Summary
During mitosis equal segregation of chromosomes depends on proper kinetochore–microtubule attachments. Merotelic kinetochore orientation, in which a single kinetochore binds microtubules from both spindle poles [1], is a major cause of chromosome instability [2], which is commonly observed in solid tumors [3, 4]. Using the fission yeast Schizosaccharomyces pombe we show that a proper force balance between kinesin motors on interpolar spindle microtubules is critical for correcting merotelic attachments. Inhibition of the plus end directed spindle elongation motors kinesin-5 (Cut7) and kinesin-6 (Klp9) reduces spindle length, tension at kinetochores, and the frequency of merotelic attachments. In contrast, merotely is increased by deletion of the minus end directed kinesin-14 (Klp2) or overexpression of Klp9. Also, Cdk1 regulates spindle elongation forces to promote merotelic correction by phosphorylating and inhibiting Klp9. The role of spindle elongation motors in merotelic correction is conserved since partial inhibition of the human kinesin-5 homolog Eg5 using the drug monastrol reduces spindle length and lagging chromosome frequency in both normal (RPE-1) and tumor cells (CaCo-2). These findings reveal unexpected links between spindle forces and correction of merotelic attachments and show that pharmacological manipulation of spindle elongation forces might be used to reduce chromosome instability in cancer cells.
Results and Discussion
The role of aurora kinase in correction of merotelic attachments is conserved in S. pombe
Merotelic attachments have been characterized primarily in mammalian cells. However recent studies show that although wild-type S. pombe cells do not typically display merotelic attachments in anaphase, mutants defective in centromeric heterochromatin (swi6Δ) or the monopolin complex (mde4Δ or pcs1Δ) display merotelic attachments, which if not corrected lead to lagging chromosomes in anaphase and potentially unevenly segregated chromosomes in telophase ([5] [6] and Figure 1A). Monopolin mutants frequently display unevenly segregated chromosomes in telophase [7], as well as lagging chromosomes in anaphase. To determine whether lagging chromosomes lead to the uneven segregation phenotype, we analyzed chromosome segregation in mde4Δ mutants expressing histone-mRFP using time-lapse analysis. We observed that 46% (30/65) of mde4Δ cells that display lagging chromosomes in anaphase show uneven chromosome segregation in telophase, indicating that some but not all lagging chromosomes lead to segregation errors consistent with other studies [8–10]. However, because all cases (n=30) of uneven chromosome segregation appeared to be the result of earlier improper segregation of anaphase lagging chromosomes (for example see Movie S1), we combined the two classes (lagging (anaphase) and unevenly segregated (telophase) chromosomes) for subsequent analysis of fixed mde4Δ and swi6Δ cells.
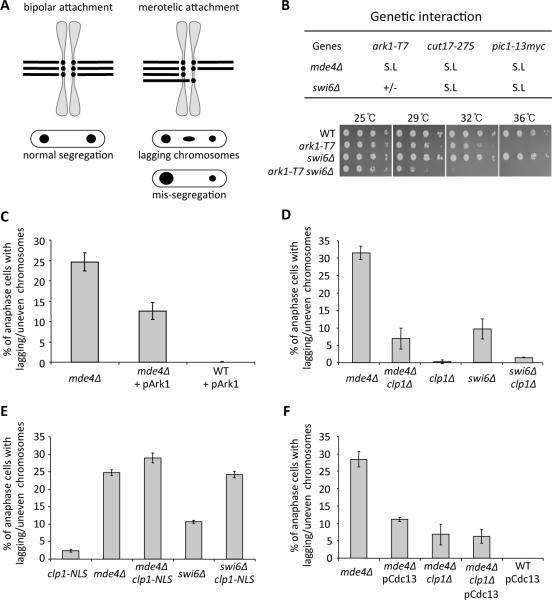
Figure 1. Aurora and Cdk1 kinases affect correction of merotelic attachments.
