Abstract
Oxidative stress has been associated with the onset and progression of mild cognitive impairment (MCI) and Alzheimer disease (AD). AD and MCI brain and plasma display extensive oxidative stress as indexed by protein oxidation, lipid peroxidation, free radical formation, DNA oxidation, and decreased antioxidants. The most abundant endogenous antioxidant, glutathione, plays a significant role in combating oxidative stress. The ratio of oxidized to reduced glutathione is utilized as a measure of intensity of oxidative stress. Antioxidants have long been considered as an approach to slow down AD progression. In this review, we focus on the elevation on glutathione through N-acytl-cysteine (NAC) and γ-glutamylcysteine ethyl ester (GCEE) as a potential therapeutic approach for Alzheimer disease.
Keywords: Alzheimer disease (AD), Mild Cognitive Impairment (MCI), Amyloid β-peptide, Glutathione (GSH), N-acetylcysteine (NAC), γ-Glutamylcysteine ethyl ester
1. Introduction
Alzheimer disease (AD) is a largely sporadic, age-related neurodegenerative disorder pathologically characterized by the accumulation of abnormal protein deposits, including extracellular amyloid plaques, intracellular neurofibrillary tangles (NFT), and loss of synaptic connections within selective brain regions [1]. One of the main components of amyloid plaques is the amyloid β-peptide (Aβ), generated by the proteolytic cleavage of the amyloid precursor protein (APP) by β- and γ-secretases. Aβ exists in many forms, such as soluble, aggregated, oligomeric, protofibrillar, and fibrillar forms [2; 3], and a number of studies have demonstrate that the oligomeric form of Aβ is highly toxic and associated with oxidative stress [4; 5; 6].
Aβ(1–42)-associated free radicals can abstract an allylic hydrogen-atom from the unsaturated acyl chains of lipid molecules within the lipid bilayer, thereby leading to the initiation of lipid peroxidation processes [7; 8]. The process of lipid peroxidation generates highly reactive products, such as 4-hydroxy-2-nonenal (HNE) and acrolein, that can further react with proteins and enzymes, effectively amplifying the effects of Aβ(1–42)-induced free radical processes [8; 9].
Under normal conditions, oxidative stress and damage are combated by endogenous antioxidant compounds and enzymes within the cell. However, the brain is particularly vulnerable to oxidative damage due to the high levels of unsaturated lipids, oxygen, redox metal ions, and relatively poor antioxidant systems. As previously reported by our laboratory and others, both AD and mild cognitive impairment (MCI) brains have significantly decreased levels of antioxidant enzymes, making the brain more vulnerable to Aβ(1–42)-induced toxic effects [10]. Oxidative stress is also evident in AD brain by marked levels of protein, lipid, DNA, and RNA oxidation, neuronal dysfunction and death [11; 12]. Consequently, one way of boosting defenses in the brain is by assisting the antioxidant defense system particularly endogenous glutathione (GSH) and glutathione-related enzymes.
2. Glutathione (GSH)
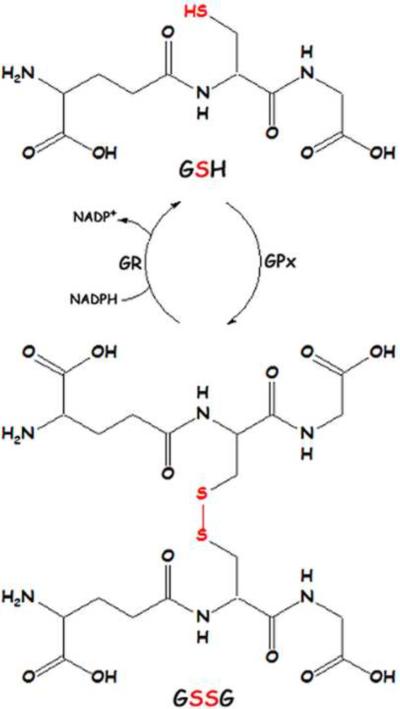
The most prevalent antioxidant in the brain, glutathione, is found in millimolar concentrations in most cells. A thiol-containing molecule, GSH is capable of reacting with reactive oxygen species (ROS) and nucleophilic compounds such as HNE and acrolein, lipid peroxidation products that react with thiols in proteins. Reduced GSH reacts with free radicals to form oxidized glutathione (GSSG), which can be catalyzed by the enzyme glutathione peroxidase (GPx) or occur independently. GSSG is recycled back to two GSH molecules by GSH reductase (GR) utilizing the reducing equivalents of NADPH (Figure 1). Glutathione S-transferases (GST) are a group of enzymes that catalyze the reaction between GSH and nucleophilic compounds such as HNE and acrolein. The resulting glutathione-S-conjugates are effluxed from the cell by the multidrug resistance protein-1 (MRP-1) [13; 14]. In AD hippocampus, GST and MRP-1 are covalently bound by the lipid peroxidation product HNE, rendering them inactive [13; 15]. Thus, glutathione-S-conjugates are not readily formed or exported, possibly increasing HNE levels in the cell [16].
Figure 1.
Recycling of glutathione (GSH) and oxidized glutathione (GSSG).
Post-translational modification of proteins by glutathionylation is reversible by glutaredoxin, a thiol transferase [17]. Redox sensitive proteins could be protected from oxidative stress by glutathionylation. Indeed, several proteins in AD inferior parietal lobule (IPL), including glyceraldehyde-3-phosphate dehydrogenase (GAPDH), α-enolase, and p53, were identified as glutathionylated [18; 19]. GAPDH and α-enolase also have decreased activity in AD brain, and were previously reported to be oxidatively modified [20; 21; 22]. GAPDH and α-enolase are enzymes in the energy producing glycolytic pathway; oxidative modification and decreased activity may contribute to the alteration in glucose metabolism noted in AD [23]. Moreover, both enzymes have pro-survival functions in addition to roles in glycolysis. Oxidative dysfunction of these enzymes is deleterious to neurons [24; 25].
GSH levels are decreased in diseases with oxidative stress - including AD - and with age [26]. In AD peripheral lymphocytes, GSH levels are decreased and GSSG levels are increased, consistent with increased oxidative stress [27]. The ratio of GSSG to GSH is used as a marker of redox thiol status and oxidative stress. Indeed, with increasing progression of AD, GSSG and GSSG/GSH levels are found to increase. Lloret and colleagues found a linear correlation between increased GSSG levels and decreased cognitive status of AD patients using the Mini Mental Status examination (MMSE) [28].
