Abstract
We previously demonstrated the involvement of the tyrosine kinase receptor c-Met in medulloblastoma malignancy. The nonreceptor tyrosine kinases FAK and Pyk2, are key players in the progression of different cancers. However, their role in medulloblastoma malignancy is not well understood. In this study, using a protein array approach, we found that c-Met phosphorylates FAK and Pyk2 in medulloblastoma cells. We therefore studied the interactions between c-Met and FAK/Pyk2 and their implications for medulloblastoma therapy. We found that c-Met activates FAK and Pyk2 in several medulloblastoma cell lines. We also found that FAK and Pyk2 mediate the malignant effects of c-Met on medulloblastoma cell proliferation, migration and invasion. Based on these findings, we hypothesized that combined c-Met and FAK inhibitions would have additive effects on the inhibition of medulloblastoma malignancy. To test this hypothesis, we assessed the effects on medulloblastoma malignancy parameters of single or combined treatments of medulloblastoma cells with c-Met and FAK small molecule kinase inhibitors. We found a significant increase in the inhibitory effect of both inhibitors on medulloblastoma cell migration and cell invasion as compared to single inhibitions (p<0.05). Additionally, oral gavage treatment with c-Met inhibitor of mice bearing medullobastoma xenografts significantly reduced in vivo tumor growth. Therefore, combining c-Met inhibitors with FAK inhibitors constitutes a new potential strategy for medulloblastoma therapy. Altogether, our study describes a role for FAK and Pyk2 in medulloblastoma malignancy, uncovers new interactions between c-Met and FAK/Pyk2, and proposes for the first time combining anti-c-Met and anti-FAK inhibitors as a new strategy for medulloblastoma therapy.
Keywords: c-Met, hepatocyte growth factor, scatter factor, focal adhesion kinase, Pyk2, medulloblastoma, migration, invasion
INTRODUCTION
Medulloblastoma is the most common brain tumor in children with an incidence of 0.6 per 100,000 patient-years according to the Central Brain Tumor Registry of the United States. It is an embryonal brain tumor that arises in the cerebellum, where it is thought to originate from primitive pluripotent precursor cells of the ventricular zone and cerebellar external germinal layer (1). Multiple signaling pathways have been associated with medulloblastoma formation and growth. These include the developmental pathways Hedgehog (Hh), Notch, and Wnt as well as the receptor tyrosine kinases (RTK) erbB2, IGF-R and TrkC, and the oncoprotein Myc (2).
Our laboratory recently demonstrated the involvement of the receptor tyrosine kinase c-Met and its ligand hepatocyte growth factor (HGF) in medulloblastoma malignancy (3). Inappropriate activation of the HGF/c-Met signaling pathway has been shown to be involved in the etiology of various human cancers including brain tumors, conferring them with invasive and metastatic properties (2, 4, 5). Based on the widespread and profound involvement of c-Met in cancer, several c-Met pathway inhibitors have been recently developed. These include ribozymes, HGF kringle variants/NK4, decoy receptors, HGF or c-Met neutralizing antibodies, and small molecule kinase inhibitors (4, 6, 7). One such small molecule kinase inhibitor, PF-2341066, was recently identified as a potent, orally available, ATP-competitive and selective inhibitor of the catalytic activity of the c-Met receptor (8). PF-2341066 strongly inhibits c-Met phosphorylation and signal transduction, as well c-Met oncongenic phenotypes of tumor cells and endothelial cells, and exerts its cytoreductive effect through antiproliferative and antiangiogenic mechanisms in different cancers (9).
The nonreceptor tyrosine kinases, focal adhesion kinase (FAK) and the proline-rich tyrosine kinase-2 (Pyk2) have emerged as key players in the progression of different cancers. FAK and Pyk2 are important signaling effectors linking integrins and growth factor signaling to cell adhesion, invasion, proliferation, migration, survival, and apoptosis in many cancers (10). Similar to FAK, which undergoes autophosphorylation at the Tyrosine397 (Tyr397) residue, autophosphorylation of Pyk2 at Tyr402 residue leads to the recruitment of Src-family kinases, activation of extracellular signal-regulated kinase (ERKs), regulation of ion channels, cell adhesion and motility (11). FAK expression and/or phosphorylation is elevated in a variety of cancers and frequently correlates with malignant or metastatic disease and poor patient prognosis (12). Many studies have shown the association between FAK expression and malignancy grade, angiogenesis, invasion and migration in gliomas (13–15), However, their role in invasive medulloblastoma is not well understood. Recently, a novel small molecule FAK inhibitor, PF-573228 was identified through a combination of high throughput screening, structure based drug design, and conventional medicinal chemistry approaches. Treatment of cells with PF-573228 blocked FAK phosphorylation on Tyr397 and concomitantly reduced the phosphorylation of the well-recognized downstream effector of FAK signaling, paxillin (16).
In the present study, using a protein array approach, we found that c-Met stimulation by HGF phosphorylates FAK and Pyk2 in medulloblastoma cell lines. Therefore, we hypothesized that FAK/Pyk2 cooperate with c-Met-induced meduloblastoma malignancy and studied the interactions between them. We found that c-Met activates FAK and Pyk2 and that FAK and Pyk2 mediate the effects of c-Met on medulloblastoma cell migration, invasion and proliferation. We also showed that combined targeting of c-Met and FAK could be advantageous for medulloblastoma therapy.
