Abstract
Objective
Our aim was to investigate how semantic and phonological information is processed in adults who stutter (AWS) preparing to name pictures, following-up a report that event-related potentials (ERPs) in AWS evidenced atypical semantic picture-word priming (Maxfield et al., 2010).
Methods
Fourteen AWS and 14 typically-fluent adults (TFA) participated. Pictures, named at a delay, were followed by probe words. Design elements not used in Maxfield et al. (2010) let us evaluate both phonological and semantic picture-word priming.
Results
TFA evidenced typical priming effects in probe-elicited ERPs. AWS evidenced diminished Semantic priming, and reverse Phonological N400 priming.
Conclusions
Results point to atypical processing of semantic and phonological information in AWS. Discussion considers whether AWS ERP effects reflect unstable activation of target label semantic and phonological representations, strategic inhibition of target label phonological neighbors, and/or phonological label-probe competition.
Significance
Results raise questions about how mechanisms that regulate activation spreading operate in AWS.
Keywords: lexical, activation spreading, ERP, N400, picture-word priming, adults who stutter
Introduction
Psycholinguistic theories of stuttering (e.g., Wingate, 1988; Perkins et al., 1991; Postma and Kolk, 1993) propose that persistent stuttering may be a product of diminished linguistic processing in addition to impaired speech motor control, the latter of which is often a main target of speech therapy for people who stutter (see Bothe et al., 2006). A body of research describes psycholinguistic abilities in adults who stutter (AWS) (Bloodstein and Ratner, 2008). However, much is still unknown about how linguistic knowledge is processed in AWS preparing to speak.
The adult lexicon comprises tens of thousands of words, each associated with specific semantic and phonological features. According to some models, these different types of linguistic information are represented independently but connected in a network-like system (Dell, 1986; Levelt et al., 1999). Speech production involves activating semantic, word and phonological representations consistent with the speaker’s intended message. Picture naming can engage this process (Glaser, 1992). As pictures are decoded visually, semantic representations activate which, in turn, activate word entries (Bierwisch and Schreuder, 1992; Roelofs, 1992, 1997). We refer to this as semantic activation spreading. As word entries activate, phonological representations associated with those words activate (Berg and Schade, 1992) which, in turn, activate still other, phonologically-related words via “bottom-up” connections (Dell, 1985, 1986). We refer to this as phonological activation spreading. Although multiple word entries may initially accrue some activation, a single word entry typically accrues greatest activation strength and is selected for naming. In typically-fluent adults (TFA), these processes usually unfold quickly, automatically and accurately (see Levelt et al., 1999).
As reviewed next, research to date offers limited insight into how these mechanisms operate in AWS. In (Maxfield et al., 2010), we began using event-related potentials (ERPs) to investigate semantic activation spreading in AWS. Here, we continue this line of research and extend our focus to phonological activation spreading in AWS.
Evidence on Semantic Activation Spreading in AWS
Existing evidence reveals clues about how semantic activation spreading operates in AWS. On tests of word association, AWS responded equally fast (Crowe and Kroll, 1991; Taylor et al., 1970) or faster (Jensen et al., 1986) than TFA, suggesting that semantically-appropriate words can activate efficiently in AWS. However, AWS were more variable in the word associations they produced and in the time taken to produce them (Crowe and Kroll, 1991), suggesting that semantic activation spreading operates less consistently in AWS.
On a task eliciting word definitions, AWS used fewer synonyms than TFA (Wingate, 1988). On a picture naming task, AWS produced more naming errors than TFA (Newman and Ratner, 2007; also see Van Lieshout et al., 1996). These results suggest that words associated with increasingly specific concepts activate at a deficit in AWS, who also seem to encounter this type of difficulty on receptive language tasks (see Watson et al., 1994; Bosshardt and Fransen, 1996; Prins et al., 1997). Error patterns produced during naming, investigated by (Newman and Ratner, 2007), offer clues about how semantic activation spreading may be affected in AWS. In addition to more outright naming errors, AWS tended to replace target labels with synonyms or near-synonyms. This pattern suggests that in AWS target labels may engage in high competition with (and sometimes lose competition to) unrelated words and, occasionally, synonymous words. In contrast, AWS tended not to substitute target labels with lower-frequency semantic associates, suggesting that target labels may not receive high competition from distant semantic neighbors. Results from a picture-word interference study involving AWS (Hennessey et al., 2008) support this latter conclusion too. One aim of the current study was to continue refining our understanding of semantic activation spreading in AWS.
Evidence on Phonological Activation Spreading in AWS
Existing evidence also reveals clues about how phonological activation spreading operates in AWS. Burger and Wijnen (1999), as well as Hennessey et al. (2008), examined spoken reaction times (SRT) of AWS and TFA with versus without phonological priming. Reductions in SRT with phonological priming were similar in magnitude between groups, suggesting that longer SRTs observed in AWS versus TFA, without phonological priming, are not attributable to diminished activation of phonological representations. Newman and Ratner (2007) also found no evidence that phonological representations activate atypically in AWS.
Other evidence suggests that phonological activation spreading operates at a deficit in AWS. In (Sasisekaran et al., 2006), AWS and TFA monitored tacitly-generated picture names for target phonemes, and completed other tasks eliciting reaction times during simple motor movements, monitoring of tone sequences, and overt naming. AWS performed on-par with TFA for all but phoneme monitoring, during which they were slower, suggesting that phonological representations are slow to activate (also see Bosshardt and Nandyal, 1988; Postma et al., 1990). AWS also had slower naming latencies than TFA for lower-frequency words (Prins et al., 1997; Newman and Ratner, 2007), which are vulnerable to competition from higher-frequency, form-matching words (Dell, 1990). Finally, AWS stutter more often on lower- versus higher-frequency words (Hubbard and Prins, 1994; Newman and Ratner, 2007), and make more sound errors than TFA on tongue twisters (Postma et al., 1990; Eldridge and Felsenfed, 1998; Brocklehurst and Corley, 2011). These effects suggest that, in addition to slow activation of phonological representations, phonological competition resolves slower and less accurately in AWS. Our second aim was to extend our understanding of phonological activation spreading in AWS.
A Brain Electrophysiological Approach to the Study of Activation Spreading in AWS
In order to extend our understanding of semantic and phonological activation spreading in AWS, we turned to ERPs elicited in a picture-word priming task. A picture shown on each trial is sometimes followed by a probe word to which ERP activity is measured. Jescheniak et al. (2002) hypothesized that when picture label and probe word are unrelated, probe-elicited N400 activity should be relatively large in amplitude. If, on the other hand, picture label and probe are related, activation spreading operations triggered by naming should be potent enough to prime activation of the related probe, attenuating probe-elicited N400 activity. These predictions are consistent with the premise that N400 amplitude varies inversely with the strength of activation emerging from a priming context (Van Petten and Kutas, 1991; Rosler and Hahne, 1992). The authors confirmed these effects, reporting that in TFA semantic picture-word priming (e.g., picture of grass, probe word mower) and phonological picture-word priming (e.g., picture of grass, probe word grab) attenuated probe-elicited N400 relative to no priming.
Maxfield et al. (2010) adopted a modified version of the picture-word priming task in order to assess, specifically, semantic activation spreading in AWS. ERPs were recorded to probe words semantically-related (but phonologically-unrelated) to the labels of corresponding pictures, and to those same probes when they were reassigned to pictures with semantically- and phonologically-unrelated labels. Phonological processing of the probes was deemphasized. TFA produced a Semantic N400 priming effect, while in AWS N400 activity was larger in amplitude to semantically-related versus unrelated probes. Three interpretations of this reverse Semantic N400 priming effect were considered, all pointing to the same general conclusion that AWS processed target label semantic representations atypically in that picture-word context.
Our next step was to investigate how AWS process target label phonological representations in addition to target label semantic representations in a picture-word task. Crucially, Jescheniak et al. (2002) only observed a Phonological N400 priming effect in TFA when their task included a robust phonological priming condition and required participants to actively attend to both the target picture labels and auditory probe words. While some of these elements were omitted from the design in (Maxfield et al., 2010) in order to deemphasize phonological processing of the probe words, all of these elements featured prominently here. Our research questions were: 1) Do AWS evidence a Phonological N400 priming effect similar to that seen for TFA in a picture-word task? 2) Do AWS continue to evidence atypical Semantic N400 priming in a picture-word task that emphasizes phonological processing?
Method
Participants
Participants included 14 AWS (11 male, 3 female, mean age 32.43 years, SD=11.42) and 14 TFA (12 female, 2 male, mean age 23.64 years, SD=5.18). We note that three AWS were also participants in the Maxfield et al. (2010) study, while the other 11 AWS were newly-recruited. None of the 14 TFA participated in Maxfield et al. (2010). All participants gave written informed consent to participate, completed a medical and language history questionnaire, and were paid 10 dollars per hour. A speech sample was collected from each AWS. The first author, a speech-language pathologist with a Certificate of Clinical Competency from the American Speech-Language-Hearing Association, confirmed their diagnosis of stuttering.
All participants were monolingual speakers of English with normal or corrected-to-normal vision, no hearing deficit, and a history of normal language function. None of the TFA took medications that affect cognitive function, and none had a history of neurological injury. One AWS reported taking a small dosage of prescription medication, Propranolol, to manage stage fright. Propranolol crosses the blood-brain barrier (Neil-Dwyer et al., 1981). Although it can impair memory during emotionally-arousing events, it does not have sedating or attentional effects, and it does not impair memory during emotionally-neutral events (see Cahill et al., 1994). We were unable to identify research documenting effects of Propranolol on N400 activations. None of the AWS had a history of neurological injury.
Stimuli
Picture stimuli
The picture stimuli were 54 black-line drawings of common objects, selected from the International Picture Naming Project (IPNP) (Szekely et al., 2004). The Supplementary Appendix A lists the target labels for all of the pictures used in this task. The most frequently-used label for each drawing, according to IPNP norms, was a noun. Target labels were one or two syllables long, and averaged 3.89 phonemes long.
Probe words
Each picture was assigned two probe words that shared a linguistic relationship with the target picture label. One probe word was the strongest semantic free associate of the target picture label, determined using the University of South Florida Free Association Norms (Nelson et al., 1998). Each of these probes was Semantically-Related but phonologically unrelated to the target label of the picture to which it was assigned. The second probe word assigned to each picture contained the same initial consonant-vowel (CV) onset combination as the target picture label. Each of these probes was Phonologically-Related but semantically unrelated to the target label of the picture to which it was assigned. Words with second syllable stress were not included in either probe set.