(A) The diagram depicts both normal (bipolar) and aberrant (merotelic) attachment of sister kinetochores to microtubules in metaphase (top) and the resulting segregation pattern of each type of attachment in anaphase (bottom). (B) Genetic interactions between mde4Δ, swi6Δ and mutations in the chromosome passenger complex are shown (top). The mde4Δ and swi6Δ mutants were crossed with the chromosome passenger complex mutants, Aurora kinase (ark1-T7), Survivin (cut17-275) and INCENP (pic1-13myc). Genetic interactions are shown as synthetic lethality (S.L) and weak growth (+/−). (bottom) swi6Δ ark1-T7 double mutant cells show a synthetic growth defect compared to either single mutant. The indicated strains were grown in YE at 25°C, and then serial dilutions were spotted on YE plates and incubated at the indicated temperatures for 3–5 days. (C) The frequency of lagging or unevenly segregated chromosomes was scored for asynchronously growing anaphase and telophase mde4Δ and wild-type cells that mildly express Ark1-GFP under leaky expression of the nmt1 promoter in the presence of thiamine. Bars represent the mean +/− SD (n=3; 100 cells each). (D–F)The frequency of lagging or mis-segregated chromosomes was determined for the following asynchronously growing cells: (D) mde4Δ and swi6Δ mutants in the presence or absence of clp1, (E) mde4Δ and swi6Δ mutants with and without low levels of clp1-NLS expression (i.e., leaky expression from the nmt1 promoter in the presence of thiamine), and (F) mde4Δ, mde4Δ clp1Δ and wild-type cells that mildly overexpress cyclin B (pCdc13) using a genomic clone on a multicopy plasmid. Cells were fixed with methanol and DNA was stained with DAPI. Bars represent the mean +/− SD (n=3; 100 cells each). See also Figure S1 and Movie S1.
We next determined whether mde4Δ and swi6Δ mutants could serve as useful models for characterizing merotelic correction mechanisms relevant to mammalian cells. In particular, because the kinase Aurora B is important for correction of merotelic attachments in mammalian cells [11, 12], we tested if this was also true for the S. pombe aurora kinase homolog Ark1. Indeed, double mutants that combined the temperature sensitive ark1–T7 mutation or the aurora associated chromosomal passenger complex proteins INCENP (pic1–13Myc) and survivin (cut17–275) with the mde4Δ or swi6Δ mutations showed strong synergistic phenotypes and increased lagging and unevenly segregated chromsomes (Figure 1B; Figure S1A). Conversely, overexpression of Ark1 reduced the frequency of lagging chromosomes in mde4Δ mutant cells (Figure 1C). Together these results show that the role of aurora kinase in correction of merotelic attachments is conserved in S. pombe and mammalian cells.
Phosphorylation of Cdk1 substrates promotes correction of merotelic attachments
In the course of making double mutants between mde4Δ and various genes involved in chromosome segregation, we discovered that deletion of the Cdc14-family phosphatase gene clp1 significantly reduced the lagging chromosome frequency in both monopolin (mde4Δ; P<0.01) and heterochromatin (swi6Δ; P<0.01) mutants (Figure 1D; Figures S1B and S1C). Conversely, increasing Clp1 activity in the nucleus using the clp1-NLS allele [13] caused an increase in the lagging chromosome frequency (Figure 1E), showing that Clp1 antagonizes correction of merotelic attachments. Because Clp1 and other Cdc14-family members oppose cyclin dependent kinase (Cdk1) by preferentially dephosphorylating sites phosphorylated by Cdk1, these results suggested that increased phosphorylation of some Cdk1 substrate(s) promotes correction of merotelic attachments. This hypothesis is supported by the observation that mild overexpression of cyclin B (Cdc13), which increases Cdk1 activity [7, 14], also causes a reduction in lagging chromosome frequency in mde4Δ mutant cells (Figure 1F).
Deletion of Clp1 corrects merotelic attachments by reducing spindle elongation forces
We investigated two possible mechanisms for how deletion of clp1 reduced the lagging chromosome frequency in mde4Δ and swi6Δ mutants. Deletion of clp1 could prolong pro/metaphase to allow more time for correction, analogous to previous findings in mammalian cells [15]. Alternatively deletion of clp1 could cause changes in spindle architecture and/or affect tension at kinetochores, which is critical for generating normal bipolar attachments [16]. As judged by time-lapse analysis, the clp1Δ mutation did not significantly change metaphase length in mde4Δ cells (Figure S1D; P=0.22). Similarly, synchronous cell experiments showed that the time from mitotic entry to anaphase onset was the same for mde4Δ and mde4Δ clp1Δ cells (Figure S1E). However, the time-lapse analysis did reveal clp1 dependent changes in spindle architecture. Namely, whereas mde4Δ mutant cells display an increase in metaphase spindle length [7], we found that deletion of clp1 in mde4Δ cells reduced this length to that of wild type (Figure S2A). (Note that although other mutants with defects in attachment of kinetochores to microtubules also show increased metaphase spindle length [17], the reason for the increase is unclear but may reflect reduced inward forces opposing spindle elongation.) As a separate method to accurately measure spindle length at the precise time of anaphase onset, we developed a method using asynchronous cells in which we simultaneously scored spindle length plus the presence or absence of the pre-anaphase marker Cut2 (securin) (see Experimental Procedures and Figures S2B, S2C, and S2D). Similar to our results using time-lapse analysis, this method also showed that deletion of clp1 significantly reduced the metaphase spindle length in mde4Δ (p<0.05), swi6Δ (p<0.05) cells (Figure 2A). Deletion of clp1 also reduced the spindle length in wild-type cells but the reduction did not quite reach the level of significance (p=0.072). Furthermore, expression of Clp1-NLS increased the length of the metaphase spindle (Figure 2B) in accord with its increase in the lagging chromosome frequency described above (Figure 1E). In summary, the genetic manipulations that reduce merotely also reduce spindle length, and vice versa.
