Abstract
Inbred strain variants of the Cdh23 gene have been shown to influence the onset and progression of age-related hearing loss (AHL) in mice. In linkage backcrosses, the recessive Cdh23 allele (ahl) of the C57BL/6J strain, when homozygous, confers increased susceptibility to AHL, while the dominant allele (Ahl+) of the CBA/CaJ strain confers resistance. To determine the isolated effects of these alleles on different strain backgrounds, we produced the reciprocal congenic strains B6.CBACa-Cdh23Ahl+and CBACa.B6-Cdh23ahl and tested 15-30 mice from each for hearing loss progression. ABR thresholds for 8 kHz, 16 kHz, and 32 kHz pure-tone stimuli were measured at 3, 6, 9, 12, 15 and 18 months of age and compared with age-matched mice of the C57BL/6J and CBA/CaJ parental strains. Mice of the C57BL/6N strain, which is the source of embryonic stem cells for the large International Knockout Mouse Consortium, were also tested for comparisons with C57BL/6J mice. Mice of the C57BL/6J and C57BL/6N strains exhibited identical hearing loss profiles: their 32 kHz ABR thresholds were significantly higher than those of CBA/CaJ and congenic strain mice by 6 months of age, and their 16 kHz thresholds were significantly higher by 12 months. Thresholds of the CBA/CaJ, the B6.CBACa-Cdh23Ahl+, and the CBACa.B6-Cdh23ahl strain mice differed little from one another and only slightly increased throughout the 18-month test period. Hearing loss, which corresponded well with cochlear hair cell loss, was most profound in the C57BL/6J and C57BL/6NJ strains. These results indicate that the CBA/CaJ-derived Cdh23Ahl+ allele dramatically lessens hearing loss and hair cell death in an otherwise C57BL/6J genetic background, but that the C57BL/6J-derived Cdh23ahl allele has little effect on hearing loss in an otherwise CBA/CaJ background. We conclude that although Cdh23ahl homozygosity is necessary, it is not by itself sufficient to account for the accelerated hearing loss of C57BL/6J mice.
Keywords: age-related hearing loss, Cdh23, ahl, C57BL/6J, C57BL/6N, CBA/CaJ, inbred mouse strains, congenic mouse strains, cochleograms, ABR thresholds, hair cells
1. Introduction
Age related hearing loss (AHL), or presbycusis, poses a serious human health concern as it negatively affects the life of many elderly individuals (Dalton et al., 2003). In a 2003 CDC study, presbycusis was found to be second only to arthritis as a handicapping condition within the elderly segment of our society (Bielefeld et al., 2010). Susceptibility to AHL has a significant genetic component although AHL presents with a complex pathophysiology (DeStefano et al., 2003). A range of environmental factors, including exposure to loud noises or ototoxic drugs, can exacerbate and accelerate AHL. The high degree of genetic complexity in human populations coupled with highly variable environmental factors make it difficult to identify the genetic components underlying AHL in the human population, although some limited success has been achieved (Bared et al., 2010; DeStefano et al., 2003; Friedman et al., 2009; Garringer et al., 2006; Rodriguez-Paris et al., 2008; Unal et al., 2005).
Laboratory mice are more amenable to studies of AHL predisposition because of their well-defined genetics, short life span and the ability of researchers to control for environmental factors. Like humans, many inbred mouse strains express variable degrees of AHL, and at least ten quantitative trait loci associated with AHL have been identified in mice (Latoche et al., 2011; Noben-Trauth et al., 2009). C57BL/6J (B6J) and CBA/CaJ (CBA) are the two most widely used inbred strains in hearing research. The B6J strain has been used extensively as a model of early onset AHL (Henry et al., 1980; Hequembourg et al., 2001b; Mikaelian, 1979; Wang et al., 2008; Willott, 1986). B6J mice exhibit a high frequency hearing loss by 3-6 months of age that progresses to a profound impairment by 15 months. In contrast, CBA mice remain resistant to AHL until 15 months of age or older and are often used as good hearing controls (Henry et al., 1980; Li et al., 1991; Zheng et al., 1999). B6J and CBA mice also exhibit dramatically different rates of cochlear hair cell loss (Spongr et al., 1997b).
One of the major genetic factors contributing to hearing loss in B6J mice is the ahl locus on Chromosome 10 (Johnson et al., 1997), which has been shown to be a splice variant of the cadherin 23 gene (Cdh23) (Noben-Trauth et al., 2003). The CBA strain does not have this predisposing Cdh23 splice variant, consistent with its much slower rate of hearing loss. Cadherin 23 has been shown to be a component of the tip links of hair cell stereocilia, along with protocadherin 15 (Kazmierczak et al., 2007). Mutations in the genes encoding these two proteins affect tip link formation and stability, which is necessary for normal hair cell mechanotransduction (Alagramam et al., 2011). Mice with an ENU-induced missense mutation in the ectodomain of Cdh23 show a loss of tip links that correlates with a progressive hearing loss that is similar to, but more severe than the hearing loss associated with the ahl splice variant of Cdh23 (Schwander et al., 2009) .
Here we report on the characterization of two reciprocal congenic strains that we developed to evaluate the isolated effects of Cdh23 allelic variants when on different strain backgrounds. The CBACa.B6-Cdh23ahl congenic stain (CBA.B6-ahl) is homozygous for the recessive AHL susceptibility allele (ahl) of Cdh23, which we transferred from the B6J strain onto the CBA background. The reciprocal B6.CBACa-Cdh23Ahl+ congenic strain (B6.CBA-Ahl+) is homozygous for the dominant AHL resistance allele (Ahl+) of Cdh23, which we transferred from the CBA strain onto the B6J background. To follow the progression of hearing loss in the congenic strain mice and mice of the B6J and CBA parental strains, auditory brainstem response (ABR) thresholds were measured at 3, 6, 9, 12, 15 and 18 months of age. After the final ABR test, inner ears of mice from each strain were examined for cochlear hair cell loss to determine its correspondence with hearing loss..
We also report on hearing loss in mice of the C57BL/6N strain (B6N), an NIH subline of C57BL/6 separated from B6J in 1951. A complete description of the B6N strain at The Jackson Laboratory (C57BL/6NJ, Stock #005304) and its development is given in the JAX mice database (http://jaxmice.jax.org/strain/005304.html). Like B6J, the B6N strain carries the Cdh23ahl hearing loss susceptibility allele, but it has not previously been assessed for AHL. Because the B6N strain is the source of embryonic stem cells for the International Knockout Mouse Consortium (http://www.knockoutmouse.org/), establishment of a hearing profile for this control strain is important for assessing hearing-related phenotypes of the many knockout mice being generated by this program.
2. Materials and methods
2.1 Mice and congenic strain development
All mice examined in this study originated from The Jackson Laboratory (http://www.jax.org/), and all procedures involving their use were approved by the Institutional Animal Care and Use Committee. The Jackson Laboratory is accredited by the American Association for the Accreditation of Laboratory Animal Care.
We use the following abbreviated strain designations throughout the paper, which are followed in parentheses by their corresponding full inbred strain names and Jackson Laboratory Stock Numbers: CBA (CBA/CaJ, Stock #000654), B6J (C57BL/6J, Stock #000664), B6N (C57BL/6NJ, Stock #005304), B6.CBA-Ahl+ (B6.CBACa-Cdh23CBA/CaJ, Stock #10615) and CBA.B6-ahl (CBACa.B6-Cdh23ahl, Stock #10614). The region of the Cdh23 gene that is associated with AHL in inbred mouse strains (Noben-Trauth et al., 2003) was amplified by PCR from the genomic DNA of each of these strains, and Cdh23ahl and Cdh23Ahl+genotypes were confirmed by DNA sequence analysis (Supplementary Fig.1).
