Abstract
The growth factor myostatin (MSTN) negatively regulates skeletal muscle mass. Mstn gene deletion in mice causes increased muscle mass, reduced adiposity and resistance to genetic or diet-induced obesity. Pharmacologic MSTN inhibition in mice also causes increased muscle mass and resistance to diet-induced obesity. To test whether MSTN inhibition causes weight loss in mice that are already obese, we inhibited MSTN in mice fed a high-fat diet. Mice were fed a diet containing 60% kcal from fat for 12 weeks followed by treatment with a soluble MSTN receptor derived from the activin receptor type IIB extracellular domain. During the next 12 weeks of soluble receptor treatment and high-fat diet feeding, lean mass increased without a loss of adipose mass. Glucose metabolism was also similar between groups. Our results suggest that MSTN inhibition may be ineffective at inducing weight loss in obese patients.
Introduction
Recent studies in rodents have suggested that increasing muscle mass could potentially be useful in treating obesity or insulin resistance [1]. One plausible means of achieving increased muscle mass in patients is by inhibition of myostatin (MSTN), a member of the transforming growth factor β family of secreted factors [2]. The Mstn gene is expressed predominantly in skeletal muscle and, to a lesser extent, adipose tissue [3]. Mstn-/- mice have a profound increase in muscle mass, a reduction in adiposity, resistance to genetic or diet-induced obesity (DIO) and increased insulin sensitivity [4]. These effects are mediated by the actions of MSTN on skeletal muscle rather than adipose tissue because inhibition of MSTN signaling specifically in muscle causes the same metabolic phenotypes as global Mstn gene deletion [5].
The highest affinity MSTN receptor is the activin receptor type IIB (ACVR2B, also known as ACTRIIB) which also mediates signaling for several other family members [2, 6]. A soluble ACVR2B consisting of the extracellular domain of the receptor fused to an immunoglobulin Fc region increases muscle mass and fiber area in normal adult mice [7] and can therefore be considered a resistance exercise mimetic. ACVR2B:Fc also increases muscle mass in Mstn-/- mice demonstrating that MSTN and other ACVR2B ligands are functionally redundant in muscle [7].
ACVR2B:Fc treatment given simultaneous with the onset of high-fat diet (HFD) feeding reduces the amount of fat mass gained at 10 weeks of treatment, although no differences in fat mass are seen at 4 weeks [8]. This effect on adiposity is likely preventative given that ACVR2B:Fc-induced muscle hypertrophy reaches a maximum by 2 weeks of treatment [7]. In mice that are already obese, induction of muscle hypertrophy has yielded mixed results. A neutralizing MSTN monoclonal antibody causes muscle hypertrophy but does not cause weight loss in obese leptin mutants (Lepob/ob) [9]. In contrast, an inducible constitutively active Akt transgene expressed specifically in muscle causes muscle hypertrophy followed by fat loss in mice previously made obese by HFD feeding [10]. We sought to test the hypothesis that an increase in skeletal muscle mass would cause fat loss using an experimental design similar to a plausible pharmacological approach for treating human obesity. To this end, we treated DIO mice with ACVR2B:Fc and measured body composition for 12 weeks.
Materials and Methods
Materials
A Chinese hamster ovary cell line expressing the extracellular domain of mouse ACVR2B fused to a mouse Fc domain were a gift from Se-Jin Lee. ACVR2B:Fc was purified from conditioned medium using protein A/G sepharose (Pierce) and dialyzed against PBS.
Animal treatment
All animal procedures were approved by Animal Care and Use Committee of the NIDDK, NIH. Sixteen week old C57BL/6J male mice fed Research Diets #12492 (5.24 kcal/g; 60% kcal fat; 20% kcal carbohydrate; 20% kcal protein) starting from 6 weeks of age were purchased from Jackson Labs. Mice were maintained on the same diet at NIH and housed with a 12-hr light/dark cycle.
After 2 weeks of acclimation (12 weeks fed the HFD), mice were injected weekly with 10 mg/kg body weight ACVR2B:Fc or equivalent volume of PBS control. After 4 weeks, mice were injected with 5 mg/kg body weight ACVR2B:Fc or PBS for another 8 weeks. Body composition was measured at weeks 0, 4, 6, 8, 10 and 12 after receiving ACVR2B:Fc by quantitative magnetic resonance in an EchoMRI 3-in-1™ (Echo Medical Systems).
Serum was collected by cardiac puncture under pentobarbital anesthesia (80 mg/kg body weight) in the early afternoon.
Insulin and glucose measurements
Serum insulin (Millipore) was determined by radioimmunoassay kit according to the manufacturer’s instructions.
Glucose tolerance tests were performed after 12 weeks of ACVR2B:Fc treatment (24 weeks on HFD) after a 16-hour overnight fast as described [11]. Blood glucose was measured by tail bleeding at indicated time points using a Contour Glucometer (Bayer).
