Abstract
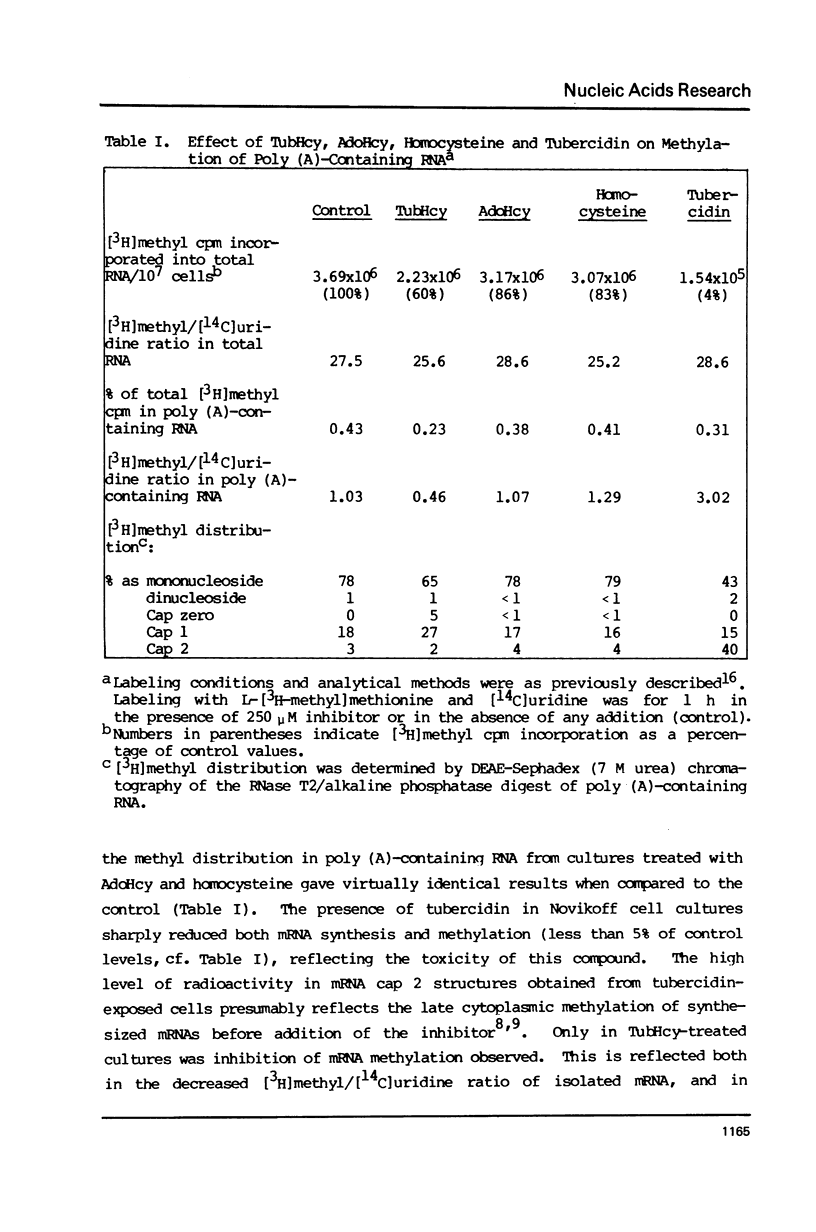
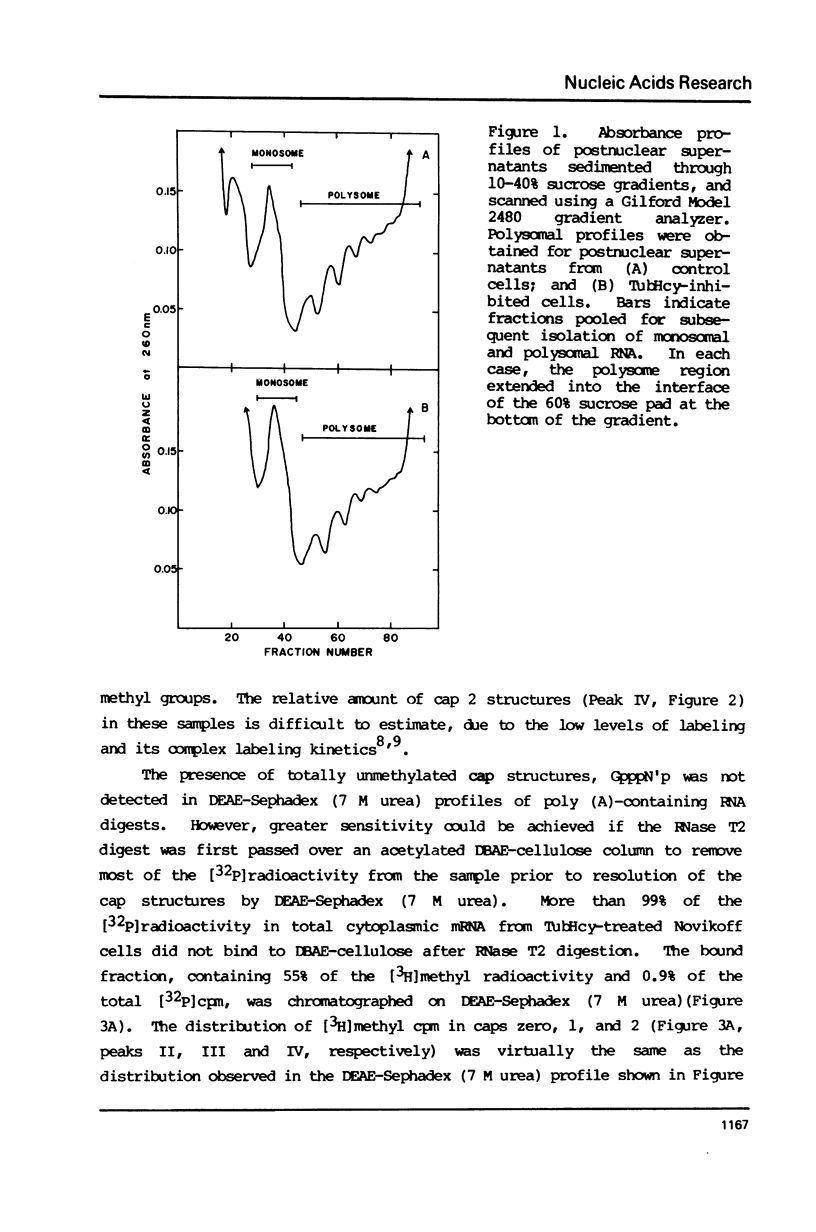
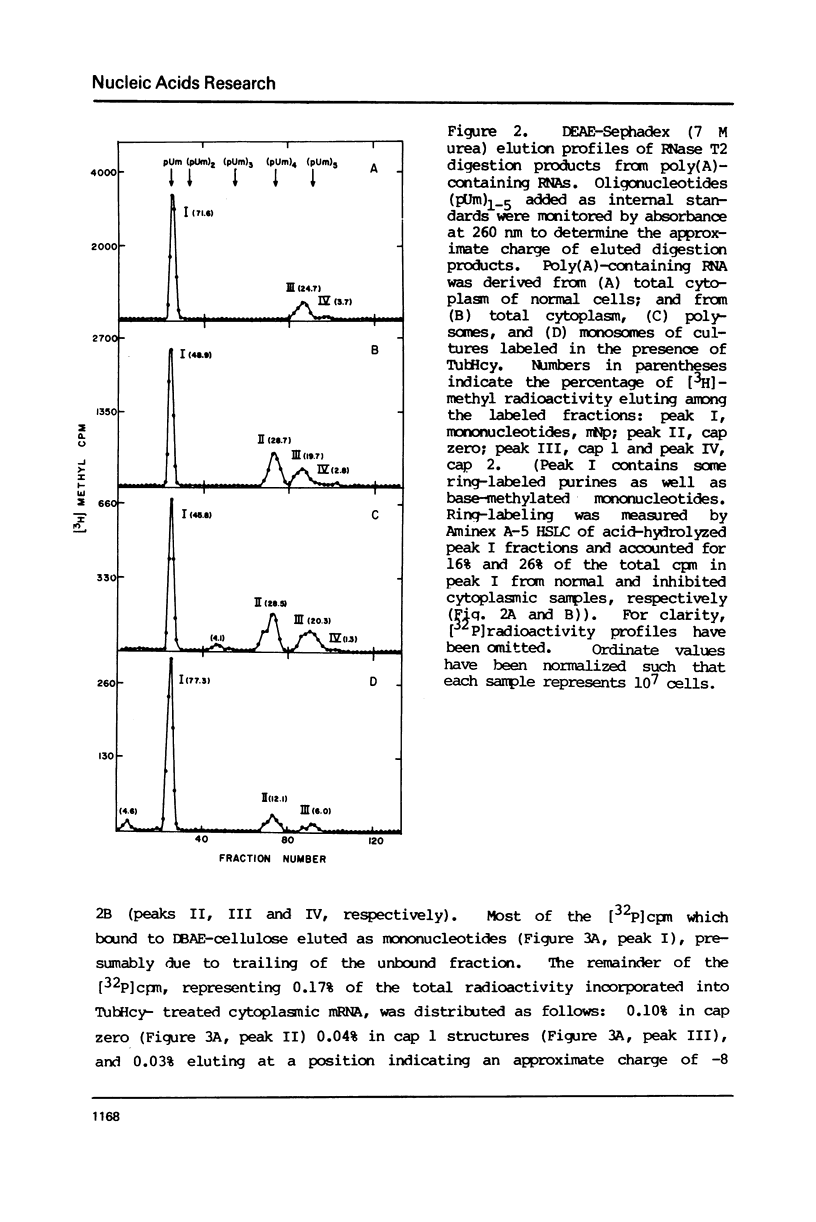
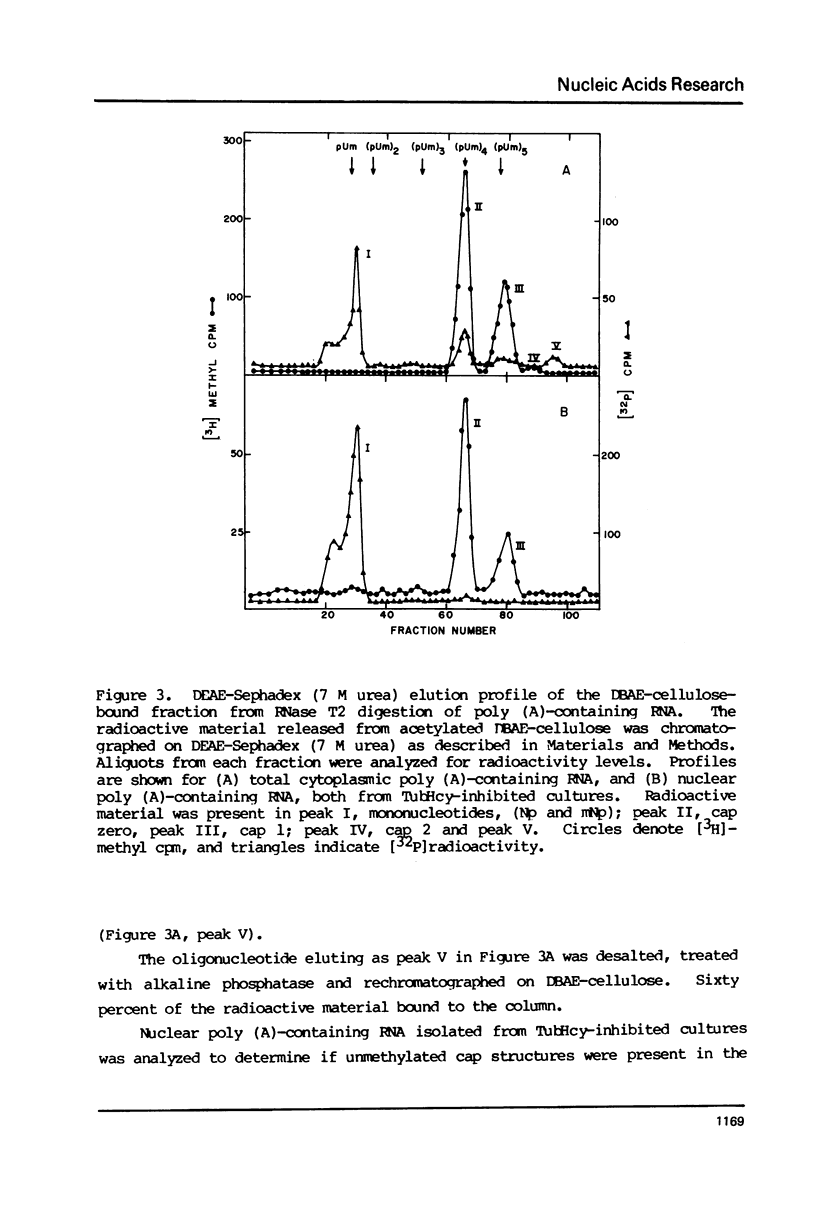
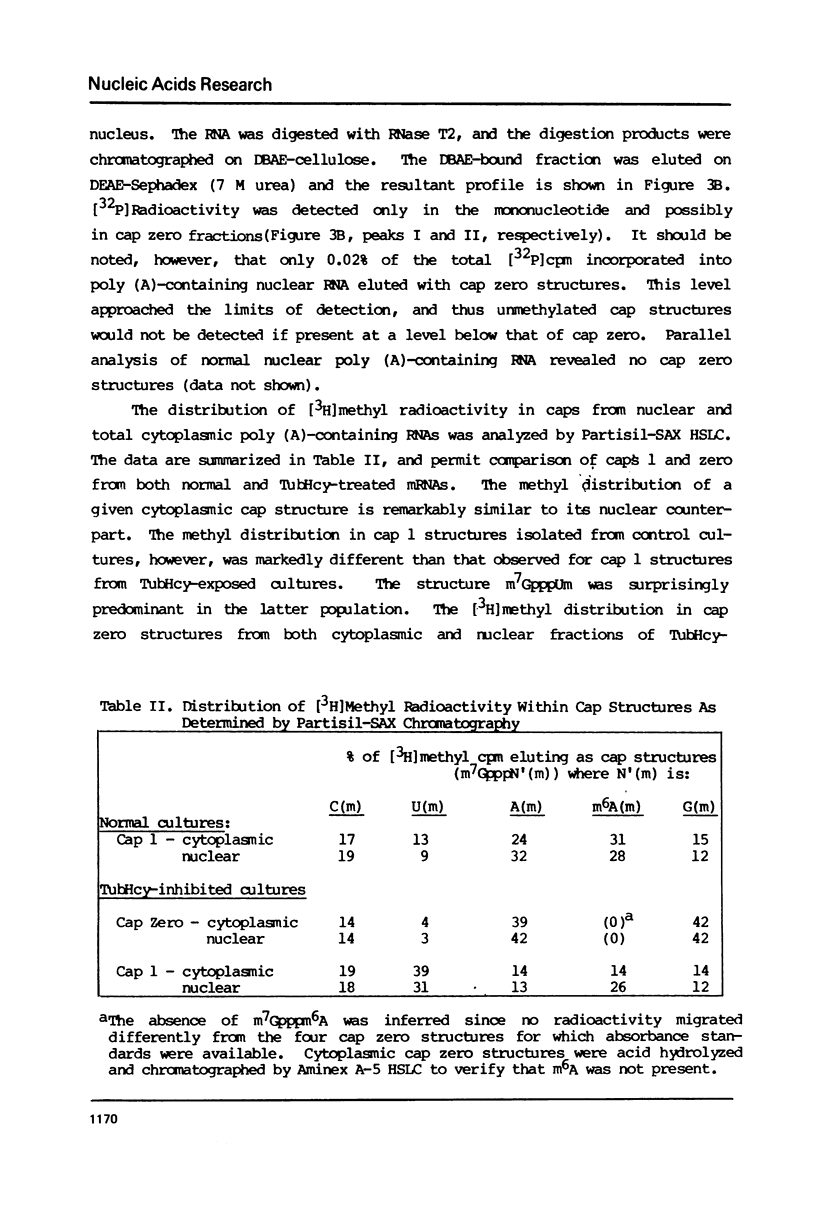
Novikoff cells in culture were labeled with L-[methyl-3H]methionine and [U-14C]uridine in the presence of (a) TubHcy2, (b) AdoHcy, (c) homocysteine, (d) tubercidin, or (e) without any additions. Only in cultures labeled in the presence of TubHcy were undermethylated cap structures observed to represent a significant portion of [3H]methyl radioactivity. Novikoff cells in culture were then simultaneously labeled with L-[methyl-3H]methionine and [32P]orthophosphate in the presence or absence of TubHcy. Total cytoplasmic, polysomal and monosomal poly(A)-containing RNAs were analyzed. Both monosomal and polysomal mRNA fractions from TubHcy-treated cells contain partially methylated cap structures, suggesting that 2'-O-methylation of the nucleoside adjacent to the pyrophosphate linkage in caps is not required for transport, ribosomal binding or translation. Comparison of nuclear and cytoplasmic cap structures from normal and inhibited cultures indicate that an altered mRNA population is generated in the presence of TubHcy.