The B6.CBA-Ahl+and CBA.B6-ahl reciprocal congenic strains were developed by genetic introgression of B6J- and CBA-derived segments of Chromosome 10 (containing the Cdh23 gene) into the genomes of CBA and B6J, respectively. To accomplish this introgression, mice from the B6J and CBA parental strains were first intercrossed to generate F1 hybrids. The F1 hybrids were then backcrossed to B6J mice to produce N2 generation mice for development of the B6.CBA-Ahl+ strain and to CBA mice to produce N2 mice for development of the CBA.B6-ahl strain. Repeated backcrossing of hybrid progeny (selected on the basis of Chromosome 10 marker genotypes) to the parental (B6J or CBA) strain continued until 10 backcross generations (N10) were completed. Finally, the N10 generation mice of each line were interbred to produce the two homozygous congenic strains. To define the extent of the congenic regions in each strain, single nucleotide polymorphisms (SNPs) along the length of Chromosome 10 were genotyped. The CBA-derived congenic interval of B6.CBA-Ahl+ extends from 53.46 Mb (SNP rs3696307) to 105.44 Mb (SNP rs13480749), and the B6J-derived congenic interval of CBA.B6-ahl extends from 38.69 Mb (SNP rs13480581) to 74.61 Mb (SNP rs13480654).
2.2 Hearing assessment: auditory-evoked brainstem response
Hearing in mice was assessed by ABR threshold analysis, essentially as previously described (Zheng et al., 1999). Mice were anesthetized with an intraperitoneal injection of tribromoethanol (0.2 ml of 12.5 mg/ml stock per 10 g of body weight), and then placed onto a 37 °C heating pad in a sound-attenuating chamber. The evoked brainstem responses to 8, 16 and 32kHz tone-bursts stimuli were amplified and averaged. By varying the sound pressure level (SPL) in 5 dB increments, ABR thresholds were defined as the lowest dB SPL level at which an ABR pattern could be clearly recognized with peaks 1-2 being the most prominent at high levels and still recognizable at intensities near threshold. Stimulus presentation, data acquisition and analysis were performed using computerized equipment from Intelligent Hearing Systems (IHS; Miami, Florida). 100 dB was the maximum SPL presented for all stimuli. With our testing system, average ABR thresholds (in dB SPL) for normal hearing mice are about 40 dB for 8 kHz, 20 dB for 16 kHz, and 45 dB for 32 kHz stimuli.
2.3 Cochlear histology
Cochleae were prepared and evaluated as described in detail in earlier publications (Ding et al., 2001; Johnson et al., 2010; Zheng et al., 2009). Mice were euthanized by CO2 asphyxiation, and decapitated. The temporal bones were removed, immersed in 4% paraformaldehyde, and shipped to the University of Buffalo for analysis. Cochleae were stained with Ehrlich's hematoxylin solution, the organ of Corti carefully microdissected out into two or three segments and mounted as a flat surface preparation in glycerin on glass slides. One person, blind to the ABR results, dissected the cochleas and prepared the surface preparation. A second person blind to the experimental conditions made the hair cell counts and had no prior knowledge of the ABR results. Surface preparations were examined with a light microscope (Zeiss Standard, 400X magnification). With careful inspection, the hair cells can be readily distinguished from support cells in hematoxylin stained specimens. By moving the focal plane of the microscope up and down, the person making the hair cell counts can determine if the stereocilia bundle, the cuticular plate and the hair cell nucleus, located slightly below the cuticular plate are present. A hair cell was counted as present if both the cuticular plate and hair cell nucleus were present; if either were missing the cell was counted as missing. Alternatively, if the hair cell is missing, the vacated space is replaced by a phalangeal scar. Inner hair cells (IHC) and outer hair cells (OHC) were counted along successive 0.12-0.24 mm intervals of the organ of Corti beginning at the apex. Cochleograms showing percent hair cells missing as a function of percent total distance from the apex were constructed for each animal. Percent hair cell losses were based on laboratory norms for young CBA/CaJ mice (Ding et al., 2001). Locations along the cochlear duct corresponding to acoustic frequencies were determined using a mouse frequency-place map (Muller et al., 2005).
2.4 Statistical analysis
ABR data analysis was performed using the JMP 7.0 interactive statistics and graphics software program (www.JMP.com). Statistical significance of the differences among means was determined by one-way ANOVA with Tukey test corrections for multiple pair-wise comparisons. Hair cell loss data were analyzed using a 2-way ANOVA with cochlear location a repeated measure. A Tukey post-hoc analysis was used to identify significant differences associated with strain and cochlear location (SigmaStat 3.5 for Windows, version 3.5).
3. Results
3.1 Age-related hearing loss
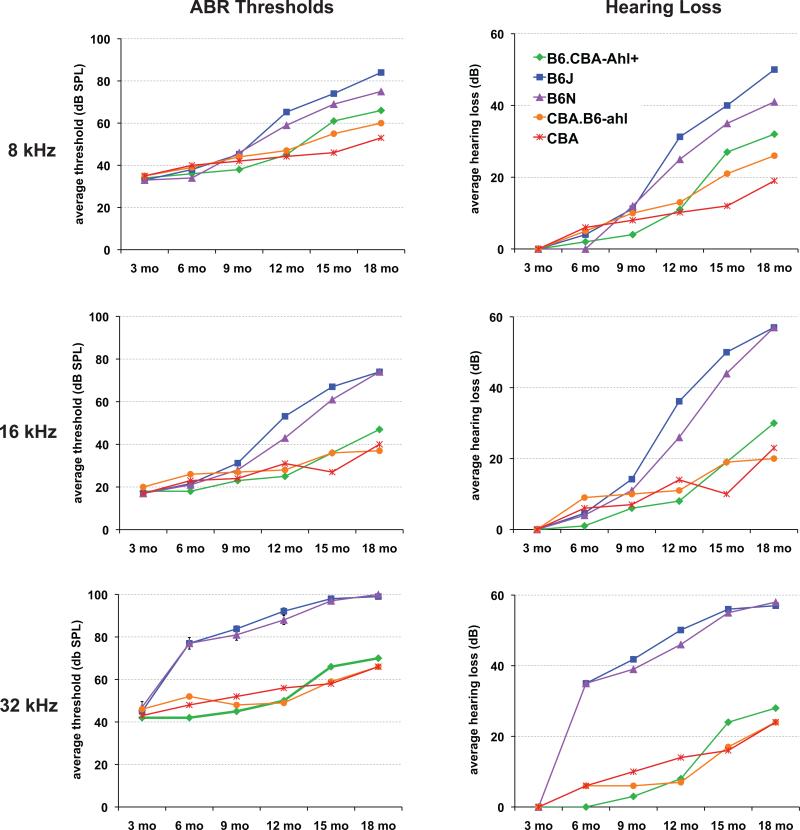
Hearing assessment by ABR threshold analysis was performed for all five strains at 3, 6, 9, 12, 15 and 18 months of age. Table 1 lists the ABR threshold means for each auditory stimulus, the numbers of mice tested, and threshold variance estimates (standard deviations) for each strain and test age. The changes in ABR thresholds for each strain over time are illustrated graphically in Figure 1 for each test stimulus. At 3 months of age all of the strains exhibited normal ABR thresholds for all three of the test frequencies (8, 16, and 32 kHz). B6J and B6N strain mice exhibit a moderate high frequency hearing loss (32 kHz, + 30 dB) by 6 months of age that progresses to almost complete high frequency deafness by 18 months. The 8 kHz and 16 kHz thresholds of B6J and B6N mice remain nearly normal until 9 months of age, but by 12 months of age they are moderately elevated (+ 25-45 dB). Hearing loss in CBA, B6.CBA-Ahl+ and CBA.B6-ahl strain mice occurs later and progresses more slowly than that observed for B6J and B6N mice. CBA, B6.CBA-Ahl+ and CBA.B6-ahl mice exhibit only a mild hearing loss at 12 months of age, which progresses to a moderate impairment (+20-30 dB) by 18 months of age. The degree of ABR threshold variation seen within each strain for all test ages and frequencies (Table 1) reflects the complex nature of AHL, a quantitative trait for which environmental and stochastic factors, as well as genetics, affect the onset time and progression of hearing loss.