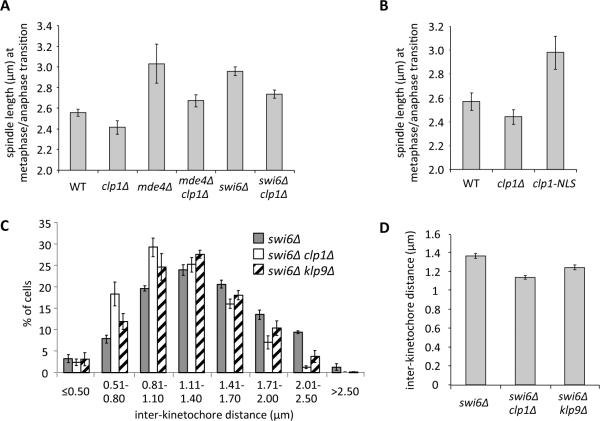
Figure 2. Lack of Clp1 corrects merotelic attachments by reducing spindle elongation forces.
(A–B) The spindle length at the metaphase/anaphase transition was determined by ROC analysis (see Experimental Procedures for details) and compared between the indicated strains. Bars represent the mean +/− SD (n=3; 100 cells each). The differences between all pairwise combinations in each graph were statistically significant by unpaired t test (p<0.05), except for between WT and clp1Δ in (B) (p=0.072). (C) Strains expressing the SPB marker Cdc11-GFP and carrying GFP-marked centromere 2 (Cen2-GFP) were arrested at metaphase by overexpressing Mad2 from the thiamine repressible nmt1 promoter for 17h and fixed. The distance between Cen2-GFP dots was measured. Bars represent the mean +/− SD (n=3; >280 cells each). (D) A comparison of average inter-kinetochore distances obtained from panel (C). The values for the swi6Δ clp1Δ and swi6Δ klp9Δ showed statistically very significant differences compared to the swi6Δ parent by an unpaired t test (p<0.0001). The bars represent the mean +/− the standard error of the mean (n=3; >280 cells each). See also Figure S2.
We hypothesized that the reduction in spindle length in clp1Δ cells could reflect decreased spindle elongation forces, which would in turn result in reduced tension on kinetochores. The reduction in kinetochore tension could then promote merotelic correction by destabilizing kinetochore microtubule attachments through aurora dependent [16] or independent mechanisms [18]. Therefore we tested whether the decrease in metaphase spindle length observed in clp1Δ mutants affects the tension on sister kinetochores at metaphase. To assess this, we measured inter-kinetochore distance in swi6Δ (Figures 2C and 2D) or wild-type cells (Figures S2E and S2F) using a GFP-marked centromere in fixed cells arrested at metaphase. Overall the clp1Δ mutation shifted the distribution towards a shorter separation between sister kinetochores (Figure 2C; Figure S2E) and significantly reduced average inter-kinetochore distance (Figure 2D (p<0.0001); Figure S2F (p<0.0001)). These data suggest that reduction in metaphase spindle length caused by deletion of clp1 results in decreased inter-kinetochore tension.
