Abstract
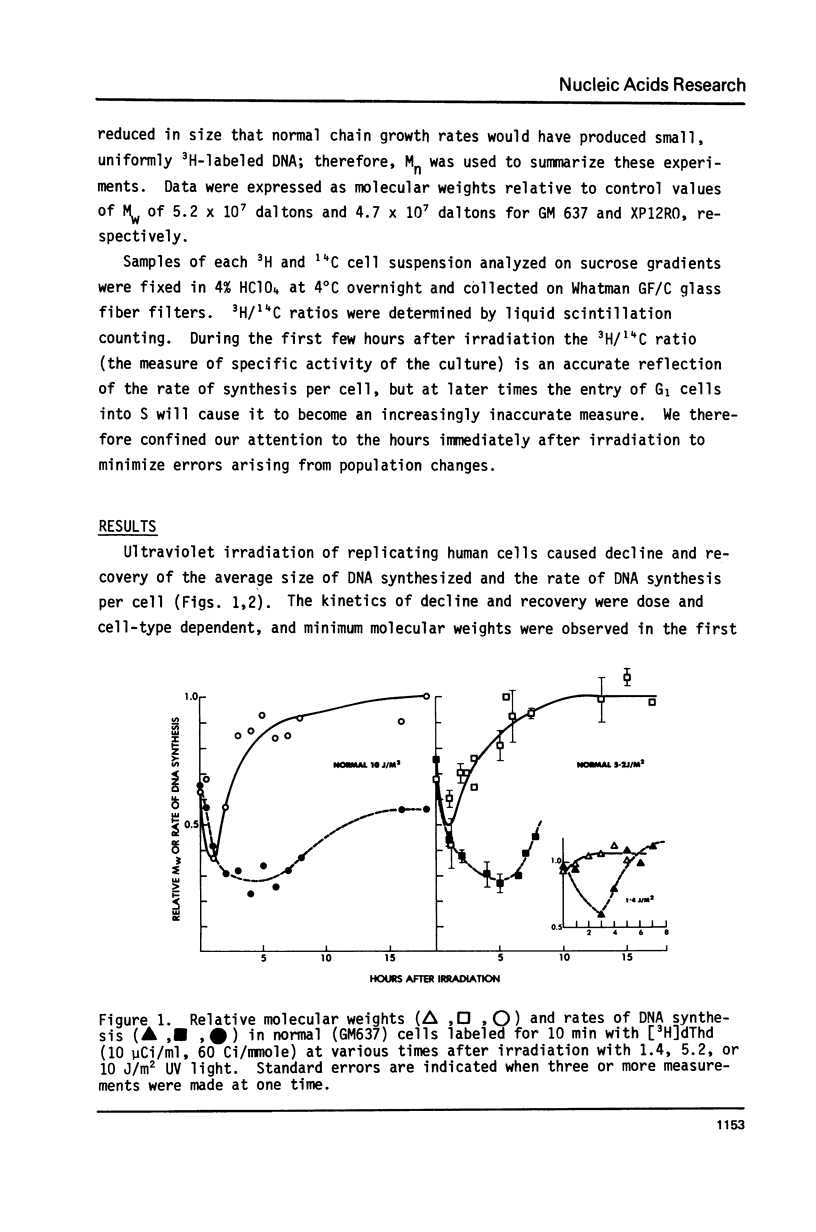
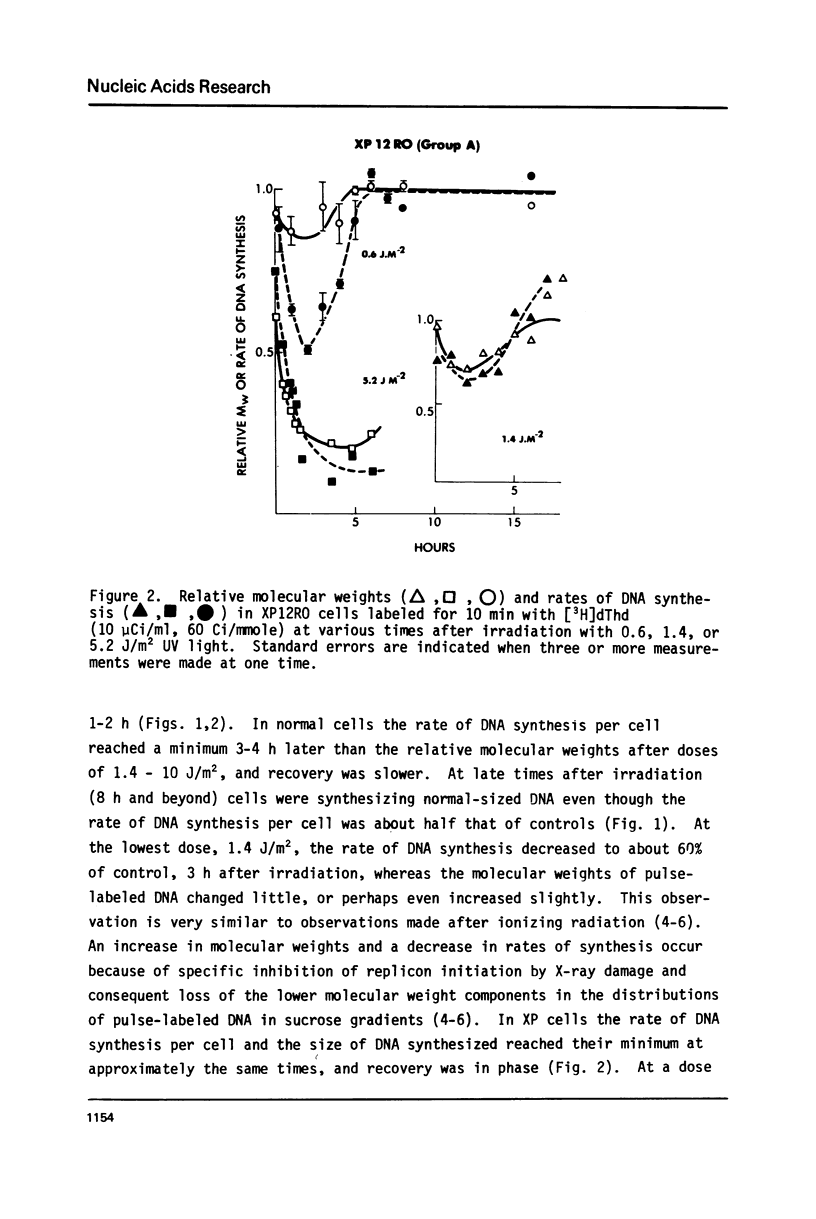
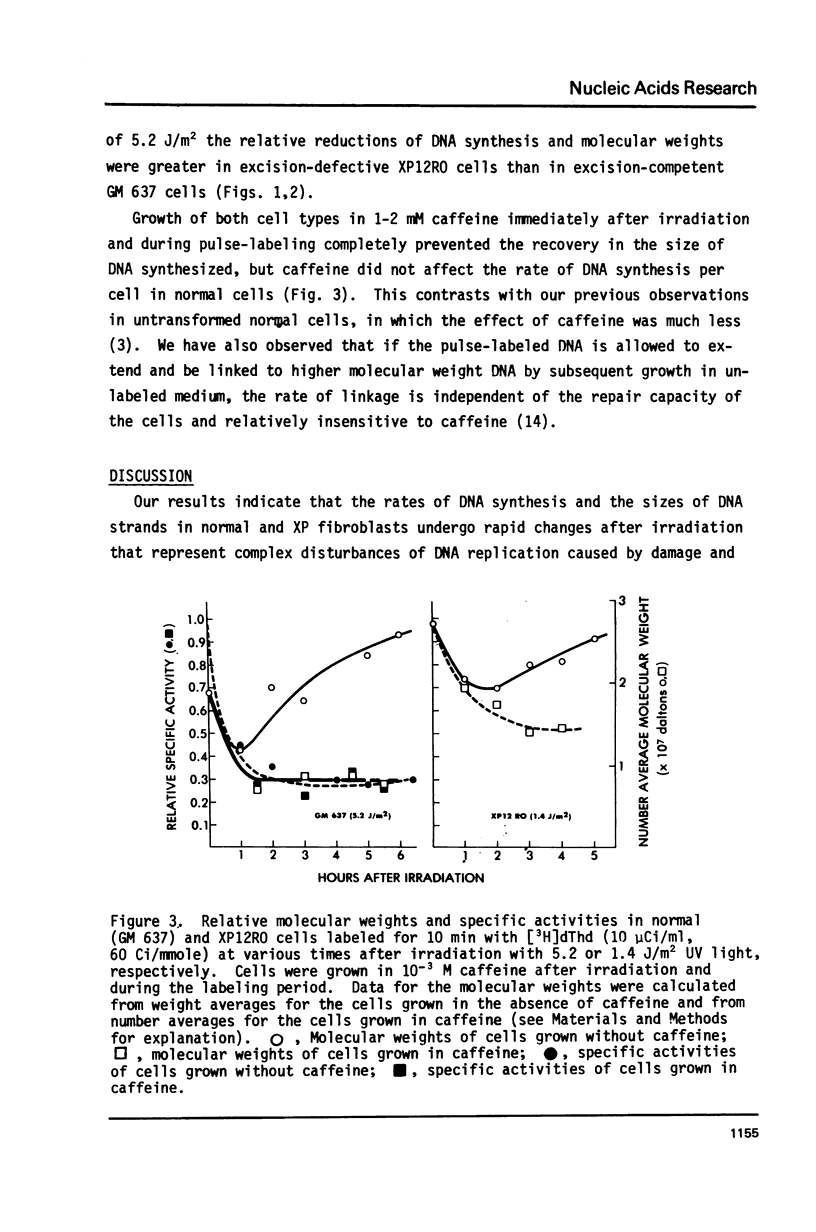
Normal human and xeroderma pigmentosum (XP, excision-defective group A) cells (both SV40-transformed) pulse-labeled with [3H]thymidine at various times after irradiation with ultraviolet light showed a decline and recovery of both the molecular weights of newly synthesized DNA and the rates of synthesis per cell. At the same ultraviolet dose, both molecular weights and rates of synthesis were inhibited more in XP than in normal cells. This indicates that excision repair plays a role in minimizing the inhibition of chain growth, possibly by excision of dimers ahead of the growing point. The ability to synthesize normal-sized DNA recovered more rapidly than rates of synthesis in normal cells, but both parameters recovered in phase in XP cells. During recovery in normal cells there are therefore fewer actively replicating clusters of replicons because the single-strand breaks involved in the excision of dimers inhibit replicon initiation. XP cells have few excision repair events and therefore fewer breaks to interfere with initiation, but chain growth is blocked by unexcised dimers. In both cell types recovery of the ability to synthesize normal-sized DNA was prevented by growing cells in caffeine after irradiation, possibly because of competition between the DNA binding properties of caffeine and replication proteins.
Our observations imply that excision repair and semiconservative replication interact strongly in irradiated cells to produce a complex spectrum of changes in DNA replication which may be confused with parts of alternative systems such as post-replication repair.