Abstract
The National Alliance for Medical Image Computing (NA-MIC), is a multi-institutional, interdisciplinary community of researchers, who share the recognition that modern health care demands improved technologies to ease suffering and prolong productive life. Organized under the National Centers for Biomedical Computing 7 years ago, the mission of NA-MIC is to implement a robust and flexible open-source infrastructure for developing and applying advanced imaging technologies across a range of important biomedical research disciplines. A measure of its success, NA-MIC is now applying this technology to diseases that have immense impact on the duration and quality of life: cancer, heart disease, trauma, and degenerative genetic diseases. The targets of this technology range from group comparisons to subject-specific analysis.
Keywords: Brachytherapy, gynecologic, image analysis, knowledge bases, open source software, radiation therapy, registration, segmentation, shape analysis, slicer, 3D slicer
Practising clinicians and biomedical researchers are intimately aware of gaps in their ability to apply medical knowledge to the needs of individual patients. An abundance of electronic clinical data are produced over the course of an individual's disease progression and treatment, representing an enormous opportunity for improving medical care. The task of interpreting this mass of clinical data, however, has become correspondingly complex, often confounding this opportunity. To borrow a phrase from the intelligence community, the medical community is facing a challenge of ‘not enough eyeballs per pixel’. It is the mission of the National Alliance for Medical Image Computing (NA-MIC) to provide technologies, through the NA-MIC kit, that facilitate analysis and decision-making using this increasing medical information available to biomedical researchers and clinicians. Figure 1 illustrates a particularly data-intensive study in which several aspects of the NA-MIC kit were used together to study shape alterations in the striatum in chorea acanthocytosis, a neurological disorder that affects movement in different parts of the body.1
Figure 1.
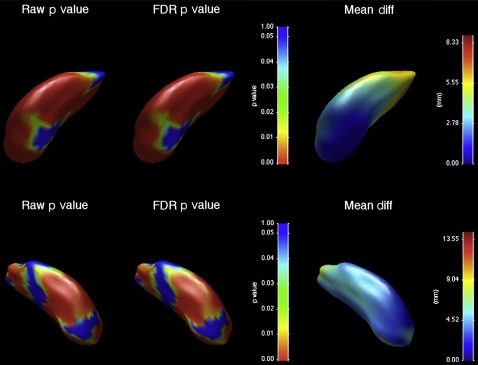
Chorea-acanthocytosis (ChAc) is an uncommon autosomal recessive disorder due to mutations of the VPS13A gene, which encodes for the membrane protein chorein. ChAc presents with progressive limb and orobuccal chorea, but there is often a marked dysexecutive syndrome. ChAc may first present with neuropsychiatric disturbance such as obsessive-compulsive disorder, suggesting a particular role for disruption to striatal structures involved in non-motor frontostriatal loops, such as the head of the caudate nucleus. We investigated morphometric changes in 13 patients with genetically or biochemically confirmed ChAc and 26 age and gender-matched controls. Subjects underwent MRI and manual segmentation of the caudate nucleus and putamen, and shape analysis using a non-parametric spherical harmonic technique. Both structures showed significant and marked reductions in volume compared with controls, with reduction greatest in the caudate nucleus. Both structures showed significant shape differences, particularly in the head of the caudate nucleus. No significant correlation was shown between the duration of illness and striatal volume or shape, suggesting that much structural change may have already taken place at the time of symptom onset. Our results suggest that striatal neuron loss may occur early in the disease process, and follows a dorsal–ventral gradient that may correlate with early neuropsychiatric and cognitive presentations of the disease. Left (top) and right (bottom) putamen changes, showing differences between ChAc patients and controls. On the left, uncorrected raw p value map; middle, FDR-corrected p value map; right, between-group displacement map. Legend to p value map shows that regions with p>0.05 are colored blue, with significant values on a spectrum from green (p=0.05) to red (p=0). Legend to displacement map shows displacement of the ChAc group from the control group in millimeters.