Phospho-regulation of Klp9 by Cdk1 and Clp1 controls metaphase spindle length and merotelic correction
Although our results indicated that Clp1 (and Cdk1) likely regulates merotelic correction through effects on metaphase spindle length, the relevant target(s) was not known. Spindle length in metaphase is determined at least in part by a balance between sliding forces generated by plus end and minus end directed kinesin motors on overlapping interpolar microtubules, as well as resistance from sister chromatid cohesion [19]. In S. pombe, the kinesin-5 Cut7 (Eg5 in humans) and the kinesin-6 Klp9 drive outward sliding of interpolar microtubules [20, 21]. Although both kinesin-5 and kinesin-6 are regulated by Cdk1 in humans [22] [23], only the kinesin-6 Klp9 appears to be a Cdk1 target in S. pombe [20] [24]. Cdk1 phosphorylation of Klp9 inhibits its localization to the spindle in early mitosis, and Clp1-mediated dephosphorylation of Klp9 allows it to promote spindle elongation in anaphase [20], but it was not clear whether Klp9 functions in spindle length control in metaphase. We hypothesized that increased Cdk1 activity or reduced Clp1 activity in metaphase might cause a reduction in spindle length by inhibiting Klp9. Indeed, deletion of klp9 reduced the metaphase spindle length in wild type, mde4Δ, and swi6Δ cells (Figure 3A) and caused a reduction in kinetochore tension (Figures 2C and 2D; Figures S2E and S2F). In contrast, metaphase spindle length increased upon over expression of Klp9 and to a greater extent nonphosphorylatable Klp9 (Klp9 (S/A)), in which the Cdk1 consensus sites are mutated to alanines [20], (Figure 3B). In accord with the effects on spindle length, manipulation of Klp9 activity yielded corresponding effects on merotelic correction. The klp9Δ mutation reduced the lagging chromosome frequency in both mde4Δ and swi6Δ mutants (Figure 3C; Figures S1B and S1C). In contrast, overexpression of Klp9 increased the frequency of lagging chromosomes, and overexpression of Klp9 (S/A) showed an even greater increase (Figure 3D). Overexpression of Klp9 (S/A) caused lagging chromosomes even in wild type cells (Figure 3D) and most of those lagging chromosomes are single chromatids consistent with them being caused by merotelic attachments (Figure S3A). Collectively, these findings suggest that Cdk1-mediated inhibition of Klp9 activity during early mitosis is important to prevent merotelic attachments.
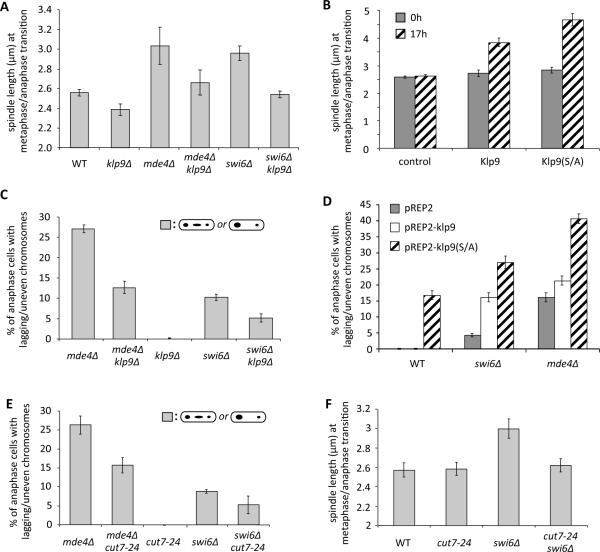
Figure 3. Correction of merotelic attachments can be modulated through manipulation of the activities of metaphase spindle elongation motors.
(A) Spindle lengths of the indicated strains at the metaphase/anaphase transition are shown (The differences between all pairwise combinations of the indicated strains were statistically significant by unpaired t test (p<0.05), except for between WT and mde4Δ klp9Δ or swi6Δ klp9Δ.). (B) Spindle lengths at the metaphase/anaphase transition was determined for wild-type cells before (0h) and after (17h) induction of expression of Klp9, Klp9 (S/A), or no protein (control) for 17h from the nmt1 promoter. Bars represent the mean +/− SD (n=3; 100 cells each). (C) The effect of klp9 on the frequency of lagging or mis-segregated chromosomes in the indicated strains is shown. (D) The effect of overexpression of Klp9 (pREP2-klp9), non-phosphorylatable Klp9 (pREP2-klp9(S/A)), or no protein (pREP2) for 17h using the nmt1 promoter in the indicated strains is shown. Bars represent the mean +/− SD (n=3; 110 cells each). (E) The frequency of lagging chromosomes in mde4Δ and swi6Δ mutants in the presence or absence of cut7-24 mutation is shown. (F) Spindle lengths at the metaphase/anaphase transition for the indicated strains were shown. Cells in parts E and F were grown at 25°C, the permissive temperature for the cut7-24 mutation. The differences between all pairwise combinations of strains were statistically significant by unpaired t test (p<0.05), except for between WT and cut7-24 (p=0.827), and WT and cut7-24 swi6Δ (p=0.435). See also Figure S3.