Each of the two related probe words assigned to each picture was also reassigned to a different picture having a semantically- and phonologically-unrelated label. For example, the picture “shoe” was paired with the Phonologically-Related probe “shoots” and with the Semantically-Related probe “foot.” These same probes, “shoots” and “foot”, were also each reassigned to another picture whose label was unrelated in form or meaning. Phonologically-Related probe words reassigned to pictures with unrelated labels are, hereafter, called P-Unrelated. Semantically-Related probe words reassigned to pictures with unrelated labels are, hereafter, called Unrelated. Each probe word appeared twice: Once in a related condition, once in an unrelated condition. The Supplementary Appendix A lists the probe words assigned to each picture.
All of the target picture labels and probe words were checked for familiarity using the Hoosier Mental Lexicon (Nusbaum et al., 1984). This corpus provides an index of word familiarity, ranging from 1 (least familiar) to 7 (most familiar). The mean familiarity rating was 6.59 (SD=0.1) for the target picture labels, 6.98 (SD=.05) for the semantically-related probes, and 6.61 (SD=0.33) for the phonologically-related probes (see the Supplementary Appendix A).
Auditory probe word preparation
A female, native speaker of English read aloud each probe word, several times consecutively. All readings were recorded to digital audiotape, digitized at a sampling rate of 44.1 kHz, and then processed using Sony Sound Forge 8.0 sound editing software. The spoken exemplar of each word judged most intelligible by both the first and second authors was selected, and its waveform spliced from the original recording and saved as a sound file (.WAV format). The intensity of each word was normalized to an RMS amplitude of 15 dB, and a noise gate was used to reduce high-frequency (“hiss”) noise.
Probe verification stimuli
An additional set of words was created for use in a probe verification task. At the end of each Experimental trial, described below, participants saw a printed word that was either identical to the probe word or rhymed with it. The rhyming verification words had a mean familiarity rating of 6.89 (SD=.24) (see the Supplementary Appendix A).
Procedure
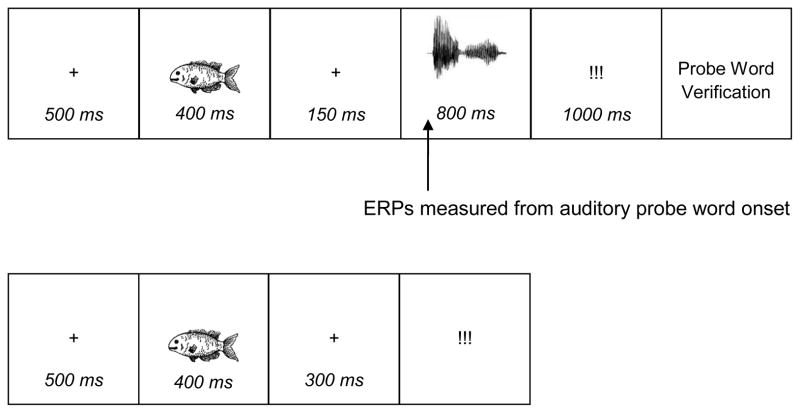
Prior to testing, participants were familiarized with the picture stimuli, making sure they knew the target label for each picture, and instructed that two types of trials would be shown during testing. On one trial type (Filler trials, see Figure 1), they saw a picture and were to name it aloud after a naming cue (i.e., “!!!”) appeared on the screen. AWS were instructed to say the word completely if they encountered a moment of stuttering. On the second trial type (Experimental trials, see Figure 1), a picture appeared, followed by a word presented through earphones. Participants were instructed to name the picture after a naming cue (“!!!”) was displayed, after which they were to use a response box to indicate their answer to a probe word verification question asking: “Is the word you heard [printed verification word shown here]?” Here, again, the AWS were told to completely finish a moment of stuttering (if any); that is, to stutter through the picture label and utter it completely before verifying the probe word.
Figure 1.
Trial structure for Experimental trials (top) and Filler trials (bottom).
Each participant received a total of 324 trials (54 Phonologically-Related trials, 54 P-Unrelated trials, 54 Semantically-Related trials, 54 Unrelated trials, and 108 Filler trials). The items were presented in a single, large block of trials with a break at roughly the halfway mark. Trials for each of the five different conditions were presented in random order. Each of the 54 pictures appeared a total of six different times during testing: Twice in Filler trials, and once in each of the four probe word (Experimental) conditions.
Apparatus and Recording
Each participant sat in a dimly-lit, sound-attenuating booth, facing a 19-inch LCD monitor. The pictures were presented in the center of the monitor. The maximum onscreen height of the pictures was 10.7 centimeters (cm); maximum onscreen width also measured 10.7 cm. Viewing distance was 90 cm. The visual angle of the pictures subtended ~6.8 degrees. Probe words were presented auditorily via insert earphones (Etymotic, Model E-2). Participants signaled the experimental software (Eprime, Psychological Software Tools, Version 1.1) to progress from trial-to-trial using a push-button response box (Psychological Software Tools).
During testing, each participant wore a nylon QuikCap (Neuroscan). The cap was fitted with 62 active recording electrodes positioned according to the International 10–20 system (Klem et al., 1999), as well as one reference electrode (positioned on the midline halfway between Cz and CPz) and one ground electrode (positioned on the midline anterior to Fz). Four additional electrodes recorded electro-ocular activity (two bipolar-referenced VEOG electrodes, and two bipolar-referenced HEOG electrodes). The electrodes were constructed of Ag/AgCl. During the experiment, EEG was recorded continuously at a sampling rate of 500 Hz. SCAN software, Version 4.3 (Neuroscan), controlled EEG recording. For most electrodes, impedance was below 5 kOhm; although impedance was as high as 30 kOhm at some electrodes (see Ferree et al., 2001). The continuous EEG data were low-pass filtered online, at a corner frequency of 100 Hz (time constant: DC).
EEG-to-Average ERP Data Reduction
The continuous EEG record of each participant was segmented into epochs. Each epoch was comprised of EEG data recorded from each electrode during presentation of the probe word on each Experimental trial, beginning 300 ms before word onset and terminating 1000 ms after word onset. Epochs of the same duration were also created for each Filler trial, beginning 300 ms before picture onset and terminating 1000 ms after picture onset. Although the latter epoch types were not ultimately analyzed, including them beneficially increased the amount of data used as input to an ocular artifact correction procedure, described next. Experimental trials eliciting incorrect picture names and/or incorrect probe word verifications were excluded. The ERP data were truncated (−100 to 800 ms relative to probe word onset) after averaging. We began with an extended epoch (−300 to 1000 ms) to ensure that artifacts on the leading and trailing edges of the shorter epoch interval were corrected or rejected.
Ocular artifact correction
In order to salvage as many trials as possible (Picton et al., 2000), we used an Independent Component Analysis (ICA)-based, ocular artifact correction procedure (see Glass et al., 2004) implemented in Matlab. After ICA decomposition (Bell and Sejnowski, 1995) of each EEG record into 64 independent components (ICs), the inverse weights of each IC, describing its topographic distribution, were correlated with a topographic template of blink activity generated by averaging at each channel the peak activity of two blink exemplars sampled from each participant. Any IC correlating with the blink template, r=.9 or better, was treated as a blink component. The activity related to each blink component was removed from each trial if it reduced the overall EEG variance for that trial. For 12 of the 14 AWS, and for 10 of the 14 TFA, a blink component was identified and their data corrected. For the other six individuals, traditional artifact rejection was used (see EEG trial rejection, next).
EEG trial rejection
For the six individuals whose data were not corrected for blink activity, an artifact rejection procedure implemented in Matlab was used whereby any trial with activity greater than or equal to 100 microvolts at EOG leads was rejected (see Picton et al., 2000). None of the six individuals lost more than one-third of their trials due to ocular artifact.
After ICA blink correction (for n=22 participants) and ocular artifact rejection (for n=6 participants), the data for all 28 participants were further checked for trials with noisy active recording electrodes. Channels whose fast-average amplitude exceeded 200 microvolts (large drift) were marked bad, as were channels whose differential amplitude exceeded 100 microvolts (high-frequency noise). Any EEG trial with more than three bad channels (5% of the total number of channels) was rejected from further analysis. No participant lost more than 20% of their trials for any condition due to bad channel artifact; most lost well under 10% of their trials.
Final EEG processing
For any accepted trial with channels marked bad (<=3), the EEG activity at those channels was replaced using a three-dimensional spline interpolation procedure implemented in Matlab (Nunez and Srinivasan, 2006, see Appendices J1–J3). Accepted EEG trials were then averaged together, separately for each Experimental condition. As a result, each participant had four sets of ERP averages: Semantically-Related, Unrelated, Phonologically-Related, and P-Unrelated. For each participant, no fewer than 34 trials went into the set of ERP averages for each Experimental condition. The averaged ERP data were low-pass filtered at a corner frequency of 40 Hz, truncated to the critical time window (−100 to 800 ms), re-referenced to the left mastoid electrode, and baseline-corrected (−100 to 0 ms). A left mastoid reference allowed us to compare our current results with our previous results (Maxfield et al., 2010), and with those of (Jescheniak et al., 2002) whose research design we adopted. ERP data referenced to left mastoid were reported in both of those previous studies.
Analysis
Analysis of behavioral data
Naming responses were scored for accuracy. Naming on each trial was judged correct if the participant named the picture using the target one-word label. Naming was judged as incorrect if no response was given, the participant began responding before the prompt (“!!!”), a whole-word substitution was generated (e.g., “stone” for “rock”), or a phonological error was generated (e.g., “thesk” for “desk”). The percentage of correct naming trials was computed for each participant in each condition, and submitted to a repeated-measures ANOVA with Condition entered as a within-subjects factor having five levels (Filler, Semantically-Related, Unrelated, Phonologically-Related, P-Unrelated) and Group entered as a between-subjects factor having two levels (AWS, TFA).
Additionally, probe word verification responses were scored for accuracy automatically by E-Prime. The percentage of correct word verifications was computed for each participant in each condition, and submitted to a repeated-measures ANOVA with Condition entered as a within-subjects factor with four levels (Semantically-Related, Unrelated, Phonologically-Related, P-Unrelated) and Group entered as a between-subjects factor with two levels (AWS, TFA).
For any test violating the sphericity assumption we report p-values based on adjusted degrees of freedom (Greenhouse and Geiser, 1959) and original F-values. Statistically significant Condition and Condition-by-Group effects were followed with Bonferroni pair-wise comparisons.