MATERIALS AND METHODS
Cell culture and reagents
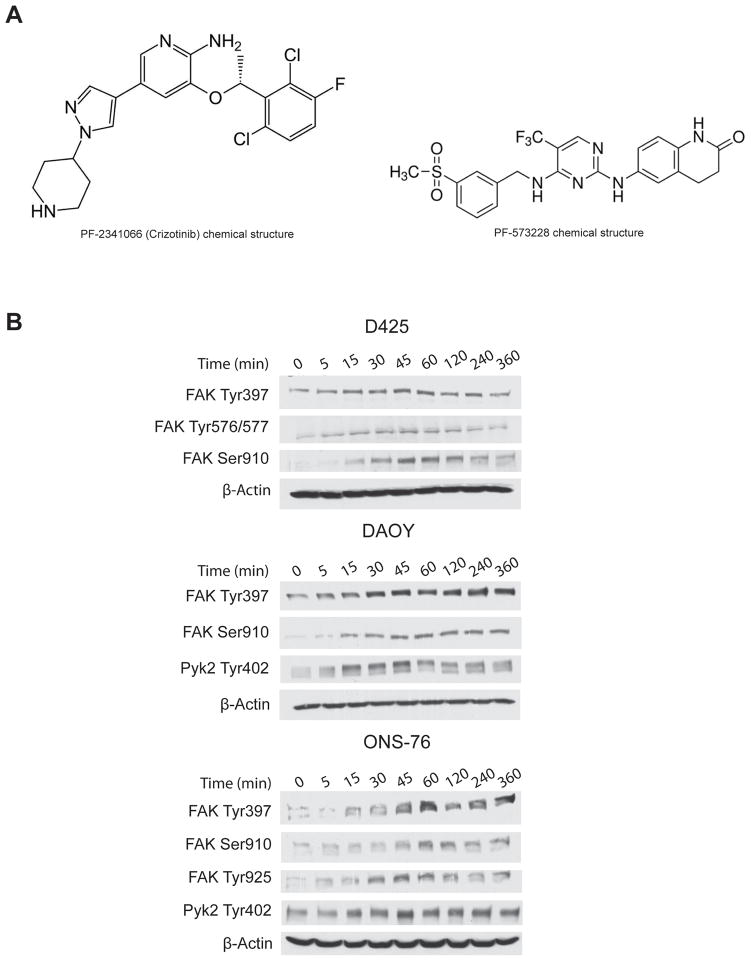
Three human medulloblastoma cell lines were used for this study. DAOY and ONS-76 were grown in RPMI-1640 media supplemented with 10% FBS. D425 cells were grown in Improved Modified Eagle Medium, Zinc option and 20% Fetal Bovine Serum (FBS). HGF-overexpressing DAOY cells (DAOY-HGF) were generated in our laboratory, and were cultured in Improved Modified Eagle Medium Zinc option, supplemented with 10% Fetal Bovine Serum (FBS) and the selection antibiotic Zeocin (1 μg/ml) (17). All cells were grown at 37°C in 5% CO2. HGF was purchased from R&D systems (Minneapolis, MN). Human type IV collagen was from Sigma-Aldrich (St. Louis, MO). The scrambled siRNA/control-siRNA, FAK-siRNA and Pyk2-siRNA were purchased from Santa-Cruz Biotechnology (Santa Cruz, CA). Oligofectamine and SDS-polyacrylamide gels were from Invitrogen (Carlsbad, CA). The small molecule c-Met kinase inhibitor PF-2341066 (METi), also clinically known as crizotinib, (R)-3-[1-(2,6-dichloro-3-fluoro-phenyl)-ethoxyl]-5-(1-piperidin-4-yl-1H-pyrazol-4-yl)-pyridin-2-ylamin was from Pfizer (Pfizer, La Jolla, CA). The FAK inhibitor PF-573228 (FAKi), 3,4-Dihydro-6-[[4-[[[3(methylsulfonyl)phenyl]methyl]amino]-5-(trifluoromethyl)-2-pyrimidinyl]amino](1H)quinolinone was purchased from Tocris bioscience (Ellisville, MO). The chemical structures of PF-2341066 and PF-573228 are shown in Figure 1A. Panorama antibody microarray cell signaling kit, Cy3 and Cy5 fluorescence dyes, and all chemicals and solvents were from Sigma-Aldrich (St. Louis, MO).
Figure 1.
A) Chemical structures of PF-2341066 and PF-573228. B) HGF induces the activation of FAK and Pyk2 in medulloblastoma cells in a time-dependent manner Immunoblots showing the time-dependent phosphorylation of FAK and Pyk2 on various tyrosines (Tyr) and serines (Ser) in medulloblastoma cells treated 20 ng/ml HGF for 5 min - 6 hours. The blots were stripped and hybridized for β-Actin as a loading control.
Protein microarrays
Protein arrays were performed to identify cell signaling molecules that are modulated by HGF in medulloblastoma cells. The Panorama antibody microarray contains 336 antibodies representing cell signaling molecules in their total and active (usually phosphorylated) forms. The antibodies are printed in duplicates on 4×8 grids. Each grid contains seven antibody duplicates plus a Cy3- and Cy5-conjugated BSA positive control as well a non-labeled BSA negative control resulting in a total of 512 spots. ONS-76 medulloblastoma cells were treated with HGF (20 ng/ml) for 30 min and untreated cells were used as a control. The cells were processed for the microarray hybridization according to the manufacturer’s instruction. Briefly, proteins were extracted by an extracting/labeling buffer containing Benzonase, protease inhibitors, and phosphatase inhibitors. 1 mg of protein extract from HGF-treated and non-treated cells were labeled with Cy5 or Cy3, respectively, according to the manufacturer’s instructions, and used at Dye/Protein ratio >2. Free non-incorporated Cy3 and Cy5 dyes were separated by applying the labeled extracts to SigmaSpin Post-Reaction Clean-Up Columns. An equal amount of labeled protein of both extracts (10 μg/ml) was incubated on the Panorama Ab Microarray slide for 30 min; all washes were done in PBS-Tween 0.05%. The slides were air-dried before scanning with the GSI 4000 scanner, and images were generated with the Panorama software (GSI Lumonics Warwickshire, UK). The slide scanning was performed using a microarray scanner (ProScanArray Scanner, Perkin Elmer). The results were analyzed using standard median normalization between both channels Cy3 and Cy5 using the “ScanArray Express Software”. All experiments were performed in triplicates.
Immunoblotting
Immunoblotting was performed using antibodies specific for phosphorylated and non- phosphorylated forms of FAK, Pyk2 (Cell signaling, Carlsbad, CA), and β-actin (Santa Cruz Biotechnologies, Santa Cruz, CA), the loading control. To assess the effects of HGF on FAK and Pyk2 activations, D425, DAOY and ONS medulloblastoma cells were treated with 20 ng/ml HGF for various time points (5 min - 6 hours) and subsequently immunoblotted for phospho-FAK on different phosphorylation sites Tyr397, Tyr576/577, Tyr925, Ser910, or phospho-Pyk2 on Tyr402 site. The cells were subsequently lysed with RIPA buffer (1% Igepal, 0.5% sodium deoxycholate and 0.1% SDS in PBS) and lysates were collected and cleared by centrifugation. The protein concentration of the supernatant was determined, equal amounts of protein were electrophoretically separated in polyacrylamide gels, and then trans-blotted to a nitrocellulose membrane. The membrane was then incubated overnight with primary antibodies at 4°C. The primary antibody-bound membranes were washed three times with Tween-PBS before incubation with the corresponding horseradish-conjugated secondary antibodies. After a final wash, the immunoreactive signals were detected by enhanced chemiluminescence., and quantified by densitometry on film using computer-assisted image analysis.
siRNA silencing
FAK-siRNA and Pyk2-siRNA were used to knockdown FAK and Pyk2 expressions in DAOY and D425 cells. Cells were transfected with either FAK-siRNA or Pyk2-siRNA using Oligofectamine transfection reagent according to the manufacturer’s instructions. Random/scrambled siRNA (cont-siRNA) was used as a control. The knockdown of FAK and Pyk2 was confirmed by immunoblotting using the FAK and Pyk2 antibodies as described above.