Table 1. ABR threshold means, standard deviations, and numbers of mice tested.
8 kHz, 16 kHz and 32 kHz threshold means (in dB SPL) and standard deviations (SD) are given for each strain and age along with the number (N) of mice tested and the gender mix (F, female; M, male). Means presented in bold are 15 dB or greater above those of 3 month old mice.
| B6.CBA-Ahl+ | B6J | B6N | CBA.B6-ahl | CBA/CaJ | ||
|---|---|---|---|---|---|---|
| 3 month test age | N=18 (8F, 10M) | N = 19 (5F, 14M) | N = 30 (14F, 16M) | N = 22 (11F, 11M) | N = 18 (10F, 8M) | |
| 8 kHz | mean | 33.61 | 33.42 | 32.83 | 35.23 | 35.00 |
| SD | 7.63 | 4.43 | 6.39 | 4.99 | 5.15 | |
| 16 kHz | mean | 18.33 | 16.58 | 17.00 | 20.00 | 16.67 |
| SD | 4.85 | 2.91 | 8.37 | 6.17 | 4.85 | |
| 32 kHz | mean | 42.22 | 45.00 | 47.35 | 46.36 | 42.50 |
| SD | 8.61 | 6.24 | 7.52 | 7.43 | 5.49 | |
| 6 month test age | N = 21 (10F, 11M) | N = 16 (10F, 6M) | N = 20 (10F, 10M) | N = 20 (10F, 10M) | N = 23 (11F, 12M) | |
| 8 kHz | mean | 35.95 | 37.50 | 34.00 | 38.75 | 40.43 |
| SD | 5.15 | 6.58 | 5.76 | 5.82 | 6.56 | |
| 16 kHz | mean | 18.10 | 21.56 | 21.25 | 26.00 | 23.48 |
| SD | 5.59 | 9.08 | 8.41 | 6.81 | 8.45 | |
| 32 kHz | mean | 42.38 | 78.13 | 76.75 | 51.54 | 48.04 |
| SD | 6.25 | 10.94 | 5.91 | 7.74 | 8.22 | |
| 9 month test age | N = 21 (5F, 16M) | N = 19 (10F, 9M) | N = 18 (9F, 9M) | N = 19 (10F, 9M) | N = 26 (15F, 11M) | |
| 8 kHz | mean | 37.86 | 45.26 | 46.11 | 43.68 | 41.54 |
| SD | 6.81 | 10.20 | 8.14 | 7.23 | 6.75 | |
| 16 kHz | mean | 22.62 | 30.53 | 27.50 | 26.58 | 24.23 |
| SD | 7.68 | 14.52 | 9.74 | 7.27 | 10.55 | |
| 32 kHz | mean | 45.24 | 83.42 | 81.39 | 47.63 | 52.12 |
| SD | 7.33 | 6.25 | 10.82 | 8.06 | 12.01 | |
| 12 month test age | N = 23 (5F, 18M) | N = 19 (10F, 9M) | N = 17 (8F, 9M) | N = 17 (10F, 7M) | N = 25 (14F, 11M) | |
| 8 kHz | mean | 45.22 | 65.26 | 59.06 | 47.35 | 43.60 |
| SD | 10.50 | 10.86 | 16.75 | 8.31 | 10.66 | |
| 16 kHz | mean | 25.22 | 53.16 | 43.24 | 27.94 | 31.00 |
| SD | 8.59 | 16.52 | 21.65 | 5.88 | 11.81 | |
| 32 kHz | mean | 50.22 | 92.11 | 87.94 | 48.53 | 55.80 |
| SD | 8.19 | 6.73 | 8.85 | 8.06 | 12.13 | |
| 15 month test age | N = 18 (4F, 14M) | N = 16 (10F, 6M) | N = 16 (7F, 9M) | N = 14 (8F, 6M) | N = 11 (6F, 5M) | |
| 8 kHz | mean | 61.39 | 74.38 | 70.63 | 55.36 | 46.36 |
| SD | 10.55 | 8.34 | 7.50 | 10.28 | 5.95 | |
| 16 kHz | mean | 35.83 | 68.13 | 61.25 | 36.07 | 27.27 |
| SD | 9.28 | 5.44 | 11.90 | 15.21 | 2.61 | |
| 32 kHz | mean | 65.56 | 97.19 | 97.81 | 58.93 | 57.73 |
| SD | 13.49 | 4.46 | 2.56 | 8.36 | 7.86 | |
| 18 month test age | N = 13 (2F, 11M) | N = 13 (8F, 5M) | N = 15 (7F, 8M) | N = 11 (7F, 4M) | N = 6 (5F, 1M) | |
| 8 kHz | mean | 66.15 | 84.23 | 75.00 | 59.55 | 52.50 |
| SD | 10.64 | 5.34 | 7.56 | 7.57 | 9.35 | |
| 16 kHz | mean | 46.54 | 74.23 | 74.33 | 37.27 | 40.00 |
| SD | 15.33 | 7.03 | 12.23 | 9.32 | 8.37 | |
| 32 kHz | mean | 70.00 | 99.23 | 99.67 | 66.36 | 65.83 |
| SD | 15.56 | 1.88 | 1.29 | 8.97 | 13.93 | |
Figure 1. Hearing loss progression.
Mice of the B6J, B6N, B6.CBA-Ahl+, CBA.B6-ahl, and CBA strains were tested at 3, 6, 9, 12, 15, and 18 months of age, and ABR thresholds were obtained for pure-tone 8 kHz, 16 kHz and 32 kHz auditory stimuli. For each auditory stimulus, plots of ABR threshold means (dB SPL) for each strain at successive test ages are shown on the left and corresponding plots of hearing loss means (dB) on the right. Numerical values, variance estimates, and numbers of mice tested are given in Table 1, and statistical analyses of mean differences among strains are presented in Table 2 and in Supplementary Figure 2.
Statistical comparisons of ABR threshold means are presented in Table 2 and detailed in Supplementary Fig 2. The threshold means of B6J and B6N strain mice did not significantly differ from one another at any of the ages and frequencies tested, but they significantly diverged from those of the other three strains by 6 months of age for high frequency stimuli (32 kHz), and by 12 months of age for lower frequencies (8 and 16 kHz) as well. Throughout the test period, no statistically significant differences in threshold means were detected between CBA.B6-ahl congenic strain mice and CBA mice. At 15 and 18 months of age, mean thresholds of B6.CBA-Ahl+ mice were greater than those of CBA mice for all test frequencies (Fig. 1), and statistically significantly different for the 8 kHz stimulus (Table 2).