Analysis of ERP Data
The aims of this analysis were to test for N400 amplitude differences between conditions (Semantically-Related versus Unrelated N400 amplitude differences and, separately, Phonologically-Related versus P-Unrelated N400 amplitude differences) within each group, and to compare N400 effects between groups. We used Principal Component Analysis (PCA), which takes as input a data set comprised of many dimensions and reduces it by forming linear combinations of the original variables. In this case, the variables used as input were time points. PCA was used to identify distinct windows of time in the ERP averages (hereafter, temporal factors or “virtual windows”) during which similar voltage variance was registered across consecutive time points (see Dien and Frishkoff, 2004).
The averaged ERP data for the Semantically-Related and Unrelated conditions1 were combined into a data matrix comprised of 401 columns (one column for each of the sampling points spanning from 0 to 800 ms) and 3,416 rows (the averaged ERP voltages for 28 participants, at each of the 61 active recording electrodes excluding the Left Mastoid reference, in each of the two conditions). This matrix was used as input to a covariance-based, temporal PCA. In order to determine how many dominant-variance components were extracted by the PCA, we used Rule M (Preisendorfer and Mobley, 1998). Rule M estimates how many components extracted from a real data set account for more variance than corresponding components extracted from a data set of normally-distributed, randomly-sampled noise having the same dimensions as the real data set. All components meeting this criterion for each PCA were retained and rotated to simple structure using Promax (Hendrickson and White, 1964) with Kaiser normalization and k=2 (Richman, 1986; Tataryn, Wood, and Gorsuch, 1999). All PCA procedures were completed using the Matlab-based PCA Toolbox (Dien, 2010).
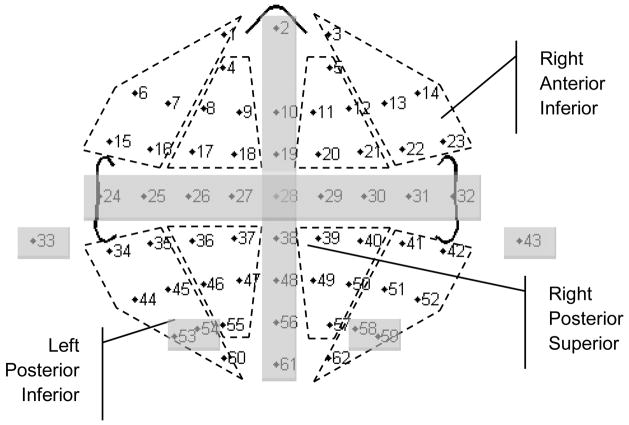
Each of the temporal factors extracted from the data set describes a virtual time window during which a distinct pattern of voltage was active. The time-course of each temporal factor is described by a set of factor loadings. The ERP variance captured by each temporal factor is described by a set of factor scores. The factor scores summarize the average amplitude of ERP activity within the virtual time window formed by each temporal factor. Factor scores associated with specific temporal factors (i.e., those with a time-course consistent with the N400 component) were computed for each participant, in each condition, at each electrode. The scores associated with specific groups of electrodes were then averaged, separately for each person in each condition. The electrodes were grouped into eight regions of interest based on recommendations from Dien and Santuzzi (2004) (see Figure 2): Left Anterior Inferior (FP1,F7,F5,FT7,FC5), Left Anterior Superior (AF3,F3,F1,FC3,FC1), Left Posterior Inferior (TP7,CP5,P7,P5,O1), Left Posterior Superior (CP3,CP1,P3,P1,PO3), Right Anterior Inferior (FP2,F8,F6,FT8,FC6), Right Anterior Superior (AF4,F4,F2,FC4,FC2), Right Posterior Inferior (TP8,CP6,P8,P6,O2), and Right Posterior Superior (CP4,CP2,P4,P2,PO4). The averaged factor scores were then submitted to a repeated-measures ANOVA with Hemisphere entered as a within-subjects factor having two levels (Left, Right), Dorsality entered as a within-subjects factor having two levels (Inferior, Superior), Anteriority entered as a within-subjects factor having two levels (Anterior, Posterior), Condition entered as a within-subjects factor having two levels (Related, Unrelated), and Group entered as a between-subjects factor having two levels (AWS, TFA). For any test violating the assumption of sphericity we report p-values based on adjusted degrees of freedom (Greenhouse and Geiser, 1959) and original F-values. When a statistically significant interaction was detected, Bonferroni-corrected pair-wise comparisons were made. The analysis path followed here resembles that used by Jescheniak et al. (2002), who chose time windows of interest based on visual inspection of grand averages, and then investigated ERP variance for priming effects using windowed amplitude measurements with topographic factors included. Temporal PCA, as incorporated here, helped define time windows of interest while minimizing ERP component overlap (see Kayser and Tenke, 2003; Dien and Frishkoff, 2004).
Figure 2.
Eight topographic regions of interest (ROIs), bounded by hashed lines, each defined by five electrodes (each indicated here by a number; standard electrode names are given in the text). Electrodes obscured by shading were not included in any ROI.
RESULTS
Behavioral Data
Naming accuracy
Picture naming was highly accurate in both groups (see Table 1). AWS were less accurate than TFA (F[1,26]=4.59,p=0.042), but the effect size was small (partial eta-squared=0.15). Naming accuracy was not affected by Condition (F[4,104=0.847,p=0.498), or by the interaction of Condition and Group (F[4,104]=0.600,p=0.637).
Table 1.
Mean percent correct (and SD) for naming accuracy by group and condition.
| Group | Filler | Semantically-Related | Unrelated | Phonologically-Related | P-Unrelated | |
|---|---|---|---|---|---|---|
| AWS | Mean | 96.03 | 96.03 | 95.50 | 95.77 | 96.56 |
| SD | 3.26 | 2.70 | 3.75 | 3.66 | 2.98 | |
| TFA | Mean | 98.01 | 97.62 | 98.15 | 97.62 | 98.15 |
| SD | 1.49 | 1.69 | 2.41 | 2.23 | 2.06 |
Probe word verification accuracy
Probe word verifications were also highly accurate in both groups (see Table 2). Verification accuracy was not affected by Condition (F[3,78]=.316,p=.761), Group (F[1,26]=2.795,p=.107), or by the interaction of Condition and Group (F[3,78]=2.189,p=.114).
Table 2.
Mean percent correct (and SD) for probe word verification accuracy by group and condition.
| Group | Semantically-Related | Unrelated | Phonologically-Related | P-Unrelated | |
|---|---|---|---|---|---|
| AWS | Mean | 98.94 | 97.62 | 97.62 | 98.68 |
| SD | 2.02 | 4.38 | 2.85 | 1.53 | |
| TFA | Mean | 98.68 | 99.47 | 99.47 | 99.20 |
| SD | 1.98 | 0.87 | 0.87 | 1.20 |
Visual Inspection and PCA of ERP Data for Semantic Picture-Word Priming
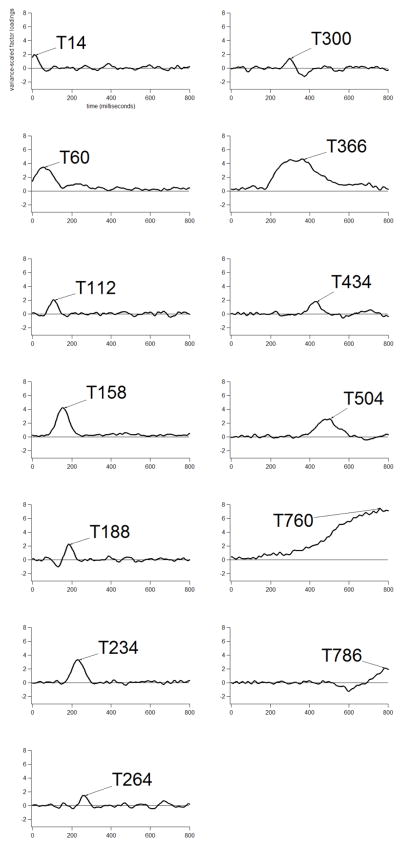
Grand average ERP waveforms are shown at three midline electrodes for Semantically-Related versus Unrelated probe words for AWS in Figure 3 (left panel) and TFA in Figure 4 (left panel). Temporal PCA generated 13 virtual time windows, accounting for 71.72% of the variance. The time-course of each virtual window is defined by variance-scaled temporal factor loadings (see Figure 5). Each factor will be referred to by its peak latency, the time point with the largest factor loading (e.g., T112). As shown in Figure 5, the temporal factors had peak latencies ranging from 14 to 786 ms after probe word onset. Jescheniak et al. (2002) reported that TFA in their study produced semantic picture-word N400 priming between ~400–800 ms after probe word onset. Similarly, inspection of our grand averages suggested that semantic priming was evident during a relatively late, broad time interval; particularly at the anterior region for our TFA group (see Figure 4, left panel, Fz plot). Thus, we analyzed the ERP variance associated with T434 (this factor explained 2.8% total variance, ~1% unique variance), T504 (12.3% total variance, 1.4% unique variance), T760 (66% total variance, 29% unique variance) and T786 (1.4% total variance, ~1% unique variance), respectively. It is noteworthy that T760 resembles a factor sometimes generated by temporal PCA, which is thought to reflect slow drift in baseline-corrected ERPs (see Wastell, 1981; Van Boxtel, 1998; Kayser and Tenke, 2003). However, it is possible for temporal factors defined by relatively late, high loadings to pick-up late-appearing experimental effects (see, for example, Foti, Hajcak and Dien, 2009). Because our grand averages revealed possible late semantic priming effects, and because Jescheniak et al. (2002) observed late semantic picture-word priming effects in their TFA, we targeted T760 for analysis, along with the three other temporal factors with peak latencies in the 400–800 ms time range. Statistically significant semantic priming was only detected for the T760 window.
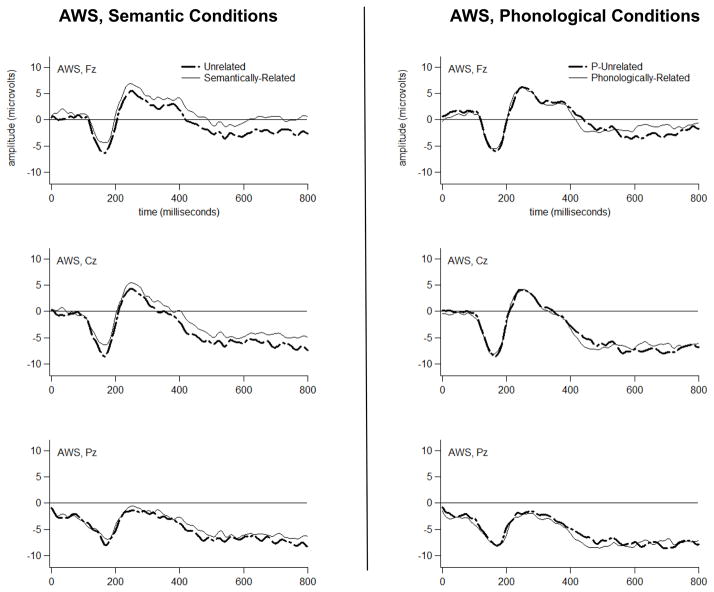
Figure 3.
