Abstract
Oxidative stress results from an imbalance between production of reactive oxygen and nitrogen species (ROS and RNS, respectively) and endogenous antioxidant defense mechanisms. Increased generation of ROS/RNS is implicated in the pathogenesis of a variety of human diseases, including neurodegenerative disease, atherosclerosis, cancer and aging. However, measuring oxidative stress in biological systems is complex and requires accurate quantification of either free radicals or damaged biomolecules. One method to quantify oxidative injury is to measure lipid peroxidation. Lipids are readily attacked by free radicals, resulting in the formation of a number of peroxidation products. F2-isoprostanes (F2-IsoPs) are one group of these compounds and they are derived by the free radical peroxidation of arachidonic acid (AA). The F2-IsoPs, prostaglandine F2-like compounds, provide an accurate measure of oxidative stress both in vitro and in vivo. This protocol details current methodology used to quantify these molecules using gas chromatography-mass spectrometry (GC-MS).
Keywords: F2-isoprostanes, oxidative damage, lipid peroxidation, reactive oxygen species
Introduction
Oxidative stress is a prominent feature of many acute and chronic diseases including cancer, cardiovascular disease, neurodegenerative disease, lung disease and even the normal aging process (1–3). When excess formation of free radicals overwhelms the capacity of endogenous cellular antioxidant defense mechanisms, these reactive species may cause cell and organ damage by distressing the normal physiology and even activate and/or accelerate disease processes. Free radicals can be generated endogenously from various sources (for example, mitochondria and oxidative burst during phagocyte activation) or derived from exogenous sources, such as environmental toxins and cigarette smoke (4). Reactive radicals readily attack a variety of critical biological molecules, including lipids, DNA and essential cellular proteins. Hence, the high content of unsaturated lipids in the brain leads to pronounced lipid peroxidation, the central feature of oxidant injury in neuronal and glial cells.
Lipid peroxidation is the mechanism by which lipids are attacked by chemical species that have sufficient reactivity to abstract a hydrogen atom from a methylene carbon in their chain. Lipid peroxidation, through a free radical pathway, requires a polyunsaturated fatty acid (PUFA) and a reactant oxidant inducer that together form a free-radical intermediate. The free radical intermediate subsequently reacts with oxygen to generate a peroxyl radical, which, in turn, with unpaired electrons may additionally abstract a hydrogen atom from another PUFA, thus initiating a propagation reaction that spreads like a brushfire. Hence, greater number of double bonds in the molecule and higher instability of hydrogen atom adjacent to the double bond explains why unsaturated lipids are particularly susceptible to peroxidation (5,6). Such reactions have been long recognized, but the biological importance of lipid peroxidation has been explored only in the last three decades (7).
There are a number of analytical approaches, which permit quantification of lipid peroxidation, or free radical-catalyzed damage to DNA or proteins (8). However, many of these techniques suffer from lack of sensitivity and specificity. In a recent multi-investigator study, termed the Biomarkers of Oxidative Stress Study (BOSS), sponsored by the National Institutes of Health, it was found that the quantification of F2-IsoPs represents the most accurate method to assess oxidative stress status in vivo (9). F2-IsoPs are prostaglandin-like compounds which are produced by a noncyclooxygenase free radical-catalyzed mechanism involving the peroxidation of the PUFA, arachidonic acid (AA, C20:4, ω-6). Formation of these compounds initially involves the generation of four positional peroxyl radical isomers of arachidonate, which undergo endocyclization to PGG2-like compounds. These intermediates are reduced to form four F2-IsoP regioisomers, each of which can consist of eight racemic diastereomers (10). In contrast to cyclooxygenase (COX)-derived prostaglandins (PGs), non-enzymatic generation of F2-IsoPs favors the formation of compounds in which the stereochemistry of the side chains has a cis orientation in relation to the prostane ring. A second important difference between F2-IsoPs and PGs is that F2-IsoPs are formed primarily in situ, esterified to phosphplipids and subsequently released by a phospholipases (11,12), whereas PGs are generated only from free AA (13).