Table 2. Statistical Analysis of ABR thresholds.
One-way ANOVA using the Tukey test was used to determine the statistical significance of ABR threshold mean differences between strains at the 0.05 alpha level (ns, no significant difference). For each auditory stimulus, threshold means were compared between each strain pair combination at each test age.
| 8 kHz ABR thresholds | 3 months | 6 months | 9 months | 12 months | 15 months | 18 months | |
|---|---|---|---|---|---|---|---|
| B6.CBA-Ahl+ | B6J | ns | ns | p<0.05 | p<0.001 | p<0.005 | p<0.001 |
| B6.CBA-Ahl+ | B6N | ns | ns | p<0.05 | p<0.005 | ns | ns |
| B6.CBA-Ahl+ | CBA.B6-ahl | ns | ns | ns | ns | ns | ns |
| B6.CBA-Ahl+ | CBA | ns | ns | ns | ns | p<0.05 | p<0.005 |
| B6J | B6N | ns | ns | ns | ns | ns | ns |
| B6J | CBA.B6-ahl | ns | ns | ns | p<0.001 | p<0.001 | p<0.001 |
| B6J | CBA | ns | ns | ns | p<0.001 | p<0.001 | p<0.001 |
| B6N | CBA.B6-ahl | p<0.05 | ns | ns | p<0.05 | p<0.005 | p<0.001 |
| B6N | CBA | p<0.05 | ns | ns | p<0.001 | p<0.001 | p<0.001 |
| CBA.B6-ahl | CBA | ns | ns | ns | ns | ns | ns |
| 16 kHz ABR thresholds | 3 months | 6 months | 9 months | 12 months | 15 months | 18 months | |
|---|---|---|---|---|---|---|---|
| B6.CBA-Ahl+ | B6J | ns | ns | ns | p<0.001 | p<0.001 | p<0.001 |
| B6.CBA-Ahl+ | B6N | ns | ns | ns | p<0.001 | p<0.001 | p<0.001 |
| B6.CBA-Ahl+ | CBA.B6-ahl | ns | p<0.05 | ns | ns | ns | ns |
| B6.CBA-Ahl+ | CBA | ns | ns | ns | ns | ns | ns |
| B6J | B6N | ns | ns | ns | ns | ns | ns |
| B6J | CBA.B6-ahl | ns | ns | ns | p<0.001 | p<0.001 | p<0.001 |
| B6J | CBA | ns | ns | ns | p<0.001 | p<0.001 | p<0.001 |
| B6N | CBA.B6-ahl | ns | ns | ns | p<0.05 | p<0.001 | p<0.001 |
| B6N | CBA | ns | ns | ns | p<0.05 | p<0.001 | p<0.001 |
| CBA.B6-ahl | CBA | ns | ns | ns | ns | ns | ns |
| 32 kHz ABR thresholds | 3 months | 6 months | 9 months | 12 months | 15 months | 18 months | |
|---|---|---|---|---|---|---|---|
| B6.CBA-Ahl+ | B6J | ns | p<0.001 | p<0.001 | p<0.001 | p<0.001 | p<0.001 |
| B6.CBA-Ahl+ | B6N | ns | p<0.001 | p<0.001 | p<0.001 | p<0.001 | p<0.001 |
| B6.CBA-Ahl+ | CBA.B6-ahl | ns | p<0.05 | ns | ns | ns | ns |
| B6.CBA-Ahl+ | CBA | ns | ns | ns | ns | ns | ns |
| B6J | B6N | ns | ns | ns | ns | ns | ns |
| B6J | CBA.B6-ahl | ns | p<0.001 | p<0.001 | p<0.001 | p<0.001 | p<0.001 |
| B6J | CBA | ns | p<0.001 | p<0.001 | p<0.001 | p<0.001 | p<0.001 |
| B6N | CBA.B6-ahl | ns | p<0.001 | p<0.001 | p<0.001 | p<0.001 | p<0.001 |
| B6N | CBA | ns | p<0.001 | p<0.001 | p<0.001 | p<0.001 | p<0.001 |
| CBA.B6-ahl | CBA | ns | ns | ns | ns | ns | ns |
3.2 Cochlear pathology
After completion of hearing assessments for the final 18-month test age, inner ears of mice from the two congenic strains (B6.CBA-Ahl+ and CBA.B6-ahl) and the two parental strains (B6J and CBA) were transported to the University at Buffalo for analysis as noted in Section 2.3. Cochlear surface preparations were made to visualize the hair cells of mice from each of the four strains. Figure 2 shows a series of photomicrographs of hematoxylin-stained surface preparations from the apex (panel A), middle (panel B) and basal (panel C) regions of the cochlea of a B6.CBA-Ahl+ mouse; the focal plane of the images was adjusted to be near the nuclei of the OHC. In this example, most of the OHC and IHC were present near the apex and middle of the cochlear whereas many OHC and some IHC were missing in the base of the cochlea. Figure 3 shows lower magnification (200X), representative photomicrographs centered roughly 40-60% (~10-20 kHz region) of the distance from the apex of the cochlea of the four strains (Muller et al., 2005). As expected, all the IHC and OHC were present in the CBA parental stain (Fig. 3A) whereas most OHC and some IHC were missing in the B6J parental strain consistent with previously reported results (Spongr et al., 1997a). Like the CBA parental strain, all the IHC and OHC were present in the CBA.B6-ahl congenic strain (Fig. 3B). Nearly all the OHC were present in the B6.CBA-Ahl+ congenic strain; however, surprisingly some IHC were missing in this congenic strain.
Figure 2. Cochlear hair cell loss.
Representative photomicrograph of surface preparations from a nineteen-month-old B6.CBA-Ahl+ mouse taken from (A) apical, (B) middle and (C) basal region of the cochlea (400X magnification). Focal plane of the microscope set near nuclei outer hair cells (OHC) and inner hair cells (IHC). Jagged arrows point to missing OHC and IHC.
Figure 3. Strain-specific patterns of hair cell loss.
Representative photomicrographs (400X magnification) taken 40-60% (10-20 kHz region) of the distance from apex of the cochleae of (A) CBA, (B) CBA.B6-ahl, (C) B6.CBA-Ahl+ and (D) B6J mouse strains examined at 18-19 months of age. Most OHCs (yellow bracket) were present in CBA, CBA.B6-ahl and B6.CBA-Ahl+ mice, whereas most OHCs were missing in B6J mice (jagged, gold arrowhead). Nearly all IHCs were present in CBA and CBA.B6-ahl mice, whereas many IHCs were missing in B6J mice and B6.CBA-Ahl+ mice (red arrowhead). Note that there is little if any IHC or OHC loss in CBA or CBA.B6-ahl mice, that B6.CBA-Ahl+ mice show much less OHC loss than B6J mice, but more than CBA or CBA.B6-ahl mice, and that IHC loss is similar in B6.CBA-Ahl+ and B6J mice.