Grand average ERP waveforms for the AWS to Semantically-Related versus Unrelated probes (left), and to Phonologically-Related versus P-Unrelated probes (right).
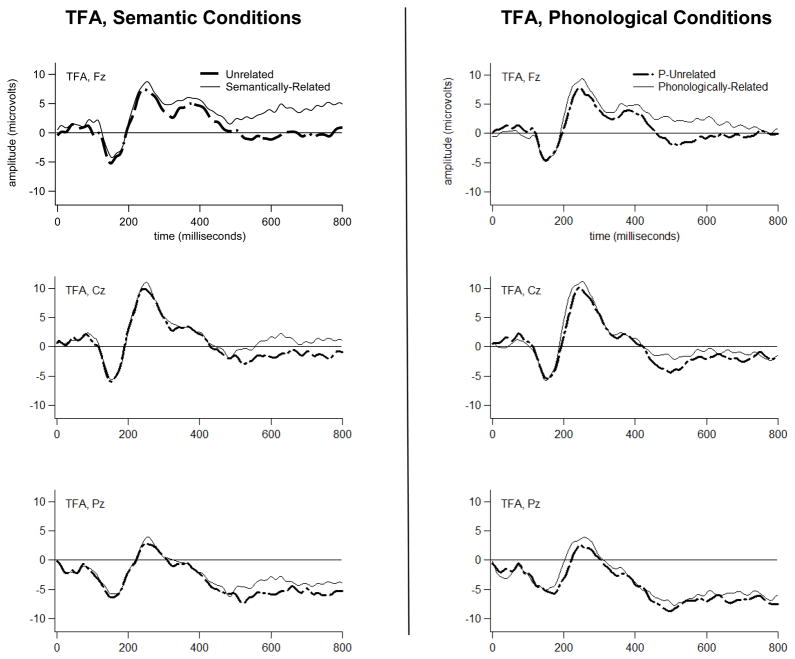
Figure 4.
Grand average ERP waveforms for the TFA to Semantically-Related versus Unrelated probes (left), and to Phonologically-Related versus P-Unrelated probes (right).
Figure 5.
Factor loadings indicating the time-course of 13 temporal factors derived from the Semantically-Related/Unrelated data set. Peak latencies are given with each label (e.g., T264 = a peak latency of 264 ms). Factors are listed in temporal order.
Analysis of T760 activity
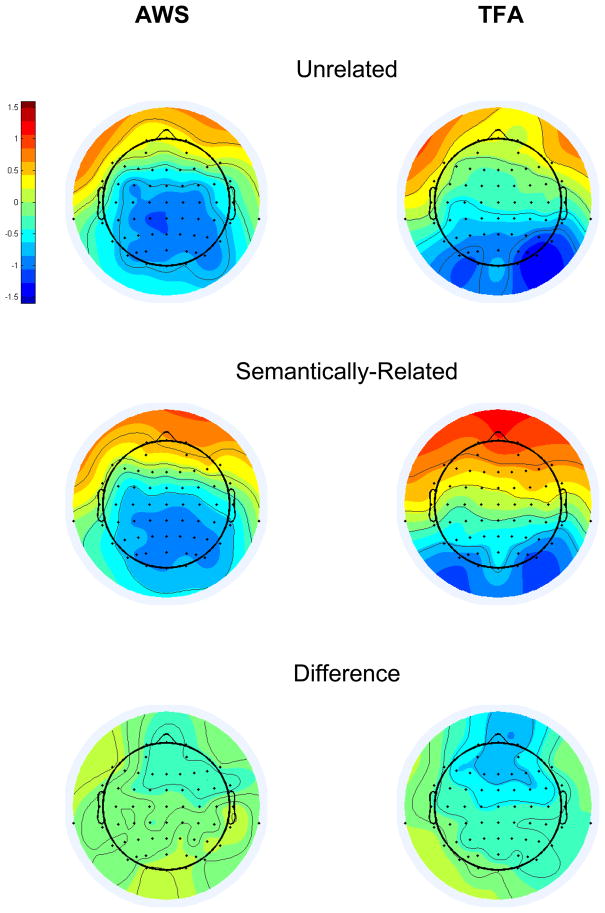
Grand average T760 scores are depicted topographically in Figure 6, separately for each group in each condition at each electrode. A five-way interaction of Group, Condition, Laterality, Dorsality, and Anteriority affected the T760 scores (F[1,26]=6.75,p=0.015). Bonferroni-corrected pair-wise comparisons revealed that, for the TFA only, Semantically-Related was attenuated versus Unrelated at the Left Anterior Superior region (p=0.024), Right Anterior Superior region (p=0.021), and (marginally) at the Right Anterior Inferior region (p=0.057). These results point to a Semantic N400-like priming effect in the T760 frontally-distributed ERP activity of the TFA (the topography of this effect, isolated via subtraction, is shown in the bottom right panel of Figure 6). Semantic priming was not detected at T760 for the AWS at any region, although Figure 6 (bottom left panel) reveals a possible, diminished anterior semantic priming effect in the T760 activity of the AWS.
Figure 6.
T760 factor scores, averaged across participants in each group, separately for Unrelated (top panels) and Semantically-Related (middle panels), at each electrode. Bottom panels show the grand average difference scores (Unrelated minus Semantically-Related) for each group at each electrode. The color scale represents the amplitude of the T760 factor scores, which are variance-corrected but not mean-centered (extreme red = 1.6, extreme blue = −1.6).
Crucially, an anonymous reviewer questioned whether preparatory processing may have influenced the T760 results. As seen in Figure 6 (right top, middle panels), the TFA did not appear to generate pronounced negative-going activity at central electrodes during T760, which might have been indicative of contingent negative variation (CNV)-like preparatory activity (see Gaillard and Beijsterveldt, 1991) in advance of delayed naming. At least for the TFA, we assume that any CNV-like activity that was present in the data was equivalent between conditions, since the interval during which preparatory processing may have built-up (between auditory probe word onset and naming cue onset) was fixed at 800 ms. AWS, who may be more prone to anticipatory anxiety during speech production (see Alm, 2004), did appear to generate pronounced negative-going activity at central-posterior electrodes (Figure 6, left top and middle panels). An interaction of Group and Dorsality was detected in the T760 variance (F[1,26]=5.78,p=.024), but Bonferroni-protected t-tests did not detect a between-groups difference at the inferior (p=.585) or superior (p=.224) region. To be sure, we compared T760 amplitudes between groups, separately for each condition at each of the eight regions of interest, and found no differences. Thus, it does not appear that AWS generated CNV-like activity more pronounced than for TFA during T760. Similarly, since no semantic priming effect was detected for AWS during T760, any preparatory processing that was active during this window did not seem to be more pronounced for semantic priming versus no priming in AWS.
Visual Inspection and PCA of ERP Data for Phonological Picture-Word Priming
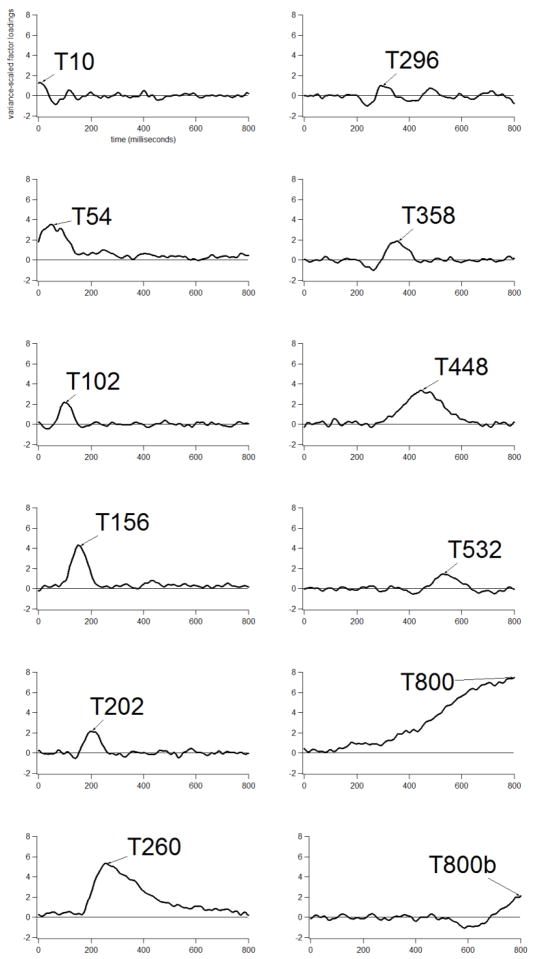
Grand average ERP waveforms are shown at three midline electrodes for Phonologically-Related versus P-Unrelated probe words for AWS in Figure 3 (right panel) and TFA in Figure 4 (right panel). Temporal PCA resulted in 12 virtual time windows, accounting for 69.47% of the variance, with peak latencies ranging from 10 to 800 ms after probe onset (see Figure 7). Jescheniak et al. (2002) reported that TFA in their study exhibited phonological picture-word N400 priming between ~250–800 ms after probe word onset. Inspection of our grand averages suggested that phonological priming was evident within a time interval spanning ~400–800 ms after probe word onset, particularly at the anterior scalp region (see Figures 3 and 4, right panel, Fz plots). Thus, we focused on the ERP variance associated with T448 (28.4% total variance, 3.1% unique variance), T532 (1.2% total variance, ~1% unique variance), T800 (66% total variance, 27.2% unique variance), and T800b (1.3% total variance, ~1% unique variance), respectively2. Although T800 resembled a baseline-drift component, noted previously, we included it for analysis, as phonological priming seemed to extend relatively late into the epoch in the Fz grand averages for each group. Of the virtual windows examined, statistically significant phonological priming was only detected in the T448 and T532 windows3.
Figure 7.
Factor loadings indicating the time-course of 12 temporal factors derived from the Phonologically-Related/P-Unrelated data set. Peak latencies are given with each label (e.g., T202 = a peak latency of 202 ms). Factors are listed in temporal order.