In practice, delivering on the promise of the information technology revolution in biomedical imaging is exceptionally difficult. The barriers to entry include the need to interoperate with scanners and other clinical systems, to organize and present clinical information according to accepted conventions, and to deploy computer systems that can efficiently process data within the time constraints of clinical practice. As many of these requirements are common across a range of clinical applications, the NA-MIC open source platform allows the most labor-intensive development and debugging tasks to be shared by the community for mutual benefit. A well defined, scalable software architecture and rigorous engineering methodology are essential to making software of this scale viable and are hallmarks of the NA-MIC approach.
The scope of NA-MIC activities includes both the highly speculative exploration of new mathematical formulations of core image analysis techniques and the ongoing effort of delivering and supporting binary distributions of software applications across a range of computing platforms. To address this continuum, the NA-MIC computer science core is organized around two teams: algorithms and engineering. These teams bring complementary skills to the technical challenges posed by the driving biological projects (DBP). The results of these technical and clinical scientists working together is the NA-MIC kit, which will be described further below.
The NA-MIC organization is guided by three principles: (1) innovation cannot and should not be managed from above; (2) communication and training across computer science and clinical research disciplines is critical to providing the highest quality biomedical image analysis research; and (3) open science concepts lower the barriers for scientific exchange and duplication of results as a quality assurance method and are therefore propagating good science. These principles are applied in a culture of shared decision-making and mutual responsibility among the leadership team.
DBP best practices
The first 6 years of NA-MIC were focused primarily on basic research in schizophrenia,2–17 autism,18 and lupus,19 in which detailed individual analyses of lesion locations,19 cortical thickness,20–23 white matter architecture,24 25 and brain morphology were studied in large populations. Statistics from these group comparisons are essential to defining the range of variation endemic in these conditions. As part of these efforts, NA-MIC invested heavily in the technologies for analysis and visualization of multimodal imaging studies of individuals. Increasingly, these tools and approaches have supported translational science in support of patient-specific clinical decision support. Although our individual computer scientists have strong credentials in this area, NA-MIC's collective involvement in translational medicine began in earnest with our work in MRI-guided prostate cancer26–29 interventions through a funded DBP. Our current DBP in Huntington's disease, traumatic brain Injury, atrial fibrillation, and head and neck cancer, reflect the goal of embodying knowledge gained from population analysis in new or modified software systems directed to the benefit of individual patients.
The overarching best practice in the NA-MIC DBP relationship is that the DBP work closely with the NA-MIC computer science team to utilize the NA-MIC kit, and in the process, they discover and drive improvements to the toolkit itself. The current DBP, for example, require improved robustness and efficiency of multimodal non-linear image registration and segmentation for almost every medical image computing application. All of the DBP work with multiple or longitudinal image data of individual subjects. These data are used to detect and characterize changes from baseline secondary to disease, trauma, or treatment. This effort requires novel image processing and visualization tools for qualitative and quantitative assessment of serial images. Quantitative evaluation of pathology, a main theme in all four DBP, necessitates new types of efficient intuitive user interaction in two and three-dimensional (3D) displays to efficiently initialize and guide image registration and segmentation procedures. To adapt these tools for clinical use, refinements are needed to render the NA-MIC kit more compatible with clinical systems, including capabilities for representing clinical data, Picture Archiving and Communication System (PACS) interfaces, and Digital Imaging and Communications in Medicine (DICOM) networking. Another technical goal common to all NA-MIC DBP is the delivery of integrated solutions of data, software, and tutorials that represent clinical best practices. In this regard, the computer science core is creating or modifying infrastructure for optimizing and validating these best practices, while the service, training, and dissemination cores are designing systems and methodologies for effectively communicating these best practices to the wider research community.