The mean (± SEM) percentages of outer hair cell (OHC) and inner hair cell (IHC) loss along the length of the cochlea were determined for each strain (Fig. 4A-D). Positions along the cochlea related to test frequencies (lower axis, Fig. 4A-D) were estimated using a mouse frequency-place map (Muller et al., 2005). B6J strain mice showed the highest percentage of OHC and IHC loss; OHC loss was typically greater than IHC (Fig. 4B). Little hair cell loss was observed in the cochleae of CBA (Fig. 3A) and CBA.B6-ahl mice (Fig 4C). B6.CBA-Ahl+ strain mice showed significantly less hair cell loss than B6J mice, but compared with CBA and CBA.B6-ahl mice, they showed a higher degree of OHC loss at the most basal and apical regions of the cochlea and greater IHC loss from the middle to the base of the cochlea. Statistical analyses of IHC and OHC losses are presented in Table 3. A two-way, repeated-measures (cochlear location) ANOVA on OHC loss (Table 3A) and IHC loss (Table 3B) showed significant main effects for genetic background, cochlear location and the interaction of genetic background and cochlear location. A Tukey post-hoc analysis showed significant differences in OHC loss (Table 3A) and IHC loss (Table 3B) among strains as a function of cochlear location.
Figure 4. Quantification of cochlear hair cell loss.
Mice of the CBA, B6J, CBA.B6-ahl, and B6.CBA-Ahl+ strains were examined at 18-19 months of age. A-D. For each strain, mean percent loss (± SEM) of IHC and OHC was plotted as a function of percent distance from the apex. E. Comparisons of mean (± SEM) percent OHC loss as a function of percent distance from the apex among strains. F. Comparisons of mean (± SEM) percent IHC loss as a function of percent distance from the apex of the cochlea among the strains. Statistical analyses of these data are presented in Table 3. Lower x-axis shows the relationship of test frequency to cochlear place (Muller et al., 2005).
Table 3. Statistical Analysis of Hair Cell Loss.
A two-way repeated measure ANOVA with Tukey post-hoc analysis was used to determine statistical significance of genetic background and cochlear location on outer and inner hair cell loss and mean differences between strains. DF, degrees of freedom; SS, sum of squares; MS, mean squares; F, F-ratio; P, probability.
| A. Outer hair cell loss: | |||||
|---|---|---|---|---|---|
| Source of Variation | DF | SS | MS | F | P |
| Genetic Background | 3 | 63624 | 21208 | 35 | <0.001 |
| Cochlear Location | 4 | 13392 | 3348 | 29 | <0.001 |
| Genetic Background × Cochlear Location | 12 | 15446 | 1287 | 11 | <0.001 |
| % Distance from Apex | |||||
|---|---|---|---|---|---|
| Strain Comparisons | 10 | 30 | 50 | 70 | 90 |
| B6.CBA-Ahl+ vs. CBA | p<0.05 | ns | ns | ns | p<0.05 |
| B6.CBA-Ahl+ vs. CBA.B6-ahl | p<0.05 | ns | ns | ns | p<0.05 |
| B6.CBA-Ahl+ vs. B6J | ns | ns | p<0.05 | p<0.05 | p<0.05 |
| B6J vs. CBA | p<0.05 | p<0.05 | p<0.05 | p<0.05 | p<0.05 |
| B6J vs. CBA.B6-ahl | p<0.05 | p<0.05 | p<0.05 | p<0.05 | p<0.05 |
| CBA.B6-ahl vs. CBA | ns | ns | ns | ns | ns |
| B. Inner Hair Cell Loss: | |||||
|---|---|---|---|---|---|
| Source of Variation | DF | SS | MS | F | P |
| Genetic Background | 3 | 37603 | 12534 | 50 | <0.001 |
| Cochlear Location | 4 | 18786 | 4697 | 37 | <0.001 |
| Genetic Background × Cochlear Location | 12 | 16635 | 1386 | 11 | <0.001 |
| % Distance from Apex | |||||
|---|---|---|---|---|---|
| Genetic Background × Cochlear Location | 10 | 30 | 50 | 70 | 90 |
| B6.CBA-Ahl+ vs. CBA | ns | p<0.05 | p<0.05 | p<0.05 | p<0.05 |
| B6.CBA-Ahl+ vs. CBA.B6-ahl | ns | p<0.05 | p<0.05 | p<0.05 | p<0.05 |
| B6.CBA-Ahl+ vs. B6J | ns | ns | ns | p<0.05 | p<0.05 |
| B6J vs. CBA | ns | p<0.05 | p<0.05 | p<0.05 | p<0.05 |
| B6J vs. CBA.B6-ahl | ns | p<0.05 | p<0.05 | p<0.05 | p<0.05 |
| CBA.B6-ahl vs. CBA | ns | ns | ns | ns | ns |
4. Discussion
4.1 CAST- and CBA-derived Cdh23 alleles reduce hearing loss in B6 congenic mice
We previously generated and analyzed a B6.CAST-Ahl+ congenic strain (Johnson et al., 1997; Keithley et al., 2004) analogous to the B6.CBA-Ahl+ congenic strain described in this paper, but with the AHL protective allele of the CAST/EiJ (CAST) strain, rather than the CBA strain, transferred to the B6J strain background. Mice of both congenic strains exhibit a much slower rate of hearing loss compared with the B6J host strain mice, demonstrating that both CAST and CBA alleles of Cdh23 can confer AHL resistance in mice with an otherwise B6J genome. The dramatic effect that the Ahl+allele has in reducing the severity of hearing loss in congenic B6J mice indicates that the ahl/ahl genotype is a major contributor to the early AHL of B6J mice.
Comparisons of our ABR threshold results for B6.CBA-Ahl+ mice with previously reported results for B6.CAST-Ahl+ mice suggest a possible difference in hearing loss profiles. At 18 months of age and older, hearing loss in B6.CAST-Ahl+ mice was intermediate to that of B6J and CAST parental strain mice (Keithley et al., 2004), whereas ABR thresholds of the 18-month-old B6.CBA-Ahl+ mice examined in the present study are only slightly higher than those of CBA parental strain mice. This apparent difference can be explained by the differences in hearing loss between the parental CAST and CBA strains. CAST mice maintain near normal hearing thresholds throughout 21 months of age (Keithley et al., 2004). In contrast, CBA mice exhibit approximately 20 dB threshold elevations by 18 months of age (Fig. 1), despite the fact that IHC or OHC loss is not evident in the corresponding cochlear regions (Fig. 4A).
4.2 The B6-derived Cdh23 allele has no effect on hearing in CBA congenic mice
We chose to use the CBA strain in this study because it is widely used in hearing research as a good hearing control strain and because CBA mice are much more docile than CAST mice, a wild-derived strain. The wild nature of CAST mice has prevented us from creating a CAST.B6-ahl congenic strain. The CBA.B6-ahl congenic strain we developed and describe here has allowed us to test whether homozygosity for the ahl variant can worsen the hearing loss in an AHL-resistant strain of mice. Somewhat unexpectedly we found that the hearing loss in CBA.B6-ahl strain mice, even at 18 months of age, remains similar to that of CBA mice and is much milder than that observed in B6J mice. This result indicates that the ahl/ahl genotype of B6J mice by itself is not sufficient to worsen hearing loss in an otherwise CBA genetic background, and that deleterious B6J alleles at additional loci must be required. Analysis of F1 hybrids between CBA.B6-ahl congenic strain and B6J mice will help determine whether these other contributing B6J alleles are recessive or dominant in nature and illuminate what genetic strategy would work best to map the additional loci.