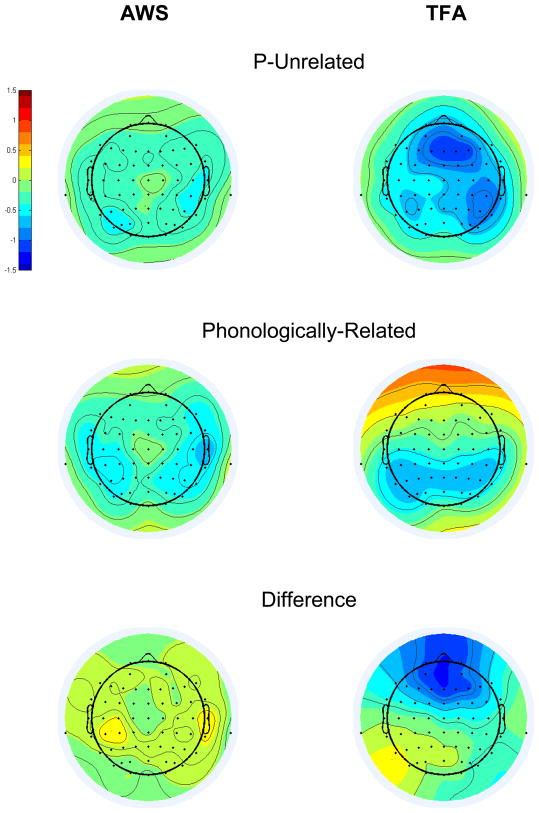
Analysis of T448 activity
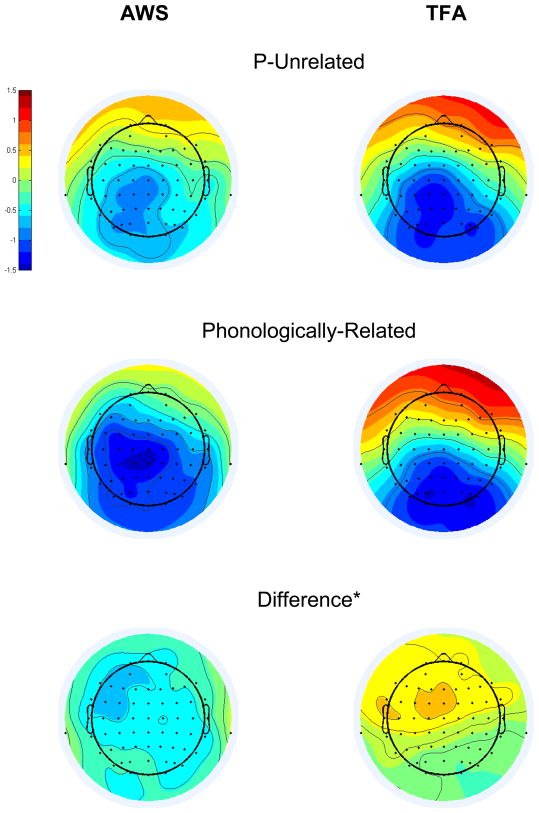
Grand average T448 scores are depicted topographically in Figure 8, separately for each group in each condition at each electrode. An interaction of Group and Condition affected the amplitude of the T448 scores (F[1,26]=6.17, p=0.02). Bonferroni-corrected pair-wise comparisons revealed that, for the AWS only, Phonologically-Related had a larger negative-going amplitude than P-Unrelated (p=0.013). These results point to a topographically-widespread, reverse Phonological N400 priming effect in the T448 ERP activity of the AWS (the topography of this effect, isolated via subtraction, is shown in the bottom left panel of Figure 8). Bonferroni-corrected t-tests, comparing Phonologically-Related versus P-Unrelated in each group at each region of interest, confirmed that reverse Phonological N400 priming was present in the AWS at all scalp regions (p<.05) except for Right Anterior Inferior (p=.114). A Phonologically-Related versus P-Unrelated effect was not detected in the T448 variance for TFA at any scalp region (p>.05), or when pooled over scalp regions (p=0.409).
Figure 8.
T448 factor scores, averaged across participants in each group, separately for P-Unrelated (top panels) and Phonologically-Related (middle panels), at each electrode. Bottom panels show the grand average difference scores (Phonologically-Related minus P-Unrelated) for each group at each electrode. The color scale represents the amplitude of the T448 factor scores, which are variance-corrected but not mean-centered (extreme red = 1.6, extreme blue = −1.6). *Note that P-Unrelated was subtracted from Phonologically-Related in order to isolate the reverse Phonological N400 priming effect generated by AWS.
One question was whether reverse Phonological N400 priming in AWS occurred because P-Unrelated amplitudes were atypically reduced in the AWS, rather than because Phonologically-Related amplitudes were atypically enhanced in the AWS? We tested this hypothesis by comparing P-Unrelated amplitudes between groups at each of the eight regions of interest and, separately, Phonologically-Related amplitudes between groups at each region of interest, using Bonferroni-protected t-tests. Despite the appearance of a group difference in T448 amplitudes for P-Unrelated (see Figure 8, top panels), a between-groups difference was not detected at any region of interest for this condition. In contrast, T448 scores had a larger, negative-going amplitude for AWS than for TFA in the Phonologically-Related condition at Left Anterior Inferior (p=.002), Left Anterior Superior (p=.01), Right Anterior Inferior (p=.022), and Right Anterior Superior (p=.031) (see Figure 8, middle panels); reinforcing the interpretation that Phonologically-Related elicited enhanced, negative-going activity in AWS during T448.
Analysis of T532 activity
Figure 9 depicts grand average T532 scores topographically, separately for each group in each condition at each electrode. A five-way interaction of Group, Condition, Laterality, Dorsality, and Anteriority affected the amplitude of the T532 scores (F[1,26]=4.27,p=0.049). Bonferroni-corrected pair-wise comparisons revealed that, for the TFA only, Phonologically-Related scores had an attenuated negative-going amplitude relative to P-Unrelated at the Left Anterior Inferior region (p=0.039), at the Left Anterior Superior region (p=0.034), and (marginally) at the Right Anterior Superior region (p=0.054). These results point to a Phonological N400-like priming effect in the T532 frontally-distributed ERP activity of the TFA (the topography of this effect, isolated via subtraction, is shown in the bottom right panel of Figure 9). Note the similarity in scalp topography of this effect and the Semantic N400-like priming effect produced by TFA (Figure 6, bottom right panel). Since both effects were localized anteriorly, and both responded to priming in TFA (albeit at different latencies), we can infer that they reflect a similar process. The topographic, time-course, and functional properties of these N400-like priming effects in TFA are further addressed in the Discussion. A Phonologically-Related versus P-Unrelated condition effect was not detected at T532 for AWS at any region.
Figure 9.
T532 factor scores, averaged across participants in each group, separately for P-Unrelated (top panels) and Phonologically-Related (middle panels), at each electrode. Bottom panels show the grand average difference scores (P-Unrelated minus Phonologically-Related) for each group at each electrode. The color scale represents the amplitude of the T532 factor scores, which are variance-corrected but not mean-centered (extreme red = 1.6, extreme blue = −1.6).
Discussion
ERP activity elicited during a picture label-probe word priming task was compared in 14 AWS versus 14 TFA. On each trial, a picture-to-be-named was sometimes followed by an auditory probe word. Some probes were Semantically-Related (but phonologically-unrelated) to the target labels of the preceding pictures. Those same probes also appeared following pictures with semantically- and phonologically-unrelated labels (Unrelated). Other probes were Phonologically-Related (but semantically-unrelated) to the target labels of their preceding pictures. Those probes, too, appeared following pictures with semantically- and phonologically-unrelated labels (P-Unrelated). Probe-elicited ERP activity was used to gauge the extent to which probe word activation was primed by self-generated picture naming.
Summary of ERP Priming Effects
The TFA evidenced a Semantic priming effect, spanning the interval between ~500–800 ms and peaking in activity at ~760 ms after probe word onset. This effect appeared primarily at right and left anterior-superior scalp regions. The TFA also evidenced a Phonological priming effect, spanning the interval between ~500–600 ms and peaking in activity at ~532 ms after probe word onset. This effect appeared primarily at two left anterior scalp regions (Left Anterior Inferior and Superior). We interpret these priming effects as reflecting Phonological and Semantic priming of a frontally-distributed, N400-like ERP component. We use the term “N400-like” because the anterior topography of these effects is notable. N400 amplitude typically modulates with priming at central and posterior electrodes (see, e.g., Deacon et al., 1995), although a recent study suggests that frontally-distributed N400 activity (i.e., FN400) is functionally equivalent to central and posterior N400 activity (see Voss and Federmeier, 2010). Concreteness is one factor known to modulate N400 effects specifically at anterior electrodes (see Kuonios and Holcomb, 1994; Holcomb et al., 1999; West and Holcomb, 2000; Lee and Federmeier, 2008). The concreteness effect on N400 topography seems to be tied to strategically invoking imagery in order to meet the goals of a task (see Schwanenflugel et al., 1992; West and Holcomb, 2000). A strategy used by our TFA may have involved attempts a connecting probe words to imagery - perhaps to physical features depicted in the pictures.
In general, semantic priming of the frontal N400-like ERP component in our TFA suggests that semantic representations defining the target labels accrued robust activation on the path to naming and, furthermore, that this activation spread to other, semantically-related words in the mental lexicon; making those words easier to process when they appeared as semantically-related probes. Similarly, phonological priming of the frontal N400-like ERP component in our TFA suggests that phonological representations associated with target labels accrued robust activation on-route to naming and, furthermore, that this activation spread to other, phonologically-related words in the mental lexicon; making it easier to process those words when they appeared as phonologically-related probes. Noteworthy is the earlier time-course of Phonological versus Semantic N400 priming in our TFA. This time-course pattern was also seen for TFA in (Jescheniak et al., 2002, Experiment 1), who interpreted this difference as revealing that phonologically decoding each probe word (and eliciting Phonological priming) requires less time than semantically decoding each probe word (and eliciting Semantic priming).
In contrast, our AWS group evidenced a reverse Phonological N400 priming effect. That is, N400 amplitude was significantly larger for Phonologically-Related versus P-Unrelated probes. This effect was topographically-widespread, and spanned the interval between ~300–600 ms, peaking in activity at ~448 ms after probe word onset. In addition, the AWS evidenced diminished Semantic priming. These effects can be interpreted along at least two different lines.
