With regard to hallucinogens like psilocybin—an ingredient of so-called “magic mushrooms” (e.g., Psilocybe cubensis)—it may be high time to reconsider long-standing hypotheses related to their actions in the human brain.
Although psilocin (the active metabolite of psilocybin) (Fig. 1A) and other classical hallucinogens like lysergic acid diethylamide (LSD) have complex pharmacologies with high affinities for multiple neurotransmitter receptors (1), it has long been appreciated that their psychedelic actions correlate best with 5-HT2A–serotonin receptor agonism (2). Indeed, in 5-HT2A knockout mice, classical hallucinogens are devoid of activity (3, 4). Importantly, the psychedelic actions of psilocybin in humans are abolished by pretreatment with relatively selective 5-HT2A antagonists (5, 6). Taken together, these findings support the hypothesis that psilocybin and other classical hallucinogens exert their psychedelic actions in humans via activating 5-HT2A serotonin receptors.
Fig. 1.
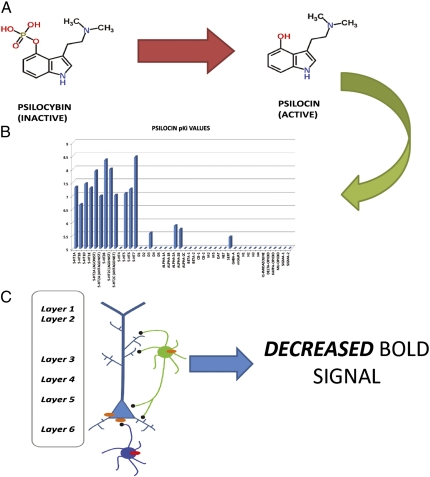
Psilocybin diminishes brain activity and connectivity. (A) Psilocybin, which is inactive, is metabolized to the active ingredient psilocin. Psilocin then activates many neurotransmitter receptors (B) to modulate activity on excitatory pyramidal and inhibitory GABA-ergic neurons (C). (B) Affinity values for psilocin are expressed as –log in nanomoles (pKi) and are from the National Institute of Mental Health Psychoactive Drug Screening Programs Ki Database (http://pdsp.med.unc.edu/kidb.php). (C) Psilocin interacts with various receptors on large excitatory pyramidal neurons and smaller inhibitory neurons. Psilocin may interact with excitatory (orange) or inhibitory (red) receptors to augment or inhibit neurotransmission. Psilocin's net effect is a decrease in neuronal activity and connectivity as measured by fMRI.
Although there is consensus regarding the pharmacological actions of classical hallucinogens, the neuronal mechanisms responsible for the psychedelic actions of hallucinogens remain controversial. Thus, some investigators have observed that LSD-like hallucinogens can enhance pyramidal neuron activity by activating 5-HT2A serotonin receptor signaling (7, 8) (Fig. 1). These findings that hallucinogens activate glutamatergic neurotransmission are consistent with many other studies demonstrating that 5-HT2A receptors were enriched on Layer V glutamatergic neurons (9) although we and others have noted that 5-HT2A receptors are also found on GABA-ergic interneurons (10–12). Indeed, 5-HT2A agonists can also augment inhibitory neuronal activity (13). Taken together, these previous findings have implied that the actions of hallucinogens such as psilocybin might be due to a mixture of actions on both excitatory (e.g., pyramidal) and inhibitory (e.g., GABA-ergic interneuronal) neuronal circuits (Fig. 1C). Conceivably, then, hallucinogens like psilocybin could induce their psychedelic effects via augmenting either excitatory or inhibitory neuronal activity in humans. Unfortunately, because of medical, legal, human use, and societal concerns, well-controlled studies of hallucinogen actions in humans have languished since the early 1960s.
In PNAS, Carhart-Harris et al. (14) successfully execute an important study that begins to fill in our gaps regarding hallucinogen actions in humans. Surprisingly, they demonstrate that psilocybin decreases surrogate markers for neuronal activity [cerebral blood flow and blood oxygen level-dependent (BOLD) signals] in key brain regions implicated in psychedelic drug actions. They also report that psilocybin appears to decrease brain “connectivity” as measured by pharmaco-physiological interaction.
To perform these studies, Carhart-Harris et al. (14) recruit 15 experienced hallucinogen users for arterial spin labeling (ASL) perfusion and BOLD fMRI studies. The individuals were scanned before and after receiving i.v. doses of placebo or psilocybin (2 mg). Individuals were also rated for the subjective effects of psilocybin or placebo. Not surprisingly, psilocybin exerted a robust psychedelic effect with individuals reporting alterations in consciousness, time perception, and visual perceptions within minutes of psilocybin administration.