Mild cognitive impairment (MCI) is often referred to as a transitional period between normal cognitive aging and mild dementia or probable AD. Many individuals with amnestic MCI develop AD, suggesting MCI is the earliest stage of AD [29; 30]. Several studies have demonstrated oxidative stress in MCI brain. In MCI hippocampus, a brain region highly affected in AD, superoxide dismutase (SOD) and GST activity is decreased, although protein expression was increased. The ratio of GSH/GSSG was decreased consistent with oxidative stress conditions. No significant difference in GPx or GR enzyme activity was noted [31]. Many enzymes are redox sensitive and easily oxidized, rendering them inactive even though protein expression level is high. Lipid and protein oxidative stress products were also elevated in the superior and middle temporal gyri of MCI brain [9; 32; 33]. Recent reports demonstrated peripheral serum levels of MCI and AD patients had significantly decreased GPx and SOD activity compared to age-matched controls, but did not differ from each other [34]. These researchers also showed increased levels of lipid peroxidation product malondialdehyde (MDA) compared to controls, with a significant increase from MCI to AD. Several previous studies also reported an increase in peripheral lipid and protein oxidation in AD and MCI patients [35; 36; 37; 38]. Decreased SOD and GPx antioxidant activity over time, leads to an accumulation of H2O2 and lipid peroxidation, possibly leading to the pathological alterations characteristic of AD. The above studies all concluded that oxidative stress conditions in early AD are already present in MCI, and the decreased antioxidant activity, particularly glutathione, may initiate the progression to AD [37]. A recent study demonstrated that MCI patients that progressed to AD displayed an increased distribution of the ApoE ε4 allele, a risk factor for sporadic AD, and displayed a significant decrease in the ratio of oxidized to reduced glutathione and vitamin E levels compared to MCI patients that remained at MCI status over time [39]. Oxidative stress indices increased over time in both MCI and MCI patients that progressed to AD, with no difference between the two groups. This study confirms that a decrease of antioxidants, particularly reduced glutathione, over time is a major contributor to the progression of MCI to AD. Increased peripheral oxidative stress indices, such as MDA, TBARS, or protein carbonyls, could potentially be used as a biomarker for diagnosing the onset of MCI, while a steady decrease of reduced glutathione may be a biomarker for progression to AD. An early diagnosis would allow early intervention utilizing appropriate antioxidants and other therapies.
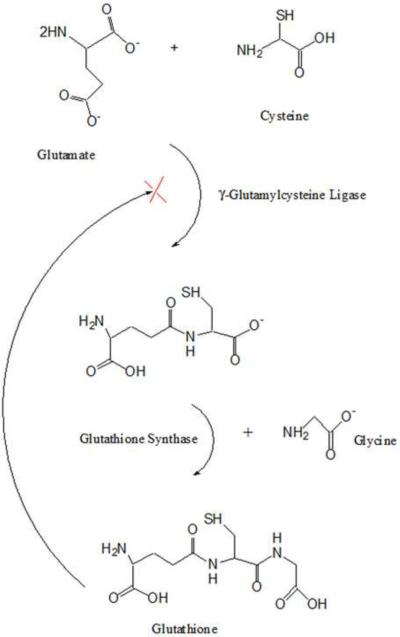
Glutathione is comprised of the amino acids glutamate, cysteine, and glycine. Glutamate and glycine are found in millimolar concentrations, whereas free cysteine is limited with most non-protein cysteine being stored within GSH. Two enzymes are involved in synthesis of GSH: γ-glutamylcysteine ligase (also called γ-glutamylcysteine synthetase) and gluthathione synthase (Fig. 2). Because the physiological amount of brain-resident cysteine limits the formation of GSH, most current research has focused on increasing cysteine levels in the brain as an indirect way to increase the levels of GSH. In particular, N-acetyl-L-cysteine (NAC) is known to directly increase brain cysteine levels, allowing for increased biosynthesis of GSH in the brain and periphery [40]. Additionally, γ-Glutamylcysteine ethyl ester (GCEE) introduces the precursor for the last step in GSH synthesis, guiding cysteine directly towards GSH synthesis in the brain and periphery and avoiding the feedback inhibition of γ-glutaminecysteine ligase.
Figure 2.
Synthesis of Glutathione
3. N-Acetyl-L-Cysteine (NAC)
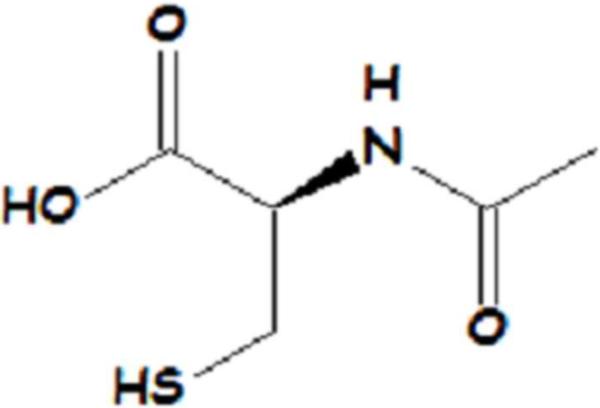
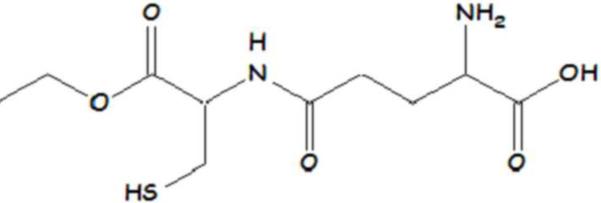
NAC (Figure 3) has been shown to be an effective precursor to GSH production and crosses the blood brain barrier (BBB) [41; 42]. NAC provides cysteine, the rate limiting substrate in glutathione synthesis. NAC acts as an antioxidant by increasing GSH levels and by directly interacting with free radicals. Intraperitoneal (i.p.) injection of NAC to rodents increased GSH in brain and synaptosomes and offered protection against peroxynitrite, hydroxyl radicals, acrolein, and oxidative stress induced by 3-nitro-propionic acid [40; 43; 44; 45]. NAC also improved neuronal survival in the hippocampus after ischemic-reperfusion [46].
Figure 3.
Structure of N-acetyl-L-cysteine (NAC).
Pretreatment with NAC in mice receiving intracerebroventricular (i.c.v.) injections of Aβ had improved learning and memory compared to vehicle-treated animals [47]. NAC also increased GSH levels, protected against Aβ-induced protein and lipid peroxidation, and decreased acetylcholine levels and choline acetyltransferase (ChAT) activity [47]. SAMP8 (Senescence Accelerated Mouse) mice overexpress APP resulting in elevated levels of Aβ in the brain. SAMP8 mice administered NAC had improved cognition in the T-maze footshock avoidance paradigm and the lever press appetitive task [42]. Recently, AD-relevant APP/PS-1 mice were orally administered NAC in drinking water for 5 months, before deposition of Aβ occurred in the brain. The antioxidant administered before Aβ induced oxidation occurred decreased protein and lipid oxidation, nitration of proteins, and increased glutathione peroxidase and reductase activity compared to age matched controls [48]. Such treatment clearly decreased oxidative stress in vivo in mice brain.