Cell proliferation assays
DAOY and D425 cells were seeded in triplicates, in medium containing 10% FBS and treated with either FAK-siRNA or Pyk2-siRNA (35 nM). Controls were treated with cont-siRNA (35 nM). Twenty-four hours later, the media was changed to low serum media (FBS 0.1%), and cells were treated with HGF (20 ng/ml). The cells were trypsinized and harvested every day, for 4 days, counted with a hemocytometer, and growth curves were established.
Migration Assays
Wound/scratch assay was performed as previously described (18). DAOY cells were seeded at 80% confluency in low serum media for 24 hours. The cells were treated with 35 nM FAK-siRNA, Pyk2-siRNA, or cont-siRNA and 24 hours later treated with 20 ng/ml HGF. The cells were assessed for migration using the scratch assay. Cells that migrated into the scratch 24 hours post-treatment were photographed at 40X magnification.
Invasion assays
Transwell invasion assays were performed to determine if FAK and/or Pyk2 mediate the effects of c-Met on medulloblastoma cell invasion. Modified Boyden Chambers (Becton Dickinson, MA) were coated with human type IV collagen (250 mg/ml). DAOY cells (2×105)were treated with 35 nM FAK-siRNA, Pyk2-siRNA, or cont-siRNA for 8 hours and then exposed to 20 ng/ml HGF for 24 hours. The following day, the cells were resuspended in serum-free media and added to each insert in the presence or absence of HGF 20 ng/ml. 600 μl of 10% FBS medium was placed in the lower chamber as a chemoattractant. After 8 hours of incubation at 37°C in 5% CO2, invading cells were stained with 0.1% crystal violet solution and photographed at 40X. The cells were then counted under a microscope in five randomly chosen fields.
Pharmacologic inhibition of FAK and c-Met
METi was dissolved in sterile water, aliquoted, filtered, and stored in the dark, at room temperature until use. FAKi was prepared in sterile DMSO, filtered, aliquoted, and stored at −80°C until use. DAOY cells or DAOY-HGF clones were treated with either FAK inhibitor FAKi (1 μM), c-Met Inhibitor METi (100 nM), a combination of both inhibitors, or control vehicle for 24 hours and subsequently treated with 20 ng/ml HGF for 24 hours for cell migration, and 6 hours for cell invasion. The cells were assessed for migration or invasion, as described above. FAK and c-Met inhibitions, as well as p-FAK and p-c-Met inhibitions, were verified by immunoblotting. Signals were quantified by densitometry and the quantification results were reported for each western blot. For proliferation, DAOY cells and DAOY-HGF clones were seeded in 6 well plates, treated with FAKi or METi or combination of both inhibitors for 24 hours at the same concentrations listed above, and subsequently treated with HGF 20 ng/ml for 24 hours. Cell growth was monitored by counting the cells for 4 days, and proliferation curves were established. The effects of single treatments on cell migration, invasion, and proliferation were compared with the effects of combined inhibitor treatment.
In vivo xenograft experiments
METi was dissolved in sterile water (stock 0.5 M), filtered and, stored in the dark at room temperature. Medulloblastoma DAOY-HGF cells (2×106) were implanted in the flanks of immunodeficient mice (n=10). METi treatment started 5 days post-tumor implantation. The animals were treated by oral gavage of 0.1 ml of 25 mM METi solution (30 mg/kg body weight) every day for three weeks. Control animals were treated with an equal volume of sterile distilled water. The flank tumors were measured with a caliper every day for two weeks. At the end of the treatment, the animals were euthanized and the tumors were removed, weighed, and a tumor volume progression curve was established.
Statistics
All experiments were performed at least in triplicates. Numerical data were expressed as mean ± standard deviation. Two group comparisons were analyzed by two-sided Student’s t test. Multiple group comparisons were analyzed with Bonferroni/Dunn multiple comparisons tests. P Values were determined for all analyses and p<0.05 was considered significant.
RESULTS
c-Met activates FAK and Pyk2 as revealed by protein arrays
We assessed the effects of c-Met activation on global cell signaling in medulloblastoma cells using antibody microarrays containing 336 cell signaling molecule probes in active and total states. We found numerous changes in the activation of signal transduction proteins in response to HGF. Many of these changes were known and expected such as the induction of cyclin D1, cyclin A, and phospho-Akt by HGF. Few other changes showed effects of HGF that had not been described before in medulloblastoma (Supplementary Table 1). One such novel and interesting effect of HGF was the phosphorylation of Focal Adhesion Kinase (FAK) at Tyr577 and the phosphorylation of the Focal adhesion kinase-related protein tyrosine kinase (Pyk2) at Tyr579 (Supplementary Table 1). Interestingly, both proteins are related to each other and play critical roles in cell migration and invasion in other cancers. We therefore hypothesized that c-Met-induced tumor cell invasion and migration could be mediated by FAK and/or Pyk2.
c-Met activates FAK and Pyk2 in medulloblastoma cells in a time-dependent manner
To confirm that c-Met activates FAK and/or Pyk2 as observed on the protein arrays, the effect of HGF on FAK and Pyk2 phosphorylations in medulloblastoma cells was assessed by immunoblotting. D425, DAOY, and ONS-76 medulloblastoma cells were treated with 20 ng/ml HGF for various time points (5 min - 6 hours) and subsequently immunoblotted for phospho-FAK Tyr397, phospho-FAK Tyr576/577, phospho-FAK Tyr925, Phospho-FAK Ser910, or phospho-Pyk2 Tyr402. HGF treatment led to FAK and Pyk2 phosphorylations at multiple sites in all medulloblastoma cells that were examined. c-Met activation with HGF induced a strong phosphorylation of FAK at Tyr397 and Tyr925, which are important for the maximal adhesion-induced activation of FAK and signaling to downstream effectors. c-Met activation with HGF also led to phosphorylation of FAK at Ser910, which is involved in modulating binding/stability of downstream signaling proteins, and phosphorylation of FAK at Tyr576/577, which is strongly associated with migration. Similarly, cell treatment with HGF resulted in a strong and fast phosphorylation of Pyk2 at Tyr402, reported to promote invasion and migration. Phosphorylations started between 5 min and 15 min after HGF treatment and lasted for several hours (Figure 1B). Phosphorylations of all aminoacids were not detected in all cell lines (not shown). Overall, the above data show that c-Met activates FAK and Pyk2 in a time-dependent manner.