Coincident with these profound perceptual alterations, decreases in cerebral blood flow were observed in key brain regions long implicated in psychedelic drug actions—the anterior and posterior cingulate cortices and thalamus. Intriguingly, the intensity of the psychedelic experience significantly correlated with decrements in blood flow in the thalamus and anterior cingulate cortex. Carhart-Harris et al. (14) also report what they refer to as decreases in “functional connectivity” between the ventral medial
Psilocybin appears to decrease brain “connectivity” as measured by pharmaco-physiological interaction.
prefrontal cortex and other regions that they interpret to indicate an overall diminished connectivity.
Overall, these findings are consistent with the hypothesis that psilocybin diminishes activity in key brain regions and networks implicated in hallucinogen actions. These provocative findings are important because they challenge many long-held models regarding hallucinogen actions that have focused mainly on their ability to enhance excitatory neurotransmission and overall brain activity.
The findings of Carhart-Harris et al. (14) are also important because they provide a nice proof that, provided appropriate safeguards are in place, psychedelic drug actions can once again be rigorously deconstructed in normal human volunteers. Psychedelic drugs are unique in their abilities to profoundly alter human awareness and perception, and these studies provide important hints regarding the neuronal substrates of human consciousness.
Acknowledgments
Work in the authors’ laboratory is supported by grants from the National Institutes of Health and the Michael Hooker Distinguished Chair of Pharmacology.
Footnotes
The authors declare no conflict of interest.
See companion article on page 2138.
References
- 1.Roth BL, et al. Salvinorin A: A potent naturally occurring nonnitrogenous kappa opioid selective agonist. Proc Natl Acad Sci USA. 2002;99:11934–11939. doi: 10.1073/pnas.182234399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glennon RA, Titeler M, McKenney JD. Evidence for 5-HT2 involvement in the mechanism of action of hallucinogenic agents. Life Sci. 1984;35:2505–2511. doi: 10.1016/0024-3205(84)90436-3. [DOI] [PubMed] [Google Scholar]
- 3.González-Maeso J, et al. Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53:439–452. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Keiser MJ, et al. Predicting new molecular targets for known drugs. Nature. 2009;462:175–181. doi: 10.1038/nature08506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Bäbler A, Vogel H, Hell D. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport. 1998;9:3897–3902. doi: 10.1097/00001756-199812010-00024. [DOI] [PubMed] [Google Scholar]
- 6.Quednow BB, Kometer M, Geyer MA, Vollenweider FX. Psilocybin-induced deficits in automatic and controlled inhibition are attenuated by ketanserin in healthy human volunteers. Neuropsychopharmacology. 2012;37:630–640. doi: 10.1038/npp.2011.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aghajanian GK, Marek GJ. Serotonin induces excitatory postsynaptic potentials in apical dendrites of neocortical pyramidal cells. Neuropharmacology. 1997;36:589–599. doi: 10.1016/s0028-3908(97)00051-8. [DOI] [PubMed] [Google Scholar]
- 8.Béïque JC, Imad M, Mladenovic L, Gingrich JA, Andrade R. Mechanism of the 5-hydroxytryptamine 2A receptor-mediated facilitation of synaptic activity in prefrontal cortex. Proc Natl Acad Sci USA. 2007;104:9870–9875. doi: 10.1073/pnas.0700436104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jakab RL, Goldman-Rakic PS. 5-Hydroxytryptamine2A serotonin receptors in the primate cerebral cortex: Possible site of action of hallucinogenic and antipsychotic drugs in pyramidal cell apical dendrites. Proc Natl Acad Sci USA. 1998;95:735–740. doi: 10.1073/pnas.95.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willins DL, Deutch AY, Roth BL. Serotonin 5-HT2A receptors are expressed on pyramidal cells and interneurons in the rat cortex. Synapse. 1997;27:79–82. doi: 10.1002/(SICI)1098-2396(199709)27:1<79::AID-SYN8>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 11.de Almeida J, Mengod G. Quantitative analysis of glutamatergic and GABAergic neurons expressing 5-HT(2A) receptors in human and monkey prefrontal cortex. J Neurochem. 2007;103:475–486. doi: 10.1111/j.1471-4159.2007.04768.x. [DOI] [PubMed] [Google Scholar]
- 12.Puig MV, Watakabe A, Ushimaru M, Yamamori T, Kawaguchi Y. Serotonin modulates fast-spiking interneuron and synchronous activity in the rat prefrontal cortex through 5-HT1A and 5-HT2A receptors. J Neurosci. 2010;30:2211–2222. doi: 10.1523/JNEUROSCI.3335-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weber ET, Andrade R. Htr2a gene and 5-HT(2A) receptor expression in the cerebral cortex studied using genetically modified mice. Front Neurosci. 2010;4 doi: 10.3389/fnins.2010.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carhart-Harris RL, et al. Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc Natl Acad Sci USA. 2012;109:2138–2143. doi: 10.1073/pnas.1119598109. [DOI] [PMC free article] [PubMed] [Google Scholar]