In AD brain and neuronal cultures exposed to Aβ, dying cells display characteristics of apoptosis [49]. A shift in redox status due to NAC changes the signaling pathways involved in the apoptosis signaling cascade [50; 51]. NAC protection against Aβ involves several signaling pathways involved in apoptosis including: activation of the Ras/ERK pathway, stimulating p35/Cdk5 activity, and reduced phosphorylation/deactivation of MLK3-MKK7-JNK3 signaling cascade [50; 51; 52]. NAC also acts as a transcription factor activating the RAS-ERK pathway, rescuing neurons from apoptotic cell death [52]. Therefore, in addition to antioxidant properties, and increasing GSH levels, NAC protects against Aβ toxicity through activation of anti-apoptotic signaling pathways.
NAC may play a role in amyloid precursor protein (APP) processing and Aβ formation. Aβ results from two proteases cleaving APP: β-secretase and γ-secretase. NAC down-regulates APP gene transcription, resulting in undetectable levels of APP mRNA in neuroblastoma cells. This activity may be related to decreased binding activity of transcription factor NF- κB, which is increased by oxidative stress and Aβ [53]. Another group demonstrated that NAC significantly decreased soluble levels of Aβ(1–40) and Aβ(1–42) and modestly reduced insoluble Aβ(1–40) in TgCRND8 transgenic mice that overexpress the APP gene [54]. Olivieri et al. (2001) showed NAC affected APP processing and increased levels of Aβ(1–40) by itself, suggesting the influence of β-secretase and γ-secretase cleavage of APP in neuroblastoma cells [55].
The role of Pin1 has been investigated in APP processing. Pin1 catalyzes the structural formation of phosphorylated Ser/Thr-Pro for dephosphorylation of APP. In AD models and AD brain, this motif remains phosphorylated resulting in increased Aβ production [56; 57]. Our laboratory demonstrated oxidation and decreased levels of Pin1 in MCI and AD brain [9; 58; 59]. Utilizing proteomics, we identify elevated levels of Pin1 in preclinical AD (PCAD) brain [60], consistent with the notion that PCAD subjects, characterized by normal scores on tests of cognition but having AD-like pathology in brain, respond to elevated Aβ by increasing expression of Pin1. Our laboratory also demonstrated, NAC treatment slightly elevated Pin1 in APP/PS1 mice over a 5 month period, possibly decreasing Aβ induced oxidative stress [48]. Results concerning NAC's effect on Aβ formation requires further study.
NAC capped quantum dots were utilized to block fibril formation of Aβ by blocking the active site of fibrils, nuclear fibrils, or protofibrils, possibly through hydrogen bonding [61]. Free NAC was unable to block Aβ fibril formation. Future antifibrilogenesis may involve quantum dot technology.
Neprilysin is a principal degrading peptidase of Aβ. In AD affected brain regions, neprilysin is oxidatively modified by HNE and has decreased levels and activity [62; 63]. Preincubation with NAC was able to prevent HNE and Aβ-induced HNE addition to neprilysin and thus maintain neprilysin activity [64]. We suggest that NAC may be protective through modulation of Aβ formation and degradation via influence on APP transcription, processing, signaling pathways, and preventing oxidative stress.
Alzheimer disease presents a prominent neuroinflammation component. Astrocytes are the main supplier of GSH to microglia and neurons. During chronic inflammation and oxidative stress, astrocytes release toxic inflammatory mediators and free radicals, accelerating activation of microglia and neurodegeneration [65]. Recently, decreased intracellular glutathione was correlated with the release of pro-inflammatory factors TNF-α, IL-6, and nitrite ions and activation of the inflammatory pathways, P38 MAP-kinase, Jun-N-terminal kinase, NF-κB, in human microglia and astrocytes [66]. Extracellular GSH attenuated the BSO-reduction of intracellular levels of GSH in the above microglia and astrocytes, suggesting involvement of a membrane channel or transporter. NAC directly inhibited inflammatory factor NF-κB and blocked production of nitric oxide from inducible nitric oxide synthase and inflammatory cytokines [67]. Increasing glutathione levels with NAC in glial cells and astrocyes may confer protection against the neuro-inflammation component of AD.
Given the multi-faceted way NAC is capable of modulating AD (see Figure 4), patient supplementation with NAC has been addressed. In a previous study by Adair et al. (2001), late-stage AD patients supplemented with NAC over a six month period not only tolerated the treatment well, but also demonstrated significantly improved performance on the Letter Fluency Task and the Wechsler Memory Scale Immediate Number Recall [68], although, measures of oxidative stress in peripheral blood did not differ significantly [68]. More recently, AD patients were given a vitamin/nutriceutical supplement that included folate, vitamin B12, α-tocopherol, S-adenosyl methionine, NAC, and acetyl-L-carnitine [69]. All cognitive endpoints were found to favor the multi-supplement. Several antioxidant clinical trials had no effects or marginal positive effects on MCI progression to AD or AD [70; 71; 72]. They did not include a multi-supplement approach or a glutathione enhancing drug. The failures in many antioxidant clinical trials likely arise from starting the therapies in the late stages of AD, not monitoring drug levels and markers for the in vivo therapeutic effect of the drug, not utilizing a multi-antioxidant approach that covers both lipophilic and hydrophilic areas of the cell or recycle the oxidized antioxidants back to the reduced state, and not taking into account the basal redox status of the subjects in the trials [10; 73; 74]. These limitations must be taken into consideration when determining if an antioxidant therapy would be beneficial in slow or preventing the progression of MCI and AD.
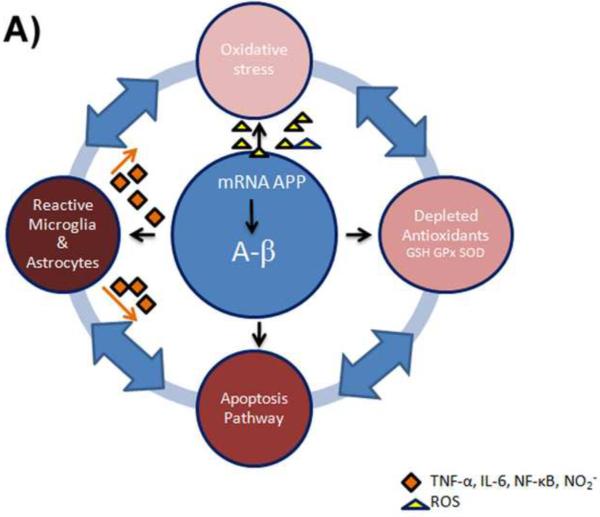
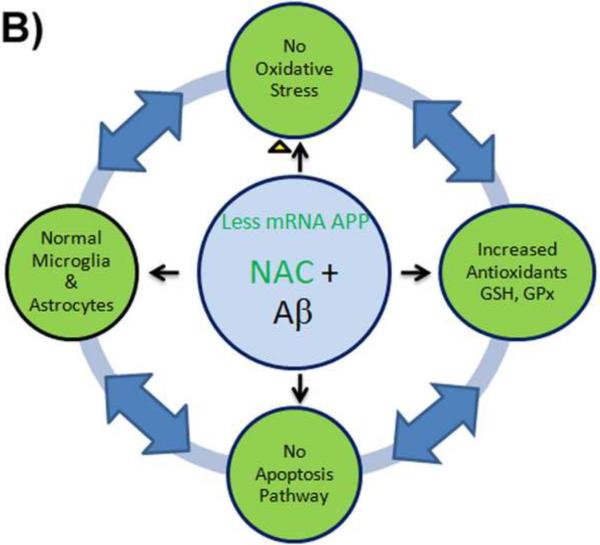
Figure 4.