FAK and Pyk2 mediate c-Met-induced cell proliferation in medulloblastoma cells
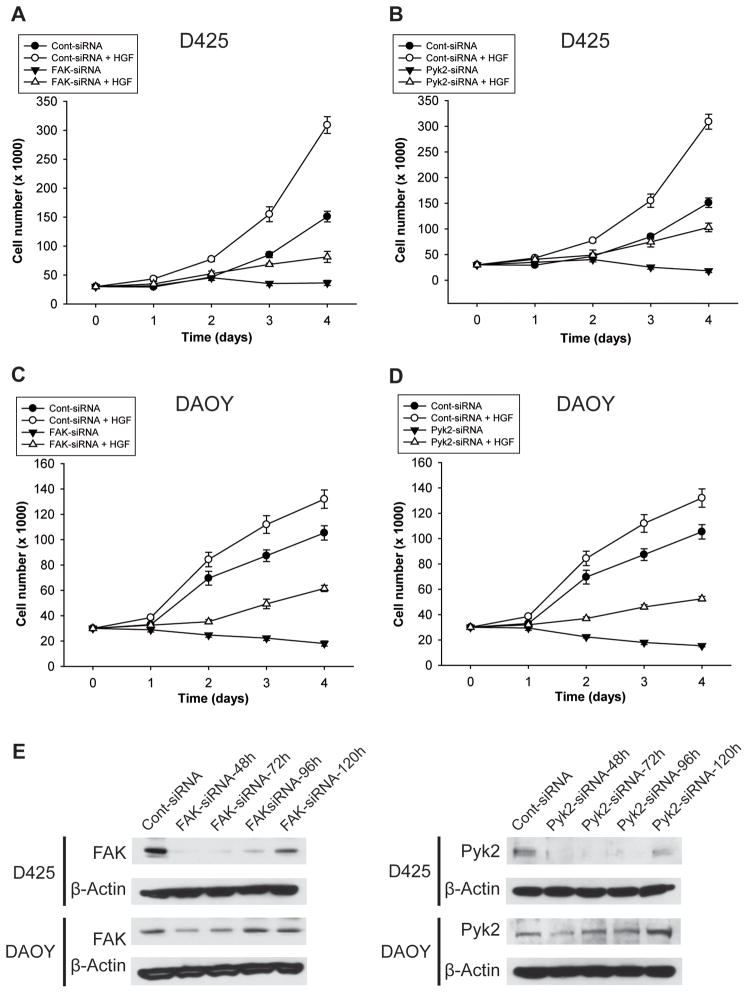
To determine if FAK and/or Pyk2 mediate c-Met-dependent cell growth, we assessed the effects of c-Met activation on DAOY and D425 medulloblastoma cell proliferation in the setting of silenced FAK or Pyk2. FAK-siRNA, Pyk2-siRNA, or scrambled control siRNA (cont-siRNA) were transfected into the cells to inhibit FAK and Pyk2 expressions. After 24 hours, the cells were treated with 20 ng/m HGF for 24 hours and cell proliferation was analyzed by cell counting over 4 days. FAK and Pyk2 silencing was verified by immunoblotting.
FAK knockdown led to significant inhibition of c-Met-dependent cell proliferation in D425 medulloblastoma cells. FAK knockdown decreased cell numbers at day 4 from 151 ± 9.29 to 36.33 ± 4.48 in HGF-untreated cells (n=3; P<0.05). FAK knockdown decreased HGF-induced cell numbers at day 4 from 309 ± 14.57 to 81.33 ± 9.56 (n=3; p<0.05) (Figure 2A). Pyk2 knockdown led to significant inhibition of c-Met-dependent cell proliferation in D425 medulloblastoma cells. At day 4 of counting, Pyk2 knockdown reduced cell numbers from 151 ± 9.29 to 18.33 ± 3.18 in HGF-untreated cells (n=3; P<0.05). Pyk2 knockdown decreased HGF-induced cell numbers at day 4 from 309 ± 14.57 to 103.00 ± 8.39 (n=3; p<0.05) (Figure 2B). FAK silencing also led to significant inhibition of c-Met-dependent cell proliferation in DAOY cells. FAK knockdown decreased cell numbers at day 4 from 105.33 ± 5.70 to 18.08 ± 3.02 in HGF-untreated cells (n=3; P<0.05). FAK knockdown decreased HGF-induced cell growth at day 4 from 132.00 ± 7.23 to 61.50 ± 2.41 (n=3; p<0.05) (Figure 2C). Pyk2 silencing led to significant inhibition of c-Met-dependent cell proliferation in DAOY cells. At day 4 of counting, Pyk2 knockdown reduced basal cell numbers from 105.33 ± 5.70 to 15.42 ± 0.87 in HGF-untreated cells (n=3; P<0.05). Pyk2 knockdown decreased HGF-induced cell numbers at day 4 from 132.00 ± 7.23 to 52.50 ± 1.84 (n=3; p<0.05) (Figure 2D). These data show that FAK and Pyk2 induce cell proliferation and mediate c-Met-dependent cell proliferation in medulloblastoma cells.
Figure 2. FAK and Pyk2 mediate the effects of c-Met on medulloblastoma cell proliferation.
FAK and Pyk2 expressions were silenced in DAOY and D425 cells by transfection with FAK-siRNA (35 nM) or Pyk2-siRNA (35 nM), respectively. Control cells were transfected with 35 nM scrambled siRNA (cont-siRNA). After 24 hours, the cells were treated with 20 ng/ml HGF and counted every day for 4 days. The results show that silencing of FAK or Pyk2 significantly decreases basal and c-Met-induced cell growth. A) D425-FAK silencing, B) D425-Pyk2 silencing, C) DAOY-FAK silencing, D) DAOY-Pyk2 silencing. E) Immunoblots show FAK and Pyk2 knockdown in cells treated with respective siRNA as described above. Results are representative of 3 different experiments.