Several methods have been developed to quantify the F2-IsoPs from biological materials (3). Our laboratory uses a gas chromatography/mass spectrometry (GC/MS) to quantify the F2-IsoPs, methodology which was originally established at our University (by the pioneering work of Dr. Roberts and Dr. Morrow, Vanderbilt University Medical School) (10,13). More specifically, after isolation and derivatization of the F2-IsoPs, we take advantage of stable isotope dilution, negative ion chemical ionization (NICI) GC/MS with select ion monitoring (SIM) for quantification. This methodology allows the lower limit of detection of the F2-IsoPs to be in the low picogram range. These properties, along with the assay’s high sensitivity and specificity, allow the F2-IsoPs to be an excellent biomarkers of and the most robust and sensitive measure of oxidative stress. Accordingly, we highlight this method and address purification and derivatization of the compounds for analysis by GC-MS.
2. Materials
Tissue or cell samples, fresh or frozen. It is important to process the samples immediately after isolation or assure their immediate storage at −80 °C for later quantification.
Blade homogenizer and sonicator are used for tissue processing.
Folch solution: 2:1 (v/v) chloroform/methanol, ice-cold, containing 0.005% (w/v) butylated hydroxytoluene (BHT). Free radical scavenging agent such as BHT is added to the organic solvent during extraction of phospholipids to prevent oxidation and formation of F2-IsoPs.
Solution of NaCl (0.9%, w/v). Stored at room temperatures.
Organic solvents including ethyl acetate, heptane, chloroform, ethanol, acetonitrile and methanol, with and without 0.005% (v/v) BHT. Solutions are prepared as volume/volume ration.
Fifteen percent potassium hydroxide solution (KOH, w/v) is used to release esterified isoprostanes.
One molar hydrochloric acid (1M HCl) and pH 3 water, adjusted by adding 1 N HCl is used to acidified the sample before solid-phase extraction (SPE) (see Note 3).
Deuterated standard, deuterium-labeled isoprostane, [2H4]15-F2t-IsoP (8-iso-PGF2α) (Cayman Chemical, Ann Arbor, MI, cat. no. 316351) (see Note 4).
Anhydrous Na2SO4 is used to dry ethyl acetate/heptane eluate from the C18 Sep-Pak (see Note 5).
Sep-Pak Plus C18 cartridge ((Waters Associates, Milford, MA, cat. no. WAT03657), Silica Sep-Pak cartridge (Waters, Milford, MA, cat. no. WAT036580) and 10-ml plastic syringe are used for normal-phase solid-phase extractions (SPE).
Pentafluorobenzyl bromide (PFBB) and N,N-diisopropylethylamine (DIPE) are prepared as 10% solutions (v/v) in anhydrous acetonitrile.
Thin-layer chromatography (TLC) plates, 5 × 20 cm glass plates covered with a 250 μm layer of silica gel particles 60 Å in diameter (Partisil LK6D; Whatman, Maidstone, England, cat. no. WC486562IV) and TLC developing chamber.
TLC standard, prostaglandin F2α (PGF2α) methyl ester is diluted in methanol (Cayman Chemical, Ann Arbor, MI, cat. no. 16011).
Ten percent phosphomolybdic acid in ethanol is used to visualize sample migration on TLC plates wormed with hot plate.
Dimethylformamide and undecane are stored over calcium hydride to prevent water accumulation (see Note 7).
Bis(trimethylsilyl)trifluoroacetamide (BSTFA, Supelco, cat. no. 33084) is stored at room temperature.
15-ml polypropylene culture tube with cap, 20-ml scintillation vial, 5-ml glass Reacti-Vial with Teflon-lined cap and 1.5-ml microcentrifuge tube are used for sample processing/chemical reactions.
Nitrogen gas and methane is used for sample evaporations and mass spectrometry.
Temperature-controlled water bath, centrifuge, 95°C oven and hair dryer.
15-m, 0.25-mm diameter, 0.25-μm film thickness, DB1701 fused silica capillary GC column and Gas chromatography (GC)/mass spectroscopy (MS) system.