A) Aβ produces ROS that eventually leads to the depletion of antioxidants and oxidative stress in Alzheimer disease. The increased oxidation induces apoptotic signaling pathways and inflammation in astrocytes. Astrocytes release toxic inflammatory mediators and free radicals, accelerating activation of microglia and neurodegeneration, connecting the cycle of negative events perpetuating AD.
B) NAC down-regulates APP gene transcription, resulting in undetectable levels of APP mRNA. Thus, since less Aβ is transcribed, fewer free radicals are produced by Aβ. NAC increases antioxidant levels of glutathione and reacts with ROS preventing oxidative stress. The decreased oxidation in the cells induces anti-apoptotic signaling pathways and prevents inflammation of the cell. NAC directly inhibits inflammatory factor NF-κB and blocks production of nitric oxide from inducible nitric oxide synthase and inflammatory cytokines.
4. γ-Glutamylcysteine Ethyl Ester (GCEE)
Another effective means for increasing biosynthesis of GSH is GCEE (Figure 5) [75]. γ-Glutamylcysteine formation is the rate-limiting step for the biosynthesis of GSH. Providing γ-glutamylcysteine bypasses the feed-back inhibition by GSH on γ-glutamylcysteine synthetase (GCS), the enzyme that catalyzes production of γ-glutamylcysteine. Attachment of an ethyl ester moiety allows γ-glutamylcysteine to more easily cross the cell membrane and blood-brain barrier (BBB). Protection against myocardial ischemic-reprefusion and mydocardial dysfunction in Se-deficient rats was afforded by GCEE [76; 77]. GCEE is able to increase brain and mitochondrial GSH levels and protect synaptosomes, neuronal cells, and mitochondria against peroxynitrite damage [78; 79]. Neuronal cells were also protected against Aβ(1–42)-induced protein oxidation, loss of mitochondrial function, and DNA fragmentaion by GCEE up-regulation of GSH. GCEE did not, however, disrupt Aβ(1–42) fibril formation [80; 81]. Aβ(1–42) is known to deplete GSH cellular levels which can lead to neuronal death. However, 24 hours after Aβ(1–42) addition, GSH and GCS levels increase intracellularly, offering protection against Aβ(1–42)-induced apoptosis in cortical neurons [82; 83; 84]. Recently, i.p. injections of GCEE protected against kainic acid induced ROS and downregulated c-fos mRNA in the cortex and hippocampus of rats [85]. GCEE may react directly with ROS due to the cysteine residue and/or increase GSH, which can protect against ROS and nucleophilic compounds.
Figure 5.
Structure of γ-glutymylcysteine ethyl ester (GCEE).
5. Conclusions
Oxidative stress is a known characteristic of MCI and AD. Up regulation of endogenous antioxidants is vital in combating oxidative stress and thus helping to slow the advancement of MCI and Alzheimer disease. Glutathione is the most abundant and versatile endogenous antioxidant with many enzyme systems to enhance its function. NAC (FDA approved) and GCEE are known to increase glutathione in the brain and periphery and protect against ROS-producing substances in vivo. More research needs to be invested in GCEE, since it has no known harmful effects and by-passes the feedback inhibition cycle of glutathione. Increasing glutathione remains a promising therapeutic strategy to slow or prevent MCI and Alzheimer disease.
Highlights
Glutathione (GSH) is the most abundant endogenous antioxidant in brain
Oxidative stress is a prominent feature of Alzheimer disease and MCI brain
Elevation of GSH in vivo protects brain against AD-relevant Abeta(1–42)
Elevation of GSH in brain induces several protective pathways
Acknowledgements
This work was supported in part by NIH grants to D.A.B. [AG-05119].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Selkoe DJ. Alzheimer's disease results from the cerebral accumulation and cytotoxicity of amyloid beta-protein. J Alzheimers Dis. 2001;3:75–80. doi: 10.3233/jad-2001-3111. [DOI] [PubMed] [Google Scholar]
- [2].Dahlgren KN, Manelli AM, Stine WB, Jr., Baker LK, Krafft GA, LaDu MJ. Oligomeric and fibrillar species of amyloid-β peptides differentially affect neuronal viability. J Biol Chem. 2002;277:32046–32053. doi: 10.1074/jbc.M201750200. [DOI] [PubMed] [Google Scholar]
- [3].Caughey B, Lansbury PT. Protofibrils, pores, fibrils, and neurodegeneration: separating the responsible protein aggregates from the innocent bystanders. Annu Rev Neurosci. 2003;26:267–298. doi: 10.1146/annurev.neuro.26.010302.081142. [DOI] [PubMed] [Google Scholar]
- [4].Fawzi NL, Okabe Y, Yap EH, Head-Gordon T. Determining the critical nucleus and mechanism of fibril elongation of the Alzheimer's Abeta(1–40) peptide. J Mol Biol. 2007;365:535–550. doi: 10.1016/j.jmb.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Walsh DM, Hartley DM, Condron MM, Selkoe DJ, Teplow DB. In vitro studies of amyloid β-protein fibril assembly and toxicity provide clues to the aetiology of Flemish variant (Ala692-->Gly) Alzheimer's disease. Biochem J. 2001;355:869–877. doi: 10.1042/bj3550869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Drake J, Link CD, Butterfield DA. Oxidative stress precedes fibrillar deposition of Alzheimer's disease amyloid beta-peptide (1–42) in a transgenic Caenorhabditis elegans model. Neurobiol Aging. 2003;24:415–420. doi: 10.1016/s0197-4580(02)00225-7. [DOI] [PubMed] [Google Scholar]
- [7].Lauderback CM, Hackett JM, Huang FF, Keller JN, Szweda LI, Markesbery WR, Butterfield DA. The glial glutamate transporter, GLT-1, is oxidatively modified by 4-hydroxy-2-nonenal in the Alzheimer's disease brain: The role of Aβ(1–42) J. Neurochem. 2001;78:413–416. doi: 10.1046/j.1471-4159.2001.00451.x. [DOI] [PubMed] [Google Scholar]