Statistical analysis
Data were analyzed by student’s t test with additional analysis of time course data by repeated measures ANOVA (SPSS v. 19). P < 0.05 was considered significant.
Results
Control mice gained ~10g more weight over an additional 12 weeks of HFD feeding after the start of injections. Body composition analysis shows that this weight gain was mainly due to an increase in fat mass (Figure 1A-C). Mice treated with ACVR2B:Fc over the same time period also increased body weight and were heavier than control mice by 8 weeks of treatment (Figure 1A). Lean mass was significantly increased in mice treated with ACVR2B:Fc, and individual muscles weighed 19-30% more than control muscles (Figure 1C, D). The ACVR2B:Fc-treated mice also continued to gain fat and there was no statistically significant difference in fat mass between treated and control mice at any time point (Figure 1C).
Figure 1.
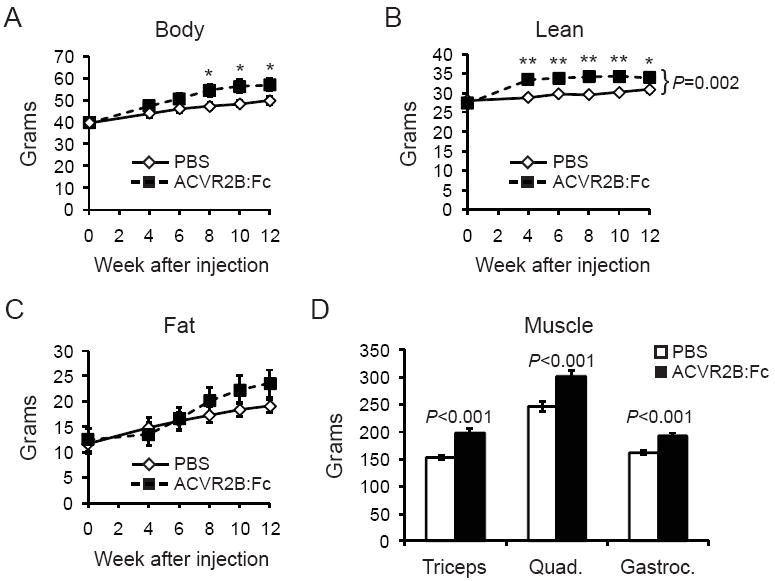
ACVR2B:Fc treatment for 12 weeks increases body weight (A) and lean mass (B) without decreasing fat mass (C) in mice fed a HFD for 12 weeks before and after treatment. The mass of triceps, quadriceps and gastrocnemius skeletal muscles (D) increased with ACVR2B:Fc treatment. Data are shown as mean ± SEM. *P < 0.05, **P < 0.01, student’s t test; bracket in (B) denotes significance by repeated measures ANOVA.
One mouse receiving soluble receptor treatment began to lose weight after 6 weeks. He appeared to have paralysis of his hindlimbs for unexplained reasons and was euthanized. His data were removed from the study. Paralysis has not been seen in other types of mice we have injected with this receptor, and to our knowledge, there are no other reports of this kind in the literature described during ACVR2B:Fc treatment.
To test whether glucose metabolism was improved, we performed glucose tolerance testing. Glucose tolerance curves were not significantly different between groups (Figure 2A). Fasting blood glucose and fed insulin levels were also not significantly different between control and ACVR2B:Fc groups (Figure 2B and C).
Figure 2.
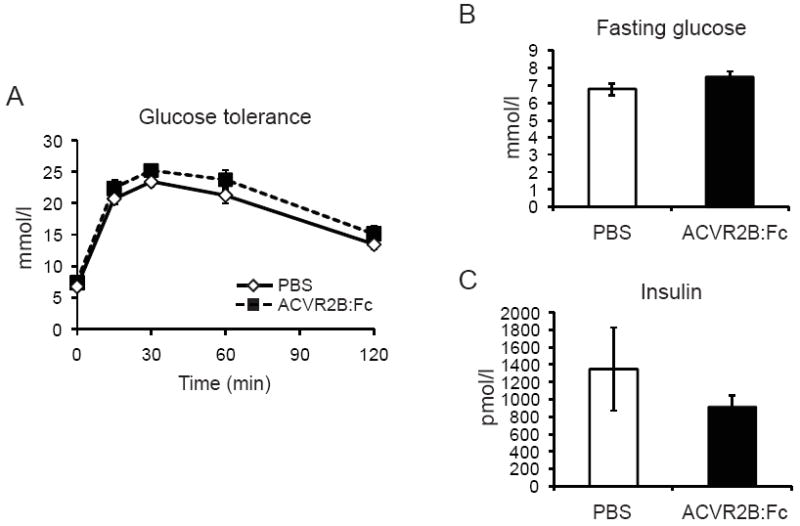
ACVR2B:Fc treatment for 12 weeks does not affect glucose tolerance (A), fasting blood glucose (B), or fed insulin (C) in mice fed a HFD for 12 weeks before and after treatment. Data are shown as mean ± SEM. Data are not significant by student’s t test and repeated measures ANOVA.