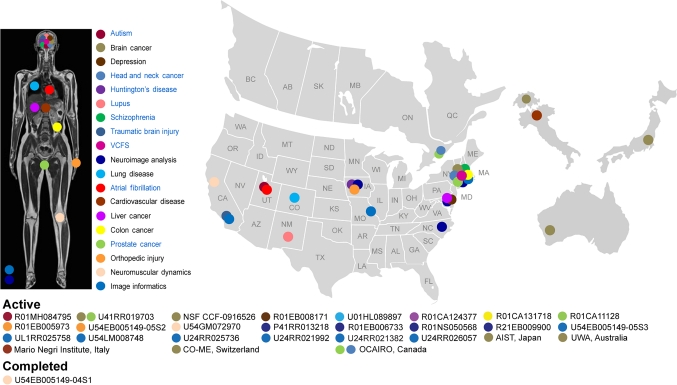
Our network of past and currently funded collaborations shown in figure 2 addresses a wide range of organ systems and pathologies. Specific focus areas include the diagnosis and therapy of schizophrenia, lupus, autism, chronic obstructive pulmonary disease, cancer of the liver, colon, and prostate, as well as musculoskeletal disorders. These are funded by 18 National Institutes of Health (NIH) grants including eight R01/R21 funded through the ‘collaboration with NCBC’ mechanism, three center grants headquartered at Brigham and Women's Hospital, and one clinical trial (for chronic obstructive pulmonary disease) that has already used the NA-MIC kit software to process 2500+ CT scans. In addition, we actively collaborate with three internationally funded efforts.
Figure 2.
This figure illustrates the network of National Alliance for Medical Image Computing (NA-MIC) collaborations that span the diagnosis and therapy of schizophrenia, lupus, autism, chronic obstructive pulmonary disease (COPD), cancer of the liver, colon, and prostate, as well as musculoskeletal disorders. These are funded by 18 National Institutes of Health grants including eight R01/R21 funded through the ‘collaboration with National Center for Biomedical Computing (NCBC’) mechanism, three center grants headquartered at Brigham and Women's Hospital, and one clinical trial (for COPD) that has already used the NA-MIC kit software to process 2500+ CT scans. In addition, we actively collaborate with three internationally funded efforts.
Best practices for DBP partnerships
In addition to maximizing the diversity of clinical challenges represented by our DBP, we used four best practices developed in our laboratory to select specific DBP teams that we invited to partner with us. The foremost selection criterion was willingness on the part of the DBP team to work in an open science community, including regular participation in community/outreach events. This was critical because open science is the key characteristic of the NA-MIC community, and open software our key deliverable; a DBP needs to adopt this mindset in order to maximize their productivity within NA-MIC. The second selection criterion was that the clinical aspect of the work be funded and at a stage at which real data are being gathered. This was important because the role of the DBP is to provide a clinical hypothesis that has been peer-reviewed by the NIH community, as well as a significant volume of data on which to test this hypothesis. The role of the NA-MIC computer science team is to provide tools to study the DBP hypothesis on their data. To ensure successful execution of the project, we stipulated that the DBP principal investigator be a scientist with an engineering background and a strong collaboration history with their own biomedical engineers. This was necessary in order to translate the clinical goals of the grant at the DBP site into technical goals that would be fulfilled by the NA-MIC partnership. We also stipulated that the team hire a software engineer (not a biomedical engineer or clinician), which was necessary for customization of the NA-MIC environment for the needs of the DBP.
Outputs of NA-MIC
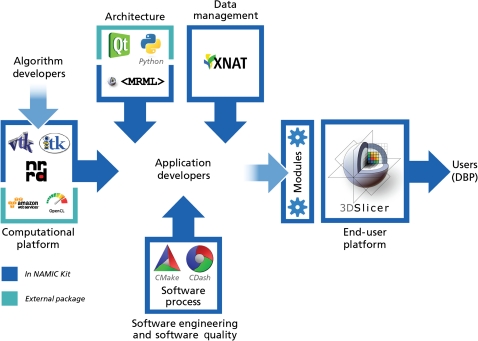
The NA-MIC kit is a free open source software platform, which is distributed under a Berkeley Software Distribution-style licence without restrictions or ‘give-back’ requirements and is intended for research, but there are no restrictions on other uses. As illustrated in figure 3, the NA-MIC kit contains a set of a modular set of interoperable free open source software packages, managed under a collaborative, high quality software engineering methodology. It consists of the 3D slicer application software, a number of tools and toolkits such as Visualization Toolkit (VTK) and Insight Segmentation and Registration Toolkit (ITK), and a software engineering methodology that enables multi-platform implementations. It also draws on other ‘best practices’ from the community to support automatic testing for quality assurance. The NA-MIC kit uses a modular approach, in which the individual components can be used by themselves or together. The NA-MIC kit is fully compatible with local installation (behind institutional firewalls) and installation as an internet service. Significant effort has been invested to ensure compatibility with standard file formats and interoperability with a large number of external applications.
