspect of the work is that NK cell deficiencies resulted in lower viral burdens. When the more aggressive conditions of infection were examined at day 10 of infection during transition to persistence, the differences in the NK cell deplete compared with the replete groups were dramatic, with up to 6 log decreases and even clearance from multiple organs. The elevated resistance to infection was accompanied by increases in the proportions of functioning, antigen-specific CD8 T cells induced at these times. The results complement those recently reported by Waggoner et al. (11). Thus, the presence of NK cells limits CD8 T-cell responses to LCMV, prevents elimination of virus, and sets up to promote viral persistence.
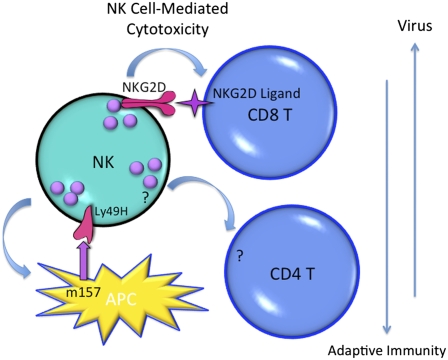
What is the mechanism used by NK cells to mediate the effects? Here, the studies from both groups conclude that NK cell cytotoxicity is responsible, but they disagree on the immune cell target. Lang et al. argue that the CD8 T cells are themselves killed off by NK cells, whereas Waggoner et al. build a case that CD4 T cells helping the CD8 T-cell responses are killed (Fig. 1). Both groups evaluated the in vivo role for NK cell cytotoxicity by examining recovery of cells adoptively transferred into perforin (prf)-deficient and control infected mice. Differences in viral replication resulting from deficiencies in CD8 T as well as NK cell-mediated cytotoxicity may have confounded both studies, but the Lang et al. and Waggoner et al. reports also examined different cells and conditions for sensitivity to in vivo NK cell-mediated killing; either congenically marked LCMV-specific T-cell receptor transgenic CD8 T cells were transferred before and evaluated at day 8 after infection, or fluorescently labeled splenic cell populations were prepared on day 4 of infection and evaluated at 5 h after transfer to day-3 infected recipient mice. Hence, the specific timing of when NK cell-mediated killing is delivered and/or when particular target cells are sensitive to the effect were not defined during the infection. As a result, the studies do not necessarily negate each other.
Fig. 1.
NK cell-mediated cytotoxicity in the regulation of immune responses to viral infections. New work is revealing a role for NK cell-mediated killing in immune regulation during LCMV infection. The effects have been reported to be delivered to either CD8 (3) or CD4 (11) T cells. The observed NK cell-mediated immunoregulatory effects are accompanied by reduced resistance and/or persistence of the viral infection. Studies of MCMV infection, with an important contribution of NK cell-mediated killing to antiviral defense, have shown increased viral persistence in the presence of NK cell defense (6). Together, these observations are establishing a paradigm with the NK cell as the “top dog” in regulating host–virus relationships and challenging the understanding of the race between the virus and the host.
Nevertheless, the target cell recognition mechanism proposed by Lang et al. and supported by both in vitro and in vivo experiments is particularly appealing. An activating receptor, expressed on all stimulated NK cells, is NKG2D (12). The ligands for this receptor are stress molecules induced under conditions of cell damage or viral infection (12), and activated T cells can express NKG2D ligands (13, 14). Under the conditions of LCMV infection used by Lang et al., the activated NK cells expressed NKG2D, antigen-specific CD8 T cells expressed ligands recognized by the receptor, and in vivo treatments with antibodies blocking NKG2D led to increased CD8 T-cell responses. The results suggest a model by which NK cells are stimulated to mediate elevated lysis and express NKG2D to kill responding CD8 T cells expressing NKG2D ligands. Thus, the studies identify a receptor–ligand pair for delivery of the NK cell-mediated immunoregulatory function and provide an important context for the cell and molecular functions not previously appreciated.
How can all of the data from the various studies be reconciled? Certainly, NK cell-mediated killing of multiple or different cell types might occur and/or might occur at different times during LCMV infection. However, the absence of a possible modest early direct NK cell-mediated antiviral effect could lead to heightened antigen presentation and stimulation of different T-cell subsets. Without NK cells and their killing functions, enhanced kinetics of T-cell responses could have resulted from any of these pathways and shifted the balance between the virus and an infected host from conditions of elevated viral replication and/or persistence to long-lived immunity or immunopathology. In fact, an increased resistance to infection resulting from elevated T-cell immunity in the absence of NK cells may have masked an early NK cell role in antiviral defense. In this regard, the previous report by Andrews et al. (6) evaluating NK cell effects on T-cell responses and viral clearance during MCMV infection is particularly interesting. The direct antiviral effect of NK cells is delivered in the
The presence of NK cells limits CD8 T-cell responses to LCMV, prevents elimination of virus, and sets up to promote viral persistence.
spleens of MCMV-infected mice, but this protective condition is accompanied by reduced CD4 and CD8 T-cell responses and development of persistent MCMV infection in the salivary glands (6). Thus, early NK cell regulation of MCMV replication is associated with viral persistence. The authors make a case for a role of NK cell cytotoxicity, depending on the Ly49H-m157 receptor–ligand pair, in limiting the exposure of T cells to antigen with evidence for prf-dependent elimination of infected dendritic/antigen presenting cells (APCs) (Fig. 1)! Here, the absence of the NK cell antiviral effects results in increased infection of APCs with increased antigen availability for T cell stimulation. Thus, there are now multiple reports of NK cell-mediated immunoregulation of T-cell responses depending on cytotoxic function, proposing different immune cell targets and activating receptor–ligand pairs. They all have difficulty, however, in excluding contributions that might be made by any of the alternative pathways.