- [8].Butterfield DA, Lauderback CM. Lipid peroxidation and protein oxidation in Alzheimer's disease brain: potential causes and consequences involving amyloid beta-peptide-associated free radical oxidative stress. Free Radic Biol Med. 2002;32:1050–1060. doi: 10.1016/s0891-5849(02)00794-3. [DOI] [PubMed] [Google Scholar]
- [9].Butterfield DA, Poon HF, St Clair D, Keller JN, Pierce WM, Klein JB, Markesbery WR. Redox proteomics identification of oxidatively modified hippocampal proteins in mild cognitive impairment: insights into the development of Alzheimer's disease. Neurobiol Dis. 2006;22:223–232. doi: 10.1016/j.nbd.2005.11.002. [DOI] [PubMed] [Google Scholar]
- [10].Pocernich CB, Bader Lange ML, Sultana R, Butterfield DA. Nutritional Approaches to Modulate Oxidative Stress in Alzheimer's Disease. Curr Alzheimer Res. 2011 doi: 10.2174/156720511796391908. [DOI] [PubMed] [Google Scholar]
- [11].Butterfield DA, Bader Lange ML, Sultana R. Involvements of the lipid peroxidation product, HNE, in the pathogenesis and progression of Alzheimer's disease. Biochim Biophys Acta. 2010;1801:924–929. doi: 10.1016/j.bbalip.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lovell MA, Markesbery WR. Oxidative damage in mild cognitive impairment and early Alzheimer's disease. J Neurosci Res. 2007;85:3036–3040. doi: 10.1002/jnr.21346. [DOI] [PubMed] [Google Scholar]
- [13].Sultana R, Butterfield DA. Oxidatively modified GST and MRP1 in Alzheimer's disease brain: implications for accumulation of reactive lipid peroxidation products. Neurochem Res. 2004;29:2215–2220. doi: 10.1007/s11064-004-7028-0. [DOI] [PubMed] [Google Scholar]
- [14].Renes J, de Vries EG, Nienhuis EF, Jansen PL, Muller M. ATP- and glutathione-dependent transport of chemotherapeutic drugs by the multidrug resistance protein MRP1. Br J Pharmacol. 1999;126:681–688. doi: 10.1038/sj.bjp.0702360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lovell MA, Xie C, Markesbery WR. Decreased glutathione transferase activity in brain and ventricular fluid in Alzheimer's disease. Neurology. 1998;51:1562–1566. doi: 10.1212/wnl.51.6.1562. [DOI] [PubMed] [Google Scholar]
- [16].Xie C, Lovell MA, Markesbery WR. Glutathione transferase protects neuronal cultures against four hydroxynonenal toxicity. Free Radic Biol Med. 1998;25:979–988. doi: 10.1016/s0891-5849(98)00186-5. [DOI] [PubMed] [Google Scholar]
- [17].Chrestensen CA, Starke DW, Mieyal JJ. Acute cadmium exposure inactivates thioltransferase (Glutaredoxin), inhibits intracellular reduction of protein-glutathionyl-mixed disulfides, and initiates apoptosis. J Biol Chem. 2000;275:26556–26565. doi: 10.1074/jbc.M004097200. [DOI] [PubMed] [Google Scholar]
- [18].Newman SF, Sultana R, Perluigi M, Coccia R, Cai J, Pierce WM, Klein JB, Turner DM, Butterfield DA. An increase in S-glutathionylated proteins in the Alzheimer's disease inferior parietal lobule, a proteomics approach. J Neurosci Res. 2007;85:1506–1514. doi: 10.1002/jnr.21275. [DOI] [PubMed] [Google Scholar]
- [19].Di Domenico F, Cenini G, Sultana R, Perluigi M, Uberti D, Memo M, Butterfield DA. Glutathionylation of the pro-apoptotic protein p53 in Alzheimer's disease brain: implications for AD pathogenesis. Neurochem Res. 2009;34:727–733. doi: 10.1007/s11064-009-9924-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Castegna A, Aksenov M, Thongboonkerd V, Klein JB, Pierce WM, Booze R, Markesbery WR, Butterfield DA. Proteomic identification of oxidatively modified proteins in Alzheimer's disease brain. Part II: dihydropyrimidinase-related protein 2, alpha-enolase and heat shock cognate 71. J Neurochem. 2002;82:1524–1532. doi: 10.1046/j.1471-4159.2002.01103.x. [DOI] [PubMed] [Google Scholar]
- [21].Castegna A, Aksenov M, Aksenova M, Thongboonkerd V, Klein JB, Pierce WM, Booze R, Markesbery WR, Butterfield DA. Proteomic identification of oxidatively modified proteins in Alzheimer's disease brain. Part I: creatine kinase BB, glutamine synthase, and ubiquitin carboxy-terminal hydrolase L-1. Free Radic Biol Med. 2002;33:562–571. doi: 10.1016/s0891-5849(02)00914-0. [DOI] [PubMed] [Google Scholar]
- [22].Sultana R, Perluigi M, Butterfield DA. Redox proteomics identification of oxidatively modified proteins in Alzheimer's disease brain and in vivo and in vitro models of AD centered around Abeta(1–42) J Chromatogr B Analyt Technol Biomed Life Sci. 2006;833:3–11. doi: 10.1016/j.jchromb.2005.09.024. [DOI] [PubMed] [Google Scholar]
- [23].Vanhanen M, Soininen H. Glucose intolerance, cognitive impairment and Alzheimer's disease. Curr Opin Neurol. 1998;11:673–677. doi: 10.1097/00019052-199812000-00011. [DOI] [PubMed] [Google Scholar]
- [24].Butterfield DA, Hardas SS, Lange ML. Oxidatively modified glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and Alzheimer's disease: many pathways to neurodegeneration. J Alzheimers Dis. 2010;20:369–393. doi: 10.3233/JAD-2010-1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Butterfield DA, Lange ML. Multifunctional roles of enolase in Alzheimer's disease brain: beyond altered glucose metabolism. J Neurochem. 2009;111:915–933. doi: 10.1111/j.1471-4159.2009.06397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Liu R, Choi J. Age-associated decline in gamma-glutamylcysteine synthetase gene expression in rats. Free Radic Biol Med. 2000;28:566–574. doi: 10.1016/s0891-5849(99)00269-5. [DOI] [PubMed] [Google Scholar]