4.3 Correspondence of hearing loss and hair cell loss
Little hair cell loss was observed in the 18-month-old CBA mice and the CBA.B6-ahl congenic strain, except in the most apical regions where 20% OHC were missing in both strains and in the most basal region where approximately 10% and 20% OHC were missing in the CBA and CBA.B6-ahl strains respectively (Fig. 4). The location and degree of hair cell loss in the CBA parental strain and CBA.B6-ahl congenic strain mice were nearly identical to that observed in our earlier study of CBA/J and CBA/CaJ mice (Spongr et al., 1997a). Despite the fact that there was little or no hair cell loss at 8, 16 and 32 kHz regions of the cochlea (Fig. 4A,C), these frequencies all showed mild to moderate ABR threshold increases of 30 dB (Fig. 1). Hearing loss without accompanying hair cell loss in aging CBA mice may be explained by a loss of marginal cells in the stria vascularis (and consequent decline in endocochlear potential) and a loss of spiral ganglion neurons, as other investigators have previously documented in CBA/CaJ mice older than one year (Ohlemiller et al., 2010).
OHC losses in the B6J parental strain mice declined from roughly 90% near the base of the cochlea to 30-50% near the apex; IHC also declined from 90% near the base to less than 10% near the apex. The location and degree of hair cell loss in these B6J strain mice was nearly identical to our previously reported results for 18-month-old C57BL/6 mice (Spongr et al., 1997a) and consistent with the configuration of the high-frequency hearing loss seen in these mice (Fig. 1). ABR threshold shifts at 8, 16 and 32 kHz were roughly proportional to the degree of OHC and/or IHC loss, suggesting that hair cell loss is a major factor contributing to hearing loss in B6J mice. Others have noted that fibrocytes in the spiral ligament begin to degenerate before hair cells and suggested that this may be a key factor contributing to AHL (Hequembourg et al., 2001a); however, there is no significant decline in endocochlear potential in aging B6J mice (Lang et al., 2002; Ohlemiller, 2009), which argues against this proposed mechanism. Impaired mitochondrial function and energy metabolism also have been implicated in AHL. Young 1.5-month-old C57BL/6N mice expressed much lower levels of a key mitochondrial enzyme, succinate dehydrogenase, in hair cells than CBA mice, months before the onset of hearing loss (Ding et al., 1999). A deletion of the mitochondrial pro-apoptotic gene, Bak1, in B6J mice reduced age-related hair cell and spiral ganglion loss and suppressed AHL (Someya et al., 2009). Although Cdh23ahl is thought to primarily affect tip link integrity and may not directly impair energy metabolism, tip link dysfunction could increase cellular stress and contribute to the activation of apoptotic pathways.
Perhaps the most novel pathological finding in this report is the unusual pattern of IHC loss in the B6.CBA-Ahl+ congenic strain mice. These mice exhibited 30-40% IHC loss over the basal 80% of the cochlea, but little OHC loss except in the most basal and apical regions of the cochlea (Fig. 4D). The magnitude of the IHC lesion in the apical half of the cochlea matched that seen in the B6J mice; however, IHC losses in the basal 40% of the cochlea were half as great as in B6J mice (Fig. 4F). OHC losses in the middle 60% of the cochlea of the B6.CBA-Ahl+ congenic mouse strain were negligible and substantially less than in the B6J parental strain, and these results corresponded well with the ABR threshold differences between these two strains (Fig. 1). The difference in OHC loss between B6J and B6.CBA-Ahl+ congenic mice was greater than the difference in IHC loss (Fig. 4E, F), suggesting that Cdh23ahl primarily affects OHC survival, especially in the mid to basal region of the cochlea, and only partially contributes to IHC loss in the basal region. The Cdh23ahl allele appears to have little effect on either low frequency hearing loss (8 kHz, Fig. 1) or hair cell loss in the apical region of the cochlea (Fig. 4E,F). Consistent with this interpretation, Ohlemiller found an identical progression of low frequency hearing loss (< 10 kHz) in B6 and B6.CAST-Ahl+ congenic mice (Ohlemiller, 2006).
4.4 Value of the B6.CBA-Ahl+ and CBA.B6-ahl congenic strains
While we realize that the congenic segments of the B6.CBA-Ahl+ strain and CBA.B6-ahl strain contain hundreds of genes, we attribute their hearing loss effects primarily to the Cdh23 gene because of its known variants and their strong associations with the hearing loss phenotypes in inbred strain mice (Noben-Trauth et al., 2003). Although we cannot rule out the possibility of minor contributions from other genes in the congenic intervals, we are aware of no other genetic differences between the B6J and CBA strains in the Chromosome 10 congenic regions that are known or predicted to influence hearing.
B6.CBA-Ahl+ and CBA.B6-ahl congenic strain mice can serve as valuable tools, in comparisons with mice of the B6J and CBA parental strains, to deduce the specific effects of Cdh23 strain variants in a wide variety of auditory studies. In addition to studies of AHL, these two newly generated congenic strains could be used to investigate the influence of these genetic factors on noise-induce hearing loss and ototoxicity. The B6.CBA-Ahl+strain also can be used to control for the effects of the Cdh23ahl allele in hearing assessments of mutations maintained on the B6J or B6N strain background. The Ahl+ allele can easily be transferred to B6J or B6N mutant strains by first outcrossing to the B6.CBA-Ahl+strain and then intercrossing the resulting hybrids and selecting F2 mice with mutant and Ahl+ genotypes as future breeders. Although the previously developed B6.CAST-Ahl+ congenic strain can be used for the same purpose, the new B6.CBA-Ahl+strain provides some increased fecundity.
4.5 Hearing loss profile of C57BL/6N mice
Many genetically engineered mouse mutations are maintained on the B6J background, but most mice from the International Knockout Mouse Consortium are being generated on B6N, a different substrain of B6 mice. Although both B6J and B6N substrains have the same ahl susceptibility allele, it is possible that their hearing phenotypes may differ due to mutations that have occurred in other genes since their separation. Behavioral differences, for example, have been detected between B6J and B6N substrain mice (Bryant et al., 2008), and a genetic difference with phenotypic consequences has been reported. The B6J strain has a null mutation of the nicotinamide nucleotide transhydrogenase gene (Nnt) that is not present in the B6N substrain and that influences diet-induced obesity (Nicholson et al., 2010). Because of the possibility of substrain differences, we examined the hearing phenotypes of B6J and B6N mice. Our results show that B6J and B6N mice have identical hearing loss onset times and rates of progression, which allows for direct comparisons of hearing-related phenotypes of mutant mice that are maintained on either background.
Supplementary Material
Supplementary Figure 1. Confirmation of Cdh23 genotypes by DNA sequence analysis. The Cdh23 polymorphism that corresponds with AHL in inbred mouse strains (Noben-Trauth et al., 2003) was genotyped by DNA sequence analysis of PCR products obtained from genomic DNA of C57BL/6J, C57BL/6N, B6.CBACa-Cdh23Ahl+(B6.CBA-Ahl+), CBACa.B6-Cdh23ahl (CBA.B6-ahl), and CBA/CaJ inbred strains. Forward primer ATCATCACGGACATGCAAGA and reverse primer AGCTACCAGGAACAGCTTGG were used to amplify a 315 bp product containing the targeted 753A/G AHL-associated polymorphism (the last nucleotide of exon 7, transcript ENSMUST00000073242). The reverse primer was used for DNA sequence analysis of the PCR products. The recessive 753A allele (ahl), when homozygous, confers increased susceptibility to AHL, while the dominant 753G allele (Ahl+) confers resistance.