Figure 3.
This figure illustrates how the National Alliance for Medical Image Computing (NA-MIC) kit, which contains a modular set of interoperable free open source software packages, is managed under a collaborative, high quality software engineering methodology. The kit itself consists of the 3D slicer application software, a number of tools and toolkits such as Visualization Tool kit (VTK) and Insight Segmentation and Registration Tool kit (ITK), and a software engineering methodology that enables multi-platform implementations. DBP, driving biological projects.
3D slicer, the ‘keystone’ of the NA-MIC kit, is a general-purpose application for loading, viewing, analyzing, processing and interacting with biomedical imaging data. Slicer can be configured at run-time through plug-in modules, enabling algorithms developers and researchers to modify and specialize slicer to a particular application. It is available for download at http://www.slicer.org/. Current download rates are approximately 1000 downloads per month.
The NA-MIC kit has been carefully designed to accommodate a range of users from those developing algorithms, to application developers, to users especially the DBP. This enables a transition path from research scientists creating leading edge computational algorithms, through deployment via slicer modules. In particular, the slicer modular architecture enables research teams to focus on the core technology without the complexity of a full-blown application, yet package and distribute them quickly to the broader community.
Current and future goals
A key contribution of NA-MIC is the creation of an active community of scientists—both users and developers—who are committed to the concept of open science and have rallied around the NA-MIC kit. In addition to traditional tutorials and workshops, we have been organizing twice-yearly community events called ‘project weeks’, which illustrate well the culture of collaboration and technical excellence championed by NA-MIC. The concept of the project week has been to create teams from representatives of multiple cores, with experience levels ranging from expert to student. Each team identified technical challenges within NA-MIC's mandate and worked together for an intensive period ranging from an afternoon to an entire week. The ultimate goal of each project is to move from research problem to solution; with the solution implemented in our open source NA-MIC kit software suite.
Seven years and 13 project events later, it is clear that the results of the experiment have been strongly positive. Over 100 participants gather at each project week to work on over 50 projects.
Our plans in NA-MIC are to continue to keep our flagship deliverable, 3D slicer, at the cutting edge of computer science solutions for biomedical research. Our roadmap includes better integration with emerging image informatics frameworks, integration of novel hardware and software technologies, and most importantly, a long-term focus on better support for translational clinical research on high-impact health problems across clinical specialities.
Footnotes
Competing interests: None.
Provenance and peer review: Commissioned; internally peer reviewed.
References
- 1.Walterfang M, Looi JC, Styner M, et al. Shape alterations in the striatum in chorea-acanthocytosis. Psychiatry Res 2011;192:29–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitford TJ, Kubicki M, Schneiderman JS, et al. Corpus callosum abnormalities and their association with psychotic symptoms in patients with schizophrenia. Biol Psychiatry 2010;68:70–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garlinghouse MA, Roth RM, Isquith PK, et al. Subjective rating of working memory is associated with frontal lobe volume in schizophrenia. Schizophr Res 2010;120:71–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ungar L, Nestor PG, Niznikiewicz MA, et al. Color stroop and negative priming in schizophrenia: an fMRI study. Psychiatry Res 2010;181:24–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeong B, Kubicki M. Reduced task-related suppression during semantic repetition priming in schizophrenia. Psychiatry Res 2010;181:114–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeong B, Wible CG, Hashimoto R, et al. Functional and anatomical connectivity abnormalities in left inferior frontal gyrus in schizophrenia. Hum Brain Mapp 2009;30:4138–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kruck CL, Flashman LA, Roth RM, et al. Lack of relationship between psychological denial and unawareness of illness in schizophrenia-spectrum disorders. Psychiatry Res 2009;169:33–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawashima T, Nakamura M, Bouix S, et al. Uncinate fasciculus abnormalities in recent onset schizophrenia and affective