Atypical Effects in AWS as Reflecting Unstable Activation of Target Label Semantic and Phonological Representations and Phonological Center-Surround Inhibition
One interpretation is that linguistic representations associated with target picture labels were unstably activated in AWS. Specifically, diminished Semantic N400 priming may reflect that target label semantic representations did not reach an activation state stable enough to spread activation to other words sharing semantic representations with (semantically-related to) those labels in AWS. Instead, about as much processing seems to have been required in AWS for Semantically-Related probes as for Unrelated, evidenced by similar ERP amplitudes. This opens the question of whether semantic activation spreading is poorly-regulated in AWS?
Feedback activation, self-inhibition and lateral inhibition are three mechanisms by which target label semantic representations are thought to reach a stable activation state on the path to naming (see Dell, 1985, 1986; Berg and Schade, 1992). Feedback activation seems to operate typically in AWS (see Newman and Ratner, 2007), and we know of no evidence on how self-inhibition operates in AWS (although see McKay and MacDonald, 1984). However, there is evidence to suggest that lateral inhibition operates atypically in AWS. With lateral inhibition, semantic representations that create incompatible hypotheses about what is depicted in the picture are inhibited (see Berg and Schade, 1992). AWS in our task generated more naming errors than TFA, consistent with previous findings (see Newman and Ratner, 2007). More naming errors suggest that semantic representations incompatible with pictured objects are not always laterally inhibited in AWS, resulting in the selection of semantically off-target words.
That semantic representations associated with target labels may not have accrued stable activation in AWS leads to the possibility that phonological representations associated with target labels were unstably activated, too. As outlined in the Introduction, a dedicated flow of activation through the mental lexicon is seen as driving phonological activation spreading, i.e., from semantic representations, to word entries, to phonological representations, to other (phonologically-similar) word entries. Thus, unstable activation of target label semantic representations conceivably resulted in unstable activation of target label phonemes in AWS. How might this, in turn, have led to a reverse Phonological N400 priming effect?
One mechanism known to produce reverse N400 priming in TFA is center-surround inhibition (see Mari-Beffa et al., 2005; Bertmeitinger et al., 2008; both of which were reviewed in Maxfield et al., 2010). Carr and Dagenbach (1990) characterized this as an attentional mechanism that allows people to “…exercise a degree of intentional control over which activated or partially-activated codes are most likely to gain entrance to working memory, and they do so by directing attention selectively toward the desired codes” (p. 349). Because access to (unstably-activated) target label phonological representations was necessary in order to name pictures successfully, the AWS in our task may have directed center-surround inhibition toward phonologically-related neighbors of target labels in order to stabilize activation of target label phonological representations for accurate naming. Alternatively, or in addition, difficulty coding target label phonological representations into a potentially impaired working memory system (see Bajaj, 2007) may have led AWS to direct center-surround inhibition toward phonological neighbors of the target picture labels. When those phonologically-related words appeared as probes, reactivating those words following inhibition likely required heightened processing, eliciting relatively large-amplitude N400 activity (reverse Phonological N400 priming).
While a center-surround interpretation is plausible, there is other evidence to suggest that this process might actually operate at a deficit in AWS. Weber-Fox, Spencer, Spruill, and Smith (2004) asked AWS and TFA to judge whether pairs of printed words rhymed. The words were similar orthographically and rhymed; were dissimilar orthographically and did not rhyme; rhymed but were orthographically dissimilar; or were orthographically similar but did not rhyme. AWS were significantly slower than TFA when judging the last of these types of stimulus pairs. Weber-Fox et al. (2004) interpreted this effect as suggesting that AWS are particularly sensitive to increased cognitive load which, in their study, was elicited by phonologic/orthographic incongruence. However, another interpretation is possible. In that task, phonological information was arguably most important and orthographic information secondary. Tan and Perfetti (1999) point out that whether center-surround inhibition is called-up depends on the activation state of both sought-after codes and secondary codes. When secondary codes potentially compete with sought-after codes, center-surround inhibition should suppress the former. Slower rhyme judgments for AWS on orthographically-similar yet non-rhyming words suggest that orthographic codes reached a fairly high state of activation but were not suppressed.
Inability to direct attention toward sought-after codes is consistent with other evidence that AWS have limited attentional regulation (Arends, Povel and Kolk, 1988; also see Heitmann, Asbjornsen, and Helland, 2004). However, it is still possible that AWS directed attention toward target label phonemes in our task; specifically, by withdrawing attentional resources from semantic processing. Other support for this idea can be found in the research of Bosshardt (2006), who showed that AWS reduce the frequency of stuttering during sentence production under dual-task demands by reducing the amount of “conceptual work” they do. As noted in the Introduction, reverse Semantic N400 priming was observed in AWS by (Maxfield et al., 2010), which might be indicative of semantic center-surround inhibition. In that experiment, attention to probe words was not required, as in the current task, which might have freed-up attentional resources for stabilizing activation of target label semantic representations (and/or for coding target label semantic representations into a potentially impaired working memory, as cited previously) via inhibition of semantically-related neighbors. Allocation of attentional resources away from semantic processing could explain how attentional resources were available for phonological center-surround inhibition in AWS in the current task, and why a reverse Semantic N400 priming effect was not seen here for AWS. Thus, the Weber-Fox et al. (2004) study does not necessarily derail a center-surround interpretation of reverse Phonological N400 generated by our AWS. Still, we considered whether other interpretations might account for this effect.
Atypical Effects in AWS as Reflecting Phonological Label-Probe Competition
Another interpretation is that the reverse Phonological N400 priming effect generated by AWS in our task reflects label-probe competition. This interpretation is based loosely on the results of a recent study by (Desroches et al., 2008), in which TFA saw a picture on each trial followed by an auditory probe word that was identical to the picture label (e.g., CONE-cone); rhymed (e.g., CONE-bone); shared initial consonant onset and subsequent vowel, called cohort mismatch (e.g., CONE-comb); or mismatched completely (e.g., CONE-fox). Participants judged whether label and probe were the same. Cohort mismatch elicited N400 activity larger in amplitude than identical match, an effect that was larger than the N400 effect seen for complete mismatches. The authors suggested that a lexical interpretation created for each picture (e.g., CONE) was initially confirmed (and, thus, strengthened) by the first couple of phonemes in each cohort mismatch probe word (which overlapped with the target label, e.g., comb) until a later, non-overlapping phoneme in the cohort mismatch probe was perceived. Discarding the original interpretation and activating its competitor heightened processing, increasing N400 activity.
In contrast to the task used in (Desroches et al., 2008), we did not require label-probe comparisons. Nor did we include an identity priming condition, which might have encouraged such comparisons. Still, AWS in our task may have calibrated the lexical interpretation created for each picture against probe word phonological information; perhaps in order to manage task demands. Delayed picture naming in conjunction with probe word verification were, arguably, particularly demanding on phonological processing for AWS who, as cited previously, may have working memory limitations. In order to maximize naming as well as probe verification accuracy, the AWS may have strategically monitored for, or at least maintained heightened sensitivity to, phonological similarity between target labels and probe words. The reverse Phonological N400 effect produced by AWS appeared relatively early - ~80 ms earlier than the typical Phonological N400 priming effect produced by TFA in our task - consistent with the idea that our AWS were sensitive to initial, overlapping phonemes in phonologically-related label-probe pairs. Anticipating phonological similarity, and/or recognizing it quickly on phonologically-related trials, may have helped AWS recruit attentional resources in order to maintain a target label for delayed naming while coding into working memory a phonologically-related probe word. Processing activity likely heightened as AWS recognized and disambiguated target labels from phonologically-related probes, increasing N400 activity for Phonologically-Related versus P-Unrelated probes. As noted previously, the AWS may have allocated attentional resources away from semantic processing in order to maximize phonological processing in the current task.
It is important to mention that the current and previous interpretations are not necessarily mutually exclusive. That is, AWS may have deployed phonological center-surround inhibition in order to stabilize activation of target label phonemes - as discussed previously - while having heightened sensitivity to phonological similarity between picture labels and probe words in order to meet task demands. According to this view, reverse Phonological N400 priming may reflect both difficulty activating phonological neighbors of target labels (which may have been inhibited by a center-surround mechanism before appearing as phonologically-related probes), as well as label-probe competition (as phonologically-related probes were activated while target labels were held in-mind for delayed naming). Under this assumption, the unique contributions of each process may be difficult to disentangle using the current task design.
Current Results versus Other N400 Effects in AWS
An important question is whether our results align with other research demonstrating atypical N400-like priming effects in AWS? Weber-Fox and colleagues have reported that AWS exhibit atypical ERP activity during sentence processing (Cuadrado and Weber-Fox, 2003), including atypical N400 activations (Weber-Fox, 2001; Weber-Fox and Hampton, 2008). It is important to acknowledge that activation spreading mechanisms driving N400 priming effects in the picture-word tasks we have adopted may be different from mechanisms that drive N400 effects elicited during sentence processing. As Van Petten (1995) points out, “One view is that lexical context may exert at least some of its influence through a fast and automatic mechanism such as spreading activation within the mental lexicon, whereas sentential context acts via a slower, more strategic mechanism that is part of an entirely different ‘level’ of the language processing system” (p. 520). Based on this view, the atypical N400 effects reported by Weber-Fox and colleagues for AWS during sentence processing may reflect a different, albeit still crucially important, level of deficit. However, it may be difficult to compare their results with ours.
Summary and Conclusions
The current results suggest that linguistic representations associated with target picture labels are processed atypically in AWS on the path to delayed picture naming. While TFA exhibited Semantic and Phonological priming indicative of typical semantic and phonological activation spreading, AWS exhibited diminished Semantic priming and reverse Phonological N400 priming. Unstable activation of target label semantic representations may account for diminished Semantic priming. Unstable semantic representations, in turn, may result in target label phonological representations activating unstably in AWS. The reverse Phonological N400 priming effect seen for AWS may have reflected a center-surround inhibition mechanism called-up by the attentional system to stabilize activation of target label phonemes, to which access was required for successful naming. Alternatively, or additionally, reverse Phonological N400 priming may have reflected a competition effect, as the AWS activated and disambiguated phonologically-related probes from target labels held in working memory for delayed naming.
The interpretations considered here are tentative, and based on processes known to generate reverse N400 priming effects in TFA. Other interpretations may certainly be possible. For example, one question is whether preparatory activity (in anticipation of delayed naming) drove some of the ERP variance seen in our data set; particularly in AWS, who may experience anxiety in anticipation of speech acts (see Alm, 2004)? An ERP index of preparatory processing, the centrally-maximal segment of the contingent negative variation (see Gaillard and Beijsterveldt, 1991), was not uniquely modulated between conditions in AWS, or between groups. Still, a question for future research is whether the linguistic and cognitive systems of AWS behave similarly in tasks that limit preparatory processing, i.e., do not require delayed naming, or attention to auditory probe words verified at a delay?