So, why preserve a pathway inducing NK cell-mediated cytototoxicity without benefit to antiviral defense? The work in the LCMV and MCMV systems suggests a broader question. What are the advantages to maintaining NK cell-mediated cytotoxicity for either immunoregulatory or antiviral effects if the consequences are to shift the balance in the race between the virus and adaptive immunity from clearance to persistence? NK cell-mediated killing for antiviral defense may be essential under conditions with acute disease resulting from innate responses but comes at the cost of regulating adaptive immunity. Conversely, NK cell cytotoxicity for immunoregulation may be important in setting the kinetics of T cell responses to protect from life-threatening adaptive immune-mediated pathology but interferes with an early contribution of T cells to viral clearance. Further investigations are required to clarify the overall biological advantages and disadvantages.
In summary, a central role for NK cell cytotoxicity in mediating both direct antiviral and immunoregulatory effects is emerging. Studies of various conditions of infection indicate that NK cell-mediated killing of different immune cells is important in immune regulation, with the consequences being such that adaptive responses are negatively affected to lead to increased viral burdens and viral persistence. These observations place NK cells as the “top dog” in directing endogenous responses but suggest that if the goal is to eliminate the virus, they may not be “man's best friend.” It is too early to assign relative importance to any particular immune target cell and/or relative benefit to the host, however, because there are many unanswered questions. Critical among these are the precise timing for delivery of NK cell-mediated effects during the unfolding of innate to adaptive immunity, the availability of NK-activating receptor–ligand pairs for mediating lysis of particular immune cell targets, and the best possible balance between particular hosts and viruses for both short- and long-term health. Efforts to address these questions are likely to lead to greater insights into the biological roles for the wide range of activating receptors available to NK cells and to significant challenge the current understanding of virus–host relationships.
Footnotes
The author declares no conflict of interest.
See companion article on page 1210 of issue 4 in volume 109.
References
- 1.Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: Function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 2.Vidal SM, Khakoo SI, Biron CA. Natural killer cell responses during viral infections: Flexibility and conditioning of innate immunity by experience. Curr Opin Virol. 2011;1:497–512. doi: 10.1016/j.coviro.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lang PA, et al. Natural killer cell activation enhances immune pathology and promotes chronic infection by limiting CD8+ T-cell immunity. Proc Natl Acad Sci USA. 2012;109:1210–1215. doi: 10.1073/pnas.1118834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bukowski JF, Woda BA, Habu S, Okumura K, Welsh RM. Natural killer cell depletion enhances virus synthesis and virus-induced hepatitis in vivo. J Immunol. 1983;131:1531–1538. [PubMed] [Google Scholar]
- 5.Su HC, et al. NK cell functions restrain T cell responses during viral infections. Eur J Immunol. 2001;31:3048–3055. doi: 10.1002/1521-4141(2001010)31:10<3048::aid-immu3048>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 6.Andrews DM, et al. Innate immunity defines the capacity of antiviral T cells to limit persistent infection. J Exp Med. 2010;207:1333–1343. doi: 10.1084/jem.20091193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitrovic M, et al. The NK-cell response to mouse cytomegalovirus infection affects the level and kinetics of the early CD8+ T-cell response. J Virol. 2011 doi: 10.1128/JVI.06042-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soderquest K, et al. Cutting edge: CD8+ T cell priming in the absence of NK cells leads to enhanced memory responses. J Immunol. 2011;186:3304–3308. doi: 10.4049/jimmunol.1004122. [DOI] [PubMed] [Google Scholar]
- 9.Krebs P, et al. NK-cell-mediated killing of target cells triggers robust antigen-specific T-cell-mediated and humoral responses. Blood. 2009;113:6593–6602. doi: 10.1182/blood-2009-01-201467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robbins SH, et al. Natural killer cells promote early CD8 T cell responses against cytomegalovirus. PLoS Pathog. 2007;3:e123. doi: 10.1371/journal.ppat.0030123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waggoner SN, Cornberg M, Selin LK, Welsh RM. Natural killer cells act as rheostats modulating antiviral T cells. Nature. 2011 doi: 10.1038/nature10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol. 2003;3:781–790. doi: 10.1038/nri1199. [DOI] [PubMed] [Google Scholar]
- 13.Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436:1186–1190. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cerboni C, et al. Antigen-activated human T lymphocytes express cell-surface NKG2D ligands via an ATM/ATR-dependent mechanism and become susceptible to autologous NK- cell lysis. Blood. 2007;110:606–615. doi: 10.1182/blood-2006-10-052720. [DOI] [PubMed] [Google Scholar]