Merotelic correction can be controlled through manipulation of spindle elongation motors
We next tested whether other motors that localize to interpolar microtubules and regulate spindle elongation forces could also affect merotelic correction. The kinesin-5 motor Cut7 is required for spindle pole separation prior to anaphase [20, 21, 25]. Because Cut7 is essential for bipolar spindle assembly, we examined the cut7–24 temperature sensitive mutant at permissive temperature, and found that it decreased the lagging chromosome frequency in both mde4Δ and swi6Δ cells (Figure 3E). Furthermore, the cut7–24 mutation significantly (p<0.05) reduced spindle length in swi6Δ cells (Figure 3F) and the interkinetochore distance in metaphase arrested wild-type cells (Figure S3B). Cut7 is thought to be opposed in pre-anaphase by the minus end directed kinesin-14 motor Klp2, and deletion of klp2 causes a slight elongation of the metaphase spindle (Figure S3C, and [26]). Although deletion of klp2 on its own did not enhance the lagging chromosome frequency in mde4Δ mutants, deletion of klp2 did increase the lagging chromosome frequency of mde4Δ klp9Δ mutant cells (Figure S3D) showing that a balance of plus and minus end directed motors is essential for correction of merotelic attachments.
Enhancement of merotelic correction in human cells using an Eg5 kinesin inhibitor
To test if reduction of spindle elongation forces in mammalian cells could enhance correction of merotelic attachments as we observed in yeast, we analyzed the effect of a low dose (15μM) of the drug monastrol, which inhibits the kinesin-5 motor Eg5, on normal human cells that had been induced to have lagging chromosomes due to merotelly. A previous study showed that the human cell line RPE-1 (which has normal ploidy and is chromosomally stable) displays merotelically attached lagging chromosomes following release from arrest in nocodazole [4]. Using a similar protocol we arrested RPE-1 cells in nocodazole for 7 hours and then released them into media with or without 15μM monastrol. (Note that although high doses of monastrol cause formation of monopolar spindles, lower doses lead to shorter spindles presumably because of reduced spindle elongation forces [27].) Monastrol treatment caused a major reduction in lagging chromosome frequency (Figure 4A). Using RPE-1 cells arrested in metaphase using MG132, we observed that 15μM monastrol treatment also caused a reduction in both spindle length and inter kinetochore distance (Figures 4B). Similar results were obtained using the colon adenocarcinoma cell line CaCo-2, which, like most solid tumors, has a high rate of chromosome instability due primarily to lagging chromosomes [4]. Treatment of CaCo-2 cells with 15μM monastrol reduced both average spindle length and the frequency of lagging chromosomes (Figure 4A) (interkinetochore distance was not assessed because the large number of chromosomes in CaCo-2 cells complicated the analysis). Monastrol treatment did not affect bipolar spindle assembly or cause a significant (P=0.48) delay in pro/metaphase (Figures S4A and S4B). Thus, as in yeast, partial inhibition of kinesin-5 can lead to correction of merotelic attachments.
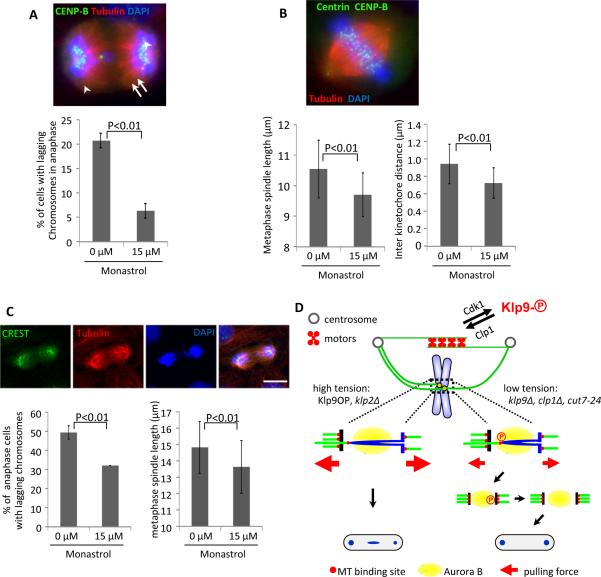
Figure 4. Merotelic correction in human cells can be controlled through manipulation of spindle elongation motors.