Also needed are investigations of whether the proposed deficits affect other aspects of psycholinguistic and/or speech motor performance in AWS. Unknown, too, is whether the atypical processing that we are seeing in AWS is present in childhood. Stuttering is a disorder of childhood, and a body of evidence reveals that cognitive and linguistic performance can differ in children who stutter versus their non-stuttering peers (recent examples include Anderson and Wagovich, 2010; Coulter et al., 2009; Ratner et al., 2009). However, as with AWS, there is limited evidence on how children who stutter process linguistic representations in real-time as they prepare to speak. Understanding more precisely how people who stutter process lexical knowledge on-route to speaking may ultimately expand and improve the scope and efficacy of diagnostic and intervention tools for stuttering.
Supplementary Material
Highlights.
Little is known about how linguistic representations are processed in adults who stutter (AWS) preparing to speak.
Event-related potentials reveal atypical picture-word priming effects in AWS.
Results suggest that target label semantic and phonological representations are processed atypically on the path to delayed picture naming in AWS.
Acknowledgments
This research was supported by a University of South Florida New Investigator Grant awarded to the first author. The first author was also supported by a grant from the National Institutes of Health - National Institute of Deafness and other Communication Disorders (NIH-NIDCD, 1R03DC011144-01) during preparation of this manuscript.
Footnotes
The ERP data related to the Phonological aspect of the task (Phonologically-Related versus P-Unrelated) were analyzed separately using this same procedure.
Although our grand average waveforms did not point very strongly to putative Phonological priming effects in either group prior to ~400 ms, we did investigate the variance associated with T260, T296, and T358, respectively; so as to be able to compare our findings fully with those of Jescheniak et al. (2002), who found Phonological priming effects in their TFA as early as ~250 ms. We found no evidence of Phonological picture-word N400 priming in the T260, T296, or T358 variance.
Although phonological priming was not detected for the T800 factor, we checked for atypical CNV activity in AWS in the T800 variance, as we did with the T760 factor in our analysis of Semantic picture-word priming effects. No evidence of atypical CNV activity was found for the AWS within the T800 window.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alm P. Stuttering, emotions and heart rate during anticipatory anxiety: A critical review. J Fluency Disord. 2004;29:123–133. doi: 10.1016/j.jfludis.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Anderson JD, Wagovich SA. Relationships among linguistic processing speed, phonological working memory, and attention in children who stutter. J Fluency Disord. 2010;35:216–234. doi: 10.1016/j.jfludis.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arends N, Povel DJ, Kolk H. Stuttering as an attentional control phenomenon. J Fluency Disord. 1988;13:141–151. [Google Scholar]
- Bajaj A. Working memory involvement in stuttering: Exploring the evidence and research implications. J Fluency Disorder. 2007;32:218–238. doi: 10.1016/j.jfludis.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Bell AJ, Sejnowski TJ. An information maximization approach to blind separation and blind deconvolution. Neural Comput. 1995;7:1129–1159. doi: 10.1162/neco.1995.7.6.1129. [DOI] [PubMed] [Google Scholar]
- Berg T, Schade U. The role of inhibition in a spreading-activation model of language production, I. The psycholinguistic perspective. J Psycholinguist Res. 1992;21:405–434. [Google Scholar]
- Bermeitinger C, Frings C, Wentura D. Reversing the N400: Event-related potentials of negative semantic priming effect. Neuroreport. 2008;19:1479–1482. doi: 10.1097/WNR.0b013e32830f4b0b. [DOI] [PubMed] [Google Scholar]
- Bierwisch M, Schreuder R. From concepts to lexical items. Cognition. 1992;42:23–60. doi: 10.1016/0010-0277(92)90039-k. [DOI] [PubMed] [Google Scholar]
- Bloodstein O, Ratner NB. A handbook on stuttering. 6. Clifton Park, NY: Delmar; 2008. [Google Scholar]
- Bosshardt HG. Cognitive processing load as a determinant of stuttering: Summary of a research programme. Clin Linguist Phon. 2006;20:371–385. doi: 10.1080/02699200500074321. [DOI] [PubMed] [Google Scholar]
- Bosshardt G, Fransen HJM. On-line sentence processing in adults who stutter and who do not stutter. J Speech Lang Hear Res. 1996;3:785–797. doi: 10.1044/jshr.3904.785. [DOI] [PubMed] [Google Scholar]
- Bosshardt HG, Nandyal I. Reading rates of stutterers and nonstutterers during silent and oral reading. J Fluency Disord. 1988;14:403–420. [Google Scholar]
- Bothe AK, Davidow JH, Bramlett RE, Ingham RJ. Stuttering treatment research 1970–2005: I. Systematic review incorporating trial quality assessment of behavioral, cognitive, and related approaches. Am J Speech Lang Pathol. 2006;15:321–341. doi: 10.1044/1058-0360(2006/031). [DOI] [PubMed] [Google Scholar]
- Brocklehurst PH, Corley M. Investigating the inner speech of people who stutter: Evidence for (and against) the Covert Repair Hypothesis. J Commun Disord. 2011;44:246–260. doi: 10.1016/j.jcomdis.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Burger R, Wijnen F. Phonological encoding and word stress in stuttering and nonstuttering subjects. J Fluency Disord. 1999;24:91–106. [Google Scholar]
- Cahill L, Prins B, Weber M, McGaugh JL. b adrenergic activation and memory for emotional events. Nature. 1994;371:702–704. doi: 10.1038/371702a0. [DOI] [PubMed] [Google Scholar]
- Carr TH, Dagenbach D. Semantic priming and repetition priming from masked words: evidence for a center-surround attentional mechanism in perceptual recognition. J Exp Psychol Learn Mem Cogn. 1990;16:341–350. [PubMed] [Google Scholar]
- Coulter CE, Anderson JD, Conture EG. Childhood stuttering and dissociations across linguistic domains: A replication and extension. J Fluency Disord. 2009;34:257–278. doi: 10.1016/j.jfludis.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe KM, Kroll RM. Response latency and response class for stutterers and nonstutterers as measured by a word-association task. J Fluency Disord. 1991;16:35–54. [Google Scholar]
- Cuadrado E, Weber-Fox C. Atypical syntactic processing in individuals who stutter: Evidence from event-related brain potentials and behavioral measures. J Speech Lang Hear Res. 2003;46:960–976. doi: 10.1044/1092-4388(2003/075). [DOI] [PubMed] [Google Scholar]
- Deacon D, Hewitt S, Yang CM, Nagata M. Event-related potential indices of semantic priming using masked and unmasked words: Evidence that the N400 does not reflect a post-lexical process. Brain Res Cogn Brain Res. 2000;9:137–146. doi: 10.1016/s0926-6410(99)00050-6. [DOI] [PubMed] [Google Scholar]
- Dell GS. Positive feedback in hierarchical connectionist models: Applications to language production. Cogn Sci. 1985;9:3–23. [Google Scholar]
- Dell GS. A spreading-activation theory of retrieval in sentence production. Psychol Rev. 1986;93:283–321. [PubMed] [Google Scholar]
- Dell GS. Effects of frequency and vocabulary type on phonological speech errors. Lang Cogn Process. 1990;5:313–349. [Google Scholar]
- Desroches AS, Newman RL, Joanisse MF. Investigating the time course of spoken word recognition: Electrophysiological evidence for the influence of phonological similarity. J Cogn Neurosci. 2009;21:1893–1906. doi: 10.1162/jocn.2008.21142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dien J. The ERP PCA Toolkit: An open source program for advanced statistical analysis of event-related potential data. J Neurosci Methods. 2010;157:138–145. doi: 10.1016/j.jneumeth.2009.12.009. (available from http://homepage.mac.com/jdien07/) [DOI] [PubMed] [Google Scholar]
- Dien J, Frishkoff GA. Principal components analysis of event-related potential datasets. In: Handy TC, editor. Event-Related Potentials: A Methods Handbook. Cambridge: MIT Press; 2004. pp. 189–208. [Google Scholar]
- Dien J, Santuzzi AM. Application of repeated measures ANOVA to high-density ERP datasets: A review and tutorial. In: Handy TC, editor. Event Related Potentials: A Methods Handbook. Cambridge: MIT Press; 2004. pp. 57–82. [Google Scholar]
- Eldridge KA, Felsenfeld S. Differentiating mild and recovered stutterers from nonstutterers. J Fluency Disord. 1998;23:173–195. [Google Scholar]
- Ferree TC, Luu P, Russell GS, Tucker DM. Scalp electrode impedance, infection risk, and EEG data quality. Clin Neurophysiol. 2001;112:536–544. doi: 10.1016/s1388-2457(00)00533-2. [DOI] [PubMed] [Google Scholar]
- Foti D, Hajcak G, Dien J. Differentiating neural responses to emotional pictures: Evidence from temporal-spatial PCA. Psychophysiology. 2009;46:521–530. doi: 10.1111/j.1469-8986.2009.00796.x. [DOI] [PubMed] [Google Scholar]
- Glaser WR. Picture naming. Cognition. 1992;42:61–105. doi: 10.1016/0010-0277(92)90040-o. [DOI] [PubMed] [Google Scholar]
- Glass K, Frishkoff GA, Frank RM, Davey C, Dien J, Maloney AD. A framework for evaluating ICA methods of artifact removal from multichannel EEG. Lect Notes Comput Sci. 2004;3195:1033–1040. [Google Scholar]
- Greenhouse SW, Geisser S. On methods in the analysis of profile data. Psychometrika. 1959;24:95–112. [Google Scholar]
- Heitmann R, Asbjørnsen A, Helland T. Attentional functions in speech fluency disorders. Logoped Phoniatr Vocol. 2004;29:119–27. doi: 10.1080/14015430410017379. [DOI] [PubMed] [Google Scholar]
- Hendrickson AE, White PO. Promax: A quick method for rotation to oblique simple structure. Brit J Statist Psych. 1964;17:65–70. [Google Scholar]