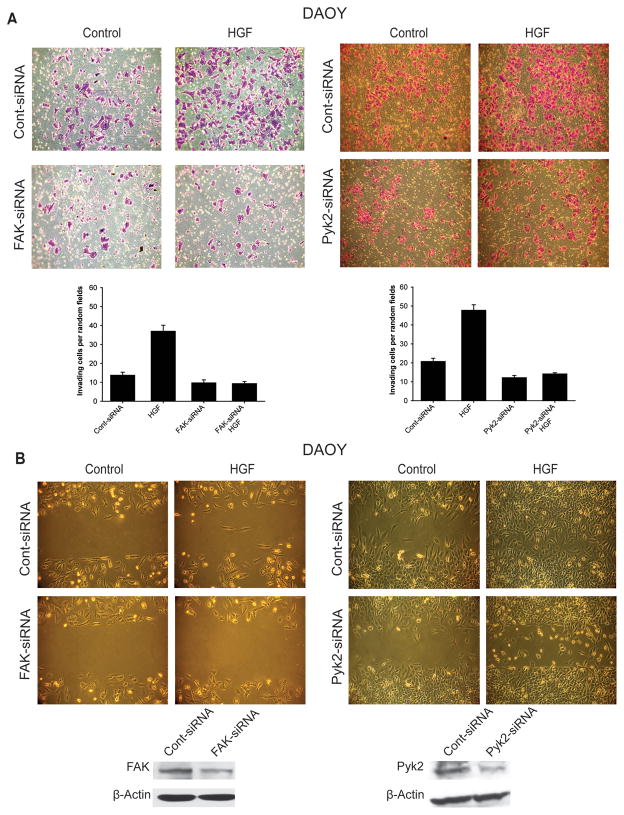
FAK and Pyk2 mediate c-Met-induced migration and invasion of medulloblastoma cells
To determine if FAK and/or Pyk2 mediate c-Met-dependent cell migration and invasion, we assessed the effects of c-Met activation on DAOY medulloblastoma cell proliferation in the setting of inhibited FAK or Pyk2 expressions. We tested the effect of FAK and Pyk2 knockdown on basal and HGF-induced cell migration and invasion using the scratch assay and transwell invasion assay, respectively. DAOY cells were treated with FAK-siRNA and FAK knockdown was confirmed by immunoblotting. The cells were then treated with 20 ng/ml HGF and invasion was quantified. HGF induced DAOY cell invasion in control (Figure 3A). FAK and Pyk2 silencing significantly decreased HGF-induced cell invasion (Figure 3A). Similarly, HGF induced DAOY cell migration in control but significantly less in FAK-siRNA or Pyk2-siRNA pretreated cells (Figure 3B). These data demonstrate that FAK and Pyk2 mediate c-Met-dependent migration and invasion in medulloblastoma cells.
Figure 3. FAK and Pyk2 mediate the effects of c-Met on medulloblastoma cell migration and invasion.
FAK and Pyk2 expressions were silenced in DAOY and D425 cells by transfection with FAK-siRNA (35 nM) or Pyk2-siRNA (35 nM), respectively. Control cells were transfected with 35 nM scrambled siRNA (cont-siRNA). After 24 hours, the cells were treated with 20 ng/ml HGF and subsequently, assessed for migration (A) and invasion (B), using the scratch assay and the boyden chamber assay, respectively. The results show that silencing of FAK or Pyk2 significantly decreases basal and c-Met-induced migration and invasion in medulloblastoma cells. The immunoblots in the lower panel show the knock-down of FAK and Pyk2 in DAOY cells treated as described above.
Combined inhibition of c-Met and FAK with small molecule kinase inhibitors inhibits medulloblastoma cell migration and invasion more than single inhibitions
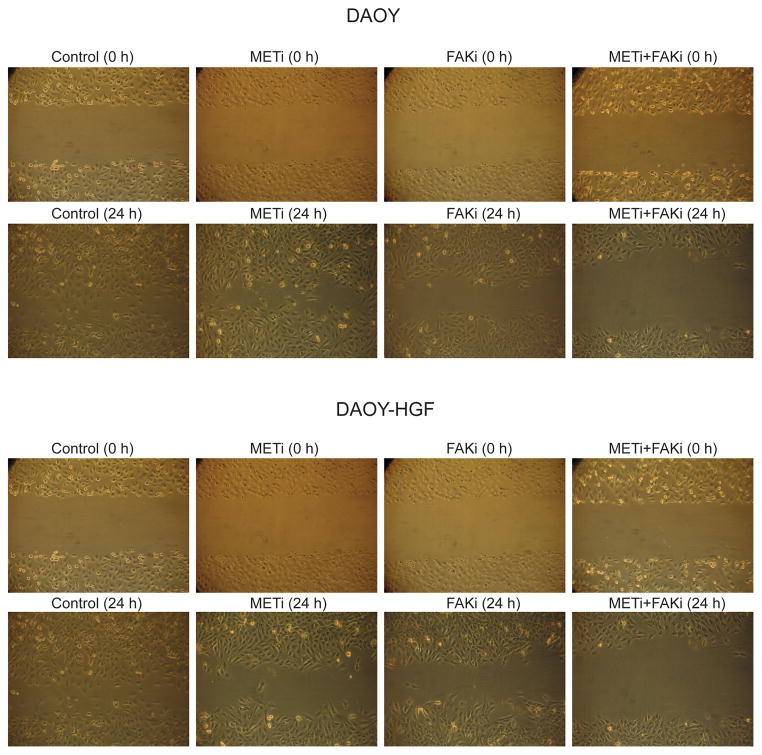
Based on the above results showing that c-Met activates FAK and Pyk2 and that FAK and Pyk2 mediate the effects of c-Met in medulloblastoma cells, we hypothesized that combined inhibition of c-Met and FAK could have greater inhibitory effects on medulloblatoma malignancy than single inhibitions. To test this hypothesis, we assessed the effects of small molecule kinase inhibitors of c-Met (METi) and FAK (FAKi) on medulloblastoma cell migration, invasion, and proliferation. We first verified the inhibitory effects of the small molecules on c-Met and FAK activation. DAOY and DAOY-HGF cells were treated with 100 nM METi and/or 1 μM FAKi for 24 hours. METi strongly inhibited HGF-induced c-Met phosphorylation in DAOY and DAOY-HGF cells (Supplementary Figure 1). FAKi inhibited HGF-induced FAK phosphorylation in DAOY and DAOY-HGF cells (Supplementary Figure 1). METi did not significantly affect FAK phosphorylation suggesting that FAK is additionally activated by factors other than c-Met (Supplementary Figure 1).