(A) RPE-1 cells stable expressing centrin-GFP were treated with nocodazole for 7h and released into media with 15μM monastrol for 40 minutes, and fixed. (Top) An example cell stained for kinetochores (CENP-B), tubulin, and DNA. (Bottom) Frequency of lagging chromosomes in anaphase. Bars represent the mean +/− SD (n=3; 100 cells each). Arrow indicates a single lagging chromosome. Only single chromosomes (as judged by CENP-B staining) were scored. (B) Comparisons of the metaphase spindle length and interkinetochore distances are shown. RPE1 centrin-GFP cells were treated with 15μM Monastrol for 16h, then 5μM MG132 for 1h and fixed. (Top) An example cell stained for kinetochores (CENP-B), tubulin, and DNA. Arrowheads indicate centrosomes, and arrows indicates paired sister kinetochores. (Bottom) Metaphase spindle length and interkinetochore distance was determined by measuring the distance between centrosomes and paired sister kinetochores respectively from cells arrested at metaphase. Bars represent the mean +/− SD (n=3; 80 cells each). (C) CaCo-2 cells were treated with 15μM Monastrol for 16h, and fixed. (Top) An example of cells stained for kinetochores (CREST), tubulin, and DNA. (Bottom left) The frequency of lagging chromosomes is shown. Only single chromosomes (as judged by CREST staining) were scored. Bars represent the mean +/− SD (n=3; 50 cells each). (Bottom right) A comparison of the metaphase spindle length is shown. Cells were treated with 15μM Monastrol for 16h, then 5μM MG132 for 1h and fixed. Metaphase spindle length was measured by distance between centrosomes from cells arrested at metaphase. Bars represent the mean +/− SD (n=3; 40 cells each). (D) A hypothetical model depicting how merotelic attachment could be corrected by manipulation of motor activities on interpolar microtubules. Phospho-regulation of Klp9 by Cdk1 and Clp1, or the indicated mutations in motor molecules, affects tension applied on sister kinetochores. In turn, tension may regulate the stability of merotelic kinetochores either directly or indirectly by affecting their exposure to the kinase Aurora B. See also Figure S4.
Conclusions
Previous studies have shown that changes in the dynamics of kinetochore microtubules can switch cells from a chromosomally stable to unstable state and vice versa [28, 29]. Our results show that changes in the force balance on the mitotic spindle can also affect chromosome stability. Specifically, increased Cdk1 activity or inhibition of motors that act on interpolar spindle microtubules causes a decrease in spindle length, tension at kinetochores, and lagging chromosomes. We show that Cdk1 may affect lagging chromosome frequency through regulation of motors acting on interpolar microtubules. It is also possible that manipulation of Cdk1 activity affects chromosome segregation through changes in microtubule dynamics. However because Klp9 and Cut7 motors are only thought to act on interpolar microtubules, they likely act by affecting tension at kinetochores through changes in spindle elongation forces. We showed that decreased spindle elongation forces caused a reduction in tension at kinetochores and reduced lagging chromosomes. There are several possible explanations for the reduction in lagging chromosome frequency. First, changes in tension could affect the strength of kinetochore microtubule attachments through aurora-dependent (Figure 4D) or independent mechanisms. For example, bipolar attachments are proposed to be stabilized because they are pulled away from a zone of high concentration of aurora, which localizes to the inner centromere of mammalian cells [30] or to the heterochromatin flanking the core centromere of S. pombe [31]. In contrast, merotelic attachments might be pulled back towards the aurora zone, making them susceptible to destabilization by aurora. However, if the spindle elongation forces were strong enough, the merotelic attachment would be pulled out of the aurora correction zone (Figure 4D, left). Reduced spindle elongation forces could relax tension on the kinetochore to allow the merotelic attachment to move back into the aurora zone, making it susceptible to destabilization (Figure 4D, right). A second possibility is that the reduction in tension could destabilize attachments independent of aurora as has been observed in budding yeast [18]. A third possibility is that reduced tension at kinetochores does not promote correction, but reduces the initial formation of merotelic attachments through an unknown mechanism.
Although spindle structure and dynamics are more complicated in mammalian cells, it is intriguing that, as in yeast, partial inhibition of a spindle elongation motor (Eg5) reduced lagging chromosome frequency, spindle length, and tension at kinetochores. It will be interesting in future studies to explore how Eg5 inhibition reduces lagging chromosome frequencies and whether it could be combined with changes in microtubule dynamics to decrease chromosome instability in tumor cells.
Supplementary Material
Highlights
-
1)
Inhibition of spindle elongation motors reduces lagging chromosome frequency.
-
2)
Cdk1 reduces lagging chromosomes by inhibiting kinesin 6 (Klp9).
-
3)
Kinesin 5 inhibition reduces chromosome instability in a human tumor cells line.