- Hennessey N, Nang C, Beilby J. Speeded verbal responding in adults who stutter: Are there deficits in linguistic encoding? J Fluency Disord. 2008;33:180–202. doi: 10.1016/j.jfludis.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Holcomb PJ, Kounios J, Anderson JE, West WC. Dual-coding, context-availability, and concreteness effects in sentence comprehension: An electrophysiological investigation. J Exp Psychol Learn Mem Cogn. 1999;25:721–742. doi: 10.1037//0278-7393.25.3.721. [DOI] [PubMed] [Google Scholar]
- Hubbard C, Prins D. Word familiarity, syllabic stress pattern, and stuttering. J Speech Lang Hear Res. 1994;37:564–571. doi: 10.1044/jshr.3703.564. [DOI] [PubMed] [Google Scholar]
- Jensen PJ, Markel NN, Beverung JW. Evidence of conversational disrhythmia in stutterers. J Fluency Disord. 1986;11:183–200. [Google Scholar]
- Jescheniak JD, Schriefers H, Garrett MF, Friederici AD. Exploring the activation of semantic and phonological codes during speech planning with event-related brain potentials. J Cogn Neurosci. 2002;14:951–964. doi: 10.1162/089892902760191162. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE. Optimizing PCA methodology for ERP component identification and measurement: Theoretical rationale and empirical evaluation. Clin Neurophysiol. 2003;114:2307–2325. doi: 10.1016/s1388-2457(03)00241-4. [DOI] [PubMed] [Google Scholar]
- Klem GH, Luders HO, Jasper HH, Elger C. The ten-twenty electrode system of the International Federation. Electroencephalogr Clin Neurophysiol. 1999;(Suppl 52):3–6. [PubMed] [Google Scholar]
- Kounios J, Holcomb PJ. Concreteness effects in semantic processing: ERP evidence supporting dual-coding theory. J Exp Psychol Learn Mem Cogn. 1994;20:804–823. doi: 10.1037//0278-7393.20.4.804. [DOI] [PubMed] [Google Scholar]
- Lee CL, Federmeier KD. To watch, to see, and to differ: An event-related potential study of concreteness effects as a function of word class and lexical ambiguity. Brain Lang. 2008;104:145–158. doi: 10.1016/j.bandl.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levelt WJM, Roelofs A, Meyer AS. A theory of lexical access in speech production. Behav Brain Sci. 1999;22:1–75. doi: 10.1017/s0140525x99001776. [DOI] [PubMed] [Google Scholar]
- MacKay DG, MacDonald M. Stuttering as a sequencing and timing disorder. In: Perkins WH, Curlee R, editors. Nature and treatment of stuttering: New directions. San Diego: College Hill Press; 1984. pp. 261–282. [Google Scholar]
- Marí-Beffa P, Valdés B, Cullen D, Catena A, Houghton G. ERP analyses of task effects on semantic processing from words. Brain Res Cogn Brain Res. 2005;23:293–305. doi: 10.1016/j.cogbrainres.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Maxfield N, Huffman J, Frisch S, Hinckley J. Neural correlates of semantic activation spreading on the path to picture naming in adults who stutter. Clin Neurophysiol. 2010;121:1447–1463. doi: 10.1016/j.clinph.2010.03.026. [DOI] [PubMed] [Google Scholar]
- Neil-Dwyer G, Bartlett J, McAinsh J, Cruickshank JM. b-Adrenoreceptor and the blood–brain barrier. Br J Clin Pharmacol. 1981;11:549–553. doi: 10.1111/j.1365-2125.1981.tb01169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DL, McEvoy CL, Schreiber TA. The University of South Florida word association, rhyme, and word fragment norms. 1988 doi: 10.3758/bf03195588. Retrieved from: http://www.usf.edu/FreeAssociation/ [DOI] [PubMed]
- Newman R, Ratner N. The role of selected lexical factors on confrontation naming accuracy, speed, and fluency in adults who do and do not stutter. J Speech Lang Hear Res. 2007;50:196–213. doi: 10.1044/1092-4388(2007/016). [DOI] [PubMed] [Google Scholar]
- Nunez PL, Srinvasan R. Electrical fields of the brain: The neurophysics of EEG (Appendices J1–J3) New York: Oxford University Press; 2006. [Google Scholar]
- Nusbaum HC, Pisoni DB, Davis CK. Research on Speech Perception Progress Report. Vol. 10. Speech Research Laboratory; Bloomington, IN: 1984. Sizing up the Hoosier Mental Lexicon: Measuring the familiarity of 20,000 words; pp. 357–376. [Google Scholar]
- Perkins WH, Kent RD, Curlee RF. A theory of neuropsycholinguistic function in stuttering. J Speech Lang Hear Res. 1991;34:734–752. doi: 10.1044/jshr.3404.734. [DOI] [PubMed] [Google Scholar]
- Picton TW, Bentin S, Berg P, Donchin E, Hillyard SA, Johnson R, Jr, et al. Committee report: Guidelines for using human event-related potentials to study cognition: Recording standards and publication criteria. Psychophysiology. 2000;37:127–152. [PubMed] [Google Scholar]
- Postma A, Kolk H. The covert repair hypothesis: Prearticulatory repair processes in normal and stuttered disfluencies. J Speech Lang Hear Res. 1993;36:472–487. [PubMed] [Google Scholar]
- Postma A, Kolk H, Povel DJ. Speech planning and execution in stutterers. J Fluency Disord. 1990;15:49–59. [Google Scholar]
- Preisendorfer RW, Mobley CD. Principal component analysis in meteorology and oceanography. New York: Elsevier; 1988. [Google Scholar]
- Prins D, Main V, Wampler S. Lexicalization in adults who stutter. J Speech Lang Hear Res. 1997;40:373–384. doi: 10.1044/jslhr.4002.373. [DOI] [PubMed] [Google Scholar]
- Ratner NB, Newman R, Strekas A. Effects of word frequency and phonological neighborhood characteristics on confrontation naming in children who stutter and normally fluent peers. J Fluency Disord. 2009;34:225–241. doi: 10.1016/j.jfludis.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Richman M. Rotation of principal components. J Clim. 1986;6:293–335. [Google Scholar]
- Roelofs A. A spreading-activation theory of lemma retrieval in speaking. Cognition. 1992;42:107–142. doi: 10.1016/0010-0277(92)90041-f. [DOI] [PubMed] [Google Scholar]
- Roelofs A. A case for nondecomposition in conceptually driven word retrieval. J Psycholinguist Res. 1997;36:33–67. [Google Scholar]
- Rosler F, Hahne A. Hirnelektrische correlate des sprachverstehens zur psycholinguistischen bedeutung der N400-komponente im EEG. Z Psychol Z Angew Psychol. 1992;11:140–161. [Google Scholar]
- Sasisekaran J, De Nil L, Smyth R, Johnson C. Phonological encoding in the silent speech of persons who stutter. J Fluency Disord. 2006;31:1–21. doi: 10.1016/j.jfludis.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Schwanenflugel PJ, Akin C, Luh WM. Context availability and the recall of abstract and concrete words. Mem Cognit. 1992;20:96–104. doi: 10.3758/bf03208259. [DOI] [PubMed] [Google Scholar]
- Szekely A, Jacobsen T, D’Amico S, Devescovi A, Andonova E, Herron D, et al. A new on-line resource for psycholinguistic studies. J Mem Lang. 2004;51:247–250. doi: 10.1016/j.jml.2004.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan LH, Perfetti CA. Phonological and associative inhibition in the early stages of English word identification: Evidence from backward masking. J Exp Psychol Hum Percept Perform. 1999;25:382–393. [Google Scholar]
- Tataryn DJ, Wood JM, Gorsuch RL. Setting the value of k in promax: A Monte Carlo study. Educ Psychol Meas. 1999;59:384–391. [Google Scholar]
- Taylor WL, Lore JI, Waldman IN. Latencies of semantic aphasics, stutterers, and normal controls to cloze items requiring unique and non-unique oral responses. Proc Annu Conv Am Psychol Assoc. 1970;78:75–76. [Google Scholar]
- Van Boxtel GJM. Computational and statistical methods for analyzing event-related potential data. Behav Res Meth Ins C. 1998;30:87–102. [Google Scholar]
- Van Lieshout PHHM, Hulstijn W, Peters HFM. Speech production in people who stutter: Testing the motor plan assembly hypothesis. J Speech Lang Hear Res. 1996;39:76–92. doi: 10.1044/jshr.3901.76. [DOI] [PubMed] [Google Scholar]
- Van Petten C. Words and sentences: Event-related brain potential measures. Psychophysiology. 1995;32:511–525. doi: 10.1111/j.1469-8986.1995.tb01228.x. [DOI] [PubMed] [Google Scholar]
- Van Petten C, Kutas M. Electrophysiological evidence for the flexibility of lexical processing. In: Simpson GB, editor. Understanding word and sentence. Amsterdam: Elsevier; 1991. pp. 129–174. [Google Scholar]
- Voss JL, Federmeier KD. FN400 potentials are functionally identical to N400 potentials and reflect semantic processing during recognition testing. Psychophysiology. 2011;48:532–546. doi: 10.1111/j.1469-8986.2010.01085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wastell DG. On the correlated nature of evoked brain activity: Biophysical and statistical considerations. Biol Psychol. 1981;13:51–69. doi: 10.1016/0301-0511(81)90027-2. [DOI] [PubMed] [Google Scholar]
- Watson B, Freeman F, Devous M, Chapman S, Finitzo T, Pool K. Linguistic performance and regional cerebral blood flow in persons who stutter. J Speech Lang Hear Res. 1994;37:1221–1228. doi: 10.1044/jshr.3706.1221. [DOI] [PubMed] [Google Scholar]
- Weber-Fox C. Neural systems for sentence processing in stuttering. J Speech Lang Hear Res. 2001;44:814–82. doi: 10.1044/1092-4388(2001/064). [DOI] [PubMed] [Google Scholar]
- Weber- Fox C, Hampton A. Stuttering and natural speech processing of semantic and syntactic constraints on verbs. J Speech Lang Hear Res. 2008;51:1058–1071. doi: 10.1044/1092-4388(2008/07-0164). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber-Fox C, Spencer RMC, Spruill JE, Smith A. Phonological processing in adults who stutter: Electrophysiological and behavioral evidence. J Speech Lang Hear Res. 2004;47:1244–1258. doi: 10.1044/1092-4388(2004/094). [DOI] [PubMed] [Google Scholar]
- West WC, Holcomb PJ. Imaginal, semantic, and surface-level processing of concrete and abstract words: An electrophysiological investigation. J Cogn Neurosci. 2000;12:1024–1037. doi: 10.1162/08989290051137558. [DOI] [PubMed] [Google Scholar]
- Wingate ME. The structure of stuttering: A psycholinguistic approach. New York: Springer-Verlag; 1988. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.