3. Methods
Measurement of F2-IsoPs has revolutionized our ability to quantify oxidative injury in cell/tissue samples. F2-IsoPs are stable, robust molecules and are detectable not only in cells and tissues but also in biological fluids, such as plasma, urine, cerebrospinal fluid and bronchoalveolar lavage fluid. As F2-IsoPs can be readily generated during purification/derivatization of biological materials containing arachidonoyl-containing lipids, it is important to process the samples immediately after isolation or assure their immediate storage at −80 °C for later quantification. Formation of F2-IsoPs does not occur if a free radical scavenging agent like BHT is added to the organic solvent during extraction of phospholipids or if the samples are rapidly frozen in liquid nitrogen prior to placement at −80 °C.
3.1. Lipid extraction and hydrolysis of F2-IsoPs-containing phospholipids in cell/tissue samples
Formation of F2-IsoPs occurs in situ in the phospholipid bilayer and then subsequently released in free form. This creates two forms of F2-IsoPs, one that remains esterified in the membrane and a second that is hydrolyzed and released in free form. To quantify total F2-IsoPs formation, both free and esterified F2-isoPs are analyzed. It is necessary to extract the phospholiopids from the cell/tissue and release the F2-isoPs from the phospholipids via base hydrolysis.
Fresh or frozen samples (0.05 –0.25 g) are added in ice-cold 5 ml of Folch solution containing 0.005% BHT in a polypropylene culture tube with cap. The sample is then homogenized with a blade homogenizer for approximately 30 sec. The second aliquot of ice-cold Folch solution, added to a separate culture tube, is used to wash the blade homogenizer and to ensure that all sample tissue is recovered as tissue can adhere to or become lodged inside the blade of the homogenizer. The two aliquots are then combined, covered with a nitrogen blanket, and mixed every 10 min over 30 min at 25 °C to allow maximal extraction of lipids from homogenized tissue (see Note 1).
The lipid extracts are mixed vigorously with 2.0 ml NaCl (0.9%, wt/vol), and the phases separated by centrifugation at 800 × g for 10 min at 25 °C. After centrifugation, the upper aqueous layer is discarded and the lower organic layer is carefully separated from the intermediate semisolid proteinaceous layer. The organic layer is then evaporated to dryness under a stream of nitrogen (see Note 2).
Total lipids are dissolved in 0.5 ml methanol containing BHT (0.005%), stored at −80 °C or if further processed, 0.5 ml of aqueous KOH (15%) added to the residue, and thus lipid extracts are saponified to release esterified isoprostanes. The mixture is sonicated and mixed vigorously until thoroughly suspended and heated at 37 °C for 30 min to affect hydrolysis and release of the F2-IsoPs. The mixture is then acidified to pH 3 with 1 M HCl (cca 1.2 ml) and diluted to a final volume of 10 ml with pH 3 water in preparation for purification of F2-IsoPs with solid-phase extraction (SPE) (see Note 3).
3.2. Sample purification for mass spectrometric analysis
Following acidification of the sample to pH 3 with 1 M HCl, 200 to 1000 pg of deuterated standard is added. The mixture is vortexed and F2-IsoPs isolated using reversed-phase and normal-phase solid-phase extractions (SPE) (see Note 4).
A 10-ml plastic syringe is used to elute the sample and subsequent solvents through the Sep-Pak cartridge. For reverse phase, Sep Pak Plus C18 columns (each cartridge contains 500 mg of C18) are preconditioned with 5 ml methanol (flow rate, ~1.0 ml/min) and 7.0 ml H2O (adjusted to pH 3.0 with 1 N HCl). Once the sample has been added, the column is washed sequentially with 10 ml of water (pH 3) and 10 ml of heptane, which removes non-polar contaminates including un-oxidized AA. The F2-IsoPs are eluted with 10 ml of ethyl acetate/heptane (50:50, vol/vol) into a 20-ml scintillation vial.
The ethyl acetate/heptane eluate from the C18 Sep-Pak is then dried over anhydrous Na2SO4 and applied to a silica Sep-Pak cartridge (each cartridge contains 500 mg of silica), which has been preconditioned with 5 ml of ethyl acetate. Once the sample has been added, the column is washed with 5 ml of ethyl acetate and the F2-IsoPs are eluted with 5 ml of ethyl acetate/methanol (50:50, vol/vol) into a 5-ml glass react-a-vial (with Teflon-lined cap). For normal-phase SPE, Sep Pak Plus Silica columns are used with a flow rate of ~0.5 ml/min throughout (see Note 5).