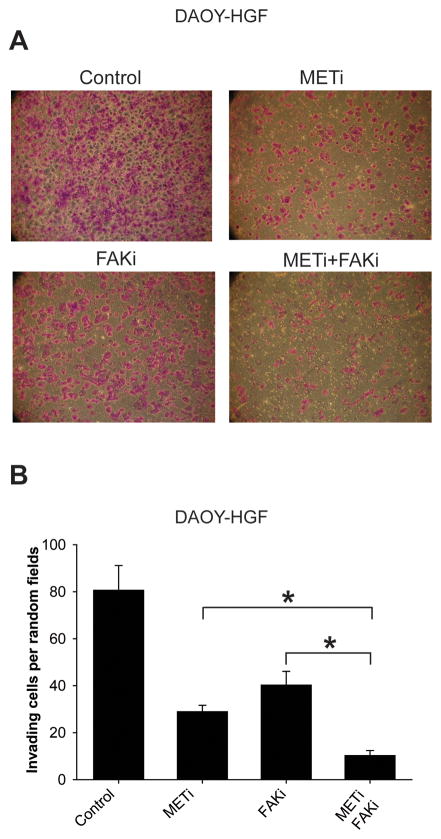
We then assessed the effects of single and combined c-Met and FAK inhibitions on cell migration, invasion, and proliferation using a scratch assay, a transwell invasion assay, and cell counting, respectively. Single METi and FAKi treatments inhibited basal and HGF-induced the migration of DAOY and DAOY-HGF cells. Combined METi and FAKi treatments had greater inhibitory effect on medulloblastoma cell migration and invasion than single inhibitions (Figure 4). FAKi reduced the number of DAOY-HGF invading cells through the collagen IV matrix from 80.6 ± 10.5 to 40.33 ± 5.77. METi reduced the number of DAOY-HGF invading cells through the collagen IV matrix, from 80.6 ± 10.5 to 29 ± 2.64. The combined effect of both inhibitors significantly decreased the number of invading cells to 10.33 ± 2.08 (p<0.01) (Figure 5). These data suggest that combining anti-c-Met and anti-FAK approaches might have experimental therapeutic advantage in medulloblastoma. However, such additive effect could not be detected on cell growth in eitherDAOY or DAOY-HGF cells (supplementary Figure 2)
Figure 4. Combined inhibition of FAK and c-Met by small molecule kinase inhibitors inhibits medulloblastoma cell migration more than single inhibitions.
DAOY or DAOY-HGF cells were treated with either 1 μM FAKi, 100 nM METi, a combination of both, or control vehicle for 1 hour prior to treatment with or without 20 ng/ml HGF (DAOY only), for 24 hours. The cells were assessed for migration using the scratch assay. Cells that migrated into the scratch were photographed at 40X magnification. The results show that combined inhibition of FAK and c-Met has greater inhibitory effects on medulloblastoma cell migration than single inhibitions.
Figure 5. Combined inhibition of FAK and c-Met by small molecule kinase inhibitors inhibits medulloblastoma cell invasion more than single inhibitions.
DAOY-HGF cells were treated with either 1 μM FAKi, 100 nM METi, a combination of both, or control for 24 hours. The cells were assessed for invasion using a transwell assay. Cells that invaded through the membrane after 6 hours were fixed and stained with crystal violet. The wells were photographed at 40X magnification and five random fields were quantified. The results show that combined inhibition of FAK and c-Met has greater inhibitory effects on medulloblastoma cell invasion than single inhibitions. A) Representative invasion assay. B) Quantification of invasion assay. * =p< 0.05.
Oral delivery of METi inhibits the in vivo growth of human medulloblastoma xenografts
Our original goal was to test the combined effects of METi and a clinically applicable FAK inhibitor on in vivo medulloblastoma growth. However, the clinically applicable FAK inhibitor is not commercially available and we were not able to obtain it from the manufacturing pharmaceutical company. We therefore assessed the effects of in vivo c-Met inhibition on medulloblastoma xenograft growth.
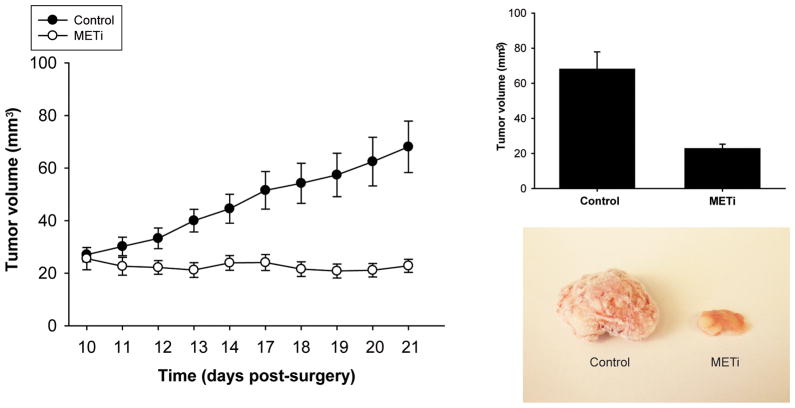
To test the in vivo anti-tumor properties of METi, we generated medulloblastoma xenografts by implantation of DAOY-HGF medulloblastoma cells in the flanks of immunodeficient mice. Tumor bearing animals were treated by oral delivery of METi via gavage for three weeks. While control animals developed very large tumors averaging 68.082 ± 9.794 mm3 in volume, the METi-treated group showed a significant inhibition of tumor growth averaging 22.806 ± 2.483 mm3 (n=10, p<0.01) (Figure 6). No obvious drug toxicity was observed during the treatment. For the first time, these data show that inhibition of c-Met kinase by a small molecule inhibitor leads to inhibition of in vivo medulloblastoma tumor growth. The data also shows the feasibility of oral delivery of small molecule kinase inhibitors of c-Met and suggest that these molecules can be combined with FAK inhibitors for improved future medulloblastoma therapies.
Figure 6. Oral delivery of METi significantly inhibits the in vivo growth of human medulloblastoma xenografts.
Human medulloblastoma xenografts were generated by flank implantation of 2×106 HGF-DAOY cells in immunodeficient mice. Five days post-tumor implantation, the animals were treated with METi by oral gavage (30 mg/kg body weight) once per day, for 3 weeks. The control group was treated with equal volume of sterile distilled water. At the end of the treatment, animals were sacrificed and tumor size was assessed by measuring tumor volume. The results show that METi treatment significantly inhibits in vivo medulloblastoma growth (p<0.01).
DISCUSSION
Our study shows for the first time that c-Met activates FAK and Pyk2 and that FAK and Pyk2 mediate the effects of c-Met in medulloblastoma. The study also establishes a role for FAK and Pyk2 in medulloblastoma malignancy. Furthermore, our study shows for the first time the anti-oncongenic effects of small molecule kinase inhibitors of c-Met and FAK in medulloblastoma and suggests that combined targeting of c-Met and FAK can be advantageous for medulloblastoma therapy.
Medulloblastoma is the most common malignant brain tumor of childhood arising in the cerebellum (19),(20). It has the highest rates of metastasis outside the nervous system (21–23) and tends to spread hematogenously into bones, bone marrow, lymphatic nodes, liver and lungs (24, 25). To date, surgery and radiation therapy remain the most effective treatment for medulloblastoma. However, craniospinal radiotherapy increase is frequently accompanied by life-long brain damage (20, 23). Targeting molecular pathways that govern medulloblastoma malignancy is a promising approach for achieving future improved clinical outcomes. We identified c-Met and FAK as two such pathways that can be simultaneously targeted for greater anti-medulloblastoma effects.