Supplementary Figure 2. Statistical comparisons of ABR threshold means among strains. Mice of strains B6.CBACa-Cdh23Ahl+ (B6.CBA-Ahl in figure), C57BL/6J, C57BL/6NJ, CBACa.B6-Cdh23ahl (CBA.B6-ahl in figure), and CBA/CaJ were tested at 3, 6, 9, 12, 15, and 18 months of age, and ABR thresholds were obtained for pure-tone 8 kHz, 16 kHz and 32 kHz auditory stimuli. For each strain, black dots represent individual data points and green diamonds indicate the mean values (mid horizontal line) and confidence intervals (vertical span). The Tukey test, which controls for multiple pair-wise comparisons, was used to determine the statistical significance of mean differences at the alpha = 0.01 level. For each age and stimulus combination, strain means that are significantly different are given different letters (in red), and those with non-significant differences are given the same letter. A strain mean given two letters is not statistically different from the means of other strains having either of these letters.
Highlights.
>We investigated auditory effects of Cdh23 variants in both C57BL/6J and CBA/CaJ genetic backgrounds.
>The CBA/CaJ-derived Cdh23 allele lessens hearing loss and decreases hair cell loss in a C57BL/6J genetic background.
> The C57BL/6J-derived Cdh23 allele has no effect on hearing loss or hair cell death in a CBA/CaJ genetic background.
> Deleterious alleles at other loci in addition to Cdh23 must contribute to the accelerated hearing loss of C57BL/6J mice.
> Mice of the C57BL/6J and C57BL/6N substrains have identical hearing loss profiles.
Acknowledgements
We thank Patsy Nishina and David Bergstrom of The Jackson Laboratory for their critical review of this manuscript. We also thank Sandra Gray for her skilled mouse colony management and assistance in the development of the congenic strains. This research was supported by R01 grant DC005827 (KRJ) from the National Institutes of Health (NIH), National Institute on Deafness and Other Communication Disorders (NIDCD). The Jackson Laboratory institutional shared services are supported by NIH National Cancer Institute support grant CA34196.
Abbreviations
- ABR
auditory brainstem response
- AHL
age-related hearing loss
- IHC
inner hair cell
- OHC
outer hair cell
- ANOVA
analysis of variance
- st dev
standard deviation
- SEM
standard error of the mean
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- Alagramam KN, Goodyear RJ, Geng R, Furness DN, van Aken AF, Marcotti W, Kros CJ, Richardson GP. Mutations in protocadherin 15 and cadherin 23 affect tip links and mechanotransduction in Mammalian sensory hair cells. PLoS One. 2011;6:e19183. doi: 10.1371/journal.pone.0019183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bared A, Ouyang X, Angeli S, Du LL, Hoang K, Yan D, Liu XZ. Antioxidant enzymes, presbycusis, and ethnic variability. Otolaryngol. Head Neck Surg. 2010;143:263–8. doi: 10.1016/j.otohns.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielefeld EC, Tanaka C, Chen GD, Henderson D. Age-related hearing loss: is it a preventable condition? Hear Res. 2010;264:98–107. doi: 10.1016/j.heares.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant CD, Zhang NN, Sokoloff G, Fanselow MS, Ennes HS, Palmer AA, McRoberts JA. Behavioral differences among C57BL/6 substrains: implications for transgenic and knockout studies. J. Neurogenet. 2008;22:315–31. doi: 10.1080/01677060802357388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton DS, Cruickshanks KJ, Klein BE, Klein R, Wiley TL, Nondahl DM. The impact of hearing loss on quality of life in older adults. Gerontologist. 2003;43:661–8. doi: 10.1093/geront/43.5.661. [DOI] [PubMed] [Google Scholar]
- DeStefano AL, Gates GA, Heard-Costa N, Myers RH, Baldwin CT. Genomewide linkage analysis to presbycusis in the Framingham Heart Study. Arch Otolaryngol Head Neck Surg. 2003;129:285–9. doi: 10.1001/archotol.129.3.285. [DOI] [PubMed] [Google Scholar]
- Ding D, McFadden SL, Salvi R. Cochlear Hair Cell Densities and Inner-Ear Staining Techniques. In: Willott JF, editor. Handbook of Mouse auditory Research from Behavior to Molecular Biology. CRC Press; Boca Raton: 2001. pp. 189–204. [Google Scholar]
- Ding DL, McFadden SL, Wang J, Hu BH, Salvi RJ. Age- and strain-related differences in dehydrogenase activity and glycogen levels in CBA and C57 mouse cochleas. Audiology and Neuro-Otology. 1999;4:55–63. doi: 10.1159/000013822. [DOI] [PubMed] [Google Scholar]
- Friedman RA, Van Laer L, Huentelman MJ, Sheth SS, Van Eyken E, Corneveaux JJ, Tembe WD, Halperin RF, Thorburn AQ, Thys S, Bonneux S, Fransen E, Huyghe J, Pyykko I, Cremers CW, Kremer H, Dhooge I, Stephens D, Orzan E, Pfister M, Bille M, Parving A, Sorri M, Van de Heyning PH, Makmura L, Ohmen JD, Linthicum FH, Jr., Fayad JN, Pearson JV, Craig DW, Stephan DA, Van Camp G. GRM7 variants confer susceptibility to age-related hearing impairment. Hum Mol Genet. 2009;18:785–796. doi: 10.1093/hmg/ddn402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garringer HJ, Pankratz ND, Nichols WC, Reed T. Hearing impairment susceptibility in elderly men and the DFNA18 locus. Arch Otolaryngol Head Neck Surg. 2006;132:506–10. doi: 10.1001/archotol.132.5.506. [DOI] [PubMed] [Google Scholar]
- Henry KR, Chole RA. Genotypic differences in behavioral, physiological and anatomical expressions of age-related hearing loss on the laboratory mouse. Audiology. 1980;19:369–383. doi: 10.3109/00206098009070071. [DOI] [PubMed] [Google Scholar]
- Hequembourg S, Liberman MC. Spiral ligament pathology: a major aspect of age-related cochlear degeneration in C57BL/6 mice. J Assoc Res Otolaryngol. 2001a;2:118–29. doi: 10.1007/s101620010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hequembourg S, Liberman MC. Spiral ligament pathology: a major aspect of age-related cochlear degeneration in C57BL/6 mice. J Assoc Res Otolaryngol. 2001b;2:118–29. doi: 10.1007/s101620010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KR, Erway LC, Cook SA, Willott JF, Zheng QY. A major gene affecting age-related hearing loss in C57BL/6J mice. Hear Res. 1997;114:83–92. doi: 10.1016/s0378-5955(97)00155-x. [DOI] [PubMed] [Google Scholar]
- Johnson KR, Yu H, Ding D, Jiang H, Gagnon LH, Salvi RJ. Separate and combined effects of Sod1 and Cdh23 mutations on age-related hearing loss and cochlear pathology in C57BL/6J mice. Hear Res. 2010 doi: 10.1016/j.heares.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmierczak P, Sakaguchi H, Tokita J, Wilson-Kubalek EM, Milligan RA, Muller U, Kachar B. Cadherin 23 and protocadherin 15 interact to form tip-link filaments in sensory hair cells. Nature. 2007;449:87–91. doi: 10.1038/nature06091. [DOI] [PubMed] [Google Scholar]
- Keithley EM, Canto C, Zheng QY, Fischel-Ghodsian N, Johnson KR. Age-related hearing loss and the ahl locus in mice. Hear Res. 2004;188:21–8. doi: 10.1016/S0378-5955(03)00365-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang H, Schulte BA, Schmiedt RA. Endocochlear potentials and compound action potential recovery: functions in the C57BL/6J mouse. Hear Res. 2002;172:118–26. doi: 10.1016/s0378-5955(02)00552-x. [DOI] [PubMed] [Google Scholar]