3.3. Conversion of F2-IsoPs to corresponding pentafluorbenzyl (PFB) esters
Isoprostanes isolated in ethyl acetate/methanol eluate by SPE are dried at 37 °C under a nitrogen stream, and derivatized to pentafluorbenzyl esters. Samples are vigorously mixed with 40 μl pentafluorobenzyl bromide: anhydrous acetonitrile (10:90, vol/vol) plus 20 μl diisopropylethylamine: anhydrous acetonitrile (10:90, vol/vol). Following reaction at 37 °C for 20 min, the esters are dried under a nitrogen stream and dissolved in 50 μl chloroform:methanol (2:3, vol/vol).
3.4. Tin layer chromatography
Thin-layer chromatography (TLC) is accomplished with 5 cm × 20 cm glass plates covered with a 250 μm layer of silica gel particles 60 Å in diameter. Just before use, the plates are washed with ethyl acetate:ethanol (90:10, vol/vol), activated at 95 °C for 20 min, and cooled in a dessicator. A TLC chamber is lined with filter paper and conditioned 30 min with 100 ml chloroform: ethanol (93:7, vol/vol).
Dissolved samples in chloroform: methanol (50 μl) are applied to the upper half of pre-adsorbent in four pre-scored lanes, and dried 5–10 s with a hair dryer. Sample plates are added to both ends of the chamber. In contrast, TLC standard (5 μg of the methyl ester of PGF2α/5 μl CH3OH) is applied to a separate plate that is positioned towards the center of the TLC chamber. After the chamber is rapidly closed, solvent is allowed to migrate 13 cm, and the plates removed.
Samples are scraped from silica plates in the region of the TLC standard and visualized by spraying with a 10% solution of phosphomolybdic acid in ethanol followed by heating. The areas 1 cm below and 1 cm above PGF2α (Rf ~0.15) are scraped and extracted from the silica with 1 ml of ethyl acetate.
Following centrifugation at 13,000 × g for 3 min at 4 °C, isoprostane pentafluorobenzyl esters in the ethyl acetate are transferred into a virgin microcentrifuge tube and stored at −80 °C or samples further processed for the GC-MS analysis (see Note 6).
3.5. Formation of trimethylsilyl ether derivatives and quantification of F2-IsoPs
Once dried under a nitrogen stream, samples are dissolved in 8 μl dimethylformamide (DMF) and mixed with 20 μl bis(trimethylsilyl)trifluoroacetamide (BSTFA) to covert the residue to the trimethylsilyl ether derivatives.
After heating for 5.0 min at 37 °C, silylated samples are dried at 37 °C under a nitrogen stream, redissolved in 20 μl of undecane, which has been dried over calcium hydride, and transferred into autosampler vial for GC-MS analysis (see Note 7).
-
For quantification of F2-IsoPs, we routinely use a Hewlett Packard 5982A GC/MS system interfaced with an IBM Pentium computer. GC is performed using a 15-m, 0.25-mm diameter, 0.25-μm film thickness, DB1701 fused silica capillary column. The column temperature is programmed from 190 to 290° at 20°/min. Methane is used as the carrier gas for NICI at a flow rate of 1 ml/min. Ion source temperature is 250 °C, electron energy is 70 eV, and the filament current is 0.25 mA.