FAK activation has been associated with a variety of cancers and frequently correlates with malignancy, metastasis, and poor patient prognosis (12). A recent study has shown the involvement of integrinβ1/FAK signaling in migration and invasion in medulloblastoma (26). Few small molecules inhibitors of FAK have been developed. PF-562271 has shown a highly selective and potent pharmacological inhibitory effect on FAK catalytic activity, allowing it to be a first in class inhibitor reaching clinical trial testing for the cancer therapy (27). Unfortunately, this inhibitor is not commercially available and we were unable to obtain it from the manufacturer. We therefore used the commercially available PF-573228 which cannot be used for in vivo animal experimentation (16). Our study shows for the first time that FAK kinase activity and FAK-associated migration and invasion are inhibited by PF-573228. Therefore FAK and Pyk2 inhibitions represent a potential novel strategy for medulloblastoma therapy.
Given the critical role of the receptor tyrosine kinase c-Met, and its ligand HGF in the progression of different human cancers including brain tumors, different approaches to inhibiting HGF and c-Met have been developed (4). Among these, the small molecule kinase inhibitor of c-Met, PF-2341066 has reached Phase I and II of clinical trials, and therefore represents a promising therapeutic approach to inhibiting c-Met in cancer and medulloblastoma. Our study demonstrates for the first time the usefulness of PF-2341066 in inhibiting c-Met activation and c-Met-dependent oncogenic effects in medulloblastoma cells and tumors.
Our study shows for the first time that HGF/c-Met activates FAK and Pyk2 in medulloblastoma. A connection between c-Met and FAK has been previously described in some cancers such as ovarian cancer (28), breast cancer (29), (30), but not in medulloblastoma. Furthermore, to date, no connection between c-Met and Pyk2 has been described in any cancer. Our findings suggest either a direct or an indirect FAK/Pyk2 activation by c-Met kinase. We used immunoprecipitation of FAK and c-Met upon HGF stimulation of medullobalstoma cells to explore the possibility of direct binding of FAK and c-Met. We could not detect any direct interaction between the two proteins (data not shown). The indirect interaction could occur via activation of scaffolding or key adaptor proteins known to mediate FAK effects such as Paxillin and p130CAS (31, 32). Considering that the FAK-Src complex mediates the phosphorylation of paxillin and p130CAS, and since c-Met is known to signal both upstream and downstream from c-Src, this latter is a possible candidate for mediating FAK activation by c-Met.
Considering that multiple molecular pathways are involved in growth, survival, migration, and invasion of tumor cells, anti-tumor activity could be improved by simultaneous use of individual agents targeting different pathways such as Raf/MEK/ERK or c-Met to allow vertical or horizontal inhibition of relevant pathways (33, 34). Such simultaneous targeting would have to be based on molecular findings indicating cooperation, redundancy or compensatory mechanisms involving the targeted molecules. Our study suggests for the first time that combining c-Met and FAK/Pyk2 inhibitors as another approach for achieving improved anti-tumor effects in medulloblastoma. Our intent was to test this combination approach in vivo in a medulloblastoma animal model, but we were unable to obtain the only FAK inhibitor with proven bioavailabilty from the manufacturer. However, we did show for the first time the in vivo usefulness of a small molecule inhibitor of c-Met on medulloblastoma tumor growth in paving the road for a potential testing of combined anti-Met/anti-FAK therapy in medulloblastoma.
Overall, our study uncovers previously unknown interactions between c-Met and FAK/Pyk2 in medulloblastoma and suggests that simultaneous targeting of these molecules might be advantageous for medulloblastoma therapy.
Supplementary Material
Acknowledgments
Supported by NIH RO1 NS045209 (R. Abounader) and NIH R01 CA134843 (R. Abounader).
References
- 1.Yokota N, Aruga J, Takai S, Yamada K, Hamazaki M, Iwase T, et al. Predominant expression of human zic in cerebellar granule cell lineage and medulloblastoma. Cancer Res. 1996;56:377–83. [PubMed] [Google Scholar]
- 2.Guessous F, Li Y, Abounader R. Signaling pathways in medulloblastoma. J Cell Physiol. 2008;217:577–83. doi: 10.1002/jcp.21542. [DOI] [PubMed] [Google Scholar]
- 3.Li Y, Guessous F, Johnson EB, Eberhart CG, Li XN, Shu Q, et al. Functional and molecular interactions between the HGF/c-Met pathway and c-Myc in large-cell medulloblastoma. Lab Invest. 2008;88:98–111. doi: 10.1038/labinvest.3700702. [DOI] [PubMed] [Google Scholar]
- 4.Abounader R, Laterra J. Scatter factor/hepatocyte growth factor in brain tumor growth and angiogenesis. Neuro-oncol. 2005;7:436–51. doi: 10.1215/S1152851705000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915–25. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- 6.Abounader R, Ranganathan S, Lal B, Fielding K, Book A, Dietz H, et al. Reversion of human glioblastoma malignancy by U1 small nuclear RNA/ribozyme targeting of scatter factor/hepatocyte growth factor and c- met expression. J Natl Cancer Inst. 1999;91:1548–56. doi: 10.1093/jnci/91.18.1548. [DOI] [PubMed] [Google Scholar]
- 7.Abounader R, Lal B, Luddy C, Koe G, Davidson B, Rosen EM, et al. In vivo targeting of SF/HGF and c-met expression via U1snRNA/ribozymes inhibits glioma growth and angiogenesis and promotes apoptosis. Faseb J. 2002;16:108–10. doi: 10.1096/fj.01-0421fje. [DOI] [PubMed] [Google Scholar]
- 8.Christensen JG, Burrows J, Salgia R. c-Met as a target for human cancer and characterization of inhibitors for therapeutic intervention. Cancer Lett. 2005;225:1–26. doi: 10.1016/j.canlet.2004.09.044. [DOI] [PubMed] [Google Scholar]
- 9.Zou HY, Li Q, Lee JH, Arango ME, McDonnell SR, Yamazaki S, et al. An orally available small-molecule inhibitor of c-Met, PF-2341066, exhibits cytoreductive antitumor efficacy through antiproliferative and antiangiogenic mechanisms. Cancer Res. 2007;67:4408–17. doi: 10.1158/0008-5472.CAN-06-4443. [DOI] [PubMed] [Google Scholar]