- Latoche JR, Neely HR, Noben-Trauth K. Polygenic inheritance of sensorineural hearing loss (Snhl2, -3, and -4) and organ of Corti patterning defect in the ALR/LtJ mouse strain. Hear Res. 2011;275:150–9. doi: 10.1016/j.heares.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H-S, Borg E. Age-related loss of auditory sensitivity in two mouse genotypes. Acta Otolaryngol. 1991;111:827–834. doi: 10.3109/00016489109138418. [DOI] [PubMed] [Google Scholar]
- Mikaelian DO. Development and degeneration of hearing in the C57/bl6 mouse: relation of electrophysiologic responses from the round window and cochlear nucleus to cochlear anatomy and behavioral responses. Laryngoscopy. 1979;34:1–15. doi: 10.1288/00005537-197901000-00001. [DOI] [PubMed] [Google Scholar]
- Muller M, von Hunerbein K, Hoidis S, Smolders JW. A physiological place-frequency map of the cochlea in the CBA/J mouse. Hear Res. 2005;202:63–73. doi: 10.1016/j.heares.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Nicholson A, Reifsnyder PC, Malcolm RD, Lucas CA, MacGregor GR, Zhang W, Leiter EH. Diet-induced obesity in two C57BL/6 substrains with intact or mutant nicotinamide nucleotide transhydrogenase (Nnt) gene. Obesity (Silver Spring) 2010;18:1902–5. doi: 10.1038/oby.2009.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noben-Trauth K, Johnson KR. Inheritance patterns of progressive hearing loss in laboratory strains of mice. Brain Res. 2009;1277:42–51. doi: 10.1016/j.brainres.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noben-Trauth K, Zheng QY, Johnson KR. Association of cadherin 23 with polygenic inheritance and genetic modification of sensorineural hearing loss. Nat Genet. 2003;35:21–23. doi: 10.1038/ng1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlemiller KK. Contributions of mouse models to understanding of age- and noise-related hearing loss. Brain Res. 2006;1091:89–102. doi: 10.1016/j.brainres.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK. Mechanisms and genes in human strial presbycusis from animal models. Brain Res. 2009;1277:70–83. doi: 10.1016/j.brainres.2009.02.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlemiller KK, Dahl AR, Gagnon PM. Divergent aging characteristics in CBA/J and CBA/CaJ mouse cochleae. J Assoc Res Otolaryngol. 2010;11:605–23. doi: 10.1007/s10162-010-0228-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Paris J, Ballay C, Inserra M, Stidham K, Colen T, Roberson J, Gardner P, Schrijver I. Genetic analysis of presbycusis by arrayed primer extension. Ann. Clin. Lab. Sci. 2008;38:352–60. [PubMed] [Google Scholar]
- Schwander M, Xiong W, Tokita J, Lelli A, Elledge HM, Kazmierczak P, Sczaniecka A, Kolatkar A, Wiltshire T, Kuhn P, Holt JR, Kachar B, Tarantino L, Muller U. A mouse model for nonsyndromic deafness (DFNB12) links hearing loss to defects in tip links of mechanosensory hair cells. Proc Natl Acad Sci U S A. 2009;106:5252–7. doi: 10.1073/pnas.0900691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Someya S, Xu J, Kondo K, Ding D, Salvi RJ, Yamasoba T, Rabinovitch PS, Weindruch R, Leeuwenburgh C, Tanokura M, Prolla TA. Age-related hearing loss in C57BL/6J mice is mediated by Bak-dependent mitochondrial apoptosis. Proc Natl Acad Sci U S A. 2009;106:19432–19437. doi: 10.1073/pnas.0908786106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spongr VP, Flood DG, Frisina RD, Salvi RJ. Quantitative measures of hair cell loss in CBA and C57BL/6 mice throughout their life spans. Journal of the Acoustical Society of America. 1997a;101:3546–53. doi: 10.1121/1.418315. [DOI] [PubMed] [Google Scholar]
- Spongr VP, Flood DG, Frisina RD, Salvi RJ. Quantitative measures of hair cell loss in CBA and C57BL/6 mice throughout their life spans. J. Acoust. Soc. Am. 1997b;101:3546–3553. doi: 10.1121/1.418315. [DOI] [PubMed] [Google Scholar]
- Unal M, Tamer L, Dogruer ZN, Yildirim H, Vayisoglu Y, Camdeviren H. N-acetyltransferase 2 gene polymorphism and presbycusis. Laryngoscope. 2005;115:2238–41. doi: 10.1097/01.mlg.0000183694.10583.12. [DOI] [PubMed] [Google Scholar]
- Wang J, Menchenton T, Yin S, Yu Z, Bance M, Morris DP, Moore CS, Korneluk RG, Robertson GS. Over-expression of X-linked inhibitor of apoptosis protein slows presbycusis in C57BL/6J mice. Neurobiol. Aging. 2008;31:1238–1249. doi: 10.1016/j.neurobiolaging.2008.07.016. [DOI] [PubMed] [Google Scholar]
- Willott JF. Effects of aging, hearing loss and anatomical location on thresholds of inferior colliculus neurons in C57BL/6 and CBA mice. J. Neurophysiol. 1986;56:391–408. doi: 10.1152/jn.1986.56.2.391. [DOI] [PubMed] [Google Scholar]
- Zheng QY, Johnson KR, Erway LC. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hear Res. 1999;130:94–107. doi: 10.1016/s0378-5955(99)00003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng QY, Ding D, Yu H, Salvi RJ, Johnson KR. A locus on distal chromosome 10 (ahl4) affecting age-related hearing loss in A/J mice. Neurobiol. Aging. 2009;30:1693–1705. doi: 10.1016/j.neurobiolaging.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Confirmation of Cdh23 genotypes by DNA sequence analysis. The Cdh23 polymorphism that corresponds with AHL in inbred mouse strains (Noben-Trauth et al., 2003) was genotyped by DNA sequence analysis of PCR products obtained from genomic DNA of C57BL/6J, C57BL/6N, B6.CBACa-Cdh23Ahl+(B6.CBA-Ahl+), CBACa.B6-Cdh23ahl (CBA.B6-ahl), and CBA/CaJ inbred strains. Forward primer ATCATCACGGACATGCAAGA and reverse primer AGCTACCAGGAACAGCTTGG were used to amplify a 315 bp product containing the targeted 753A/G AHL-associated polymorphism (the last nucleotide of exon 7, transcript ENSMUST00000073242). The reverse primer was used for DNA sequence analysis of the PCR products. The recessive 753A allele (ahl), when homozygous, confers increased susceptibility to AHL, while the dominant 753G allele (Ahl+) confers resistance.
Supplementary Figure 2. Statistical comparisons of ABR threshold means among strains. Mice of strains B6.CBACa-Cdh23Ahl+ (B6.CBA-Ahl in figure), C57BL/6J, C57BL/6NJ, CBACa.B6-Cdh23ahl (CBA.B6-ahl in figure), and CBA/CaJ were tested at 3, 6, 9, 12, 15, and 18 months of age, and ABR thresholds were obtained for pure-tone 8 kHz, 16 kHz and 32 kHz auditory stimuli. For each strain, black dots represent individual data points and green diamonds indicate the mean values (mid horizontal line) and confidence intervals (vertical span). The Tukey test, which controls for multiple pair-wise comparisons, was used to determine the statistical significance of mean differences at the alpha = 0.01 level. For each age and stimulus combination, strain means that are significantly different are given different letters (in red), and those with non-significant differences are given the same letter. A strain mean given two letters is not statistically different from the means of other strains having either of these letters.