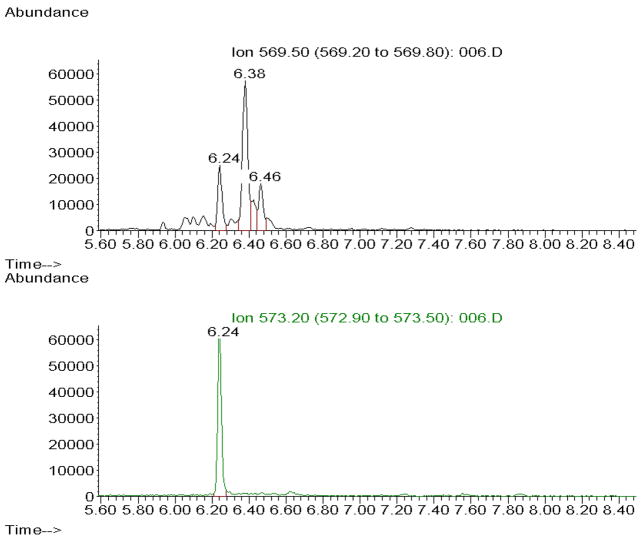
The major ions generated in the NICI mass spectra of the pentafluorobenzyl ester, tris-trimethylsilyl ether derivatives of F2-IsoP are m/z 569 and corresponding ion for the [2H4]15-F2t-IsoP internal standard, m/z 573 (Figure 1). For quantification purposes we compare the height of the pick containing derivatized F2-IsoPs (m/z 569) with the height of the deuterated internal standard peak (m/z 573). The coefficient of variance for the assay is routinely less than 8% (see Notes 8 and 9).
Figure 1.
Chromatograms of F2-IsoPs from tissue sample. Chromatograms plot abundance vs. time (min) with m/z 569 chromatogram showing F2-IsoPs and m/z 573 chromatogram showing internal standard.
Acknowledgments
This study was supported by grants from NIH NS057223 (DM), NIH NS62684 and NIH ES16754 (TM), NIEHS ES 007331, NIEHS 10563 and DoD W81XWH-05-1-0239 (MA).
Footnotes
Presence of BHT during extraction and hydrolysis is important in order to inhibit additional formation of F2-IsoPs; since polystyrene is not resistant to chloroform and its subsequent interference with the analytical procedures, it is recommended that lipid extraction be carried out in polypropylene tubes; Keep on ice.
The organic layer and the proteinaceous layer can be readily separated by carefully pouring off the organic layer into a new culture tube. If the proteinaceous layer is small, because of the type and size of the cell/tissue sample, it is often easier to remove the aqueous and proteinaceous layers simultaneously via suction. However, care must be taken not to compromise the organic phase if this approach is used.
It is important to dilute the methanol in this solution to 5% or less to ensure proper column extraction of free F2-IsoPs in the subsequent purification procedure.
The internal standard is a deuterium-labeled isoprostane, [2H4]15-F2t-IsoP (8-iso-PGF2a). The amount of internal standard added depends on the levels of F2-IsoPs in the sample as well as the sensitivity of the mass spectrometer. For low-level samples such as cerebrospinal fluid (CSF), less internal standard needs to be added. Samples that consist of a particularly large amount of tissue will require more internal standard. This is because complex samples such as brain tissue, despite our best purification efforts, will still contain some unwanted compounds that may potentially have the same m/z (mass-to-charge) ratio as the internal standard when analyzed by GC/MS. Increasing the amount of internal standard to 1000 pg in these samples minimizes the variability in the internal standard ion channel due to contamination in the tissue sample.
Drying of ethyl acetate/heptane eluate should be completed promptly, as Na2SO4 has been shown to adsorb lipids to some degree. Care must be taken not to transfer any Na2SO4 to the silica Sep-Pak cartridge.
Ethyl acetate should be carefully removed without disrupting the silica pellet in the bottom of the tube (silica may affect the instrument’s sensitivity). Avoid applying samples to the first 1 cm of the plate and do not spray the sample plate.
DMF should be stored over calcium hydride to prevent water accumulation. Similarly to the amount of internal standard that is added to a sample, consideration should be given to the amount of undecane that is used to dissolve the derivatized sample. The amount added will depend on the levels of F2-IsoPs. Samples that are rich in F2-IsoPs will require greater amounts of undecane to keep them from overloading the column during GC. Likewise, low-level samples will require less undecane in order for the GC/MS signal to be of sufficient intensity for optimal quantification.
Quantification of the F2-IsoPs levels may be also achieved by comparing the areas of the appropriate peaks in the m/z 569 SIM chromatogram of the F2-IsoPs to that of the peak of the internal standard in the m/z 573 SIM chromatogram (Figure 1).