Conclusions
Our results show that MSTN inhibition was ineffective in reducing fat mass when given after 12 weeks of HFD feeding. We not only failed to detect adipose tissue weight loss, we did not find evidence for prevention of adipose weight gain. Individual fat depots might by differentially affected by ACVR2B:Fc treatment without an effect on overall adiposity, but these were not measured. It is possible that ACVR2B:Fc would be more effective with longer treatment time, a different obesogenic diet, a lower level of starting obesity, or a greater increase in muscle mass via a higher dose of ACVR2B:Fc or in younger animals. Our results are consistent, however, with the lack of adipose tissue weight loss using a neutralizing monoclonal antibody that targets MSTN in a genetic obesity model, the Lepob/ob mutant [9]. Muscle hypertrophy by anti-MSTN therapy in adult mice does seem to be effective as a preventative agent for obesity [8], and may be useful for maintenance of weight loss by achieved by other means. In this regard, women who perform resistance exercise after a 12-kg weight loss maintain their weight better than non-exercisers [12].
Although fat is not reduced, anti-MSTN therapy improves glucose metabolism in Lepob/ob mice [9]. Glucose tolerance and fasting glucose were not affected here perhaps because it is difficult to get C57BL/6 mice to become very hyperglycemic using HFD feeding. We did not measure insulin sensitivity or triglycerides, although it is possible these were affected without altering glucose tolerance.
In summary, our results together with those of other recent studies [8, 9] suggest that myostatin inhibition may be more efficacious in reducing adipose weight gain in those with no or fairly mild obesity rather than in causing weight loss.
Acknowledgments
This work is supported by the Intramural Research Program of the NIH, NIDDK. We thank Se-Jin Lee and Oksana Gavrilova for generously sharing materials.
References
- 1.LeBrasseur NK, Walsh K, Arany Z. Metabolic benefits of resistance training and fast glycolytic skeletal muscle. Am J Physiol Endocrinol Metab. 2010;300:E3–10. doi: 10.1152/ajpendo.00512.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee SJ. Extracellular Regulation of Myostatin: A Molecular Rheostat for Muscle Mass. Immunol Endocr Metab Agents Med Chem. 2010;10:183–194. doi: 10.2174/187152210793663748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-β superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- 4.McPherron AC. Metabolic Functions of Myostatin and Gdf11. Immunol Endocr Metab Agents Med Chem. 2010;10:217–231. doi: 10.2174/187152210793663810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo T, Jou W, Chanturiya T, Portas J, Gavrilova O, McPherron AC. Myostatin inhibition in muscle, but not adipose tissue, decreases fat mass and improves insulin sensitivity. PLoS One. 2009;4:e4937. doi: 10.1371/journal.pone.0004937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Caestecker M. The transforming growth factor-β superfamily of receptors. Cytokine Growth Factor Rev. 2004;15:1–11. doi: 10.1016/j.cytogfr.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Lee SJ, Reed LA, Davies MV, et al. Regulation of muscle growth by multiple ligands signaling through activin type II receptors. Proc Natl Acad Sci U S A. 2005;102:18117–18122. doi: 10.1073/pnas.0505996102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akpan I, Goncalves MD, Dhir R, et al. The effects of a soluble activin type IIB receptor on obesity and insulin sensitivity. Int J Obes (Lond) 2009;33:1265–1273. doi: 10.1038/ijo.2009.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernardo BL, Wachtmann TS, Cosgrove PG, et al. Postnatal PPARdelta activation and myostatin inhibition exert distinct yet complimentary effects on the metabolic profile of obese insulin-resistant mice. PLoS One. 2010;5:e11307. doi: 10.1371/journal.pone.0011307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Izumiya Y, Hopkins T, Morris C, et al. Fast/Glycolytic muscle fiber growth reduces fat mass and improves metabolic parameters in obese mice. Cell Metab. 2008;7:159–172. doi: 10.1016/j.cmet.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao B, Wall RJ, Yang J. Transgenic expression of myostatin propeptide prevents diet-induced obesity and insulin resistance. Biochem Biophys Res Commun. 2005;337:248–255. doi: 10.1016/j.bbrc.2005.09.044. [DOI] [PubMed] [Google Scholar]
- 12.Hunter GR, Brock DW, Byrne NM, Chandler-Laney PC, Del Corral P, Gower BA. Exercise Training Prevents Regain of Visceral Fat for 1 Year Following Weight Loss. Obesity (Silver Spring) 2009;18:690–695. doi: 10.1038/oby.2009.316. [DOI] [PMC free article] [PubMed] [Google Scholar]