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buhl S. N., Setlow R. B., Regan J. D. Recovery of the ability to synthesize DNA in segments of normal size at long times after ultraviolet irradiation of human cells. Biophys J. 1973 Dec;13(12):1265–1275. doi: 10.1016/S0006-3495(73)86061-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaver J. E., Bootsma D. Xeroderma pigmentosum: biochemical and genetic characteristics. Annu Rev Genet. 1975;9:19–38. doi: 10.1146/annurev.ge.09.120175.000315. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E. Sedimentation of DNA from human fibroblasts irradiated with ultraviolet light: possible detection of excision breaks in normal and repair-deficient xeroderma pigmentosum cells. Radiat Res. 1974 Feb;57(2):207–227. [PubMed] [Google Scholar]
- Cleaver J. E., Thomas G. H., Trosko J. E., Lett J. T. Excision repair (dimer excision, strand breakage and repair replication) in primary cultures of eukaryotic (bovine) cells. Exp Cell Res. 1972 Sep;74(1):67–80. doi: 10.1016/0014-4827(72)90482-x. [DOI] [PubMed] [Google Scholar]
- Collins A. R., Schor S. L., Johnson R. T. The inhibition of repair in UV irradiated human cells. Mutat Res. 1977 Mar;42(3):413–432. doi: 10.1016/s0027-5107(77)80046-8. [DOI] [PubMed] [Google Scholar]
- Cook K. H., Friedberg E. C. Measurement of thymine dimers in DNA by thin-layer chromatography. II. The use of one-dimensional systems. Anal Biochem. 1976 Jun;73(2):411–418. doi: 10.1016/0003-2697(76)90188-3. [DOI] [PubMed] [Google Scholar]
- D'Ambrosio S. M., Setlow R. B. Enhancement of postreplication repair in Chinese hamster cells. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2396–2400. doi: 10.1073/pnas.73.7.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domon M., Barton B., Porte A., Rauth A. M. The interaction of caffeine with ultra-violet-light-irradiated DNA. Int J Radiat Biol Relat Stud Phys Chem Med. 1970;17(4):395–399. doi: 10.1080/09553007014550481. [DOI] [PubMed] [Google Scholar]
- Edenberg H. J., Huberman J. A. Eukaryotic chromosome replication. Annu Rev Genet. 1975;9:245–284. doi: 10.1146/annurev.ge.09.120175.001333. [DOI] [PubMed] [Google Scholar]