- [27].Calabrese V, Sultana R, Scapagnini G, Guagliano E, Sapienza M, Bella R, Kanski J, Pennisi G, Mancuso C, Stella AM, Butterfield DA. Nitrosative stress, cellular stress response, and thiol homeostasis in patients with Alzheimer's disease. Antioxid Redox Signal. 2006;8:1975–1986. doi: 10.1089/ars.2006.8.1975. [DOI] [PubMed] [Google Scholar]
- [28].Lloret A, Badia MC, Mora NJ, Pallardo FV, Alonso MD, Vina J. Vitamin E paradox in Alzheimer's disease: it does not prevent loss of cognition and may even be detrimental. J Alzheimers Dis. 2009;17:143–149. doi: 10.3233/JAD-2009-1033. [DOI] [PubMed] [Google Scholar]
- [29].Petersen RC. Mild cognitive impairment: transition between aging and Alzheimer's disease. Neurologia. 2000;15:93–101. [PubMed] [Google Scholar]
- [30].Portet F, Ousset PJ, Touchon J. What is a mild cognitive impairment? Rev Prat. 2005;55:1891–1894. [PubMed] [Google Scholar]
- [31].Sultana R, Piroddi M, Galli F, Butterfield DA. Protein levels and activity of some antioxidant enzymes in hippocampus of subjects with amnestic mild cognitive impairment. Neurochem Res. 2008;33:2540–2546. doi: 10.1007/s11064-008-9593-0. [DOI] [PubMed] [Google Scholar]
- [32].Keller JN, Schmitt FA, Scheff SW, Ding Q, Chen Q, Butterfield DA, Markesbery WR. Evidence of increased oxidative damage in subjects with mild cognitive impairment. Neurology. 2005;64:1152–1156. doi: 10.1212/01.WNL.0000156156.13641.BA. [DOI] [PubMed] [Google Scholar]
- [33].Reed T, Perluigi M, Sultana R, Pierce WM, Klein JB, Turner DM, Coccia R, Markesbery WR, Butterfield DA. Redox proteomic identification of 4-hydroxy-2-nonenal-modified brain proteins in amnestic mild cognitive impairment: insight into the role of lipid peroxidation in the progression and pathogenesis of Alzheimer's disease. Neurobiol Dis. 2008;30:107–120. doi: 10.1016/j.nbd.2007.12.007. [DOI] [PubMed] [Google Scholar]
- [34].Padurariu M, Ciobica A, Hritcu L, Stoica B, Bild W, Stefanescu C. Changes of some oxidative stress markers in the serum of patients with mild cognitive impairment and Alzheimer's disease. Neurosci Lett. 2010;469:6–10. doi: 10.1016/j.neulet.2009.11.033. [DOI] [PubMed] [Google Scholar]
- [35].Pratico D, Clark CM, Liun F, Rokach J, Lee VY, Trojanowski JQ. Increase of brain oxidative stress in mild cognitive impairment: a possible predictor of Alzheimer disease. Arch Neurol. 2002;59:972–976. doi: 10.1001/archneur.59.6.972. [DOI] [PubMed] [Google Scholar]
- [36].Greilberger J, Koidl C, Greilberger M, Lamprecht M, Schroecksnadel K, Leblhuber F, Fuchs D, Oettl K. Malondialdehyde, carbonyl proteins and albumin-disulphide as useful oxidative markers in mild cognitive impairment and Alzheimer's disease. Free Radic Res. 2008;42:633–638. doi: 10.1080/10715760802255764. [DOI] [PubMed] [Google Scholar]
- [37].Baldeiras I, Santana I, Proenca MT, Garrucho MH, Pascoal R, Rodrigues A, Duro D, Oliveira CR. Peripheral oxidative damage in mild cognitive impairment and mild Alzheimer's disease. J Alzheimers Dis. 2008;15:117–128. doi: 10.3233/jad-2008-15110. [DOI] [PubMed] [Google Scholar]
- [38].Sultana R, Mecocci P, Mangialasche F, Cecchetti R, Baglioni M, Butterfield DA. Increased protein and lipid oxidative damage in mitochondria isolated from lymphocytes from patients with Alzheimer's disease: insights into the role of oxidative stress in Alzheimer's disease and initial investigations into a potential biomarker for this dementing disorder. J Alzheimers Dis. 2011;24:77–84. doi: 10.3233/JAD-2011-101425. [DOI] [PubMed] [Google Scholar]
- [39].Baldeiras I, Santana I, Proenca MT, Garrucho MH, Pascoal R, Rodrigues A, Duro D, Oliveira CR. Oxidative damage and progression to Alzheimer's disease in patients with mild cognitive impairment. J Alzheimers Dis. 2010;21:1165–1177. doi: 10.3233/jad-2010-091723. [DOI] [PubMed] [Google Scholar]
- [40].Pocernich CB, La Fontaine M, Butterfield DA. In-vivo glutathione elevation protects against hydroxyl free radical-induced protein oxidation in rat brain. Neurochem Int. 2000;36:185–191. doi: 10.1016/s0197-0186(99)00126-6. [DOI] [PubMed] [Google Scholar]
- [41].Anderson ME, Luo JL. Glutathione therapy: from prodrugs to genes. Semin Liver Dis. 1998;18:415–424. doi: 10.1055/s-2007-1007174. [DOI] [PubMed] [Google Scholar]
- [42].Farr SA, Poon HF, Dogrukol-Ak D, Drake J, Banks WA, Eyerman E, Butterfield DA, Morley JE. The antioxidants alpha-lipoic acid and N-acetylcysteine reverse memory impairment and brain oxidative stress in aged SAMP8 mice. J Neurochem. 2003;84:1173–1183. doi: 10.1046/j.1471-4159.2003.01580.x. [DOI] [PubMed] [Google Scholar]
- [43].Koppal T, Drake J, Butterfield DA. In vivo modulation of rodent glutathione and its role in peroxynitrite-induced neocortical synaptosomal membrane protein damage. Biochim Biophys Acta. 1999;1453:407–411. doi: 10.1016/s0925-4439(99)00014-9. [DOI] [PubMed] [Google Scholar]
- [44].Pocernich CB, Cardin AL, Racine CL, Lauderback CM, Butterfield DA. Glutathione elevation and its protective role in acrolein-induced protein damage in synaptosomal membranes: relevance to brain lipid peroxidation in neurodegenerative disease. Neurochem Int. 2001;39:141–149. doi: 10.1016/s0197-0186(01)00012-2. [DOI] [PubMed] [Google Scholar]
- [45].LaFontaine MA, Geddes JW, Butterfield DA. 3-nitropropionic acid-induced changes in bilayer fluidity in synaptosomal membranes: implications for Huntington's disease. Neurochem Res. 2002;27:507–511. doi: 10.1023/a:1019852720521. [DOI] [PubMed] [Google Scholar]
- [46].Zhang Q, Tian H, Fu X, Zhang G. Delayed activation and regulation of MKK7 in hippocampal CA1 region following global cerebral ischemia in rats. Life Sci. 2003;74:37–45. doi: 10.1016/j.lfs.2003.06.025. [DOI] [PubMed] [Google Scholar]