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bantle J. A., Maxwell I. H., Hahn W. E. Specificity of oligo (dT)-cellulose chromatography in the isolation of polyadenylated RNA. Anal Biochem. 1976 May 7;72:413–427. doi: 10.1016/0003-2697(76)90549-2. [DOI] [PubMed] [Google Scholar]
- Brack C., Tonegawa S. Variable and constant parts of the immunoglobulin light chain gene of a mouse myeloma cell are 1250 nontranslated bases apart. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5652–5656. doi: 10.1073/pnas.74.12.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Mandel J. L., Chambon P. Ovalbumin gene is split in chicken DNA. Nature. 1977 Nov 24;270(5635):314–319. doi: 10.1038/270314a0. [DOI] [PubMed] [Google Scholar]
- Chang C. D., Coward J. K. Effect of S-adenosylhomocysteine and S-tubercidinylhomocysteine on transfer ribonucleic acid methylation in phytohemagglutinin-stimulated lymphocytes. Mol Pharmacol. 1975 Nov;11(6):701–707. [PubMed] [Google Scholar]
- Cheng T., Kazazian H. H., Jr Sequential methylation of globin mRNA in nucleated erythroid cells and reticulocytes of mice. J Biol Chem. 1978 Jan 10;253(1):246–251. [PubMed] [Google Scholar]