- Ehmann U. K., Lett J. T. Review and evaluation of molecular weight calculations from the sedimentation profiles of irradiated DNA. Radiat Res. 1973 Apr;54(1):152–162. [PubMed] [Google Scholar]
- Fujiwara Y., Tatsumi M. Replicative bypass repair of ultraviolet damage to DNA of mammalian cells: caffeine sensitive and caffeine resistant mechanisms. Mutat Res. 1976 Oct;37(1):91–110. doi: 10.1016/0027-5107(76)90058-0. [DOI] [PubMed] [Google Scholar]
- Lehmann A. R. Postreplication repair of DNA in ultraviolet-irradiated mammalian cells. J Mol Biol. 1972 May 28;66(3):319–337. doi: 10.1016/0022-2836(72)90418-4. [DOI] [PubMed] [Google Scholar]
- Painter R. B. Inhibition of initiation of HeLa cell replicons by methyl methanesulfonate. Mutat Res. 1977 Feb;42(2):299–303. doi: 10.1016/s0027-5107(77)80031-6. [DOI] [PubMed] [Google Scholar]
- Painter R. B., Young B. R. Formation of nascent DNA molecules during inhibition of replicon initiation in mammalian cells. Biochim Biophys Acta. 1976 Jan 19;418(2):146–153. doi: 10.1016/0005-2787(76)90063-0. [DOI] [PubMed] [Google Scholar]
- Paterson M. C., Lohman P. H., Sluyter M. L. Use of UV endonuclease from Micrococcus luteus to monitor the progress of DNA repair in UV-irradiated human cells. Mutat Res. 1973 Aug;19(2):245–256. doi: 10.1016/0027-5107(73)90083-3. [DOI] [PubMed] [Google Scholar]
- Povirk L. F., Painter R. B. Rate of DNA chain elongation in ultraviolet light-irradiated mammalian cells as estimated by a bromodeoxyuridine photolysis method. Biophys J. 1976 Aug;16(8):883–889. doi: 10.1016/S0006-3495(76)85738-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudé J. M., Friedberg E. C. Semi-conservative deoxyribonucleic acid synthesis in unirradiated and ultraviolet-irradiated xeroderma pigmentosum and normal human skin fibroblasts. Mutat Res. 1977 Mar;42(3):433–442. doi: 10.1016/s0027-5107(77)80047-x. [DOI] [PubMed] [Google Scholar]