- [47].Fu AL, Dong ZH, Sun MJ. Protective effect of N-acetyl-L-cysteine on amyloid beta-peptide-induced learning and memory deficits in mice. Brain Res. 2006;1109:201–206. doi: 10.1016/j.brainres.2006.06.042. [DOI] [PubMed] [Google Scholar]
- [48].Huang Q, Aluise CD, Joshi G, Sultana R, St Clair DK, Markesbery WR, Butterfield DA. Potential in vivo amelioration by N-acetyl-L-cysteine of oxidative stress in brain in human double mutant APP/PS-1 knock-in mice: toward therapeutic modulation of mild cognitive impairment. J Neurosci Res. 2010;88:2618–2629. doi: 10.1002/jnr.22422. [DOI] [PubMed] [Google Scholar]
- [49].Estus S, Tucker HM, van Rooyen C, Wright S, Brigham EF, Wogulis M, Rydel RE. Aggregated amyloid-beta protein induces cortical neuronal apoptosis and concomitant “apoptotic” pattern of gene induction. J Neurosci. 1997;17:7736–7745. doi: 10.1523/JNEUROSCI.17-20-07736.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Hsiao YH, Chen PS, Yeh SH, Lin CH, Gean PW. N-acetylcysteine prevents beta-amyloid toxicity by a stimulatory effect on p35/cyclin-dependent kinase 5 activity in cultured cortical neurons. J Neurosci Res. 2008;86:2685–2695. doi: 10.1002/jnr.21710. [DOI] [PubMed] [Google Scholar]
- [51].Xu Y, Hou XY, Liu Y, Zong YY. Different protection of K252a and N-acetyl-L-cysteine against amyloid-beta peptide-induced cortical neuron apoptosis involving inhibition of MLK3-MKK7-JNK3 signal cascades. J Neurosci Res. 2009;87:918–927. doi: 10.1002/jnr.21909. [DOI] [PubMed] [Google Scholar]
- [52].Yan CY, Greene LA. Prevention of PC12 cell death by N-acetylcysteine requires activation of the Ras pathway. J Neurosci. 1998;18:4042–4049. doi: 10.1523/JNEUROSCI.18-11-04042.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Studer R, Baysang G, Brack C. N-Acetyl-L-Cystein downregulates beta-amyloid precursor protein gene transcription in human neuroblastoma cells. Biogerontology. 2001;2:55–60. doi: 10.1023/a:1010065103073. [DOI] [PubMed] [Google Scholar]
- [54].Tucker S, Ahl M, Cho HH, Bandyopadhyay S, Cuny GD, Bush AI, Goldstein LE, Westaway D, Huang X, Rogers JT. RNA therapeutics directed to the non coding regions of APP mRNA, in vivo anti-amyloid efficacy of paroxetine, erythromycin, and N-acetyl cysteine. Curr Alzheimer Res. 2006;3:221–227. doi: 10.2174/156720506777632835. [DOI] [PubMed] [Google Scholar]
- [55].Olivieri G, Baysang G, Meier F, Muller-Spahn F, Stahelin HB, Brockhaus M, Brack C. N-acetyl-L-cysteine protects SHSY5Y neuroblastoma cells from oxidative stress and cell cytotoxicity: effects on beta-amyloid secretion and tau phosphorylation. J Neurochem. 2001;76:224–233. doi: 10.1046/j.1471-4159.2001.00090.x. [DOI] [PubMed] [Google Scholar]
- [56].Lee MS, Kao SC, Lemere CA, Xia W, Tseng HC, Zhou Y, Neve R, Ahlijanian MK, Tsai LH. APP processing is regulated by cytoplasmic phosphorylation. J Cell Biol. 2003;163:83–95. doi: 10.1083/jcb.200301115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Pastorino L, Sun A, Lu PJ, Zhou XZ, Balastik M, Finn G, Wulf G, Lim J, Li SH, Li X, Xia W, Nicholson LK, Lu KP. The prolyl isomerase Pin1 regulates amyloid precursor protein processing and amyloid-beta production. Nature. 2006;440:528–534. doi: 10.1038/nature04543. [DOI] [PubMed] [Google Scholar]
- [58].Sultana R, Boyd-Kimball D, Poon HF, Cai J, Pierce WM, Klein JB, Markesbery WR, Zhou XZ, Lu KP, Butterfield DA. Oxidative modification and down-regulation of Pin1 in Alzheimer's disease hippocampus: A redox proteomics analysis. Neurobiol Aging. 2006;27:918–925. doi: 10.1016/j.neurobiolaging.2005.05.005. [DOI] [PubMed] [Google Scholar]
- [59].Sultana R, Boyd-Kimball D, Cai J, Pierce WM, Klein JB, Merchant M, Butterfield DA. Proteomics analysis of the Alzheimer's disease hippocampal proteome. J Alzheimers Dis. 2007;11:153–164. doi: 10.3233/jad-2007-11203. [DOI] [PubMed] [Google Scholar]
- [60].Aluise CD, Robinson RA, Beckett TL, Murphy MP, Cai J, Pierce WM, Markesbery WR, Butterfield DA. Preclinical Alzheimer disease: brain oxidative stress, Abeta peptide and proteomics. Neurobiol Dis. 2010;39:221–228. doi: 10.1016/j.nbd.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Xiao L, Zhao D, Chan WH, Choi MM, Li HW. Inhibition of beta 1–40 amyloid fibrillation with N-acetyl-L-cysteine capped quantum dots. Biomaterials. 2010;31:91–98. doi: 10.1016/j.biomaterials.2009.09.014. [DOI] [PubMed] [Google Scholar]
- [62].Wang DS, Lipton RB, Katz MJ, Davies P, Buschke H, Kuslansky G, Verghese J, Younkin SG, Eckman C, Dickson DW. Decreased neprilysin immunoreactivity in Alzheimer disease, but not in pathological aging. J Neuropathol Exp Neurol. 2005;64:378–385. doi: 10.1093/jnen/64.5.378. [DOI] [PubMed] [Google Scholar]
- [63].Wang DS, Iwata N, Hama E, Saido TC, Dickson DW. Oxidized neprilysin in aging and Alzheimer's disease brains. Biochem Biophys Res Commun. 2003;310:236–241. doi: 10.1016/j.bbrc.2003.09.003. [DOI] [PubMed] [Google Scholar]
- [64].Wang R, Malter JS, Wang DS. N-acetylcysteine prevents 4-hydroxynonenal- and amyloid-beta-induced modification and inactivation of neprilysin in SH-SY5Y cells. J Alzheimers Dis. 2010;19:179–189. doi: 10.3233/JAD-2010-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Fuller S, Steele M, Munch G. Activated astroglia during chronic inflammation in Alzheimer's disease--do they neglect their neurosupportive roles? Mutat Res. 2010;690:40–49. doi: 10.1016/j.mrfmmm.2009.08.016. [DOI] [PubMed] [Google Scholar]