Acknowledgements
We thank Dr. Mitsuhiro Yanagida, Dr. J. Richard McIntosh, Dr. Shiv Grewal, Dr. Julie Cooper, Dr. Yoshinori Watanabe, Dr Kim Nasmyth, Dr. Phong Tran, and Dr. Kathleen Gould, for yeast strains and plasmids, and Alexey Khodjakov for GFP-centrin expressing cells. We are grateful to Dr. Peter Pryciak for comments on the manuscript, Dr. Nick Rhind for advice on ROC analysis, and Dr. Param Murugan for technical assistance. This work was supported by NIH grant R01GM068786 to D.M.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gregan J, Polakova S, Zhang L, Tolic-Norrelykke IM, Cimini D. Merotelic kinetochore attachment: causes and effects. Trends Cell Biol. 2011;21:374–381. doi: 10.1016/j.tcb.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cimini D, Howell B, Maddox P, Khodjakov A, Degrassi F, Salmon ED. Merotelic kinetochore orientation is a major mechanism of aneuploidy in mitotic mammalian tissue cells. J Cell Biol. 2001;153:517–527. doi: 10.1083/jcb.153.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson SL, Bakhoum SF, Compton DA. Mechanisms of chromosomal instability. Curr Biol. 2010;20:R285–295. doi: 10.1016/j.cub.2010.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson SL, Compton DA. Examining the link between chromosomal instability and aneuploidy in human cells. J Cell Biol. 2008;180:665–672. doi: 10.1083/jcb.200712029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gregan J, Riedel CG, Pidoux AL, Katou Y, Rumpf C, Schleiffer A, Kearsey SE, Shirahige K, Allshire RC, Nasmyth K. The kinetochore proteins Pcs1 and Mde4 and heterochromatin are required to prevent merotelic orientation. Curr Biol. 2007;17:1190–1200. doi: 10.1016/j.cub.2007.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rumpf C, Cipak L, Schleiffer A, Pidoux A, Mechtler K, Tolic-Norrelykke IM, Gregan J. Laser microsurgery provides evidence for merotelic kinetochore attachments in fission yeast cells lacking Pcs1 or Clr4. Cell Cycle. 2010;9:3997–4004. doi: 10.4161/cc.9.19.13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi SH, Peli-Gulli MP, McLeod I, Sarkeshik A, Yates JR, 3rd, Simanis V, McCollum D. Phosphorylation state defines discrete roles for monopolin in chromosome attachment and spindle elongation. Curr Biol. 2009;19:985–995. doi: 10.1016/j.cub.2009.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson SL, Compton DA. Chromosome missegregation in human cells arises through specific types of kinetochore-microtubule attachment errors. Proc Natl Acad Sci U S A. 2011;108:17974–17978. doi: 10.1073/pnas.1109720108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janicke MA, Lasko L, Oldenbourg R, LaFountain JR., Jr. Chromosome malorientations after meiosis II arrest cause nondisjunction. Mol Biol Cell. 2007;18:1645–1656. doi: 10.1091/mbc.E06-10-0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torosantucci L, De Santis Puzzonia M, Cenciarelli C, Rens W, Degrassi F. Aneuploidy in mitosis of PtK1 cells is generated by random loss and nondisjunction of individual chromosomes. J Cell Sci. 2009;122:3455–3461. doi: 10.1242/jcs.047944. [DOI] [PubMed] [Google Scholar]
- 11.Cimini D, Wan X, Hirel CB, Salmon ED. Aurora kinase promotes turnover of kinetochore microtubules to reduce chromosome segregation errors. Curr Biol. 2006;16:1711–1718. doi: 10.1016/j.cub.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 12.Knowlton AL, Lan W, Stukenberg PT. Aurora B is enriched at merotelic attachment sites, where it regulates MCAK. Curr Biol. 2006;16:1705–1710. doi: 10.1016/j.cub.2006.07.057. [DOI] [PubMed] [Google Scholar]
- 13.Trautmann S, McCollum D. Distinct nuclear and cytoplasmic functions of the S. pombe Cdc14-like phosphatase Clp1p/Flp1p and a role for nuclear shuttling in its regulation. Curr Biol. 2005;15:1384–1389. doi: 10.1016/j.cub.2005.06.039. [DOI] [PubMed] [Google Scholar]
- 14.Chang L, Morrell JL, Feoktistova A, Gould KL. Study of cyclin proteolysis in anaphase-promoting complex (APC) mutant cells reveals the requirement for APC function in the final steps of the fission yeast septation initiation network. Mol Cell Biol. 2001;21:6681–6694. doi: 10.1128/MCB.21.19.6681-6694.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cimini D, Moree B, Canman JC, Salmon ED. Merotelic kinetochore orientation occurs frequently during early mitosis in mammalian tissue cells and error correction is achieved by two different mechanisms. J Cell Sci. 2003;116:4213–4225. doi: 10.1242/jcs.00716. [DOI] [PubMed] [Google Scholar]