- Craig N. The effects of inhibitors of RNA and DNA synthesis on protein synthesis and polysome levels in mouse L-cells. J Cell Physiol. 1973 Oct;82(2):133–150. doi: 10.1002/jcp.1040820202. [DOI] [PubMed] [Google Scholar]
- Desrosiers R. C., Friderici K. H., Rottman F. M. Characterization of Novikoff hepatoma mRNA methylation and heterogeneity in the methylated 5' terminus. Biochemistry. 1975 Oct 7;14(20):4367–4374. doi: 10.1021/bi00691a004. [DOI] [PubMed] [Google Scholar]
- Desrosiers R., Friderici K., Rottman F. Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3971–3975. doi: 10.1073/pnas.71.10.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschenfeldt W. H., Patterson R. J. Polysome isolation of sepharose column chromatography. Prep Biochem. 1975;5(3):247–255. doi: 10.1080/00327487508061575. [DOI] [PubMed] [Google Scholar]
- Friderici K., Kaehler M., Rottman F. Kinetics of Novikoff cytoplasmic messenger RNA methylation. Biochemistry. 1976 Nov 30;15(24):5234–5241. doi: 10.1021/bi00669a006. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Flavell R. A. The rabbit beta-globin gene contains a large large insert in the coding sequence. Cell. 1977 Dec;12(4):1097–1108. doi: 10.1016/0092-8674(77)90172-6. [DOI] [PubMed] [Google Scholar]