- [66].Lee M, Cho T, Jantaratnotai N, Wang YT, McGeer E, McGeer PL. Depletion of GSH in glial cells induces neurotoxicity: relevance to aging and degenerative neurological diseases. FASEB J. 2010;24:2533–2545. doi: 10.1096/fj.09-149997. [DOI] [PubMed] [Google Scholar]
- [67].Pahan K, Sheikh FG, Namboodiri AM, Singh I. N-acetyl cysteine inhibits induction of no production by endotoxin or cytokine stimulated rat peritoneal macrophages, C6 glial cells and astrocytes. Free Radic Biol Med. 1998;24:39–48. doi: 10.1016/s0891-5849(97)00137-8. [DOI] [PubMed] [Google Scholar]
- [68].Adair JC, Knoefel JE, Morgan N. Controlled trial of N-acetylcysteine for patients with probable Alzheimer's disease. Neurology. 2001;57:1515–1517. doi: 10.1212/wnl.57.8.1515. [DOI] [PubMed] [Google Scholar]
- [69].Remington R, Chan A, Paskavitz J, Shea TB. Efficacy of a vitamin/nutriceutical formulation for moderate-stage to later-stage Alzheimer's disease: a placebo-controlled pilot study. Am J Alzheimers Dis Other Demen. 2009;24:27–33. doi: 10.1177/1533317508325094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Sano M, Ernesto C, Thomas RG, Klauber MR, Schafer K, Grundman M, Woodbury P, Growdon J, Cotman CW, Pfeiffer E, Schneider LS, Thal LJ. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer's disease. The Alzheimer's Disease Cooperative Study. N Engl J Med. 1997;336:1216–1222. doi: 10.1056/NEJM199704243361704. [DOI] [PubMed] [Google Scholar]
- [71].Le Bars PL, Velasco FM, Ferguson JM, Dessain EC, Kieser M, Hoerr R. Influence of the severity of cognitive impairment on the effect of the Gnkgo biloba extract EGb 761 in Alzheimer's disease. Neuropsychobiology. 2002;45:19–26. doi: 10.1159/000048668. [DOI] [PubMed] [Google Scholar]
- [72].Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, Galasko D, Jin S, Kaye J, Levey A, Pfeiffer E, Sano M, van Dyck CH, Thal LJ. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352:2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- [73].Kamat CD, Gadal S, Mhatre M, Williamson KS, Pye QN, Hensley K. Antioxidants in central nervous system diseases: preclinical promise and translational challenges. J Alzheimers Dis. 2008;15:473–493. doi: 10.3233/jad-2008-15314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Pratico D. Evidence of oxidative stress in Alzheimer's disease brain and antioxidant therapy: lights and shadows. Ann N Y Acad Sci. 2008;1147:70–78. doi: 10.1196/annals.1427.010. [DOI] [PubMed] [Google Scholar]
- [75].Anderson ME, Meister A. Transport and direct utilization of gamma-glutamylcyst(e)ine for glutathione synthesis. Proc Natl Acad Sci U S A. 1983;80:707–711. doi: 10.1073/pnas.80.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Hoshida S, Kuzuya T, Yamashita N, Nishida M, Kitahara S, Hori M, Kamada T, Tada M. gamma-Glutamylcysteine ethyl ester for myocardial protection in dogs during ischemia and reperfusion. J Am Coll Cardiol. 1994;24:1391–1397. doi: 10.1016/0735-1097(94)90125-2. [DOI] [PubMed] [Google Scholar]
- [77].Okamoto T, Mizuta K, Takahashi T, Kishi T, Kitahara S, Komori S, Hashimoto K, Goshima K. Protective effect of gamma-glutamylcysteinylethyl ester on dysfunction of the selenium-deficient rat heart. Biochem Pharmacol. 1999;57:955–963. doi: 10.1016/s0006-2952(98)00361-x. [DOI] [PubMed] [Google Scholar]
- [78].Drake J, Kanski J, Varadarajan S, Tsoras M, Butterfield DA. Elevation of brain glutathione by gamma-glutamylcysteine ethyl ester protects against peroxynitrite-induced oxidative stress. J Neurosci Res. 2002;68:776–784. doi: 10.1002/jnr.10266. [DOI] [PubMed] [Google Scholar]
- [79].Drake J, Sultana R, Aksenova M, Calabrese V, Butterfield DA. Elevation of mitochondrial glutathione by gamma-glutamylcysteine ethyl ester protects mitochondria against peroxynitrite-induced oxidative stress. J Neurosci Res. 2003;74:917–927. doi: 10.1002/jnr.10810. [DOI] [PubMed] [Google Scholar]
- [80].Boyd-Kimball D, Sultana R, Abdul HM, Butterfield DA. Gamma-glutamylcysteine ethyl ester-induced up-regulation of glutathione protects neurons against Abeta(1–42)-mediated oxidative stress and neurotoxicity: implications for Alzheimer's disease. J Neurosci Res. 2005;79:700–706. doi: 10.1002/jnr.20394. [DOI] [PubMed] [Google Scholar]
- [81].Boyd-Kimball D, Sultana R, Poon HF, Mohmmad-Abdul H, Lynn BC, Klein JB, Butterfield DA. Gamma-glutamylcysteine ethyl ester protection of proteins from Abeta(1–42)-mediated oxidative stress in neuronal cell culture: a proteomics approach. J Neurosci Res. 2005;79:707–713. doi: 10.1002/jnr.20393. [DOI] [PubMed] [Google Scholar]
- [82].Abramov AY, Canevari L, Duchen MR. Changes in intracellular calcium and glutathione in astrocytes as the primary mechanism of amyloid neurotoxicity. J Neurosci. 2003;23:5088–5095. doi: 10.1523/JNEUROSCI.23-12-05088.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Medina S, Martinez M, Hernanz A. Antioxidants inhibit the human cortical neuron apoptosis induced by hydrogen peroxide, tumor necrosis factor alpha, dopamine and beta-amyloid peptide 1–42. Free Radic Res. 2002;36:1179–1184. doi: 10.1080/107157602100006445. [DOI] [PubMed] [Google Scholar]
- [84].Barber VS, Griffiths HR. Is glutathione an important neuroprotective effector molecule against amyloid beta toxicity? Biofactors. 2003;17:215–228. doi: 10.1002/biof.5520170121. [DOI] [PubMed] [Google Scholar]
- [85].Turunc E, Kanit L, Yalcin A. Effect of gamma-glutamylcysteine ethylester on the levels of c-fos mRNA expression, glutathione and reactive oxygen species formation in kainic acid excitotoxicity. J Pharm Pharmacol. 2010;62:1010–1017. doi: 10.1111/j.2042-7158.2010.01122.x. [DOI] [PubMed] [Google Scholar]