- 16.Lampson MA, Cheeseman IM. Sensing centromere tension: Aurora B and the regulation of kinetochore function. Trends Cell Biol. 2011;21:133–140. doi: 10.1016/j.tcb.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goshima G, Saitoh S, Yanagida M. Proper metaphase spindle length is determined by centromere proteins Mis12 and Mis6 required for faithful chromosome segregation. Genes Dev. 1999;13:1664–1677. doi: 10.1101/gad.13.13.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akiyoshi B, Sarangapani KK, Powers AF, Nelson CR, Reichow SL, Arellano-Santoyo H, Gonen T, Ranish JA, Asbury CL, Biggins S. Tension directly stabilizes reconstituted kinetochore-microtubule attachments. Nature. 2010;468:576–579. doi: 10.1038/nature09594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goshima G, Scholey JM. Control of mitotic spindle length. Annu Rev Cell Dev Biol. 2010;26:21–57. doi: 10.1146/annurev-cellbio-100109-104006. [DOI] [PubMed] [Google Scholar]
- 20.Fu C, Ward JJ, Loiodice I, Velve-Casquillas G, Nedelec FJ, Tran PT. Phospho-regulated interaction between kinesin-6 Klp9p and microtubule bundler Ase1p promotes spindle elongation. Dev Cell. 2009;17:257–267. doi: 10.1016/j.devcel.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hagan I, Yanagida M. Kinesin-related cut7 protein associates with mitotic and meiotic spindles in fission yeast. Nature. 1992;356:74–76. doi: 10.1038/356074a0. [DOI] [PubMed] [Google Scholar]
- 22.Blangy A, Lane HA, d'Herin P, Harper M, Kress M, Nigg EA. Phosphorylation by p34cdc2 regulates spindle association of human Eg5, a kinesin-related motor essential for bipolar spindle formation in vivo. Cell. 1995;83:1159–1169. doi: 10.1016/0092-8674(95)90142-6. [DOI] [PubMed] [Google Scholar]
- 23.Mishima M, Pavicic V, Gruneberg U, Nigg EA, Glotzer M. Cell cycle regulation of central spindle assembly. Nature. 2004;430:908–913. doi: 10.1038/nature02767. [DOI] [PubMed] [Google Scholar]
- 24.Drummond DR, Hagan IM. Mutations in the bimC box of Cut7 indicate divergence of regulation within the bimC family of kinesin related proteins. J Cell Sci. 1998;111(Pt 7):853–865. doi: 10.1242/jcs.111.7.853. [DOI] [PubMed] [Google Scholar]
- 25.Hagan I, Yanagida M. Novel potential mitotic motor protein encoded by the fission yeast cut7+ gene. Nature. 1990;347:563–566. doi: 10.1038/347563a0. [DOI] [PubMed] [Google Scholar]
- 26.Troxell CL, Sweezy MA, West RR, Reed KD, Carson BD, Pidoux AL, Cande WZ, McIntosh JR. pkl1(+)and klp2(+): Two kinesins of the Kar3 subfamily in fission yeast perform different functions in both mitosis and meiosis. Mol Biol Cell. 2001;12:3476–3488. doi: 10.1091/mbc.12.11.3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodman B, Channels W, Qiu M, Iglesias P, Yang G, Zheng Y. Lamin B counteracts the kinesin Eg5 to restrain spindle pole separation during spindle assembly. J Biol Chem. 2010;285:35238–35244. doi: 10.1074/jbc.M110.140749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bakhoum SF, Genovese G, Compton DA. Deviant kinetochore microtubule dynamics underlie chromosomal instability. Curr Biol. 2009;19:1937–1942. doi: 10.1016/j.cub.2009.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bakhoum SF, Thompson SL, Manning AL, Compton DA. Genome stability is ensured by temporal control of kinetochore-microtubule dynamics. Nat Cell Biol. 2009;11:27–35. doi: 10.1038/ncb1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu D, Vader G, Vromans MJ, Lampson MA, Lens SM. Sensing chromosome bi-orientation by spatial separation of aurora B kinase from kinetochore substrates. Science. 2009;323:1350–1353. doi: 10.1126/science.1167000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawashima SA, Tsukahara T, Langegger M, Hauf S, Kitajima TS, Watanabe Y. Shugoshin enables tension-generating attachment of kinetochores by loading Aurora to centromeres. Genes Dev. 2007;21:420–435. doi: 10.1101/gad.1497307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.