In general, 12 samples can be assayed for F2-IsoPs in approximately 10 hrs by an experienced investigator. Homogenization, lipid extraction and hydrolysis of this number of samples requires ~ 3 hrs; Sep-Pac purifications takes ~ 2 hrs; drying, derivatization and TLC purification requires ~ 3 hrs; and drying and silylation requires ~ 2 hrs. Though compared to other assays of oxidative stress the time requirement for this assay is relatively large, it is noteworthy that the present assay has the greatest sensitivity and specificity for the detection of lipid peroxidation. Mass spectrometric analysis is automated and each sample requires ~ 15 min of instrument time. If the peak signal is low or if no peaks are detected by the mass spectrometer, the samples should be removed from the auto sampler vial, washed by ethyl acetate, dried under nitrogen and the conversion procedure to silylether derivative should be repeated. If the internal standard is detected at m/z 573, but there is low or non-existent peak at 569, than levels of F2-IsoPs are below the limit of detection.
References
- 1.Halliwell B, Gutteridge JMC. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- 2.Simonian NA, Coyle JT. Oxidative stress in neurodegenerative diseases. Annu Rev Pharmacol Toxicol. 1996;36:83–106. doi: 10.1146/annurev.pa.36.040196.000503. [DOI] [PubMed] [Google Scholar]
- 3.Basu S. F2-isoprostanes in human health and diseases: from molecular mechanisms to clinical implications. Antioxidants and Redox Signaling. 2008;10:1405–1434. doi: 10.1089/ars.2007.1956. [DOI] [PubMed] [Google Scholar]
- 4.Morrow JD. Quantification of isoprostanes as indices of oxidant stress and the risk of atherosclerosis in humans. Arterioscler Thromb Vasc Biol. 2005;25:279–86. doi: 10.1161/01.ATV.0000152605.64964.c0. [DOI] [PubMed] [Google Scholar]
- 5.Pratico D, Rokach J, Lawson J, FitzGerald GA. F2-isoprostanes as indices of lipid peroxidation in inflammatory diseases. Chem Phys Lipids. 2004;128:165–171. doi: 10.1016/j.chemphyslip.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Gao L, Yin H, Milne GL, Porter NA, Morrow JD. Formation of F-ring Isoprostane-like Compounds (F3-Isoprostanes) in Vivo from Eicosapentaenoic Acid. J Biol Chem. 2006;281:14092–14099. doi: 10.1074/jbc.M601035200. [DOI] [PubMed] [Google Scholar]
- 7.Gutteridge JMC, Halliwell B. The measurement and mechanism of lipid peroxidation in biological systems. Trends Biochem Sci. 1990;15:129–1365. doi: 10.1016/0968-0004(90)90206-q. [DOI] [PubMed] [Google Scholar]
- 8.Lee SH, Blair IA. Oxidative DNA damage and cardiovascular disease. Trends Cardiovasc Med. 2001;11:148–155. doi: 10.1016/s1050-1738(01)00094-9. [DOI] [PubMed] [Google Scholar]
- 9.Kadiiska MB, Gladen BC, Baird DD, Germolec D, Graham LB, Parker CE, et al. Biomarkers of Oxidative Stress Study II: Are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radic Biol Med. 2005;38:698–710. doi: 10.1016/j.freeradbiomed.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 10.Morrow JD, Hill KE, Burk RF, Nammour TM, Badr KF, Roberts LJ., 2nd A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc Natl Acad Sci U S A. 1990;87:9383–9387. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morrow JD, Awad JA, Boss HJ, Blair IA, Roberts LJ., II Non-cyclooxygenase derived prostanoids (F2-isoprostanes) are formed in situ on phospholipids. Proc Natl Acad Sci USA. 1992;89:10721–10725. doi: 10.1073/pnas.89.22.10721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Famm SS, Morrow JD. The isoprostanes: Unique products of arachidonic acid oxidation- a review. Curr Med Chem. 2003;10:1723–1740. doi: 10.2174/0929867033457115. [DOI] [PubMed] [Google Scholar]
- 13.Morrow JD, Harris TM, Roberts LJ., 2nd Noncyclooxygenase oxidative formation of a series of novel prostaglandins: analytical ramifications for measurement of eicisanoids. Analyt Biochem. 1990;184:1–10. doi: 10.1016/0003-2697(90)90002-q. [DOI] [PubMed] [Google Scholar]