- Rupp W. D., Howard-Flanders P. Discontinuities in the DNA synthesized in an excision-defective strain of Escherichia coli following ultraviolet irradiation. J Mol Biol. 1968 Jan 28;31(2):291–304. doi: 10.1016/0022-2836(68)90445-2. [DOI] [PubMed] [Google Scholar]
- SUTHERLAND E. W., RALL T. W. Fractionation and characterization of a cyclic adenine ribonucleotide formed by tissue particles. J Biol Chem. 1958 Jun;232(2):1077–1091. [PubMed] [Google Scholar]
- Scudiero D., Strauss B. Increased repair in DNA growing point regions after treatment of human lymphoma cells with N-methyl-N'-nitro-N-nitrosoguanidine. Mutat Res. 1976 Jun;35(2):311–324. doi: 10.1016/0027-5107(76)90194-9. [DOI] [PubMed] [Google Scholar]
- Setlow R. B., Regan J. D., German J., Carrier W. L. Evidence that xeroderma pigmentosum cells do not perform the first step in the repair of ultraviolet damage to their DNA. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1035–1041. doi: 10.1073/pnas.64.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slor H., Cleaver J. E. Repair replication in replicating and non-replicating DNA after irradiation with UV light. Nucleic Acids Res. 1978 Jun;5(6):2095–2098. doi: 10.1093/nar/5.6.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters R. A., Hildebrand C. E. Evidence that x-irradiation inhibits DNA replicon initiation in Chinese hamster cells. Biochem Biophys Res Commun. 1975 Jul 8;65(1):265–271. doi: 10.1016/s0006-291x(75)80088-x. [DOI] [PubMed] [Google Scholar]
- Watanabe I. Radiation effects on DNA chain growth in mammalian cells. Radiat Res. 1974 Jun;58(3):541–556. [PubMed] [Google Scholar]
- Williams J. I., Cleaver J. E. Excision repair of ultraviolet damage in mammalian cells. Evidence for two steps in the excision of pyrimidine dimers. Biophys J. 1978 May;22(2):265–279. doi: 10.1016/S0006-3495(78)85488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