- Kaehler M., Coward J., Rottman F. In vivo inhibition of Novikoff cytoplasmic messenger RNA methylation by S-tubercidinylhomocysteine. Biochemistry. 1977 Dec 27;16(26):5770–5775. doi: 10.1021/bi00645a019. [DOI] [PubMed] [Google Scholar]
- Kwan S. P., Wood T. G., Lingrel J. B. Purification of a putative precursor of globin messenger RNA from mouse nucleated erythroid cells. Proc Natl Acad Sci U S A. 1977 Jan;74(1):178–182. doi: 10.1073/pnas.74.1.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelot R. J., Lesko N., Stout R. W., Coward J. K. Effect of S-adenosylhomocysteine and S-tubercidinylhomocysteine on catecholamine methylation in neuroblastoma cells. Mol Pharmacol. 1977 Mar;13(2):368–373. [PubMed] [Google Scholar]
- Munns T. W., Podratz K. C., Katzman P. A. A method for determination of the methylated constituents of transfer ribonucleic acid. Biochemistry. 1974 Oct 8;13(21):4409–4416. doi: 10.1021/bi00718a026. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Kinetics of formation of 5' terminal caps in mRNA. Cell. 1976 Jul;8(3):433–442. doi: 10.1016/0092-8674(76)90156-2. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Methylated constituents of heterogeneous nuclear RNA: presence in blocked 5' terminal structures. Cell. 1975 Sep;6(1):13–19. doi: 10.1016/0092-8674(75)90068-9. [DOI] [PubMed] [Google Scholar]
- Pugh C. S., Borchardt R. T., Stone H. O. Inhibition of Newcastle disease virion messenger RNA (guanine-7-)-methyltransferase by analogues of S-adenosylhomocysteine. Biochemistry. 1977 Aug 23;16(17):3928–3932. doi: 10.1021/bi00636a032. [DOI] [PubMed] [Google Scholar]
- Rottman F. M., Desrosiers R. C., Friderici K. Nucleotide methylation patterns in eukaryotic mRNA. Prog Nucleic Acid Res Mol Biol. 1976;19:21–38. doi: 10.1016/s0079-6603(08)60906-x. [DOI] [PubMed] [Google Scholar]
- Salditt-Georgieff M., Jelinek W., Darnell J. E., Furuichi Y., Morgan M., Shatkin A. Methyl labeling of HeLa cell hnRNA: a comparison with mRNA. Cell. 1976 Feb;7(2):227–237. doi: 10.1016/0092-8674(76)90022-2. [DOI] [PubMed] [Google Scholar]
- Shatkin A. J. Capping of eucaryotic mRNAs. Cell. 1976 Dec;9(4 Pt 2):645–653. doi: 10.1016/0092-8674(76)90128-8. [DOI] [PubMed] [Google Scholar]
- Sripati C. E., Groner Y., Warner J. R. Methylated, blocked 5' termini of yeast mRNA. J Biol Chem. 1976 May 25;251(10):2898–2904. [PubMed] [Google Scholar]
- Thomason A. R., Friderici K. H., Velicer L. F., Rottman F. Presence of 5'-terminal cap structures in virus-specific RNA from feline leukemia virus-infected cells. J Virol. 1978 May;26(2):226–235. doi: 10.1128/jvi.26.2.226-235.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilghman S. M., Curtis P. J., Tiemeier D. C., Leder P., Weissmann C. The intervening sequence of a mouse beta-globin gene is transcribed within the 15S beta-globin mRNA precursor. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1309–1313. doi: 10.1073/pnas.75.3.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilghman S. M., Tiemeier D. C., Polsky F., Edgell M. H., Seidman J. G., Leder A., Enquist L. W., Norman B., Leder P. Cloning specific segments of the mammalian genome: bacteriophage lambda containing mouse globin and surrounding gene sequences. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4406–4410. doi: 10.1073/pnas.74.10.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker R. D., Duerre J. A. S-adenosylhomocysteine metabolism in various species. Can J Biochem. 1975 Mar;53(3):312–319. doi: 10.1139/o75-044. [DOI] [PubMed] [Google Scholar]



