Figure 2.
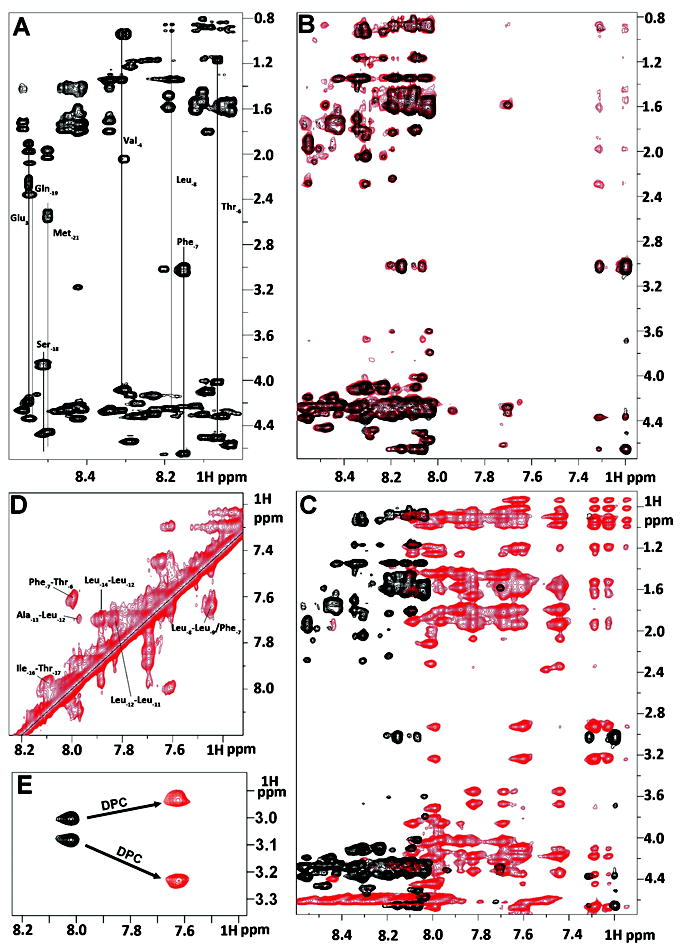
Summary of NMR spectral perturbation for KAP25 peptide upon binding to SPase I Δ2-75 or to DPC micelles. (A) 1H-1H TOCSY spectrum of KAP25 peptide. (B) transferred NOE evidence of the interaction between the KAP25 peptide and SPase I Δ2-75. Superimposition of 1H-1H NOESY spectra for KAP25 in absence (black) and presence (red) of SPase I Δ2-75 at 150:1 ratio, 22°C, 400 ms mixing time. (C) NOESY spectral perturbation upon KAP25 binding to DPC. 1H-1H NOESY spectra for KAP25 in absence (black) and presence (red) of DPC micelles at 1:1.4 molar ratio, 40°C, 200 ms mixing time. (D) The expanded amide region of the NOESY spectrum for KAP25 peptide in DPC showing few HN (i, i+1) and (i, i+2) connections. (E) NH-Hβ phenylalanine cross-peaks in 1H-1H TOCSY spectra in the absence (black) or in the presence of DPC.