psychosis: a diffusion tensor imaging study. Schizophr Res 2009;110:119–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee K, Yoshida T, Kubicki M, et al. Increased diffusivity in superior temporal gyrus in patients with schizophrenia: a diffusion tensor imaging study. Schizophr Res 2009;108:33–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fitzsimmons J, Kubicki M, Smith K, et al. Diffusion tractography of the fornix in schizophrenia. Schizophr Res 2009;107:39–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kubicki M, Styner M, Bouix S, et al. Reduced interhemispheric connectivity in schizophrenia-tractography based segmentation of the corpus callosum. Schizophr Res 2008;106:125–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenberger G, Kubicki M, Nestor PG, et al. Age-related deficits in fronto-temporal connections in schizophrenia: a diffusion tensor imaging study. Schizophr Res 2008;102:181–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nestor PG, Kubicki M, Niznikiewicz M, et al. Neuropsychological disturbance in schizophrenia: a diffusion tensor imaging study. Neuropsychology 2008;22:246–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maddah M, Kubicki M, Wells WM, et al. Findings in schizophrenia by tract-oriented DT-MRI analysis. Med Image Comput Comput Assist Interv 2008;11:917–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roth RM, Koven NS, Pendergrass JC, et al. Apathy and the processing of novelty in schizophrenia. Schizophr Res 2008;98:232–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Onitsuka T, McCarley RW, Kuroki N, et al. Occipital lobe gray matter volume in male patients with chronic schizophrenia: a quantitative MRI study. Schizophr Res 2007;92:197–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flashman LA, Roth RM, Pixley HS, et al. Cavum septum pellucidum in schizophrenia: clinical and neuropsychological correlates. Psychiatry Res 2007;154:147–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fletcher PT, Whitaker RT, Tao R, et al. Microstructural connectivity of the arcuate fasciculus in adolescents with high-functioning autism. Neuroimage 2010;51:1117–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scully M, Anderson B, Lane T, et al. An automated method for segmenting white matter lesions through multi-level morphometric feature classification with application to lupus. Front Hum Neurosci 2010;4:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Westlye LT, Walhovd KB, Dale AM, et al. Increased sensitivity to effects of normal aging and Alzheimer's disease on cortical thickness by adjustment for local variability in gray/white contrast: a multi-sample MRI study. Neuroimage 2009;47:1545–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldman AL, Pezawas L, Mattay VS, et al. Widespread reductions of cortical thickness in schizophrenia and spectrum disorders and evidence of heritability. Arch Gen Psychiatry 2009;66:467–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dickerson BC, Fenstermacher E, Salat DH, et al. Detection of cortical thickness correlates of cognitive performance: reliability across MRI scan sessions, scanners, and field strengths. Neuroimage 2008;39:10–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wisco JJ, Kuperberg G, Manoach D, et al. Abnormal cortical folding patterns within Broca's area in schizophrenia: evidence from structural MRI. Schizophr Res 2007;94:317–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oh JS, Kubicki M, Rosenberger G, et al. Thalamo-frontal white matter alterations in chronic schizophrenia: a quantitative diffusion tractography study. Hum Brain Mapp 2009;30:3812–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kubicki M, Niznikiewicz M, Connor E, et al. Relationship between white matter integrity, attention, and memory in schizophrenia: a diffusion tensor imaging study. Brain Imaging Behav 2009;3:191–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oguro S, Tokuda J, Elhawary H, et al. MRI signal intensity based B-spline nonrigid registration for pre- and intraoperative imaging during prostate brachytherapy. J Magn Reson Imaging 2009;30:1052–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tokuda J, Fischer GS, DiMaio SP, et al. Integrated navigation and control software system for MRI-guided robotic prostate interventions. Comput Med Imaging Graph 2010;34:3–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tempany C, Straus S, Hata N, et al. MR-guided prostate interventions. J Magn Reson Imaging 2008;27:356–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tokuda J, Fischer GS, Csoma C, et al. Software strategy for robotic transperineal prostate therapy in closed-bore MRI. Med Image Comput Comput Assist Interv 2008;11:701–9 [DOI] [PMC free article] [PubMed] [Google Scholar]