- 10.Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci. 2003;116:1409–16. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- 11.Boutahar N, Guignandon A, Vico L, Lafage-Proust MH. Mechanical strain on osteoblasts activates autophosphorylation of focal adhesion kinase and proline-rich tyrosine kinase 2 tyrosine sites involved in ERK activation. J Biol Chem. 2004;279:30588–99. doi: 10.1074/jbc.M313244200. [DOI] [PubMed] [Google Scholar]
- 12.Chatzizacharias NA, Kouraklis GP, Theocharis SE. Clinical significance of FAK expression in human neoplasia. Histol Histopathol. 2008;23:629–50. doi: 10.14670/HH-23.629. [DOI] [PubMed] [Google Scholar]
- 13.Natarajan M, Hecker TP, Gladson CL. FAK signaling in anaplastic astrocytoma and glioblastoma tumors. Cancer J. 2003;9:126–33. doi: 10.1097/00130404-200303000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Bigarella CL, Borges L, Costa FF, Saad ST. ARHGAP21 modulates FAK activity and impairs glioblastoma cell migration. Biochim Biophys Acta. 2009;1793:806–16. doi: 10.1016/j.bbamcr.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 15.Liu M, Yang Y, Wang C, Sun L, Mei C, Yao W, et al. The effect of epidermal growth factor receptor variant III on glioma cell migration by stimulating ERK phosphorylation through the focal adhesion kinase signaling pathway. Arch Biochem Biophys. 2010;502:89–95. doi: 10.1016/j.abb.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 16.Slack-Davis JK, Martin KH, Tilghman RW, Iwanicki M, Ung EJ, Autry C, et al. Cellular characterization of a novel focal adhesion kinase inhibitor. J Biol Chem. 2007;282:14845–52. doi: 10.1074/jbc.M606695200. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Lal B, Kwon S, Fan X, Saldanha U, Reznik TE, et al. The scatter factor/hepatocyte growth factor: c-met pathway in human embryonal central nervous system tumor malignancy. Cancer Res. 2005;65:9355–62. doi: 10.1158/0008-5472.CAN-05-1946. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Guessous F, Kwon S, Kumar M, Ibidapo O, Fuller L, et al. PTEN has tumor-promoting properties in the setting of gain-of-function p53 mutations. Cancer Res. 2008;68:1723–31. doi: 10.1158/0008-5472.CAN-07-1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crawford JR, MacDonald TJ, Packer RJ. Medulloblastoma in childhood: new biological advances. Lancet Neurol. 2007;6:1073–85. doi: 10.1016/S1474-4422(07)70289-2. [DOI] [PubMed] [Google Scholar]
- 20.Howes TL, Buatti JM, Kirby PA, Carlisle TL, Ryken TC. Radiation induced adult medulloblastoma: a case report. J Neurooncol. 2006;80:191–4. doi: 10.1007/s11060-006-9175-4. [DOI] [PubMed] [Google Scholar]
- 21.Feltbower RG, Picton S, Bridges LR, Crooks DA, Glaser AW, McKinney PA. Epidemiology of central nervous system tumors in children and young adults (0–29 years), Yorkshire, United Kingdom. Pediatr Hematol Oncol. 2004;21:647–60. doi: 10.1080/08880010490501079. [DOI] [PubMed] [Google Scholar]
- 22.Ranger A, McDonald W, Bauman GS, Del Maestro R. Effects of surgical excision and radiation on medulloblastoma cell invasiveness. Can J Neurol Sci. 2009;36:631–7. doi: 10.1017/s0317167100008155. [DOI] [PubMed] [Google Scholar]
- 23.Sun LM, Yeh SA, Wang CJ, Huang EY, Chen HC, Hsu HC, et al. Postoperative radiation therapy for medulloblastoma--high recurrence rate in the subfrontal region. J Neurooncol. 2002;58:77–85. doi: 10.1023/a:1015865614640. [DOI] [PubMed] [Google Scholar]
- 24.Lowenfels AB. Ileostomy and ileal carcinoma. Gastroenterology. 1989;96:551. doi: 10.1016/s0016-5085(89)91616-8. [DOI] [PubMed] [Google Scholar]
- 25.Mazloom A, Zangeneh AH, Paulino AC. Prognostic factors after extraneural metastasis of medulloblastoma. Int J Radiat Oncol Biol Phys. 2010;78:72–8. doi: 10.1016/j.ijrobp.2009.07.1729. [DOI] [PubMed] [Google Scholar]
- 26.Nalla AK, Asuthkar S, Bhoopathi P, Gujrati M, Dinh DH, Rao JS. Suppression of uPAR retards radiation-induced invasion and migration mediated by integrin beta1/FAK signaling in medulloblastoma. PLoS One. 2010;5:e13006. doi: 10.1371/journal.pone.0013006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts WG, Ung E, Whalen P, Cooper B, Hulford C, Autry C, et al. Antitumor activity and pharmacology of a selective focal adhesion kinase inhibitor, PF-562,271. Cancer Res. 2008;68:1935–44. doi: 10.1158/0008-5472.CAN-07-5155. [DOI] [PubMed] [Google Scholar]
- 28.Mitra AK, Sawada K, Tiwari P, Mui K, Gwin K, Lengyel E. Ligand-independent activation of c-Met by fibronectin and alpha(5)beta(1)-integrin regulates ovarian cancer invasion and metastasis. Oncogene. 2011;30:1566–76. doi: 10.1038/onc.2010.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hui AY, Meens JA, Schick C, Organ SL, Qiao H, Tremblay EA, et al. Src and FAK mediate cell-matrix adhesion-dependent activation of Met during transformation of breast epithelial cells. J Cell Biochem. 2009;107:1168–81. doi: 10.1002/jcb.22219. [DOI] [PubMed] [Google Scholar]
- 30.Garcia S, Dales JP, Jacquemier J, Charafe-Jauffret E, Birnbaum D, Andrac-Meyer L, et al. c-Met overexpression in inflammatory breast carcinomas: automated quantification on tissue microarrays. Br J Cancer. 2007;96:329–35. doi: 10.1038/sj.bjc.6603569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cary LA, Han DC, Polte TR, Hanks SK, Guan JL. Identification of p130Cas as a mediator of focal adhesion kinase-promoted cell migration. J Cell Biol. 1998;140:211–21. doi: 10.1083/jcb.140.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schaller MD, Parsons JT. pp125FAK-dependent tyrosine phosphorylation of paxillin creates a high-affinity binding site for Crk. Mol Cell Biol. 1995;15:2635–45. doi: 10.1128/mcb.15.5.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hilger RA, Scheulen ME, Strumberg D. The Ras-Raf-MEK-ERK pathway in the treatment of cancer. Onkologie. 2002;25:511–8. doi: 10.1159/000068621. [DOI] [PubMed] [Google Scholar]
- 34.Koon EC, Ma PC, Salgia R, Welch WR, Christensen JG, Berkowitz RS, et al. Effect of a c-Met-specific, ATP-competitive small-molecule inhibitor SU11274 on human ovarian carcinoma cell growth, motility, and invasion. Int J Gynecol Cancer. 2008;18:976–84. doi: 10.1111/j.1525-1438.2007.01135.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.